Determination of the Critical Voltage for the Observation of Uncoated Wood Samples in Electron Microscopy
Abstract
:1. Introduction
2. Material and Methodology
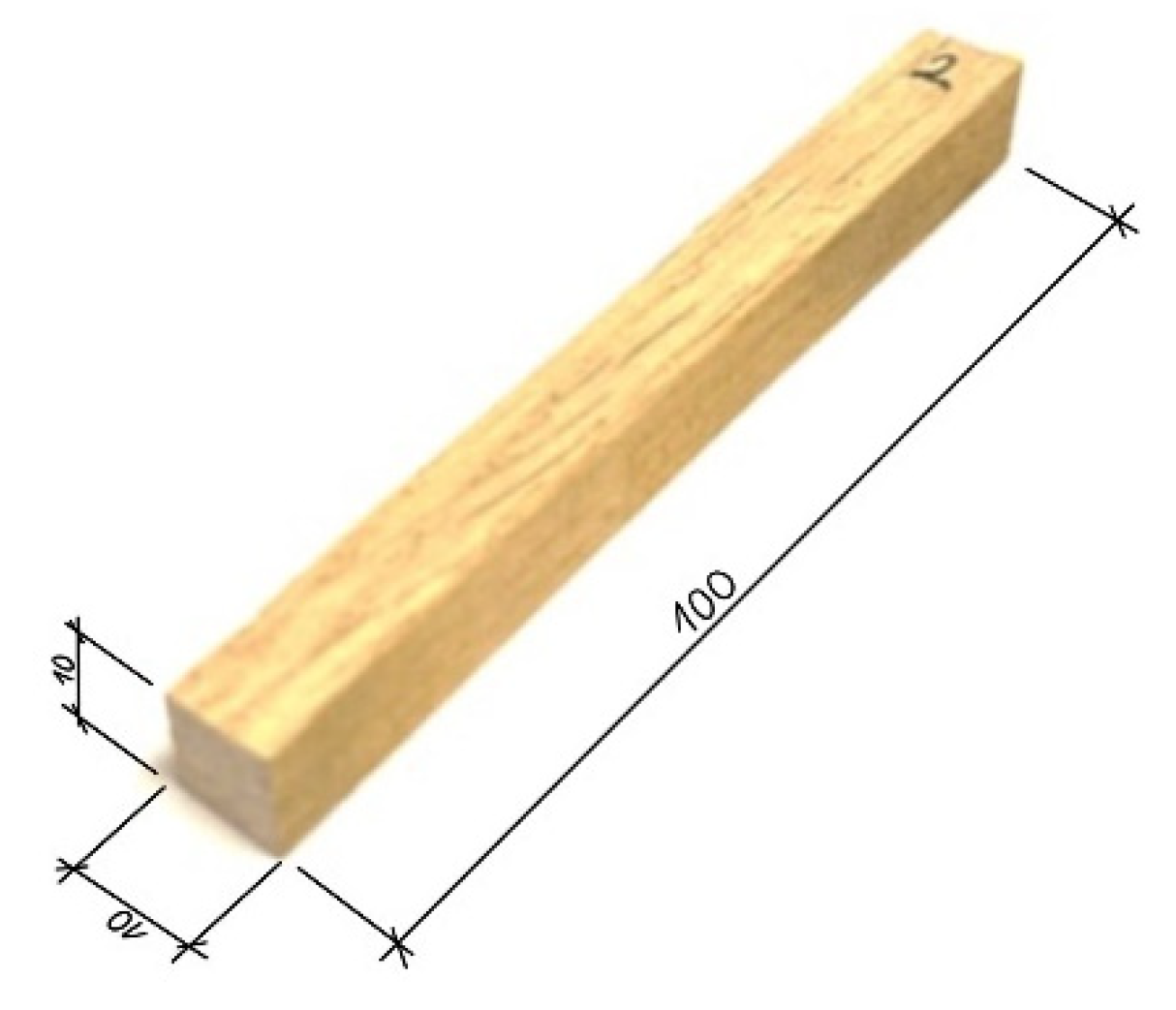
2.1. Samples
2.2. Electron Microscopy
2.3. Process/Method
3. Results and Discussion
- ✓
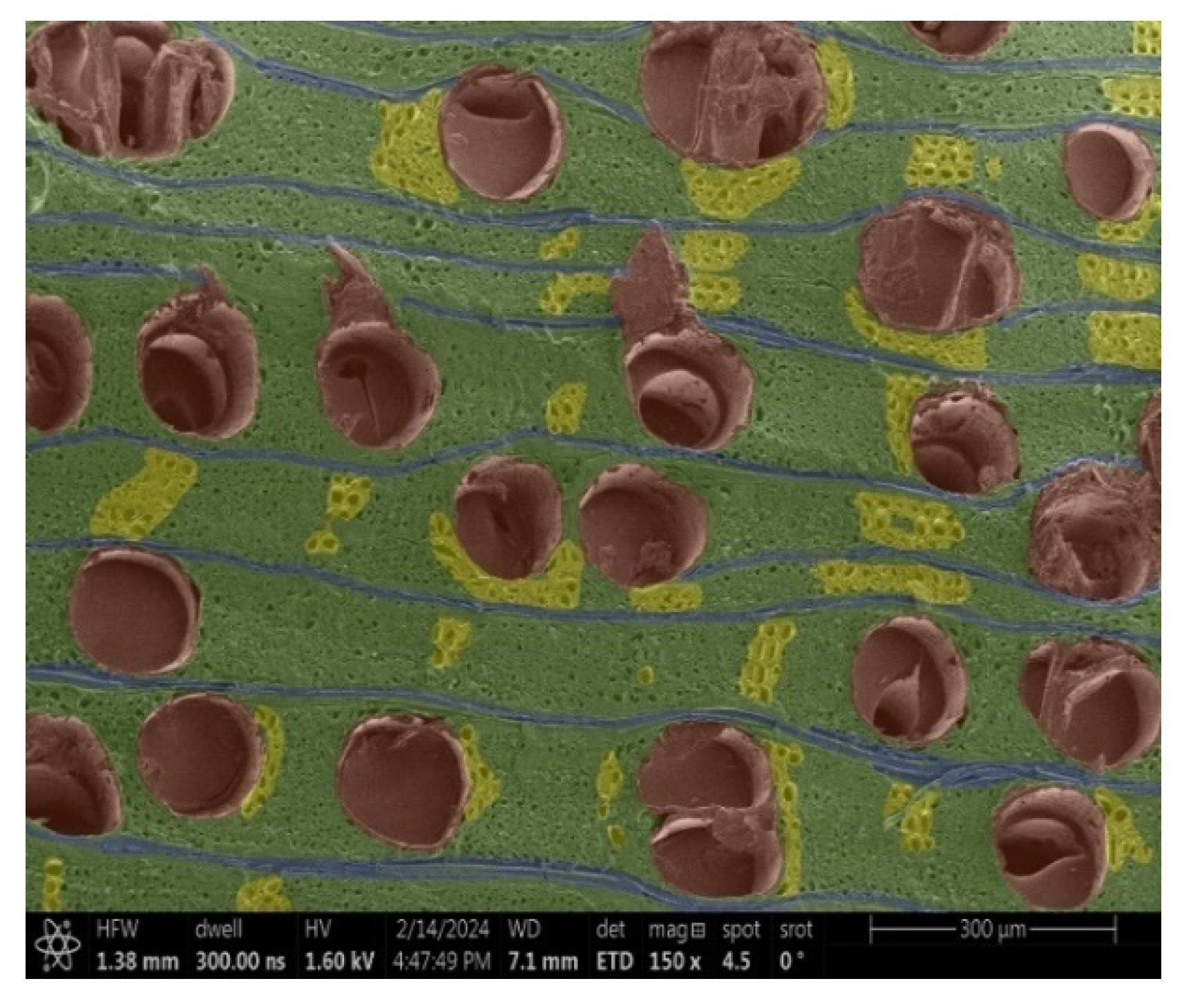
- Spruce and Thermowood™:
- Structure: Uniform tracheids that form a homogeneous network.
- Voltage: Due to the uniform structure, a uniform distribution of electric charge is assumed, but practical tests have shown that this may not be a decisive factor.
- ✓
- Acacia, Oak, Garapa, Ash, Massaranduba and Merbau:
- Structure: A combination of large vessels and smaller vessels surrounded by libriform fibers and parenchymal cells.
- Voltage: The heterogeneous structure can cause uneven charging, which can lead to image artifacts during observation.
- ✓
- Maple:
- Structure: Smaller and more uniform vessels compared to other woods.
- Voltage: A more uniform structure provides more stable electrical properties when observed, but again a direct relationship between structure and charge was not found.
- ✓
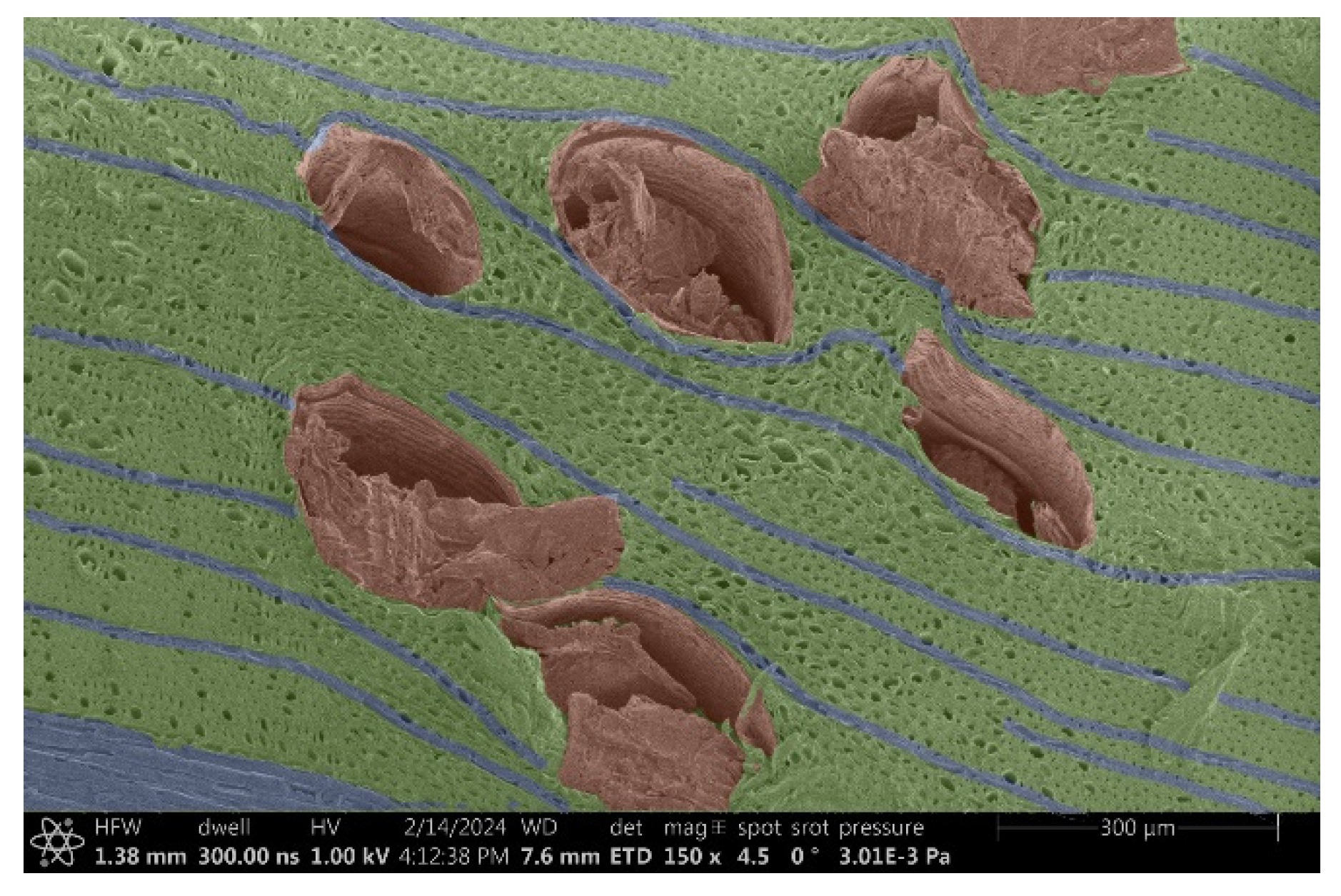
- Ipé:
- Structure: Extremely smooth, with minimal visible veins.
- Voltage: It was hypothesized that the smooth structure might minimize charging, but the results were inconclusive.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sulaiman, M.S.; Wahab, R.; Mokhtar, N.; Edin, T.; Razali, S.M.; Ghani, R.S.M. Scanning Electron MicroscopyStudy of the Effectiveness Oil Heat Treatment on 10-years old teak wood in ground contact test. Borneo J. Sci. Technol. 2021, 3, 24–32. [Google Scholar] [CrossRef]
- Adobes-Vidal, M.; Frey, M.; Keplinger, T. Atomic force microscopy imaging of delignified secondary cell walls in liquid conditions facilitates interpretation of wood ultrastructure. J. Struct. Biol. 2020, 211, 107532. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.; Abdullah, A. Scanning electron microscopy (SEM): A review. In Proceedings of the 2018 International Conference on Hydraulics and Pneumatics—HERVEX, Băile Govora, Romania, 8–10 November 2018; Volume 2018, pp. 7–9. [Google Scholar]
- Wang, C.; Wang, N.; Liu, S.; Zhang, H.; Zhi, Z. Investigation of microfibril angle of flax fibers using X-ray diffraction and scanning electron microscopy. J. Nat. Fibers 2020, 17, 1001–1010. [Google Scholar] [CrossRef]
- Toumpanaki, E.; Shah, D.U.; Eichhorn, S.J. Beyond what meets the eye: Imaging and imagining wood mechanical–structural properties. Adv. Mater. 2021, 33, 2001613. [Google Scholar] [CrossRef]
- Panshin, A.J.; Zeeuw, C. Textbook of Wood Technology: Structure, Identification, Properties, and Uses of the Commercial Woods of the United States and Canada, 4th ed.; McGraw-Hill Series in Forest Resources; McGraw-Hill: New York, NY, USA, 1980; 736p, ISBN 978-0-07-048441-2. [Google Scholar]
- Calovi, M.; Rossi, S. Impact of high concentrations of cellulose fibers on the morphology, durability and protective properties of wood paint. Coatings 2023, 13, 721. [Google Scholar] [CrossRef]
- Xu, X.; Garemark, J.; Ram, F.; Wang, Z.; Li, Y. Metallic Wood through Deep-Cell-Wall Metallization: Synthesis and Applications. ACS Appl. Mater. Interfaces 2024, 16, 22433–22442. [Google Scholar] [CrossRef]
- Lu, X.; Lagnoni, M.; Bertei, A.; Das, S.; Owen, R.E.; Li, Q.; O’Regan, K.; Wade, A.; Finegan, D.P.; Kendrick, E.; et al. Multiscale dynamics of charging and plating in graphite electrodes coupling operando microscopy and phase-field modelling. Nat. Commun. 2023, 14, 5127. [Google Scholar] [CrossRef]
- Kwon, H.M.; Kim, N.H.; Hong, S.J.; Sim, W.H.; Lee, M.; Son, S.; Bae, K.Y.; Kim, J.Y.; Youn, D.H.; Kim, Y.S.; et al. Uniform Li-metal growth on renewable lignin with lithiophilic functional groups derived from wood for high-performance Li-metal batteries. Surf. Interfaces 2024, 44, 103643. [Google Scholar] [CrossRef]
- Hasan, K.F.; Champramary, S.; Al Hasan, K.N.; Indic, B.; Ahmed, T.; Pervez, M.N.; Horváth, P.G.; Bak, M.; Sándor, B.; Hofmann, T.; et al. Eco-friendly production of cellulosic fibers from Scots pine wood and sustainable nanosilver modification: A path toward sustainability. Results Eng. 2023, 19, 101244. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, W.; Zhang, Y.; Shi, J.; Shan, S.; Cai, L. Exploring wood micromechanical structure: Impact of microfibril angle and crystallinity on cell wall strength. J. Build. Eng. 2024, 90, 109452. [Google Scholar] [CrossRef]
- Thiel, B.L.; Toth, M. Secondary electron contrast in low-vacuum/environmental scanning electron microscopy of dielectrics. J. Appl. Phys. 2005, 97, 051101. [Google Scholar] [CrossRef]
- Feria-Reyes, R.; Ramírez-Cruz, S.O.; Ruiz-Aquino, F.; Robledo-Taboada, L.H.; Sánchez-Medina, M.A.; Mijangos-Ricárdez, O.F.; Gabriel-Parra, R.; Suárez-Mota, M.E.; Puc-Kauil, R.; Porcallo-Vargas, J. Pine bark as a potential source of condensed tannin: Analysis through fourier transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM), and energy dispersive X-ray (EDX). Forests 2023, 14, 1433. [Google Scholar] [CrossRef]
- Kamdem, D.P.; Zhang, J.; Freeman, M.H. The effect of post-steaming on copper naphthenate-treated southern pine. Wood Fiber Sci. 1998, 56, 210–217. [Google Scholar]
- Sader, K.; Stopps, M.; Lesley, J.; Calder, P.; Rosenthal, B. Cryomicroscopy of radiation sensitive specimens on unmodified graphene sheets: Reduction of electron-optical effects of charging. J. Struct. Biol. 2013, 183, 531–536. [Google Scholar] [CrossRef]
- Kitin, P.; Hermanson, J.C.; Abe, H.; Nakaba, S.; Funada, R. Light microscopy of wood using sanded surface instead of slides. IAWA J. 2021, 42, 322–335. [Google Scholar] [CrossRef]
- Akahori, H.; Yoshida, H.; Amakawa, Y.; Takahashi, I.; Yamada, M. Studies on the SEM examination of uncoated non-conductive specimens prepared by plasma-ion shower method. J. Electron. Microsc. 1997, 46, 457–466. [Google Scholar] [CrossRef]
- Bozzola, J.J.; dee Russell, L. Electron Microscopy: Principles and Techniques for Biologists, 2nd ed.; Jones and Bartlett: Sudbury, MA, USA, 1999; 670p, ISBN 978-0-7637-0192-5. [Google Scholar]
- Meyer, E.; Hans, J.H.; Bennewitz, R. Scanning Probe Microscopy: The Lab on a Tip; Advanced Texts in Physics; Springer: Berlin, Germany; New York, NY, USA, 2004; 210p, ISBN 978-3-540-43180-0. [Google Scholar]
- Malac, M.; Hettler, S.; Hayashida, M.; Kano, E.; Egerton, R.F.; Beleggia, M. Phase plates in the transmission electron microscope: Operating principles and applications. Microscopy 2021, 70, 75–115. [Google Scholar] [CrossRef]
- Reimer, L.; Kohl, H. Transmission Electron Microscopy: Physics of Image Formation; Springer: New York, NY, USA, 2008; 590p. [Google Scholar] [CrossRef]
- Kaegi, R.; Holzer, L. Transfer of a single particle for continued ESEM and TEM analysis. Atmosph. Environ. 2003, 37, 4353–4359. [Google Scholar] [CrossRef]
- Merela, M.; Thaler, N.; Balzano, A.; Plavčak, D. Optimal Surface Preparation for Wood Anatomy Research of Invasive Species by Scanning Electron Microscopy. Wood Ind./Drv. Ind. 2020, 71, 117–127. [Google Scholar] [CrossRef]
- Stokes, D.J.; Mugnier, J.Y.; Clarke, C.J. Static and dynamic experiments in cryo-electron microscopy: Comparative observations using high-vacuum, low-voltage and low-vacuum SEM. J. Microsc. 2004, 213, 198–204. [Google Scholar] [CrossRef]
- Frank, L.; Mikmeková, E.; Lejeune, M. Treatment of surfaces with low-energy electrons. Appl. Surf. Sci. 2017, 407, 105–108. [Google Scholar] [CrossRef]
- Zobačová, J.; Frank, L. Specimen charging and detection of signal from non-conductors in a cathode lens-equipped scanning electron microscope. Scanning J. Scanning Microsc. 2003, 25, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Environmental Scanning Electron Microscope|Quattro ESEM. Thermo Fisher Scientific—CZ. Available online: https://www.thermofisher.com/cz/en/home/electron-microscopy/products/scanning-electron-microscopes/quattro-esem.html.html#em-contact-form (accessed on 27 July 2024).
- Mamoňová, M. Elektrónová Mikroskopia a Štúdium Drevných Štruktúr (Electron Microscopy and the Study of Wood Structures), 1st ed.; Technical University in Zvolen: Zvolen, Slovakia, 2018; 94p, ISBN 978-80-228-3150-5. [Google Scholar]
- Tokareva, E.N.; Fardim, P.; Pranovich, A.V.; Fagerholm, H.P.; Daniel, G.; Holmbom, B. Imaging of wood tissue by ToF-SIMS: Critical evaluation and development of sample preparation techniques. Appl. Surf. Sci. 2007, 253, 7569–7577. [Google Scholar] [CrossRef]
- Jansen, S.; Kitin, P.; De Pauw, H.; Idris, M.; Beeckman, H.; Smets, E. Preparation of wood specimens for transmitted light microscopy and scanning electron microscopy. Belg. J. Bot. 1998, 131, 41–49. [Google Scholar]
- Balzano, A.; Merela, M.; Čufar, K. Scanning Electron Microscopy Protocol for Studying Anatomy of Highly Degraded Waterlogged Archaeological Wood. Forests 2022, 13, 161. [Google Scholar] [CrossRef]
- Shan, X.; Li, L.; Wang, L.; Chen, Z.; Wang, X. Regulation of wood porous structure and construction of reduced graphene oxide@ wood derived carbon conducting collector. Ind. Crops Prod. 2024, 219, 119069. [Google Scholar] [CrossRef]
- de Silveira, G.; Forsberg, P.; Conners, T.E. Scanning electron microscopy: A tool for the analysis of wood pulp fibers and paper. In Surface Analysis of Paper; CRC Press: Boca Raton, FL, USA, 2020; pp. 41–71. ISBN 9780429279997. [Google Scholar] [CrossRef]
- Wentzel, M.; Koddenberg, T.; Militz, H. Anatomical characteristics of thermally modified Eucalyptus nitens wood in an open and closed reactor system. Wood Mater. Sci. Eng. 2019, 15, 223–228. [Google Scholar] [CrossRef]
- Setyawan, E.; Napitupulu, R.; Djiwo, S.; Djoko, P.; Nugroho, A. A Preliminary Study of Scanning Electron Microscopy (SEM) for Characterization of The Wood Pellet Process of Sengon Wood (Albizia chinensis). In Proceedings of the 2nd Universitas Kuningan International Conference on System Engineering, and Technology, UNISET 2021, Kuningan, Indonesia, 2 December 2021. [Google Scholar] [CrossRef]
- Chen, C.; Kuang, Y.; Zhu, S.; Burgert, I.; Keplinger, T.; Gong, A.; Li, T.; Berglund, L.; Eichhorn, S.J.; Hu, L. Structure–property–function relationships of natural and engineered wood. Nat. Rev. Mater. 2020, 5, 642–666. [Google Scholar] [CrossRef]
- Shan, X.; Wang, L.; Wu, J.; Wang, X. Preparation of Wood-based Graphene Material and Its Three-dimensional Electrical Conductivity. J. Northeast For. Univ. 2021, 50, 123–130. [Google Scholar] [CrossRef]
- van Huis, M.A.; Friedrich, H. Electron Microscopy Techniques. In Nanoparticles; De Mello Donega, C., Ed.; Springer: Cham, Switzerland, 2024. [Google Scholar] [CrossRef]
- Müller, A.; Schmidt, D.; Albrecht, J.P.; Rieckert, L.; Otto, M.; Galicia Garcia, L.E.; Fabig, G.; Solimena, M.; Weigert, M. Modular segmentation, spatial analysis and visualization of volume electron microscopy datasets. Nat. Protoc. 2024, 19, 1436–1466. [Google Scholar] [CrossRef]





| Sample Number | Type of Wood | Weight Before Drying [g] | Weight After 4 h [g] | Weight After 6 h [g] | Weight of Water [g] | Original Humidity [%] |
|---|---|---|---|---|---|---|
| 1 | Acacia | 7.910 | 7.420 | 7.420 | 0.490 | 6.60 |
| 2 | Oak | 6.980 | 6.390 | 6.390 | 0.590 | 9.23 |
| 3 | Maple | 7.715 | 7.035 | 7.035 | 0.680 | 9.67 |
| 4 | Ash | 8.770 | 8.005 | 8.005 | 0.765 | 9.56 |
| 5 | Spruce | 5.015 | 4.540 | 4.540 | 0.475 | 10.46 |
| 6 | Thermowood™ | 5.050 | 4.820 | 4.820 | 0.230 | 4.77 |
| 7 | Garapa | 8.725 | 8.015 | 8.015 | 0.710 | 8.86 |
| 8 | Massaranduba | 12.210 | 11.165 | 11.165 | 1.045 | 9.36 |
| 9 | Merbau | 8.615 | 7.915 | 7.915 | 0.700 | 8.84 |
| 10 | Ipé | 14.370 | 13.210 | 13.210 | 1.160 | 8.78 |
| Sample Number | Type of Wood | Width [cm] | Height [cm] | Length [cm] | Volume [cm3] | Weight After 6 h [g] | Density [g/cm3] |
|---|---|---|---|---|---|---|---|
| 1 | Acacia | 0.943 | 1.01 | 10.123 | 9.641 | 7.420 | 0.770 |
| 2 | Oak | 1.064 | 1.09 | 10.118 | 11.734 | 6.390 | 0.545 |
| 3 | Maple | 1.031 | 1.072 | 9.874 | 10.913 | 7.035 | 0.645 |
| 4 | Ash | 1.01 | 1.078 | 10.142 | 11.042 | 8.005 | 0.725 |
| 5 | Spruce | 1.027 | 1.197 | 10.14 | 12.465 | 4.540 | 0.364 |
| 6 | Thermowood™ | 1.051 | 1.079 | 9.61 | 10.898 | 4.820 | 0.442 |
| 7 | Garapa | 1.073 | 1.039 | 9.875 | 11.009 | 8.015 | 0.728 |
| 8 | Massaranduba | 1.082 | 1.029 | 9.764 | 10.871 | 11.165 | 1.027 |
| 9 | Merbau | 1.04 | 1.019 | 10.151 | 10.758 | 7.915 | 0.736 |
| 10 | Ipé | 1.199 | 1.041 | 9.718 | 12.130 | 13.210 | 1.089 |
| Number | Wood | Fiber Direction | Voltage Range |
|---|---|---|---|
| 1 | Acacia | parallel to the fibers | 0.5–1.4 kV |
| perpendicular to the fibers | 0.5–1.4 kV | ||
| 2 | Oak | parallel to the fibers | 0.5–1.5 kV |
| perpendicular to the fibers | 0.5–1.6 kV | ||
| 3 | Maple | parallel to the fibers | 0.5–1.6 kV |
| perpendicular to the fibers | 0.5–1.5 kV | ||
| 4 | Ash | parallel to the fibers | 0.5–1.4 kV |
| perpendicular to the fibers | 0.5–1.6 kV | ||
| 5 | Spruce | parallel to the fibers | 0.5–1.5 kV |
| perpendicular to the fibers | 0.5–1.4 kV | ||
| 6 | Thermowood™ | parallel to the fibers | 0.5–1.3 kV |
| perpendicular to the fibers | 0.5–1.4 kV | ||
| 7 | Garapa | parallel to the fibers | 0.5–1.7 kV |
| perpendicular to the fibers | 0.5–1.6 kV | ||
| 8 | Massaranduba | parallel to the fibers | 0.5–1.6 kV |
| perpendicular to the fibers | 0.5–1.3 kV | ||
| 9 | Merbau | parallel to the fibers | 0.5–1.3 kV |
| perpendicular to the fibers | 0.5–1.5 kV | ||
| 10 | Ipé | parallel to the fibers | 0.5–0.7 kV |
| perpendicular to the fibers | 0.5–1.2 kV |
| Voltage [kV] | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Type of Wood | Density [g/cm3] | 0.5 | 1.0 | 1.1 | 1.2 | 1.3 | 1.4 | 1.5 | 1.6 | 1.7 | 1.8 |
| Spruce | 0.364 | x | x | x | x | x | x | x | Yes | ||
| Spruce cross | 0.364 | x | x | x | x | x | x | Yes | |||
| Thermowood™ | 0.442 | x | x | x | x | x | Yes | ||||
| Thermowood™ cross | 0.442 | x | x | x | x | x | x | Yes | |||
| Oak | 0.545 | x | x | x | x | x | x | x | Yes | ||
| Oak cross | 0.545 | x | x | x | x | x | x | x | x | Yes | |
| Maple | 0.645 | x | x | x | x | x | x | x | x | Yes | |
| Maple cross | 0.645 | x | x | x | x | x | x | x | Yes | ||
| Ash | 0.725 | x | x | x | x | x | x | Yes | |||
| Ash cross | 0.725 | x | x | x | x | x | x | x | x | Yes | |
| Garapa | 0.728 | x | x | x | x | x | x | x | x | x | Yes |
| Garapa cross | 0.728 | x | x | x | x | x | x | x | x | Yes | |
| Merbau | 0.736 | x | x | x | x | x | Yes | ||||
| Merbau cross | 0.736 | x | x | x | x | x | x | x | Yes | ||
| Acacia | 0.770 | x | x | x | x | x | x | Yes | |||
| Acacia cross | 0.770 | x | x | x | x | x | x | Yes | |||
| Massaranduba | 1.027 | x | x | x | x | x | x | x | x | Yes | |
| Massaranduba cross | 1.027 | x | x | x | x | x | Yes | ||||
| Ipé | 1.089 | x | Yes | ||||||||
| Ipé cross | 1.089 | x | x | x | x | Yes | |||||
| Type of Wood | Cross | Axial |
|---|---|---|
| Acacia |  | 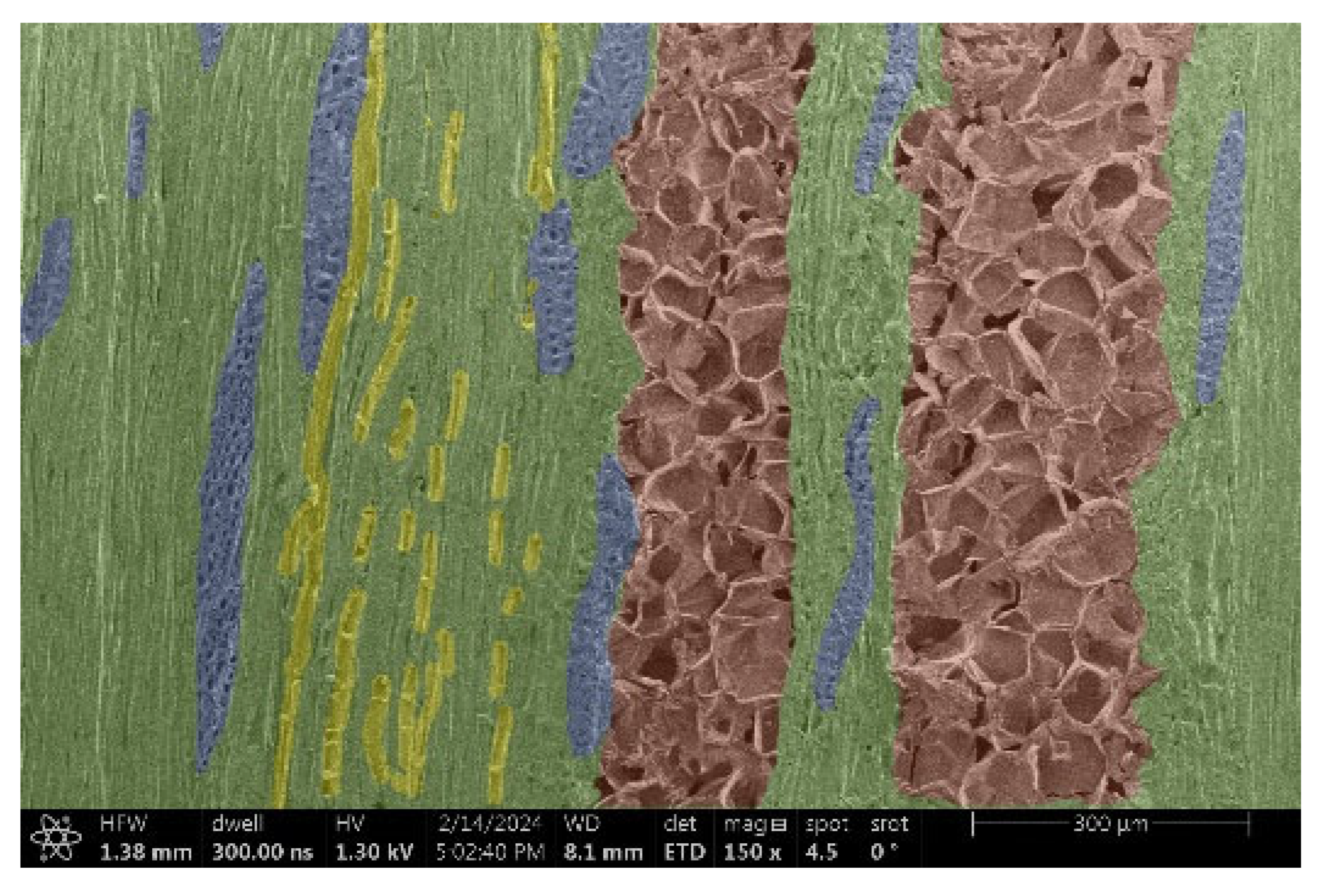 |
| Oak | 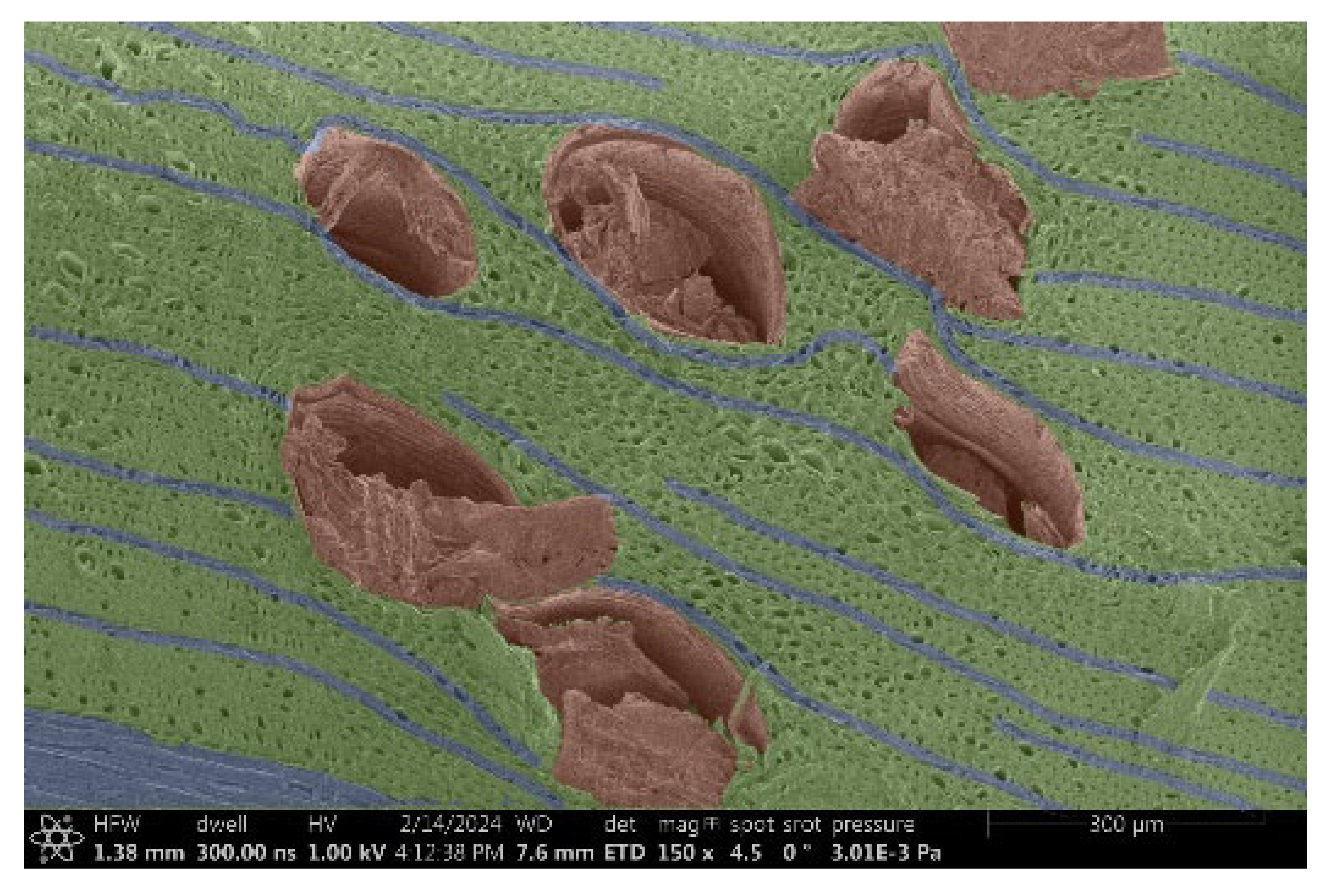 |  |
| Maple |  | 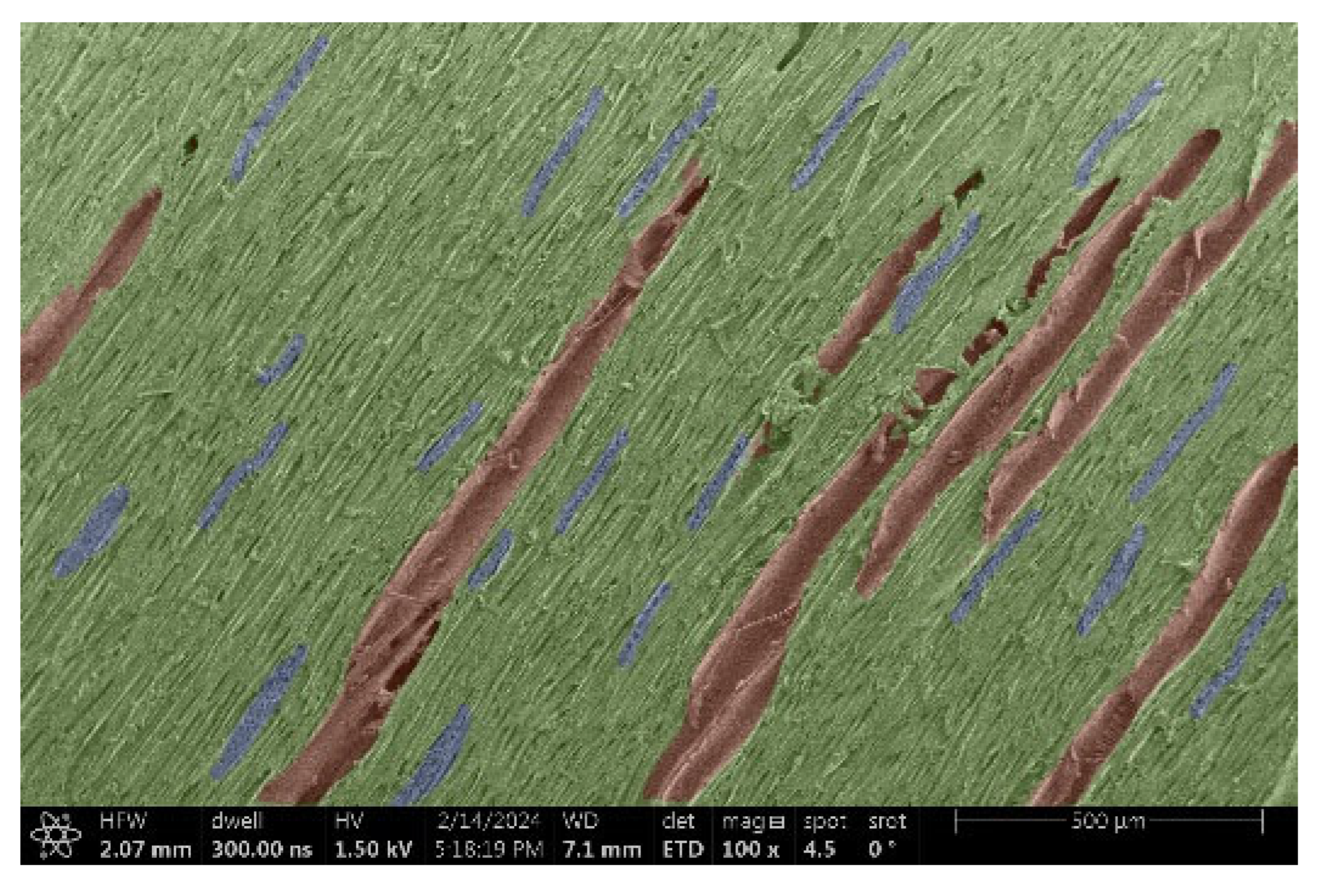 |
| Ash |  | 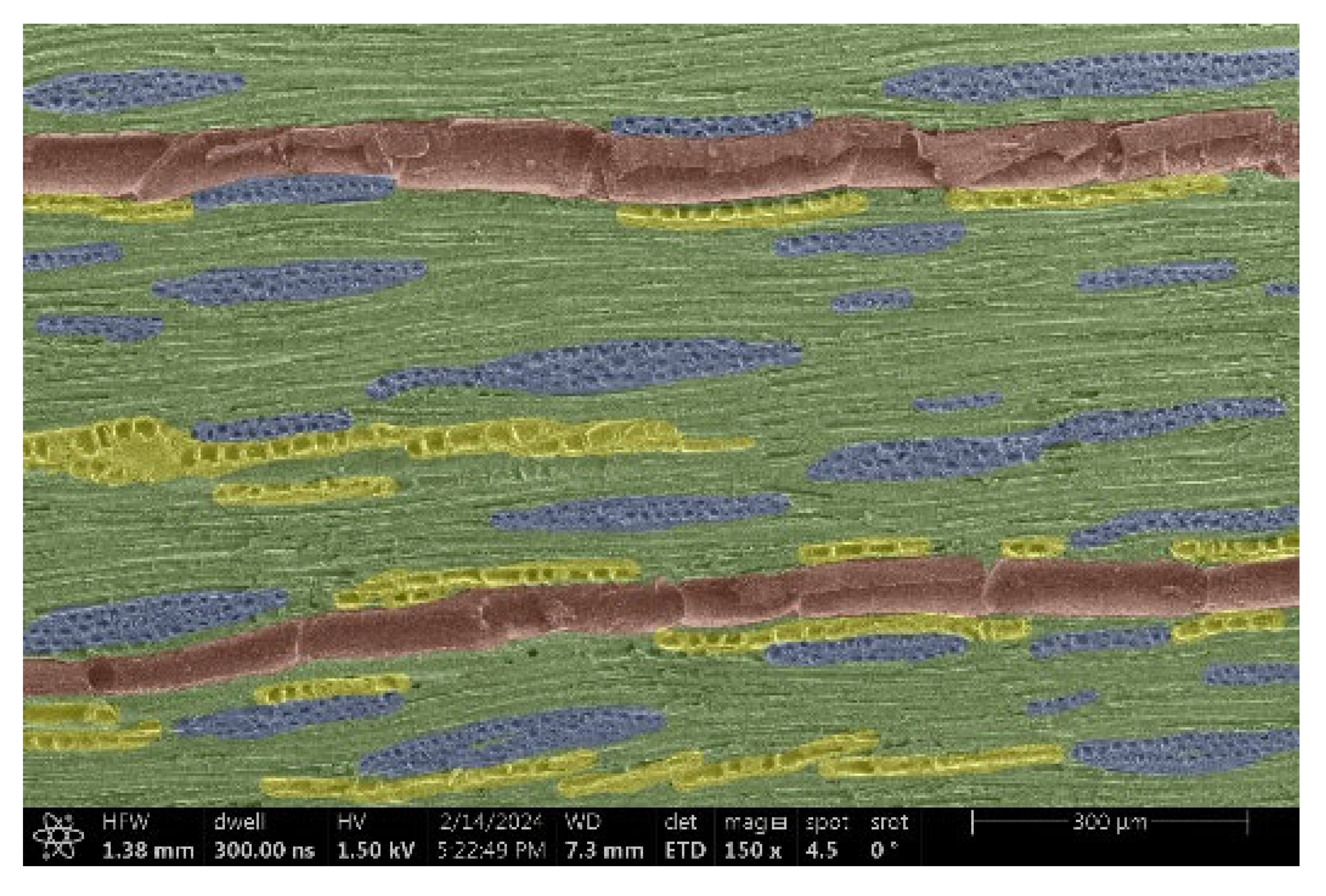 |
| Spruce | 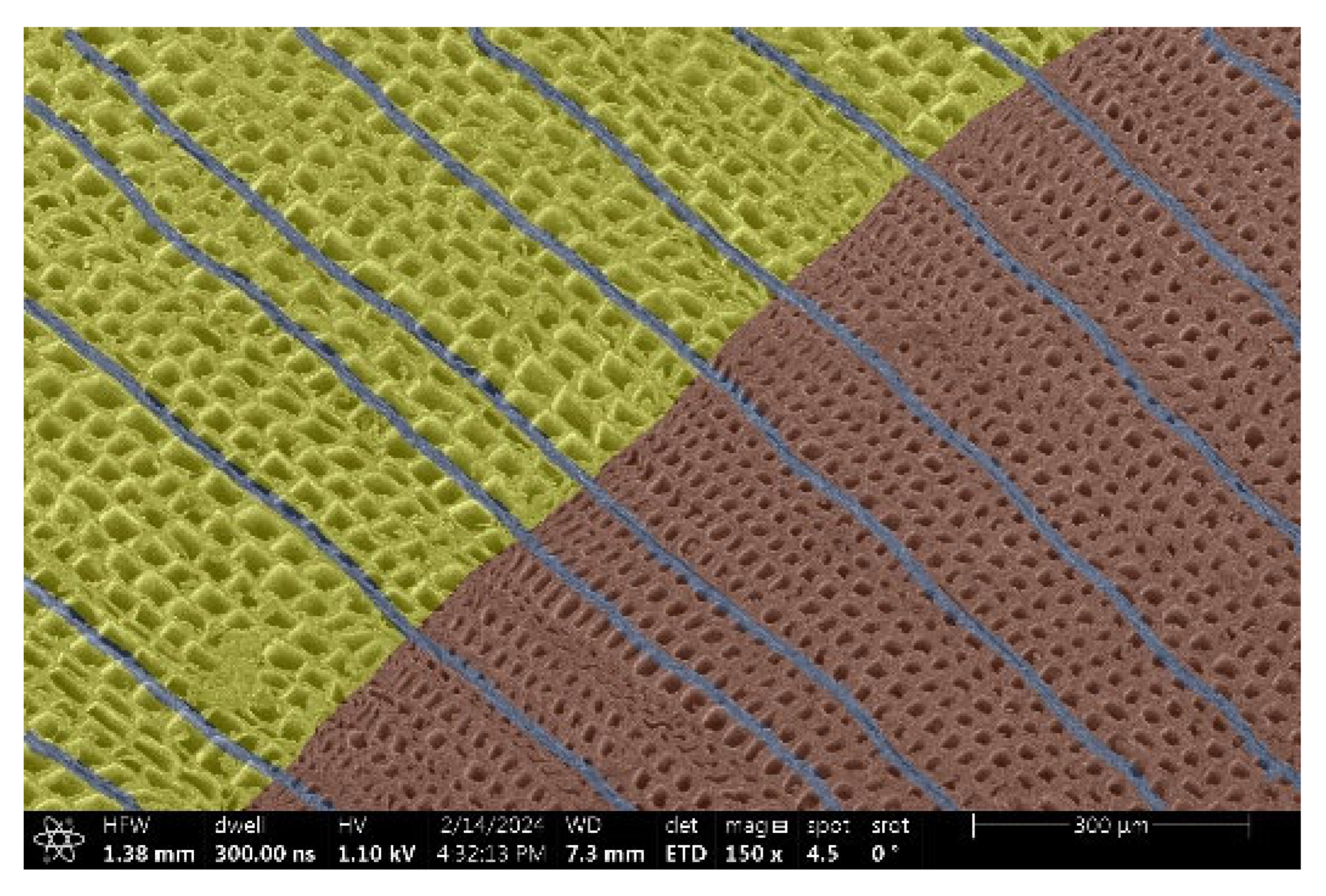 |  |
| Thermowood™ | 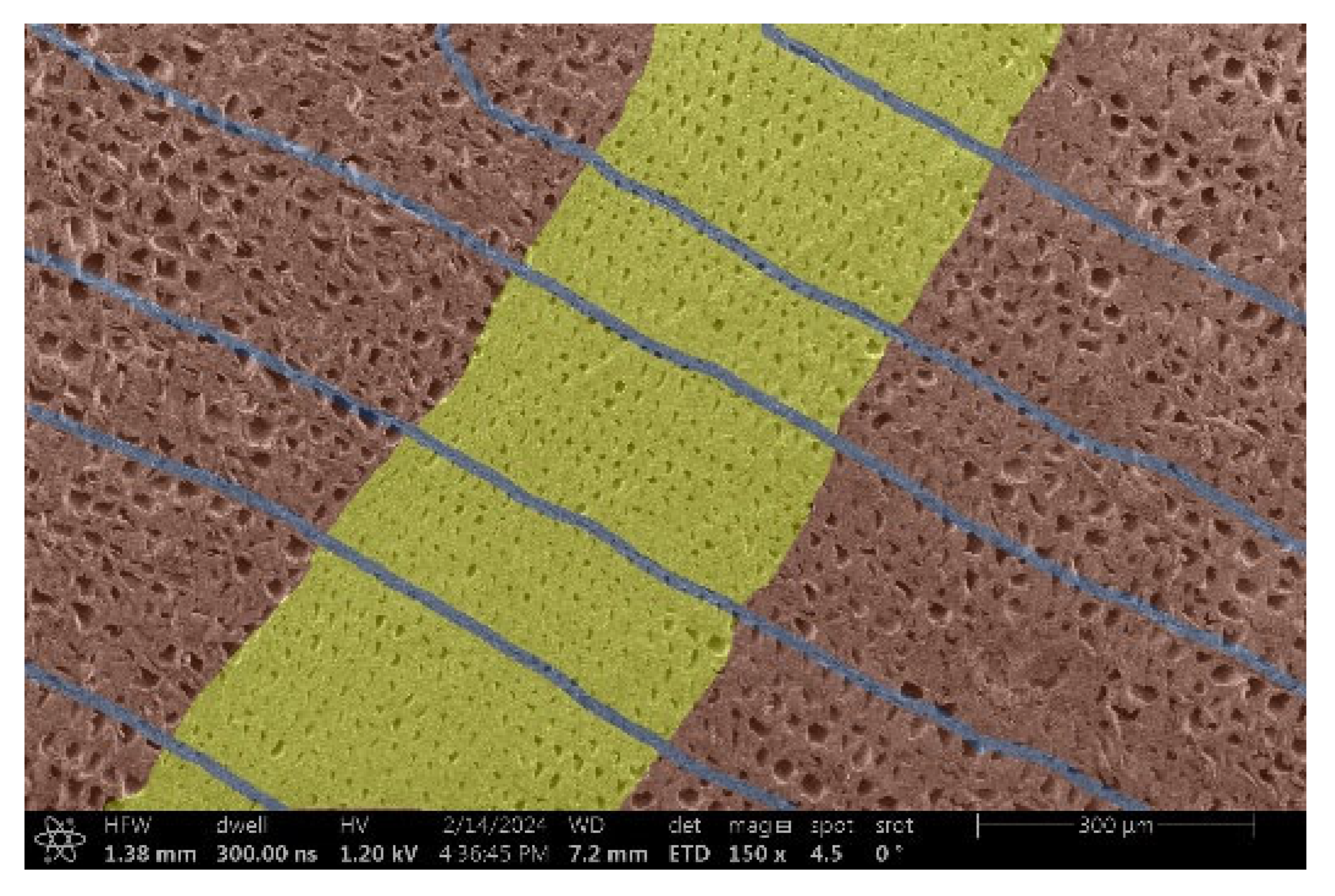 | 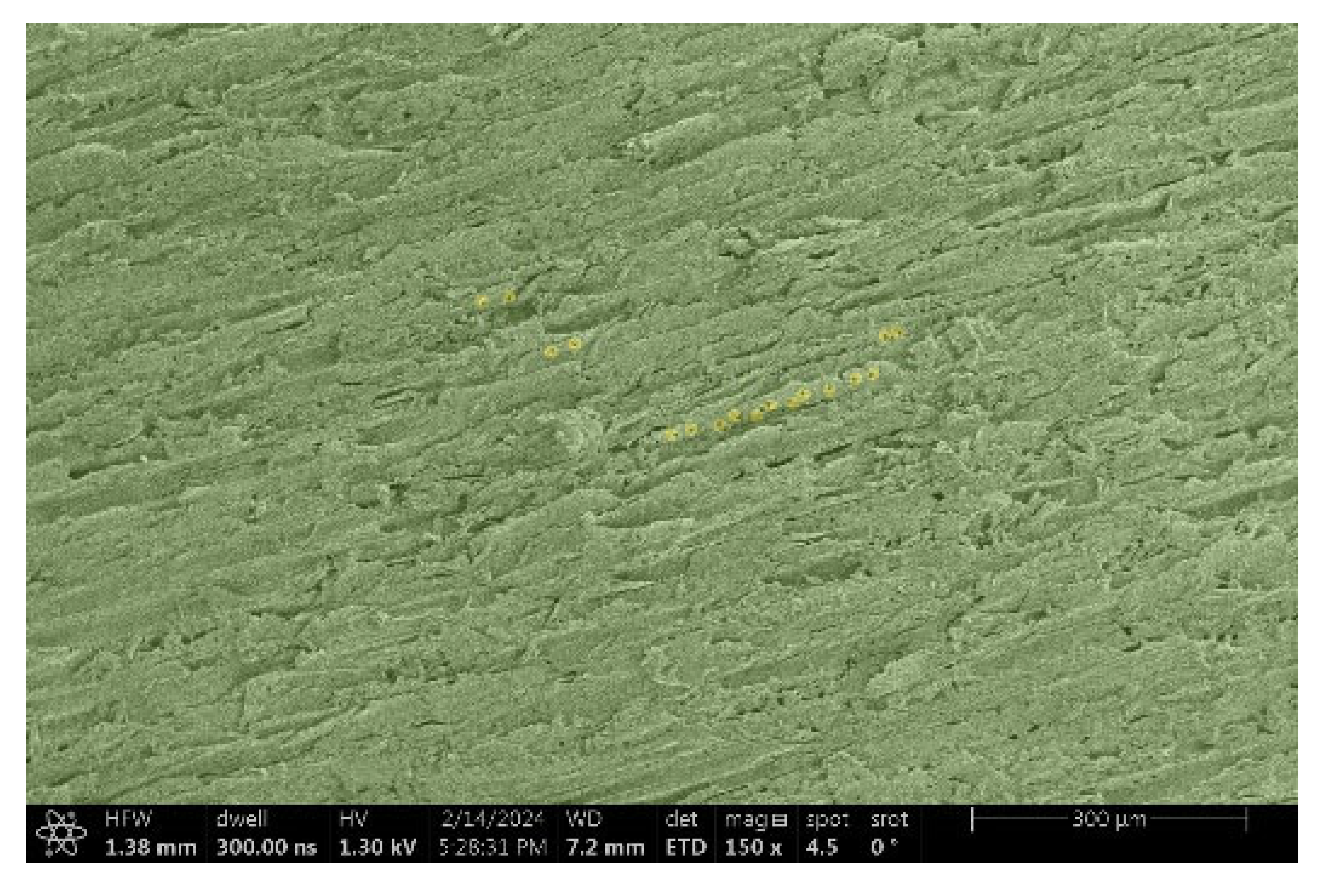 |
| Garapa |  |  |
| Massaranduba |  |  |
| Merbau |  | 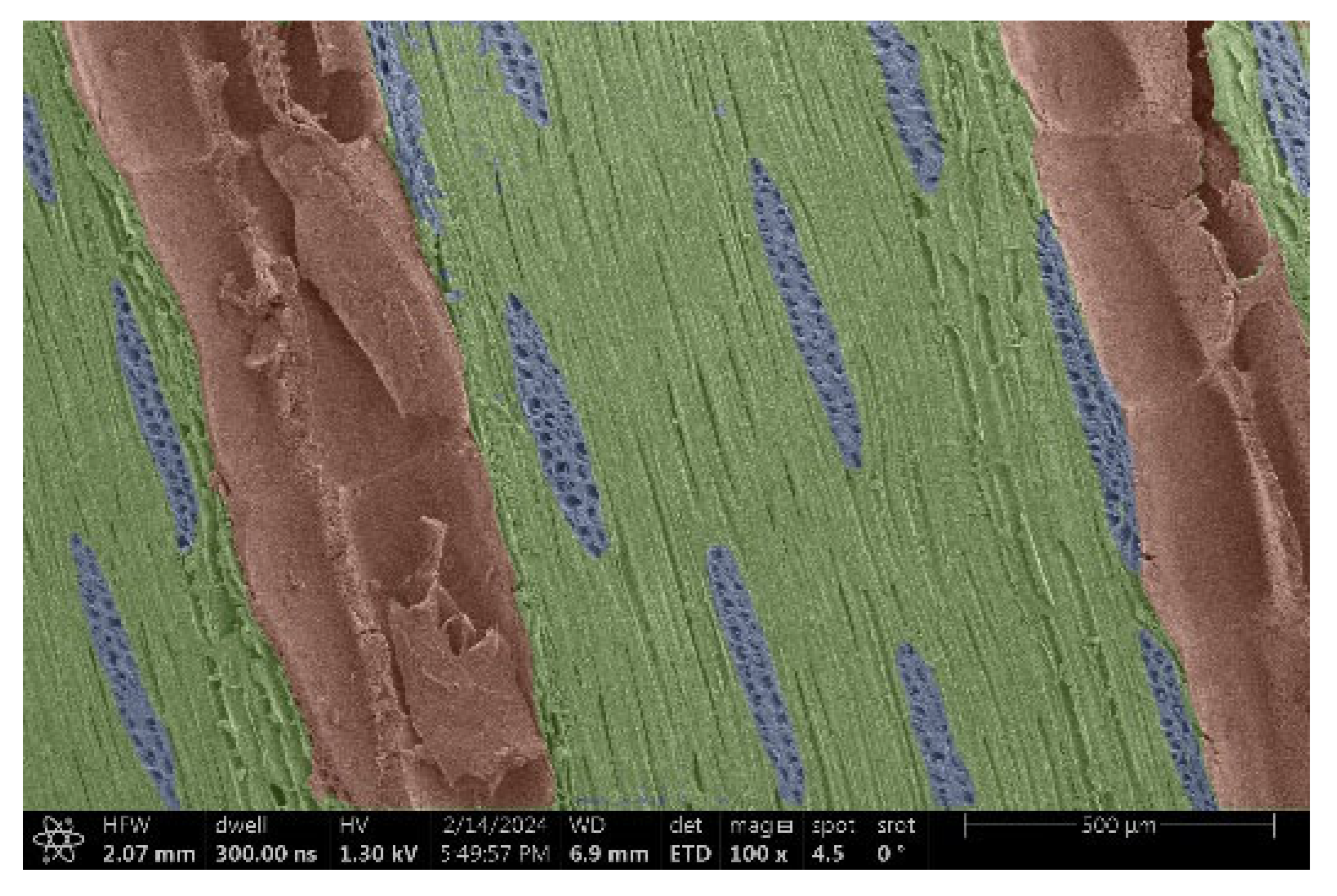 |
| Ipé | 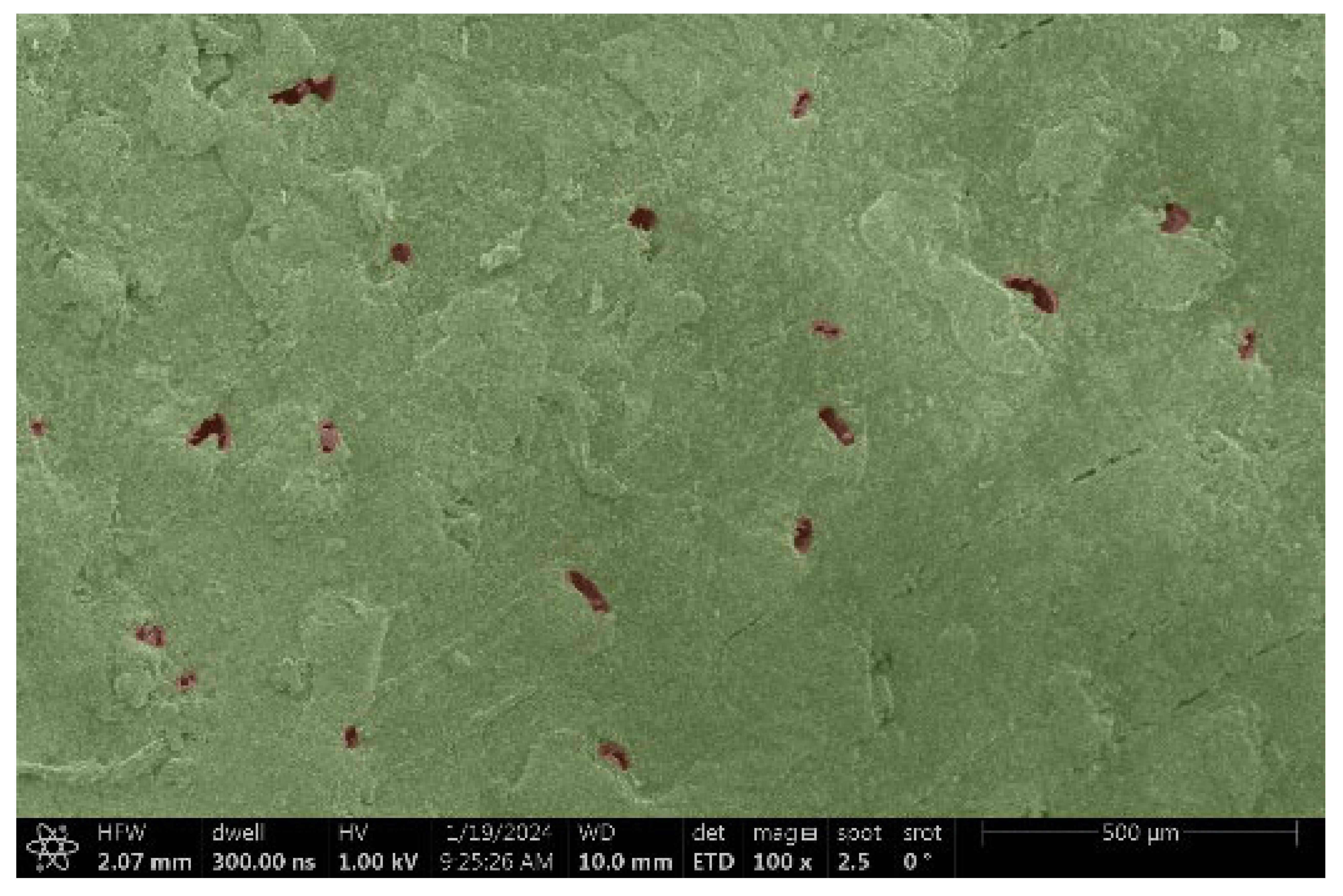 |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kvietková, M.S.; Dvořák, O.; Kubista, K.; Těhníková, K.; Lin, C.-F.; Jones, D. Determination of the Critical Voltage for the Observation of Uncoated Wood Samples in Electron Microscopy. Materials 2025, 18, 236. https://doi.org/10.3390/ma18020236
Kvietková MS, Dvořák O, Kubista K, Těhníková K, Lin C-F, Jones D. Determination of the Critical Voltage for the Observation of Uncoated Wood Samples in Electron Microscopy. Materials. 2025; 18(2):236. https://doi.org/10.3390/ma18020236
Chicago/Turabian StyleKvietková, Monika Sarvašová, Ondřej Dvořák, Kryštof Kubista, Kristýna Těhníková, Chia-Feng Lin, and Dennis Jones. 2025. "Determination of the Critical Voltage for the Observation of Uncoated Wood Samples in Electron Microscopy" Materials 18, no. 2: 236. https://doi.org/10.3390/ma18020236
APA StyleKvietková, M. S., Dvořák, O., Kubista, K., Těhníková, K., Lin, C.-F., & Jones, D. (2025). Determination of the Critical Voltage for the Observation of Uncoated Wood Samples in Electron Microscopy. Materials, 18(2), 236. https://doi.org/10.3390/ma18020236







