Effects of Biochar Addition on the Properties of Alkali-Activated Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Recipes
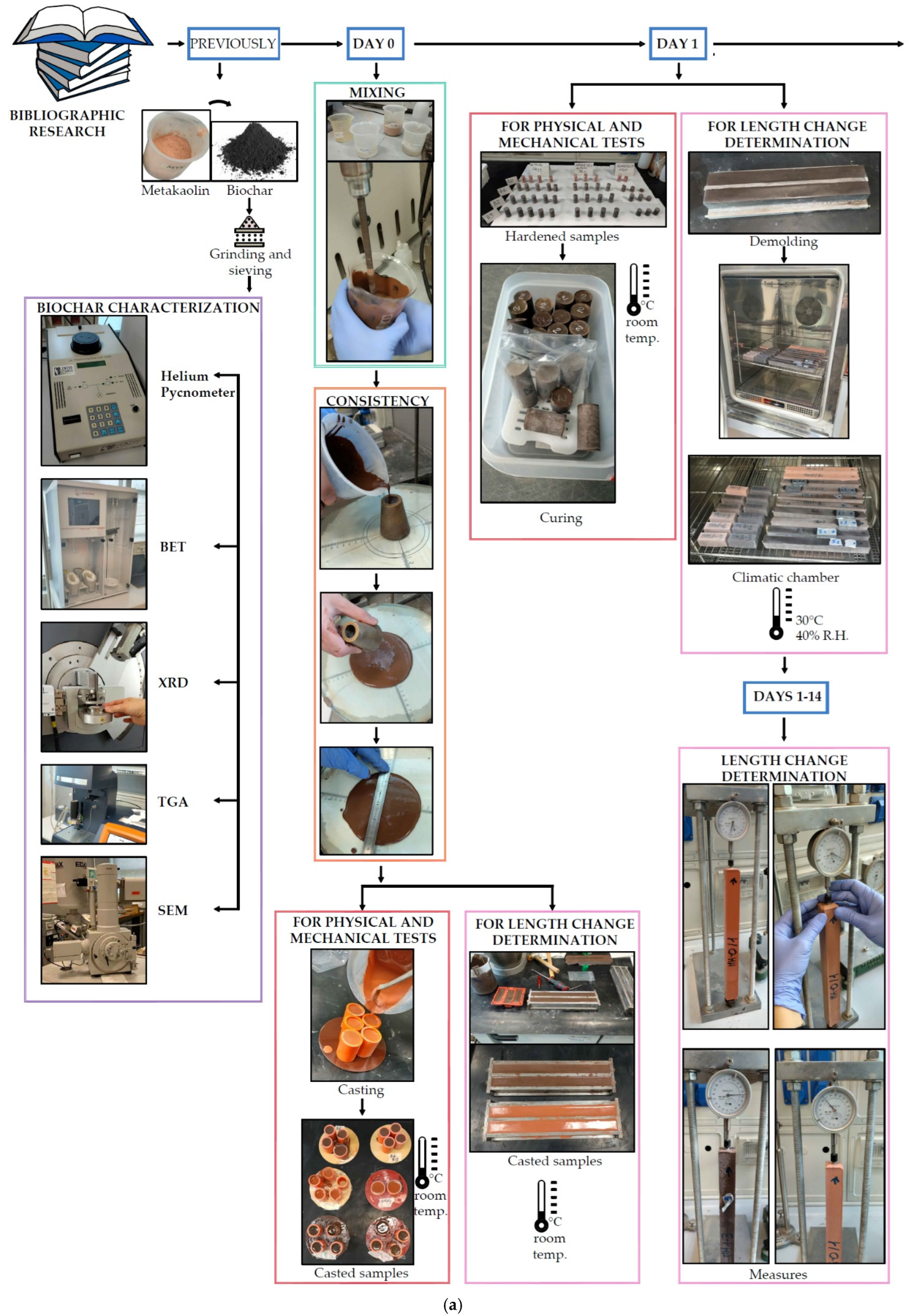
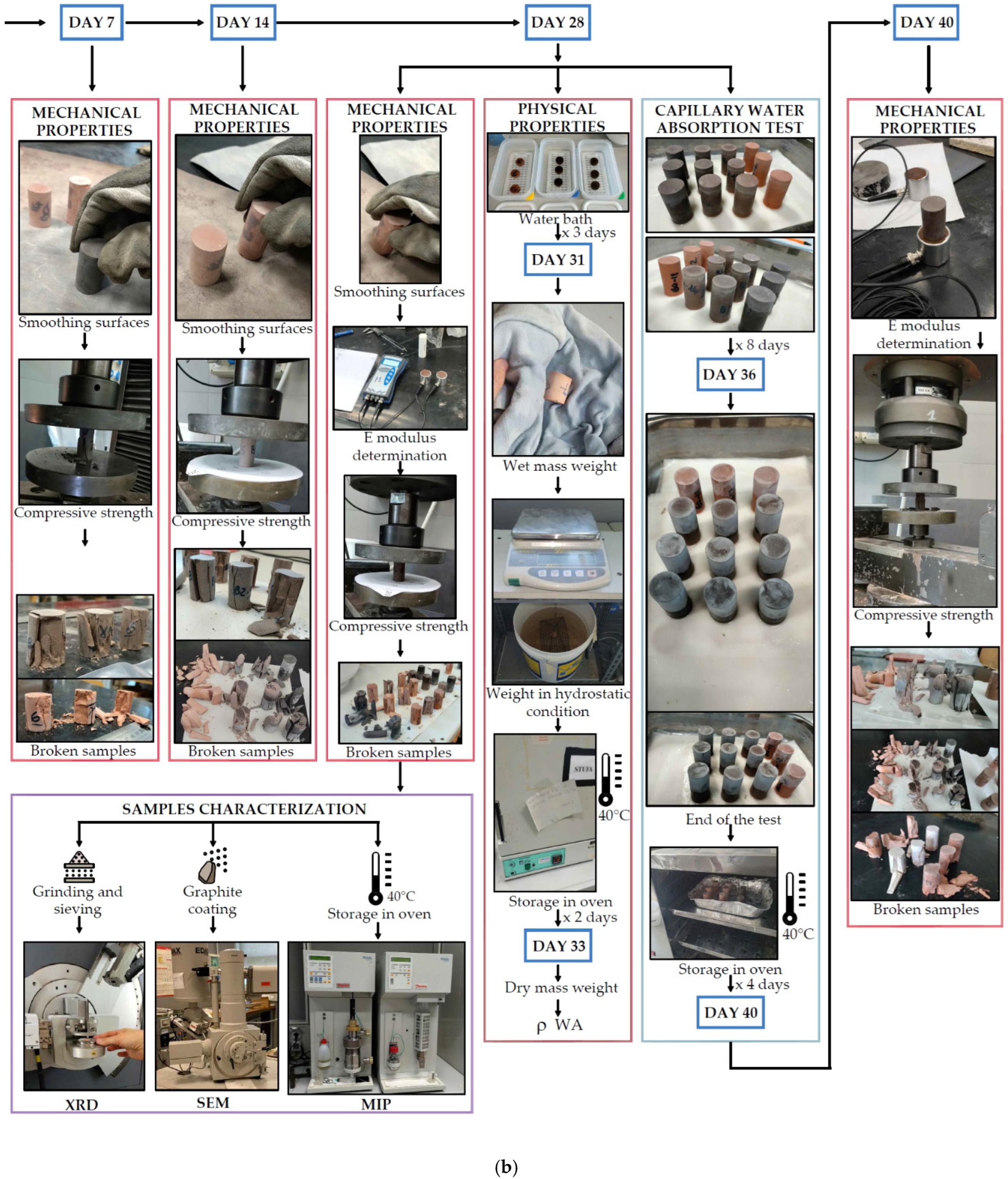
2.2. Mix Design and Sample Preparation
2.3. Methods
2.3.1. Determination of Consistency
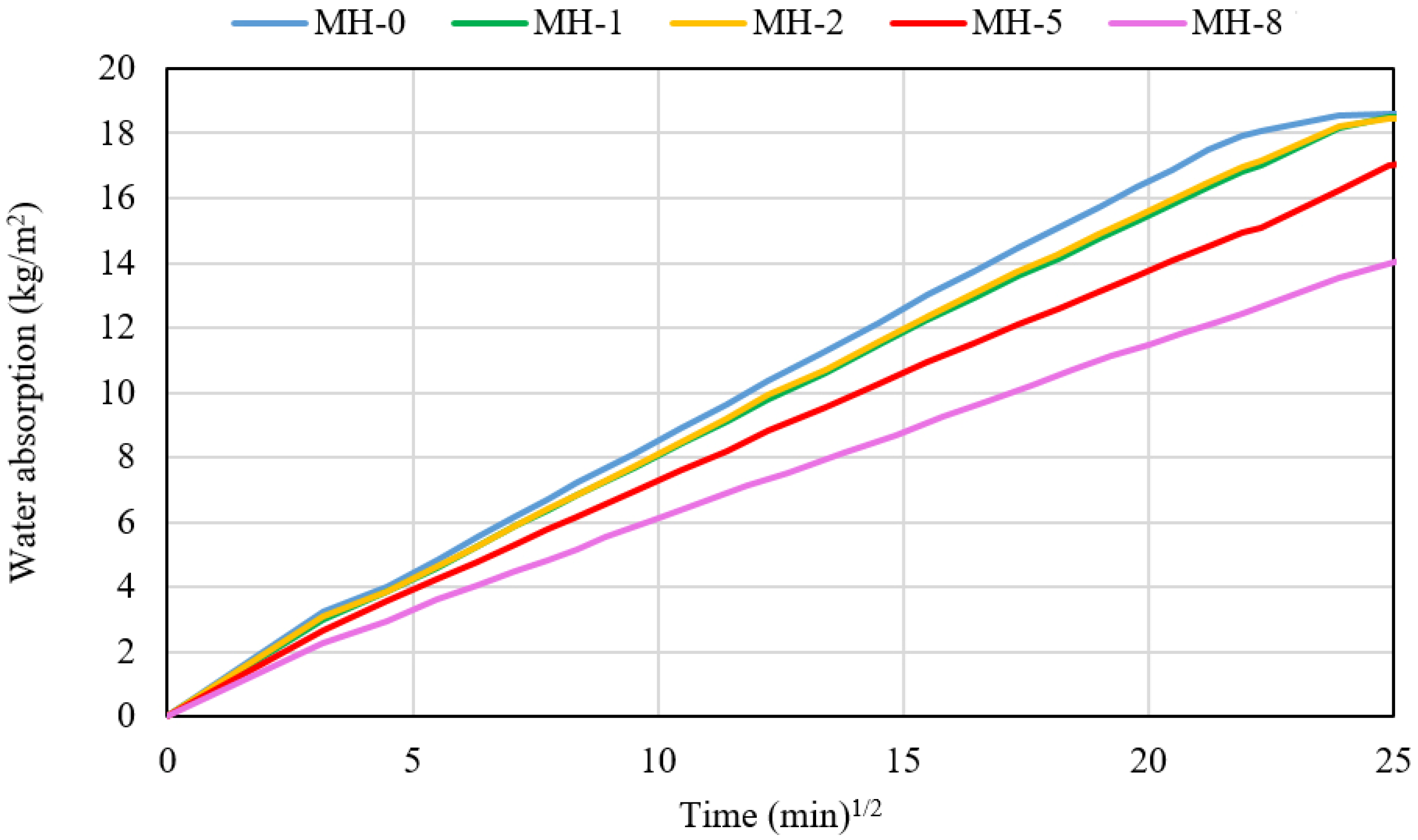
2.3.2. Physical Properties
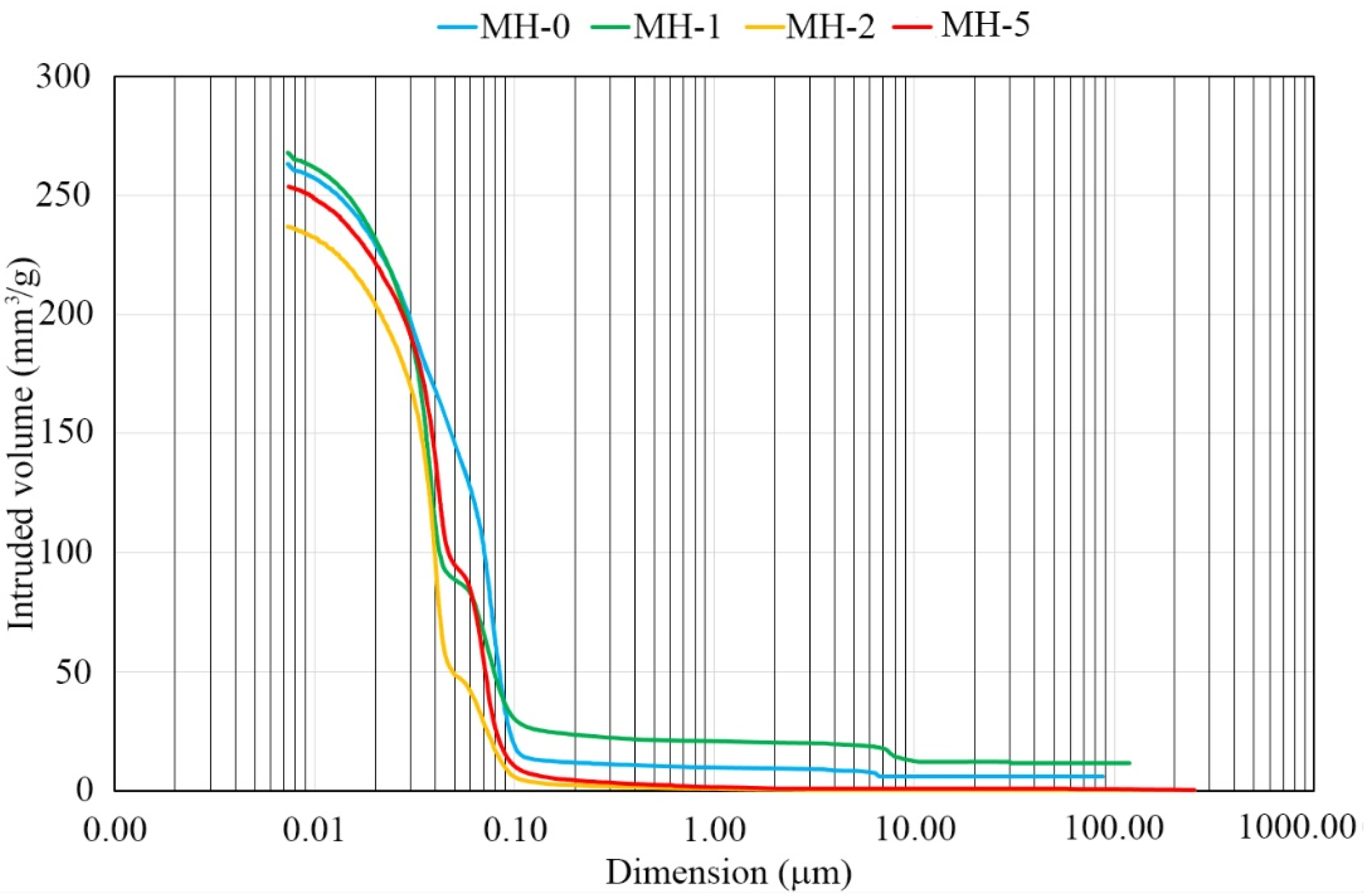
2.3.3. Pore Size Distribution
2.3.4. Mechanical Properties
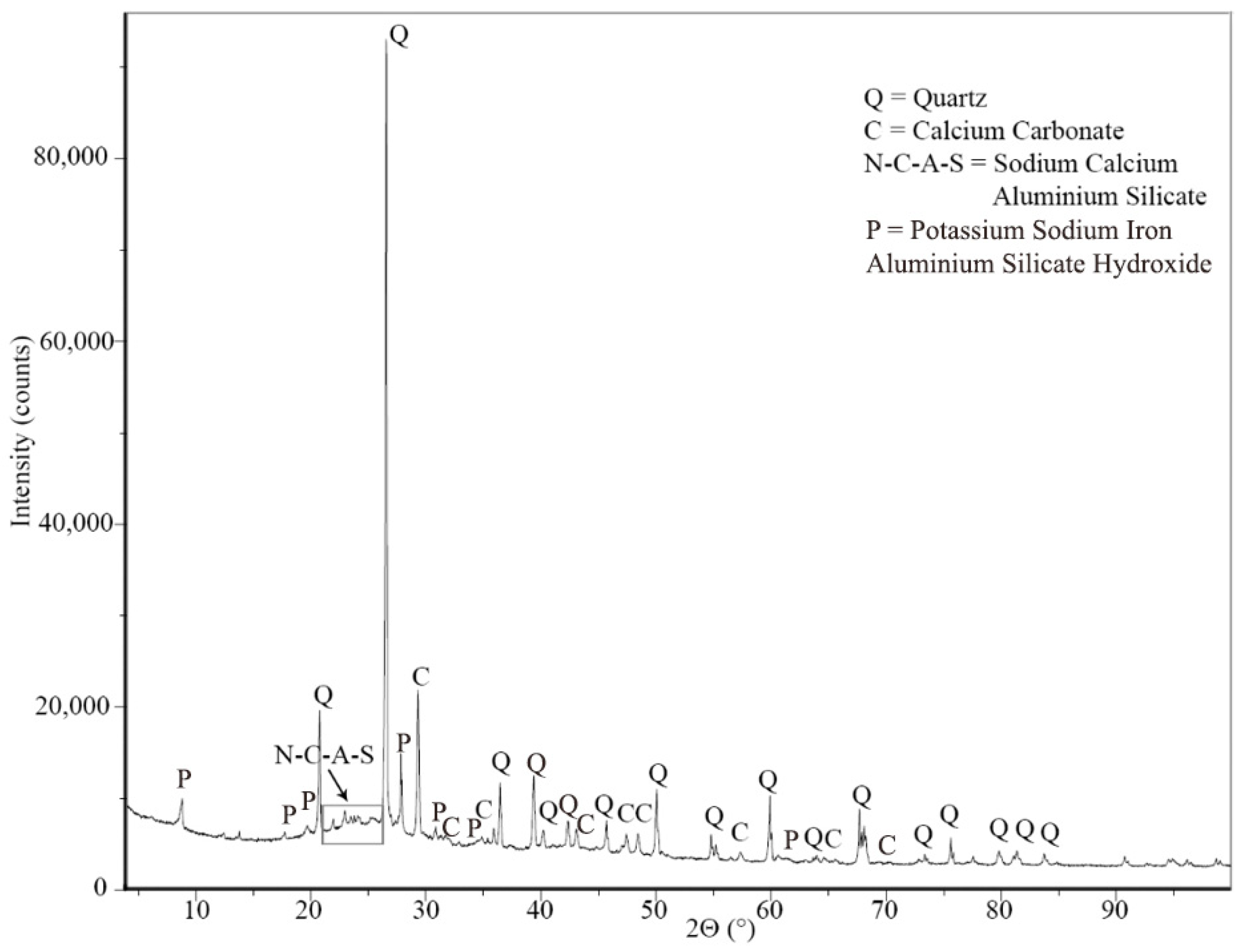
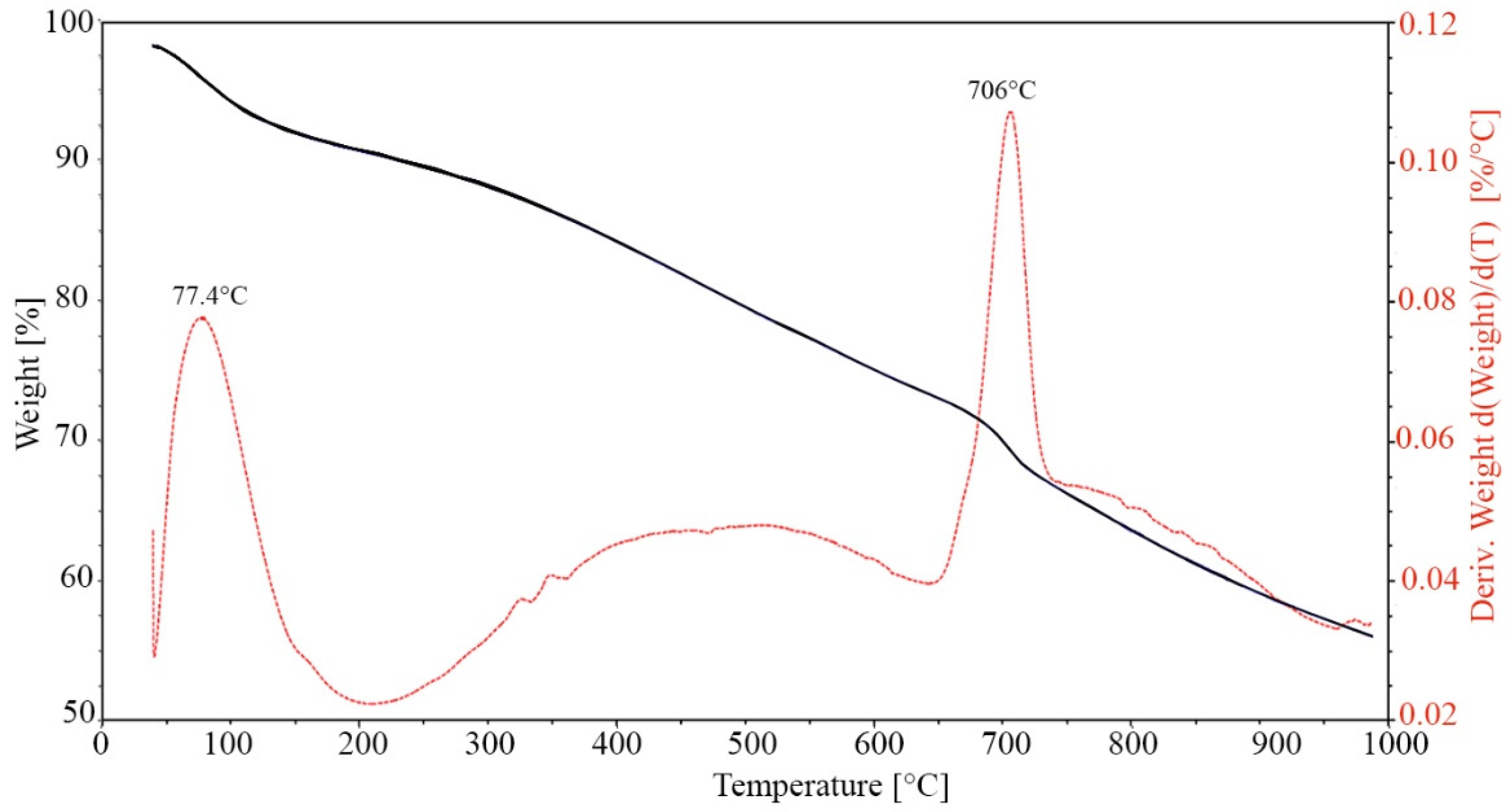
2.3.5. Microstructure
2.3.6. Determination of Length Change
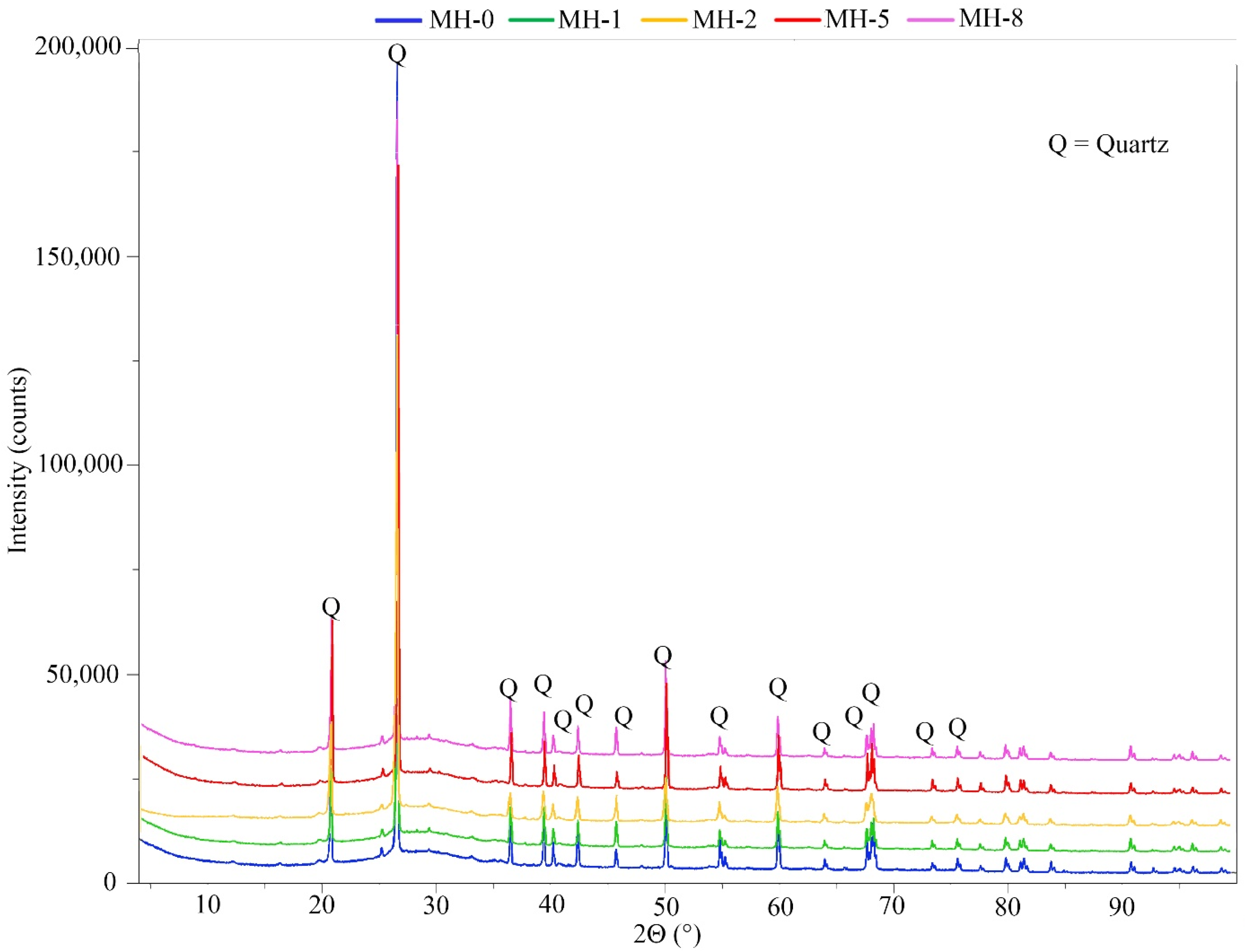
3. Results and Discussion
4. Future Scope
5. Conclusions
- -
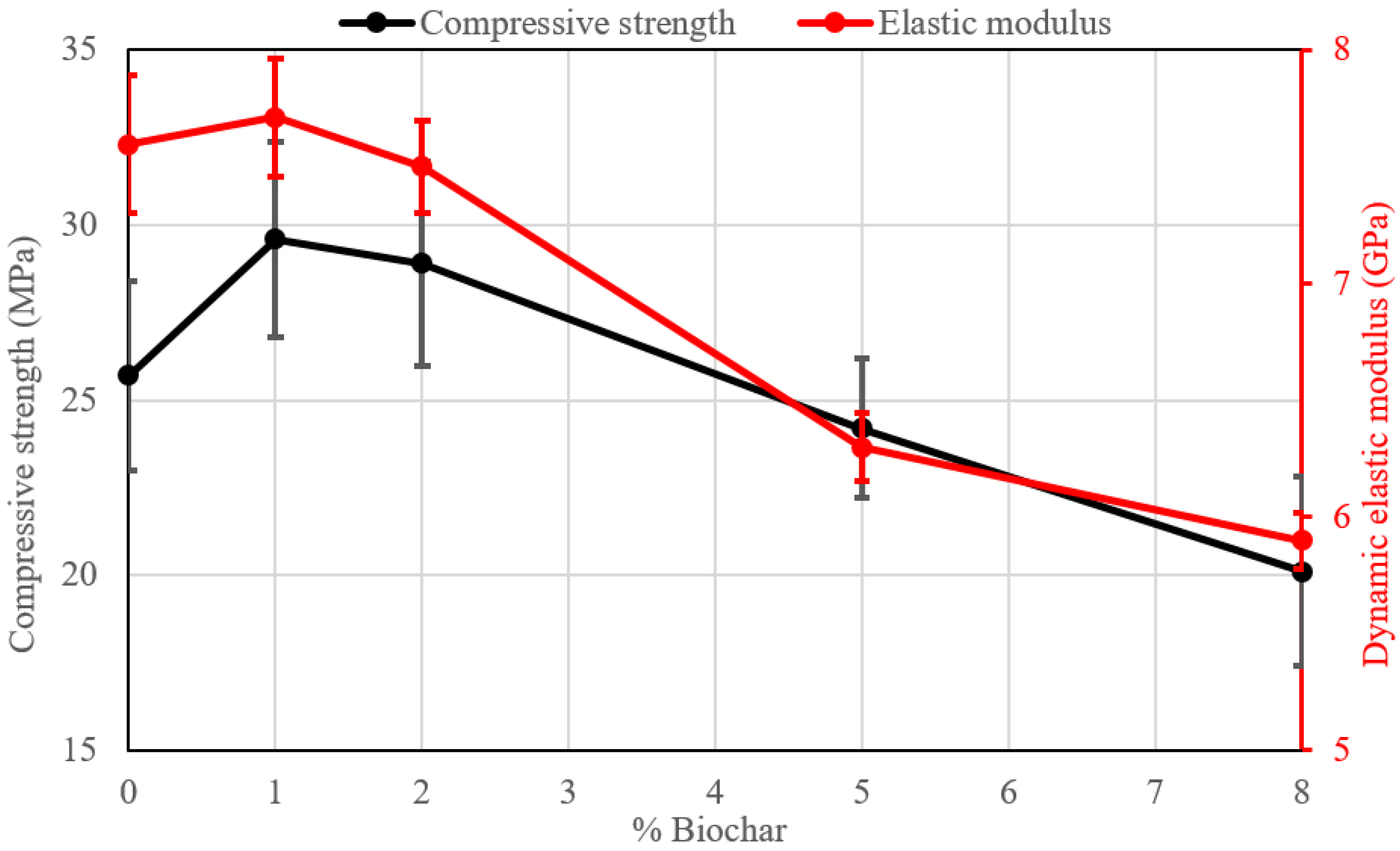
- An improvement in the mechanical properties (compressive strength and elastic modulus) is obtained with the addition of 1% of biochar (+15% for compressive strength and +0.9% for dynamic elastic modulus after 28 days). A higher amount progressively tends to decrease the mechanical performance.
- -
- Water permeability tends to decrease with the addition of biochar, while the formation of efflorescence is reduced. An increased durability of the modified materials can thus be forecast.
- -
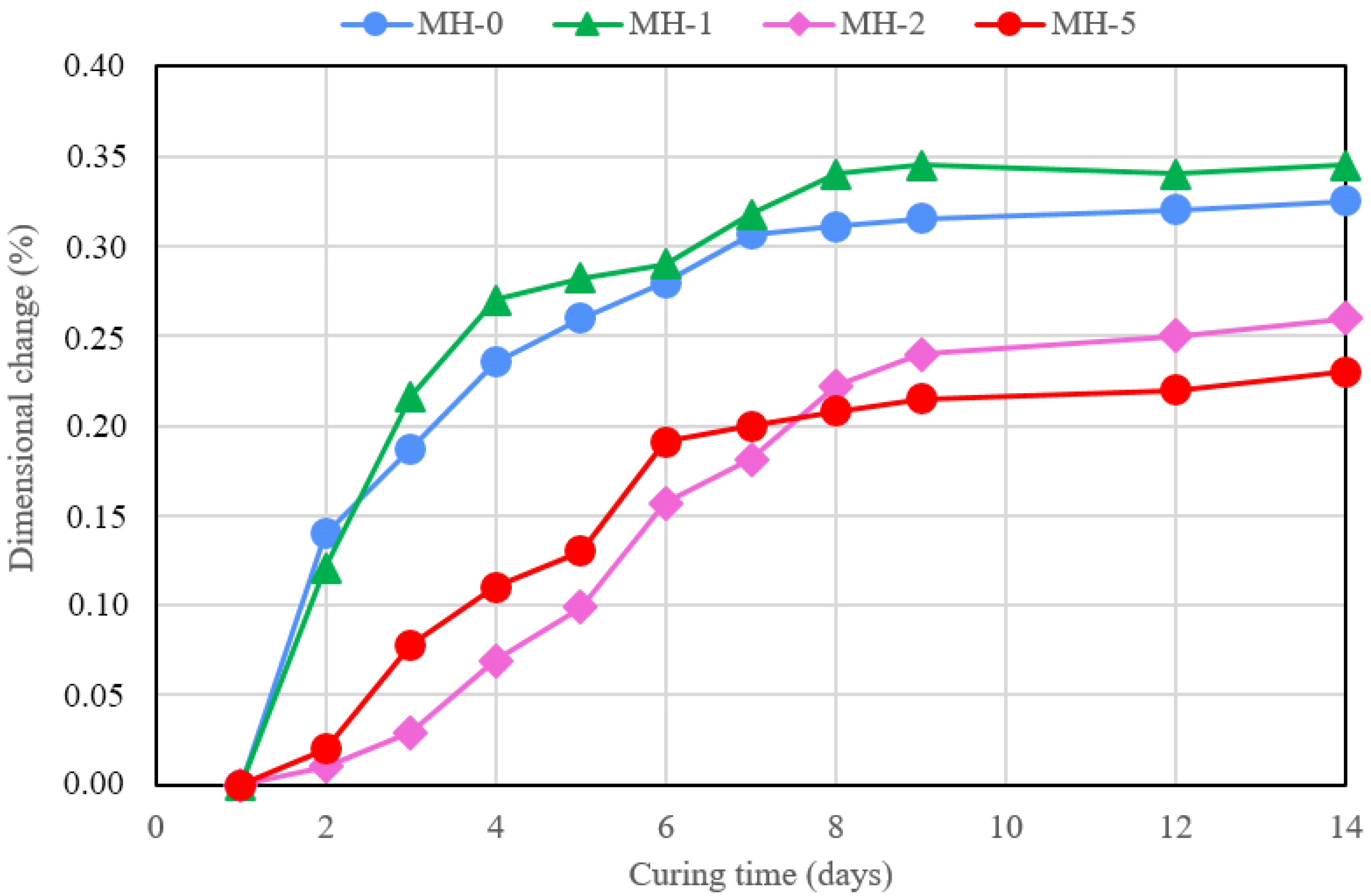
- At compositions above 2%, biochar provides higher dimensional stability to the samples, a feature that was never tested before.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mensah, R.A.; Shanmugam, V.; Narayanan, S.; Razavi, N.; Ulfberg, A.; Blanksvärd, T.; Sayahi, F.; Simonsson, P.; Reinke, B.; Försth, M.; et al. Biochar-added cementitious materials—A review on mechanical, thermal, and environmental properties. Sustainability 2021, 13, 9336. [Google Scholar] [CrossRef]
- Silvestro, L.; Piccinini Scolaro, T.; Ruviaro, A.S.; dos Santos Lima, G.T.; Gleize, P.J.P.; Pelisser, F. Use of biomass wood ash to produce sustainable geopolymeric pastes. Constr. Build. Mater. 2023, 370, 130641. [Google Scholar] [CrossRef]
- Palomo, A.; Krivenko, P.; Garcia-Lodeiro, I.; Kavalerova, E.; Maltseva, O.; Fernández-Jiménez, A. A review on alkaline activation: New analytical perspectives. Mater. Constr. 2014, 64, e022. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, T.; Yang, J.; Qi, J.; Zhang, L.; Liu, T.; Dai, S.; Zhao, Y.; Huang, Q.; Liu, Z.; et al. A review on the roles of biochar incorporated into cementitious materials: Mechanisms, application and perspectives. Constr. Build. Mater. 2023, 409, 134204. [Google Scholar] [CrossRef]
- Akinyemi, B.A.; Adesina, A. Recent advancements in the use of biochar for cementitious applications: A review. J. Build. Eng. 2020, 32, 101705. [Google Scholar] [CrossRef]
- Gupta, S.; Kashani, A.; Mahmood, A.H.; Han, T. Carbon sequestration in cementitious composites using biochar and fly ash—Effect on mechanical and durability properties. Constr. Build. Mater. 2021, 291, 123363. [Google Scholar] [CrossRef]
- Gupta, S.; Kua, H.W.; Low, C.Y. Use of biochar as carbon sequestering additive in cement mortar. Cem. Concr. Compos. 2018, 87, 110–129. [Google Scholar] [CrossRef]
- Barbhuiya, S.; Das, B.B.; Kanavaris, F. Biochar-concrete: A comprehensive review of properties, production and sustainability. Case Stud. Constr. Mater. 2024, 20, e02859. [Google Scholar] [CrossRef]
- Neve, S.; Du, J.; Barhemat, R.; Meng, W.; Bao, Y.; Sarkar, D. Valorization of vetiver root biochar in eco-friendly reinforced concrete: Mechanical, economic, and environmental performance. Materials 2023, 16, 2522. [Google Scholar] [CrossRef]
- Ryłko-Polak, I.; Komala, W.; Białowiec, A. The reuse of biomass and industrial waste in biocomposite construction materials for decreasing natural resource use and mitigating the environmental impact of the construction industry: A review. Materials 2022, 15, 4078. [Google Scholar] [CrossRef]
- Maljaee, H.; Madadi, R.; Paiva, H.; Tarelho, L.; Ferreira, V.M. Incorporation of biochar in cementitious materials: A roadmap of biochar selection. Constr. Build. Mater. 2021, 283, 122757. [Google Scholar] [CrossRef]
- Chen, X.; Li, J.; Xue, Q.; Huang, X.; Liu, L.; Poon, C.S. Sludge biochar as a green additive in cement-based composites: Mechanical properties and hydration kinetics. Constr. Build. Mater. 2020, 262, 120723. [Google Scholar] [CrossRef]
- Gupta, S.; Muthukrishnan, S.; Kua, H.W. Comparing influence of inert biochar and silica rich biochar on cement mortar—Hydration kinetics and durability under chloride and sulfate environment. Constr. Build. Mater. 2021, 268, 121142. [Google Scholar] [CrossRef]
- Gupta, S.; Kua, H.W. Carbonaceous micro-filler for cement: Effect of particle size and dosage of biochar on fresh and hardened properties of cement mortar. Sci. Total Environ. 2019, 662, 952–962. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Kua, H.W.; Pang, S.D. Biochar-mortar composite: Manufacturing, evaluation of physical properties and economic viability. Constr. Build. Mater. 2018, 167, 874–889. [Google Scholar] [CrossRef]
- Wang, L.; Chen, L.; Tsang, D.C.W.; Kua, H.W.; Yang, J.; Ok, Y.S.; Ding, S.; Hou, S.; Poon, C.S. The roles of biochar as green admixture for sediment-based construction products. Cem. Concr. Compos. 2019, 104, 103348. [Google Scholar] [CrossRef]
- Muthukrishnan, S.; Gupta, S.; Kua, H.W. Application of rice husk biochar and thermally treated low silica rice husk ash to improve physical properties of cement mortar. Theor. Appl. Fract. Mech. 2019, 104, 102376. [Google Scholar] [CrossRef]
- De Carvalho Gomes, S.; Zhou, J.L.; Zeng, X.; Long, G. Water treatment sludge conversion to biochar as cementitious material in cement composite. J. Environ. Manag. 2022, 306, 114463. [Google Scholar] [CrossRef]
- Zeidabadi, Z.A.; Bakhtiari, S.; Abbaslou, H.; Ghanizadeh, A.R. Synthesis, characterization and evaluation of biochar from agricultural waste biomass for use in building materials. Constr. Build. Mater. 2018, 181, 301–308. [Google Scholar] [CrossRef]
- Navaratnam, S.; Wijaya, H.; Rajeev, P.; Mendis, P.; Nguyen, K. Residual stress-strain relationship for the biochar-based mortar after exposure to elevated temperature. Case Stud. Constr. Mater. 2021, 14, e00540. [Google Scholar] [CrossRef]
- Marathe, S.; Sadowski, L. Developments in biochar incorporated geopolymers and alkali activated materials: A systematic literature review. J. Clean. Prod. 2024, 469, 143136. [Google Scholar] [CrossRef]
- Kim, J.-G.; Kim, H.-B.; Baek, K. Novel electrochemical method to activate biochar derived from spent coffee grounds for enhanced adsorption of lead (Pb). Sci. Total Environ. 2023, 886, 163891. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhou, X.; Moghaddam, T.B.; Zhang, F.; Otto, F. Synergistic effects of iron (Fe) and biochar on light-weight geopolymers when used in wastewater treatment applications. J. Clean. Prod. 2021, 322, 129033. [Google Scholar] [CrossRef]
- Dzoujo, H.T.; Shikuku, V.O.; Tome, S.; Akiri, S.; Kengne, N.M.; Abdpour, S.; Janiak, C.; Etoh, M.A.; Dina, D. Synthesis of pozzolan and sugarcane bagasse derived geopolymer-biochar composites for methylene blue sequestration from aqueous medium. J. Environ. Manag. 2022, 318, 115533. [Google Scholar] [CrossRef]
- Zhu, Y.; Ji, S.; Liang, W.; Li, C.; Nie, Y.; Dong, J.; Shi, W.; Ai, S. A low-cost and eco-friendly powder catalyst: Iron and copper nanoparticles supported on biochar/geopolymer for activating potassium peroxymonosulfate to degrade naphthalene in water and soil. Chemosphere 2022, 303, 135185. [Google Scholar] [CrossRef]
- Suresh, R.; Gnanasekaran, L.; Rajendran, S.; Jalil, A.A.; Soto-Moscoso, M.; Khoo, K.S.; Ma, Z.; Halimatul Munawaroh, H.S.; Show, P.L. Biomass waste as an alternative source of carbon and silicon-based absorbents for CO2 capturing application. Chemosphere 2023, 343, 140173. [Google Scholar] [CrossRef]
- Candamano, S.; Policicchio, A.; Conte, G.; Abarca, R.; Algieri, C.; Chakraborty, S.; Curcio, S.; Calabrò, V.; Crea, F.; Agostino, R.G. Preparation of foamed and unfoamed geopolymer/NaX zeolite/activated carbon composites for CO2 adsorption. J. Clean. Prod. 2022, 330, 129843. [Google Scholar] [CrossRef]
- Piccolo, F.; Andreola, F.; Barbieri, L.; Lancellotti, I. Synthesis and characterization of biochar-based geopolymer materials. Appl. Sci. 2021, 11, 10945. [Google Scholar] [CrossRef]
- Farges, R.; Gharzouni, A.; Ravier, B.; Jeulin, P.; Rossignol, S. Insulating foams and dense geopolymers from biochar by-products. J. Ceram. Sci. Technol. 2018, 9, 193–200. [Google Scholar] [CrossRef]
- Egodagamage, H.; Yapa, H.D.; Buddika, H.A.D.S.; Navaratnam, S.; Nguyen, K. Effective use of biochar as an additive for alkali-activated slag mortar production. Constr. Build. Mater. 2023, 370, 130487. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, B.; Duan, H. Effect of rice husk ash on properties of slag based geopolymer pastes. J. Build. Eng. 2023, 76, 107035. [Google Scholar] [CrossRef]
- Prabahar, J.; Vafaei, B.; Baffoe, E.; Ghahremaninezhad, A. The effect of biochar on the properties of alkali-activated slag pastes. Constr. Mater. 2022, 2, 1–14. [Google Scholar] [CrossRef]
- Sorrenti, G.; Masiello, C.A.; Dugan, B.; Toselli, M. Biochar physico-chemical properties as affected by environmental exposure. Sci. Total Environ. 2016, 563–564, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Rajamma, R.; Labrincha, J.A.; Ferreira, V.M. Alkali activation of biomass fly ash–metakaolin blends. Fuel 2012, 98, 265–271. [Google Scholar] [CrossRef]
- Abdulkareem, O.A.; Ramli, M.; Matthews, J.C. Production of geopolymer mortar system containing high calcium biomass wood ash as a partial substitution to fly ash: An early age evaluation. Compos. B Eng. 2019, 174, 106941. [Google Scholar] [CrossRef]
- Ishak, S.; Lee, H.S.; Singh, J.K.; Ariffin, M.A.M.; Lim, N.H.A.S.; Yang, H.M. Performance of fly ash geopolymer concrete incorporating bamboo ash at elevated temperature. Materials 2019, 12, 3404. [Google Scholar] [CrossRef]
- Yadav, A.L.; Sairam, V.; Srinivasan, K.; Muruganandam, L. Synthesis and characterization of geopolymer from metakaolin and sugarcane bagasse ash. Constr. Build. Mater. 2020, 258, 119231. [Google Scholar] [CrossRef]
- Rajan, H.S.; Kathirvel, P. Sustainable development of geopolymer binder using sodium silicate synthesized from agricultural waste. J. Clean. Prod. 2021, 286, 124959. [Google Scholar] [CrossRef]
- Saloni; Parveen; Pham, T.M.; Lim, Y.Y.; Pradhan, S.S.; Jatin Kumar, J. Performance of rice husk Ash-Based sustainable geopolymer concrete with Ultra-Fine slag and Corn cob ash. Constr. Build. Mater. 2021, 279, 122526. [Google Scholar] [CrossRef]
- Singh, K. Experimental study on metakolin and bagasse ash based geopolymer concrete. Mater. Today Proc. 2021, 37, 3289–3295. [Google Scholar] [CrossRef]
- Manzi, S.; Molari, L.; Bignozzi, M.C.; Masi, G.; Saccani, A. Geopolymeric Composites Containing Industrial Waste Reinforced with Arundo donax Fibers. Buildings 2024, 14, 1191. [Google Scholar] [CrossRef]
- EN 772-13; Determination of Net and Gross Dry Density. European Committee for Standardization: Brussels, Belgium, 2002.
- EN 772-21; Determination of Water Absorption. European Committee for Standardization: Brussels, Belgium, 2011.
- EN 15801; Conservation of Cultural Property—Test Methods—Determination of Water Absorption by Capillarity. European Committee for Standardization: Brussels, Belgium, 2010.
- EN 196-1; Methods of Testing Cement—Part 1: Determination of Strength. European Committee for Standardization: Brussels, Belgium, 2016.
- EN 12504-4; Testing Concrete in Structures—Part 4: Determination of Ultrasonic Pulse Velocity. European Committee for Standardization: Brussels, Belgium, 2021.
- ASTM C 490/C 490M-21; Standard Practice for Use of Apparatus for the Determination of Length Change of Hardened Cement Paste, Mortar, and Concrete. ASTM International: West Conshohocken, PA, USA, 2021.
- Ruan, S.; Chen, S.; Zhang, Y.; Mao, J.; Yan, D.; Liu, Y.; Liu, X.; Hosono, H. Molecular-level hybridized hydrophobic geopolymer ceramics for corrosion protection. Chem. Mater. 2023, 35, 1735–1744. [Google Scholar] [CrossRef]
- Manzi, S.; Baldazzi, L.; Saccani, A. Formulating geopolymer mortars through Construction and Demolition Waste (CDW) Recycling: A comprehensive case study. Materials 2023, 16, 7304. [Google Scholar] [CrossRef] [PubMed]
- Alastair, Y.H.; Marsh, T.M.; Yue, Z.; Krishnan, S.; Adu-Amankwah, S.; Bernal, S.A. Hybrid organic-inorganic blast furnace slag binders activated with alkali acetates. ACS Omega 2024, 9, 35888–35905. [Google Scholar] [CrossRef]




| Mixture | Binder | Biochar | Test Performed | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | Ref. | P | M | C | OPC | MTK | FA | GGBS | Sludge | Rice husk-Bag. | Wood -Nut | OAB (%) | Flow | σc-σf | σc Max MPa | SEM EDX | XRD | FTIR | TGA DSC | Perm. Absor. | ρ | MIP BET | LC | Dur. |
| Chen | [4] | 5 | 37 | |||||||||||||||||||||
| Barbhuiya | [8] | 1-5 | 35 | |||||||||||||||||||||
| Malijaee | [11] | 2 | 70 | |||||||||||||||||||||
| Chen | [12] | 2 | 65 | |||||||||||||||||||||
| Gupta | [13] | 1-2 | 65 | |||||||||||||||||||||
| Gupta | [14] | 1-2 | 70 | |||||||||||||||||||||
| Gupta | [15] | 1-2 | 65 | |||||||||||||||||||||
| Wang | [16] | 1-2 | 70 | |||||||||||||||||||||
| Muthukrishnan | [17] | 20 | 70 | |||||||||||||||||||||
| De Carvalho | [18] | 1-2 | 73 | |||||||||||||||||||||
| Zeidabadi | [19] | 5 | 55 | |||||||||||||||||||||
| Navaratnam | [20] | 5 | 38 | |||||||||||||||||||||
| Marathe | [21] | 2-10 | ||||||||||||||||||||||
| Piccolo | [28] | |||||||||||||||||||||||
| Egodagamage | [30] | 2 | 43 | |||||||||||||||||||||
| Zhao | [31] | 50 | 72 | |||||||||||||||||||||
| Prabahar | [32] | 1.5 | 22 | |||||||||||||||||||||
| Rajamma | [34] | 60 | 38 | |||||||||||||||||||||
| Abdulkareem | [35] | 10 | 60 | |||||||||||||||||||||
| Ishak | [36] | 5 | 41 | |||||||||||||||||||||
| Yadav | [37] | 55 | 25 | |||||||||||||||||||||
| Rajan | [38] | 33 | 65 | |||||||||||||||||||||
| Saloni | [39] | 90 | 60 | |||||||||||||||||||||
| Singh | [40] | 10 | 30 | |||||||||||||||||||||
| Silvestro | [2] | 10 | 83 | |||||||||||||||||||||
| Ref. = Bibliographical Reference P = Paste M = Mortar C = Concrete OPC = Ordinary Portland cement MTK = Metakaolin FA = Fly ash GGBS = Ground granulated blast-furnace slag | Bag. = Bagasse Nut = Nuts and coconut OAB = Optimum Amount of Biochar σc = Compressive strength σf = Flexural strength σc Max = Maximum value of compressive strength (MPa) SEM-EDX = Scanning electron microscope energy dispersive X-ray Spectroscopy XRD = X-ray diffraction FTIR = Fourier transform infrared spectroscopy | TGA = Thermogravimetry DSC = Differential Scanning Calorimetry Perm. = Permeability Absor. = Absorption ρ = Density MIP = Mercury Intrusion Porosimetry BET = Brauner -Emmet-Teller instrument LC = Length change determination Dur. = Durability tests  = perfomed = perfomed | ||||||||||||||||||||||
| Name | Metakaolin (%) | Biochar (%) | Na2SiO3 (%) | NaOH (%) | H2O (%) |
|---|---|---|---|---|---|
| MH-0 | 56.02 | 0.00 | 21.29 | 21.29 | 1.40 |
| MH-1 | 55.46 | 0.56 | 21.29 | 21.29 | 1.40 |
| MH-2 | 54.90 | 1.12 | 21.29 | 21.29 | 1.40 |
| MH-5 | 53.22 | 2.80 | 21.29 | 21.29 | 1.40 |
| MH-8 | 51.54 | 4.48 | 21.29 | 21.29 | 1.40 |
| Sample Name | dm (mm) | C (%) | ρb (Mg/m3) | WA (%) |
|---|---|---|---|---|
| MH-0 | 120 | 200 | 1.42 ± 0.01 | 27.5 ± 0.1 |
| MH-1 | 117 | 193 | 1.42 ± 0.02 | 27.5 ± 0.7 |
| MH-2 | 115 | 188 | 1.42 ± 0.01 | 27.5 ± 0.4 |
| MH-5 | 102 | 155 | 1.41 ± 0.02 | 28.1 ± 0.7 |
| MH-8 | 96 | 140 | 1.37 ± 0.01 | 28.6 ± 0.3 |
| Sample Name | σc 7 Days (MPa) | σc 14 Days (MPa) | σc 28 Days (MPa) | Ed 28 Days (GPa) |
|---|---|---|---|---|
| MH-0 | 23.6 ± 3.0 | 26.6 ± 2.9 | 25.7 ± 2.7 | 7.6 ± 0.3 |
| MH-1 | 28.3 ± 3.0 | 30.0 ± 2.4 | 29.6 ± 2.8 | 7.7 ± 0.3 |
| MH-2 | 27.6 ± 2.8 | 30.4 ± 2.8 | 28.9 ± 2.9 | 7.5 ± 0.2 |
| MH-5 | 21.1 ± 2.8 | 23.6 ± 2.2 | 24.2 ± 2.0 | 6.3 ± 0.1 |
| MH-8 | 18.2 ± 2.6 | 19.2 ± 2.5 | 20.1 ± 2.7 | 5.9 ± 0.1 |
| Sample Name | σc (MPa) | Ed (GPa) | Δ (%) |
|---|---|---|---|
| MH-0 | 22.4 ± 2.7 | 9.3 ± 0.6 | −12.8 |
| MH-1 | 29.8 ± 2.1 | 9.2 ± 0.3 | +0.7 |
| MH-2 | 30.2 ± 2.8 | 8.4 ± 0.3 | +4.5 |
| MH-5 | 26.3 ± 3.0 | 7.6 ± 0.2 | +8.7 |
| MH-8 | 21.1 ± 1.2 | 7.7 ± 0.2 | +5.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saccani, A.; Baldazzi, L.; Manzi, S. Effects of Biochar Addition on the Properties of Alkali-Activated Materials. Materials 2025, 18, 486. https://doi.org/10.3390/ma18030486
Saccani A, Baldazzi L, Manzi S. Effects of Biochar Addition on the Properties of Alkali-Activated Materials. Materials. 2025; 18(3):486. https://doi.org/10.3390/ma18030486
Chicago/Turabian StyleSaccani, Andrea, Luca Baldazzi, and Stefania Manzi. 2025. "Effects of Biochar Addition on the Properties of Alkali-Activated Materials" Materials 18, no. 3: 486. https://doi.org/10.3390/ma18030486
APA StyleSaccani, A., Baldazzi, L., & Manzi, S. (2025). Effects of Biochar Addition on the Properties of Alkali-Activated Materials. Materials, 18(3), 486. https://doi.org/10.3390/ma18030486







