Core–Shell Composite GaP Nanoparticles with Efficient Electroluminescent Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Samples
2.3. Characterizations
3. Results and Discussion
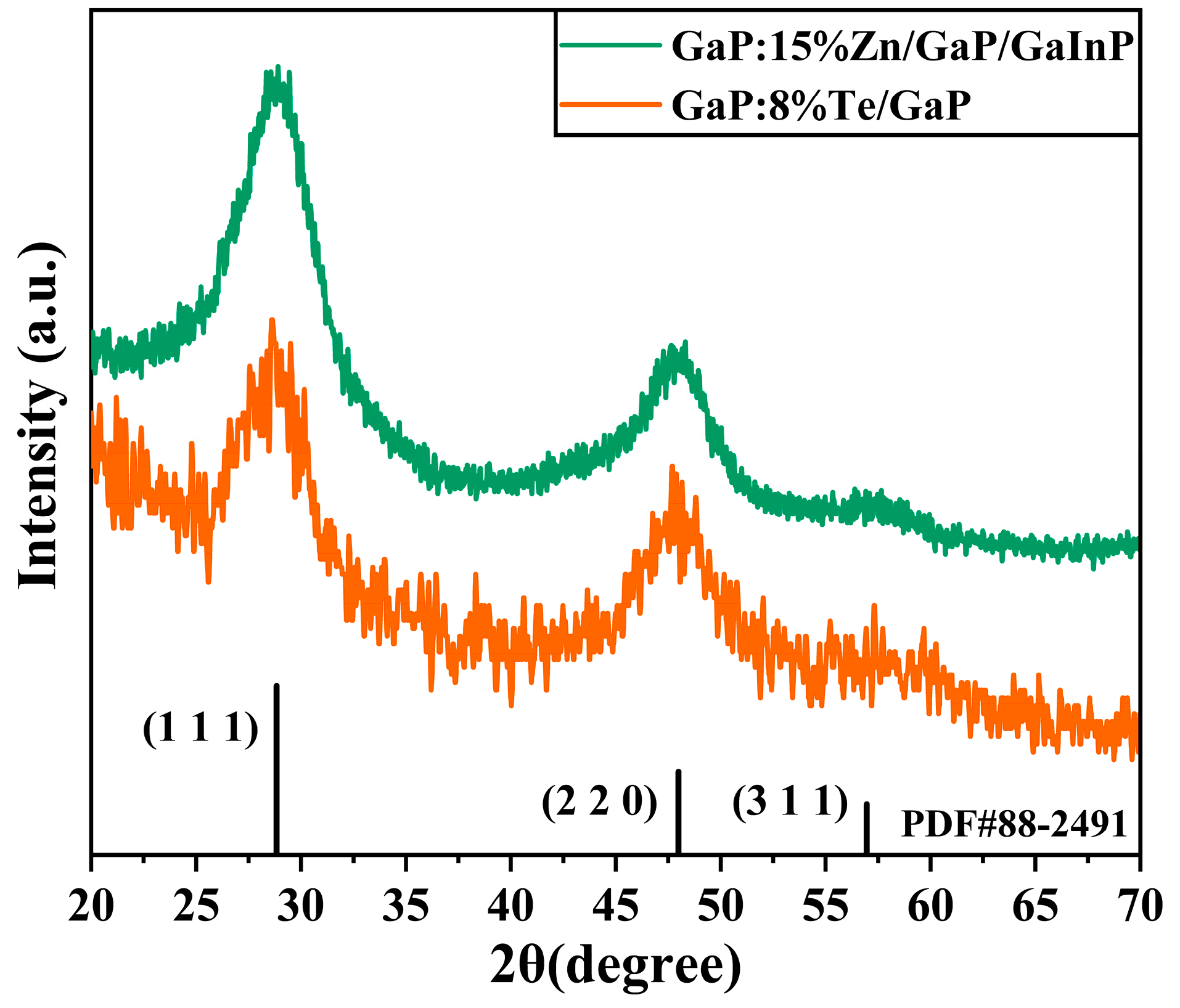
3.1. Phase Identification
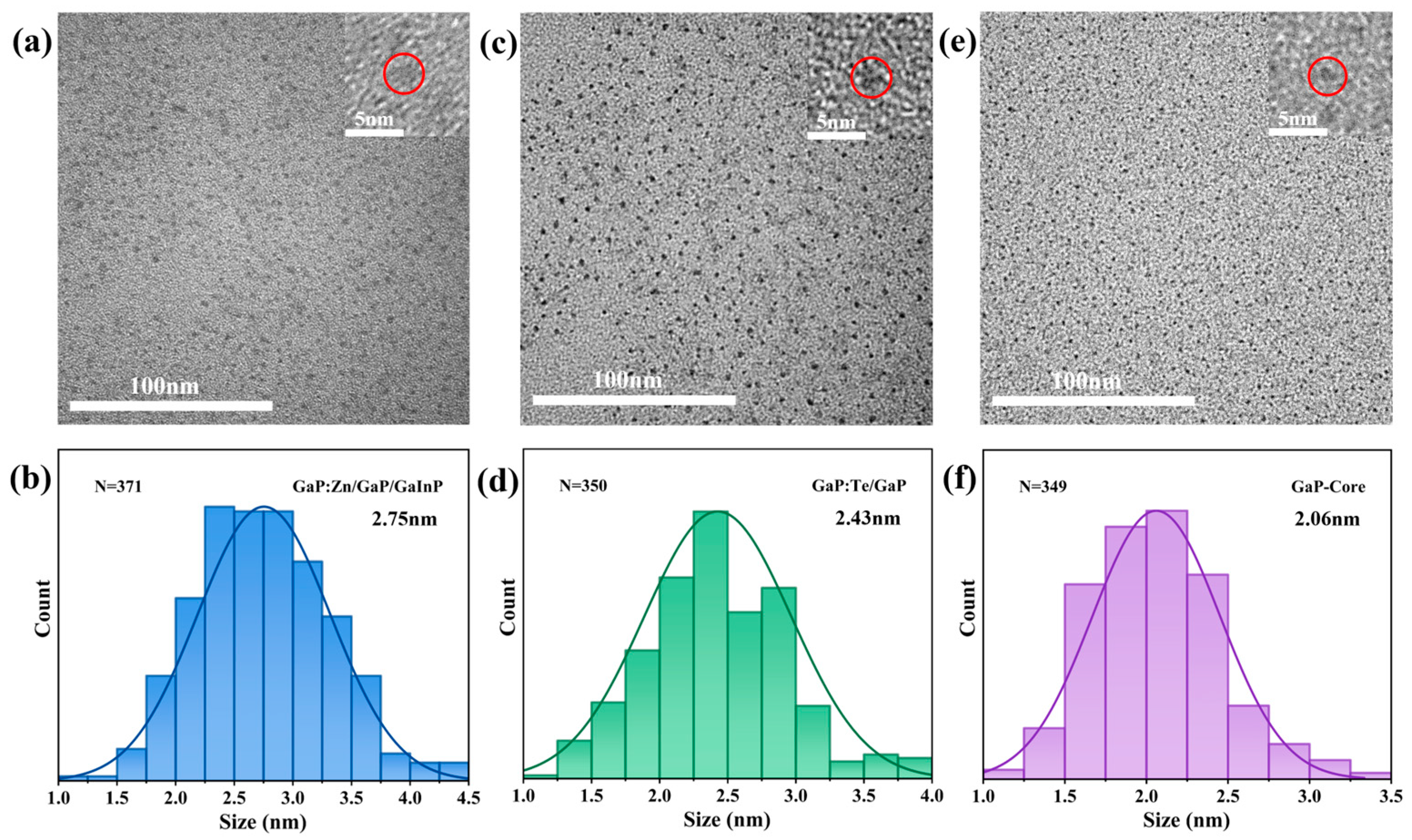
3.2. Surface Morphology
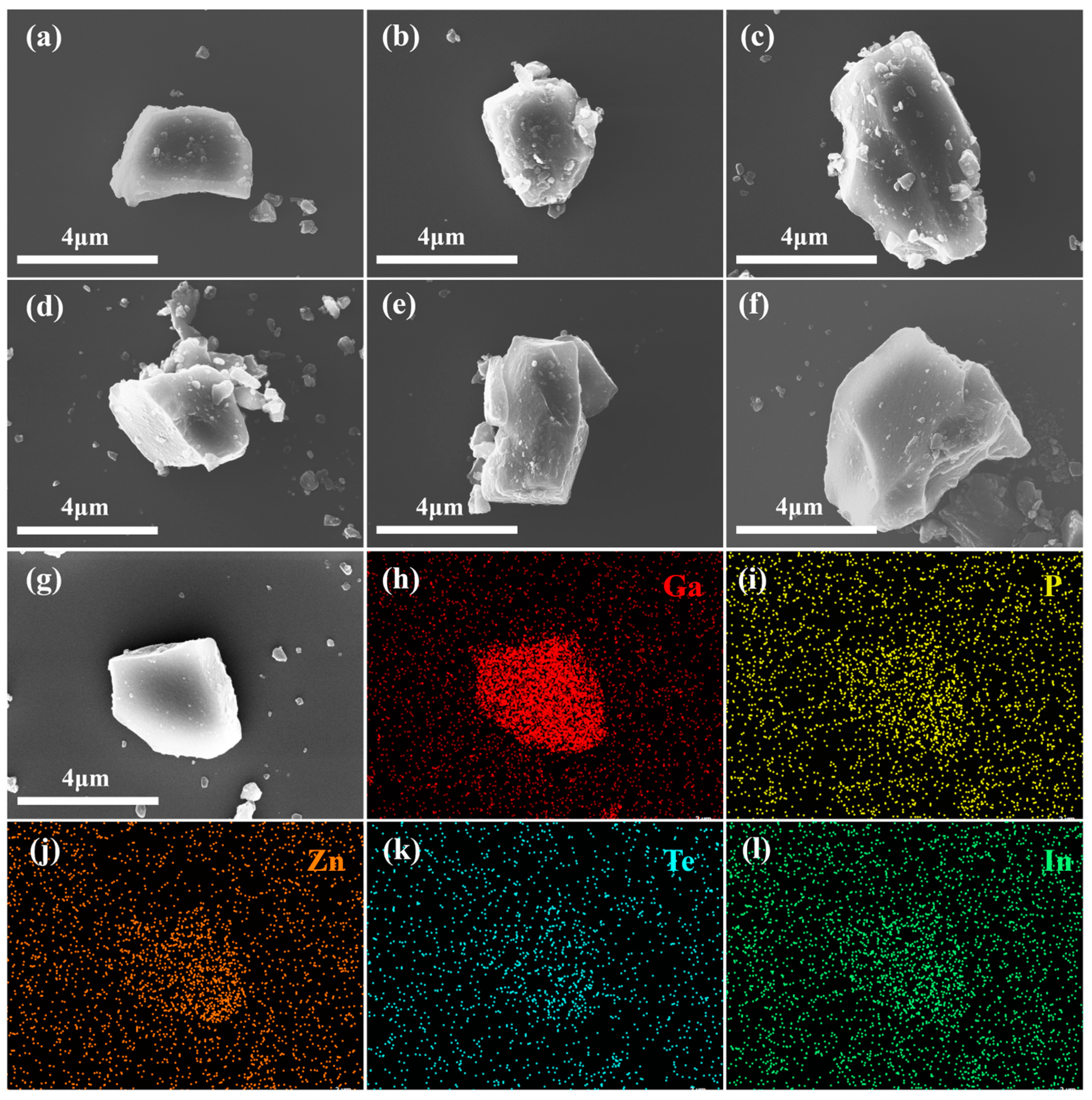
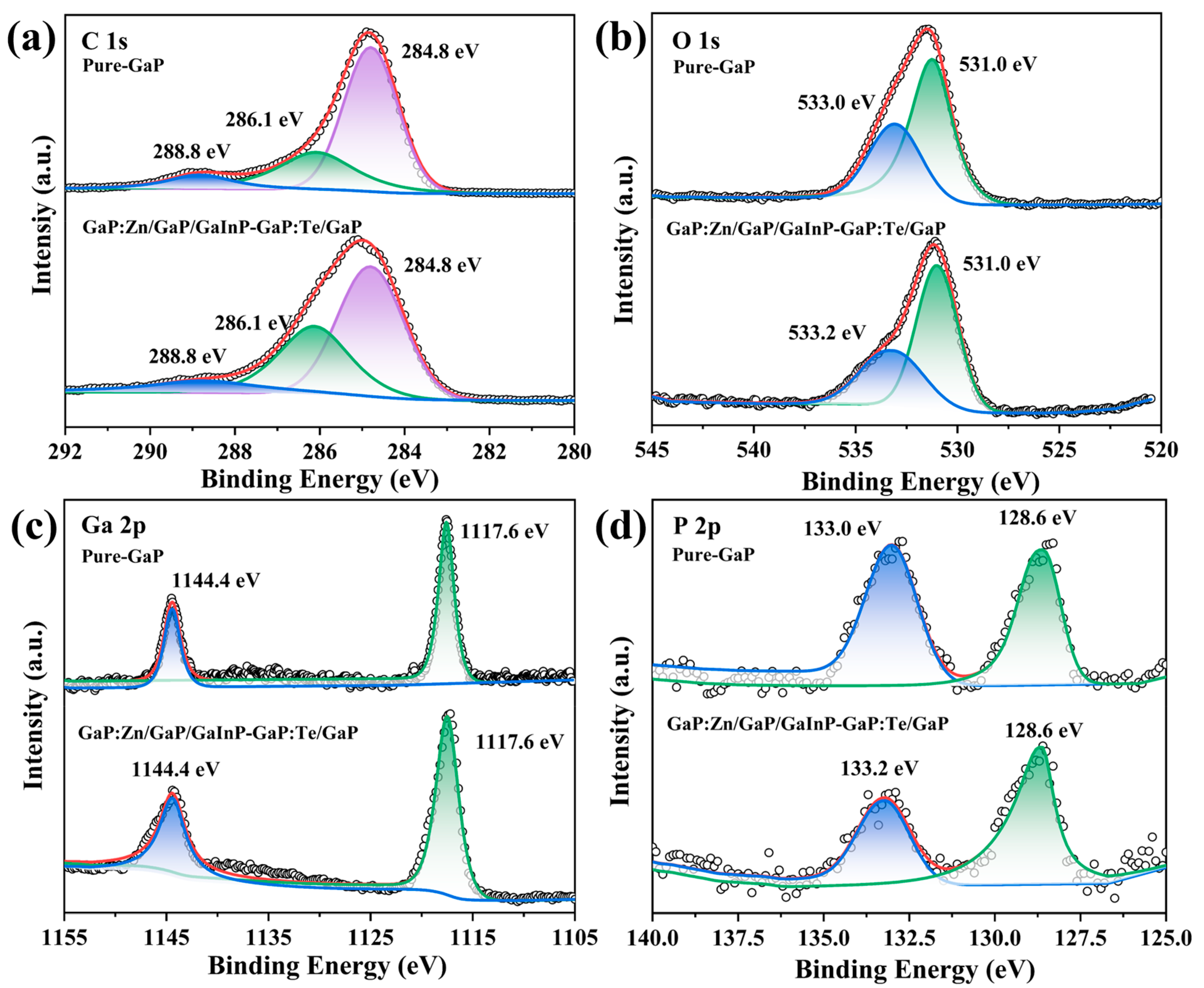
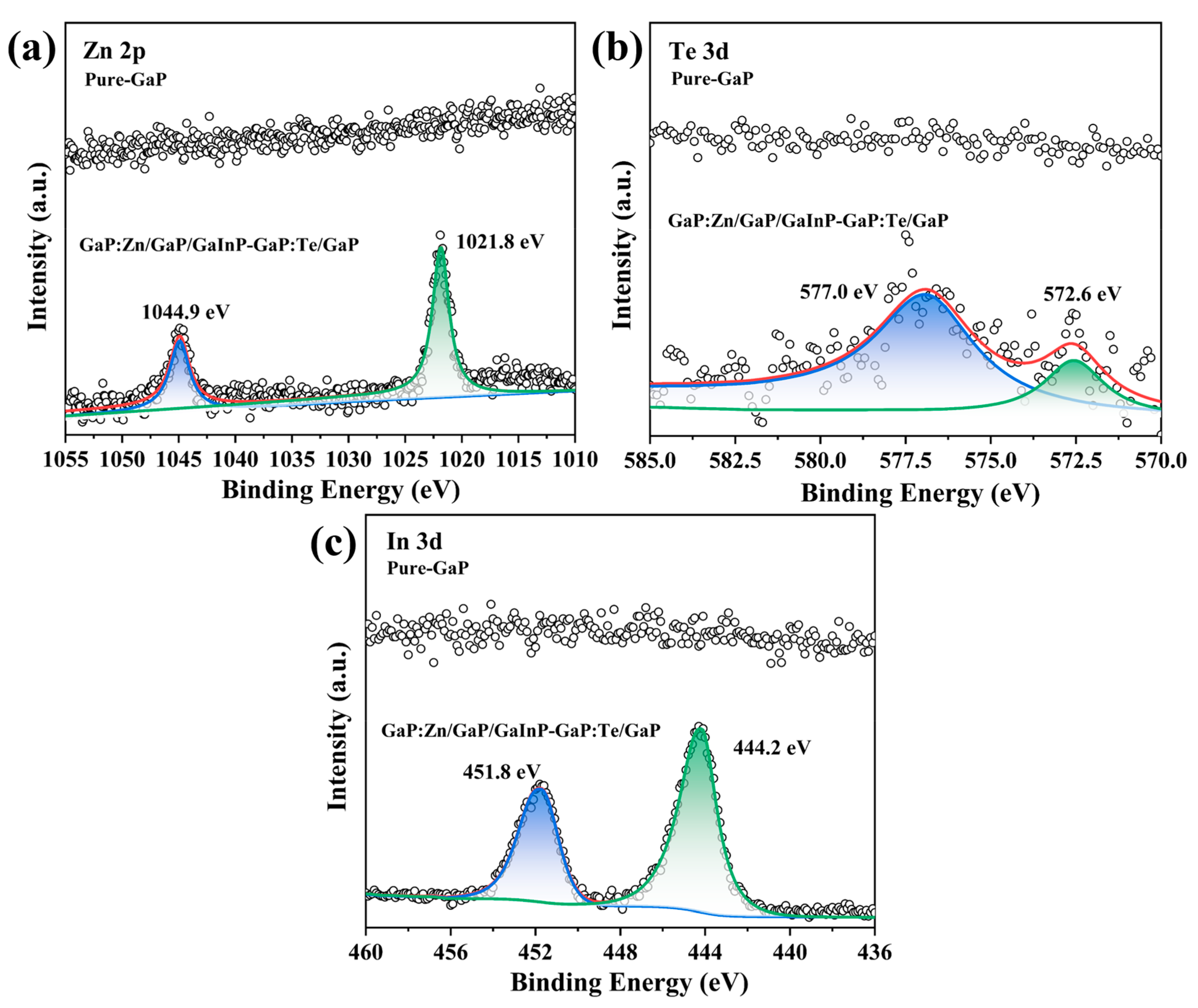
3.3. Element Composition
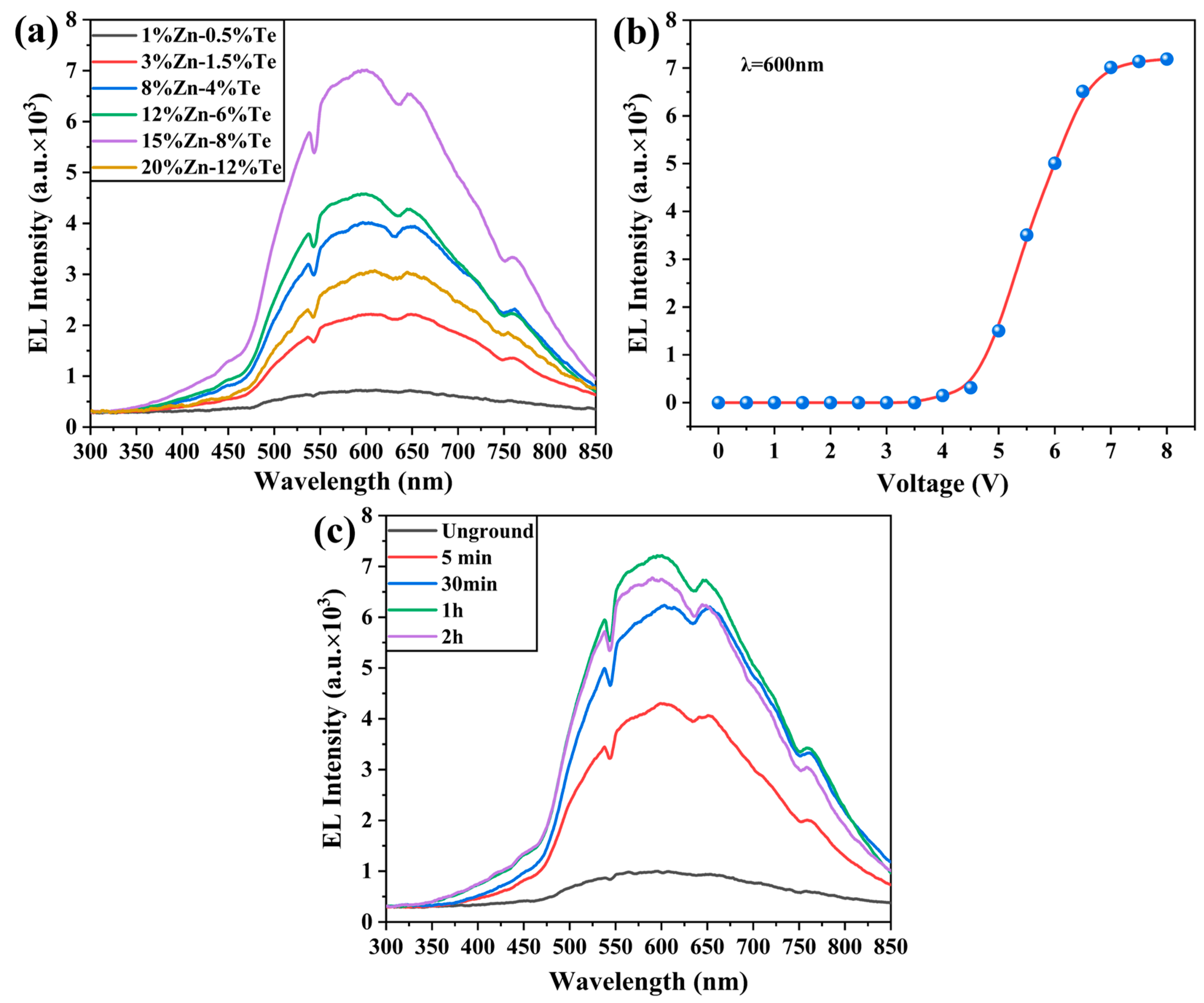
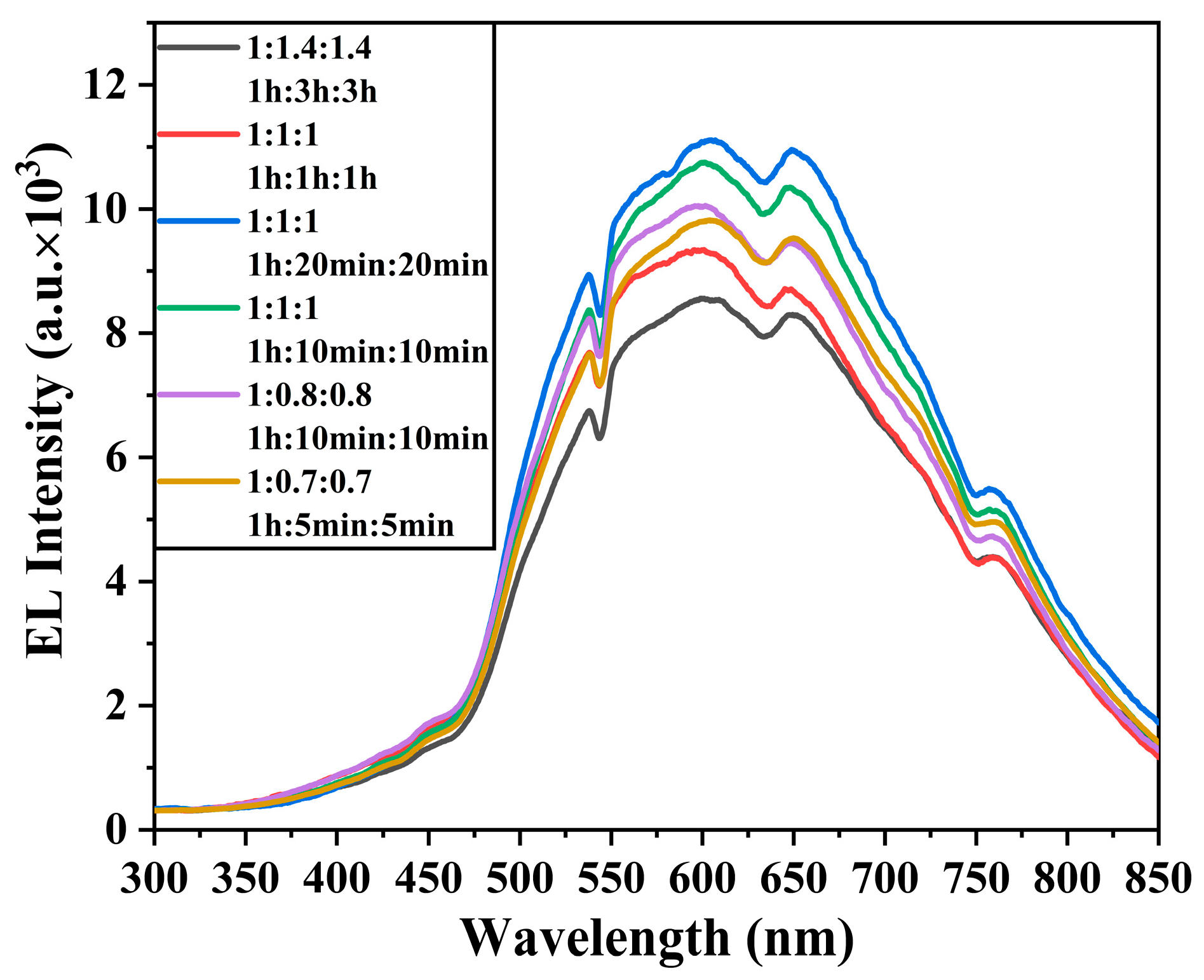
3.4. Electroluminescence
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dupuis, R.D.; Krames, M.R. History, development, and applications of high-brightness visible light-emitting diodes. J. Light. Technol. 2008, 26, 1154–1171. [Google Scholar] [CrossRef]
- Han, D.-P.; Lee, G.W. Comparative study of III-phosphide- and III-nitride-based light-emitting diodes: Understanding the factors limiting efficiency. Semicond. Sci. Technol. 2021, 36, 115004. [Google Scholar] [CrossRef]
- Piprek, J. Efficiency models for GaN-based light-emitting diodes: Status and challenges. Materials 2020, 13, 5174. [Google Scholar] [CrossRef]
- Usman, M.; Munsif, M.; Mushtaq, U.; Anwar, A.-R.; Muhammad, N. Green gap in GaN-based light-emitting diodes: In perspective. Crit. Rev. Solid State Mater. Sci. 2020, 46, 450–467. [Google Scholar] [CrossRef]
- Vanderwater, D.A.; Tan, I.H.; Hofler, G.E.; Defevere, D.C.; Kish, F.A. High-brightness AlGaInP light emitting diodes. Proc. IEEE 1997, 85, 1752–1764. [Google Scholar] [CrossRef]
- Carreira, J.F.C.; Xie, E.; Bian, R.; Herrnsdorf, J.; Haas, H.; Gu, E.; Strain, M.J.; Dawson, M.D. Gigabit per second visible light communication based on AlGaInP red micro-LED micro-transfer printed onto diamond and glass. Opt. Express 2020, 28, 12149–12156. [Google Scholar] [CrossRef]
- Li, P.; Zhang, X.; Qi, L.; Lau, K.M. Full-color micro-display by heterogeneous integration of InGaN blue/green dual-wavelength and AlGaInP red LEDs. Opt. Express 2022, 30, 23499–23510. [Google Scholar] [CrossRef]
- Qi, L.; Li, P.; Zhang, X.; Wong, K.M.; Lau, K.M. Monolithic full-color active-matrix micro-LED micro-display using InGaN/AlGaInP heterogeneous integration. Light Sci. Appl. 2023, 12, 258. [Google Scholar] [CrossRef]
- Berg, A.; Yazdi, S.; Nowzari, A.; Storm, K.; Jain, V.; Vainorius, N.; Samuelson, L.; Wagner, J.B.; Borgstrom, M.T. Radial Nanowire light-emitting diodes in the (AlxGa1-x)yIn1-yP material system. Nano Lett. 2016, 16, 656–662. [Google Scholar] [CrossRef]
- Hai, Q.; Chen, H.; Sun, C.; Chen, D.; Qi, Y.; Shi, M.; Zhao, X. Green-light GaN p-n junction luminescent particles enhance the superconducting properties of B(P)SCCO smart meta-superconductors (SMSCs). Nanomaterials 2023, 13, 3029. [Google Scholar] [CrossRef]
- Qi, Y.; Chen, D.; Li, Y.; Sun, C.; Hai, Q.; Shi, M.; Chen, H.; Zhao, X. Green-light p-n junction particle inhomogeneous phase enhancement of MgB2 smart meta-superconductors. J. Mater. Sci. Mater. Electron. 2024, 35, 424. [Google Scholar] [CrossRef]
- Qi, Y.; Chen, D.; Sun, C.; Hai, Q.; Zhao, X. The influence of electroluminescent inhomogeneous phase addition on enhancing MgB2 superconducting performance and magnetic flux pinning. Materials 2024, 17, 1903. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Hai, Q.; Shi, M.; Chen, H.; Li, Y.; Qi, Y. An improved smart meta-superconductor MgB2. Nanomaterials 2022, 12, 2590. [Google Scholar] [CrossRef]
- Binks, D.J.; Dawson, P.; Oliver, R.A.; Wallis, D.J. Cubic GaN and InGaN/GaN quantum wells. Appl. Phys. Rev. 2022, 9, 041309. [Google Scholar] [CrossRef]
- Ko, M.; Hong, E.; Eo, Y.J.; Lee, S.; Kim, S.; Kim, H.J.; Lee, K.N.; Kim, C.; Do, Y.R. Development and isolation of dot LEDs for display applications through electrochemical etching and sonochemical separation. Adv. Funct. Mater. 2023, 34, 2303727. [Google Scholar] [CrossRef]
- Park, B.; Lee, J.K.; Koch, C.T.; Wolz, M.; Geelhaar, L.; Oh, S.H. High-resolution mapping of strain partitioning and relaxation in InGaN/GaN nanowire heterostructures. Adv. Sci. 2022, 9, e2200323. [Google Scholar] [CrossRef]
- Liu, N.; Gocalinska, A.; Justice, J.; Gity, F.; Povey, I.; McCarthy, B.; Pemble, M.; Pelucchi, E.; Wei, H.; Silien, C.; et al. Lithographically defined, room temperature low threshold subwavelength red-emitting hybrid plasmonic lasers. Nano Lett. 2016, 16, 7822–7828. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, B.; Sasangka, W.A.; Bao, S.; Zhang, Y.; Demir, H.V.; Michel, J.; Lee, K.E.K.; Yoon, S.F.; Fitzgerald, E.A.; et al. High-performance AlGaInP light-emitting diodes integrated on silicon through a superior quality germanium-on-insulator. Photonics Res. 2018, 6, 290–295. [Google Scholar] [CrossRef]
- Shenai-Khatkhate, D.V.; Goyette, R.J.; DiCarlo, R.L.; Dripps, G. Environment, health and safety issues for sources used in MOVPE growth of compound semiconductors. J. Cryst. Growth 2004, 272, 816–821. [Google Scholar] [CrossRef]
- Oda, O.; Hori, M. Novel epitaxy for nitride semiconductors using plasma technology. Phys. Status Solidi A 2020, 218, 2000462. [Google Scholar] [CrossRef]
- Peaker, A.R. Light-emitting-diodes. Iee Proc. A Sci. Meas. Technol. 1980, 127, 202–210. [Google Scholar] [CrossRef]
- Beppu, T.; Toyama, M.; Kasami, A. GaP green light-emitting diodes with p-n-p-n structure. Jpn. J. Appl. Phys. 1972, 11, 1338–1346. [Google Scholar] [CrossRef]
- Peaker, A.R.; Mottram, A. Injection and quantum efficiencies of red-light-emitting gallium-phosphide diodes. J. Phys. D Appl. Phys. 1972, 5, 803. [Google Scholar] [CrossRef]
- de Boer, W.D.; Timmerman, D.; Dohnalova, K.; Yassievich, I.N.; Zhang, H.; Buma, W.J.; Gregorkiewicz, T. Red spectral shift and enhanced quantum efficiency in phonon-free photoluminescence from silicon nanocrystals. Nat. Nanotechnol. 2010, 5, 878–884. [Google Scholar] [CrossRef]
- Miranda, A.; Serrano, F.A.; Vázquez-Medina, R.; Cruz-Irisson, M. Hydrogen surface passivation of Si and Ge nanowires: A semiempirical approach. Int. J. Quantum Chem. 2010, 110, 2448–2454. [Google Scholar] [CrossRef]
- Splendiani, A.; Sun, L.; Zhang, Y.; Li, T.; Kim, J.; Chim, C.Y.; Galli, G.; Wang, F. Emerging photoluminescence in monolayer MoS2. Nano Lett. 2010, 10, 1271–1275. [Google Scholar] [CrossRef]
- Lauth, J.; Strupeit, T.; Kornowski, A.; Weller, H. A transmetalation route for colloidal gaas nanocrystals and additional III–V semiconductor materials. Chem. Mater. 2012, 25, 1377–1383. [Google Scholar] [CrossRef]
- Gao, S.M.; Lu, J.; Zhao, Y.; Chen, N.; Xie, Y. The growth process, stability of GaP nanocrystals and formation of Ga3P nanocrystals under solvothermal conditions in benzene. Eur. J. Inorg. Chem. 2003, 1822–1827. [Google Scholar] [CrossRef]
- Kher, S.S.; Wells, R.L. A straightforward, new method for the synthesis of nanocrystalline GaAs and GaP. Chem. Mater. 1994, 6, 2056–2062. [Google Scholar] [CrossRef]
- Kim, Y.H.; Jun, Y.W.; Jun, B.H.; Lee, S.M.; Cheon, J.W. Sterically induced shape and crystalline phase control of GaP nanocrystals. J. Am. Chem. Soc. 2002, 124, 13656–13657. [Google Scholar] [CrossRef]
- Macdougall, J.E.; Eckert, H.; Stucky, G.D.; Herron, N.; Wang, Y.; Moller, K.; Bein, T.; Cox, D. Synthesis and characterization of III-V semiconductor clusters - GAP in zeolite-Y. J. Am. Chem. Soc. 1989, 111, 8006–8007. [Google Scholar] [CrossRef]
- Micic, O.I.; Sprague, J.R.; Heben, M.; Lu, Z.; Curtis, C.J.; Nozik, A.J. Synthesis and characterization of InP, GaP, and GaInP2 quantum dots. J. Phys. Chem. 1995, 99, 7754–7759. [Google Scholar] [CrossRef]
- Kim, S.; Lee, K.; Kim, S.; Kwon, O.P.; Heo, J.H.; Im, S.H.; Jeong, S.; Lee, D.C.; Kim, S.-W. Origin of photoluminescence from colloidal gallium phosphide nanocrystals synthesized via a hot-injection method. RSC Adv. 2015, 5, 2466–2469. [Google Scholar] [CrossRef]
- Choi, Y.; Choi, C.; Bae, J.; Park, J.; Shin, K. Synthesis of gallium phosphide quantum dots with high photoluminescence quantum yield and their application as color converters for LEDs. J. Ind. Eng. Chem. 2023, 123, 509–516. [Google Scholar] [CrossRef]
- Almeida, G.; Ubbink, R.F.; Stam, M.; du Fossé, I.; Houtepen, A.J. InP colloidal quantum dots for visible and near-infrared photonics. Nat. Rev. Mater. 2023, 8, 742–758. [Google Scholar] [CrossRef]
- Gwak, N.; Shin, S.; Yoo, H.; Seo, G.W.; Kim, S.; Jang, H.; Lee, M.; Park, T.H.; Kim, B.J.; Lim, J.; et al. Highly luminescent shell-less indium phosphide quantum dots enabled by atomistically tailored surface states. Adv. Mater. 2024, 36, e2404480. [Google Scholar] [CrossRef]
- Won, Y.H.; Cho, O.; Kim, T.; Chung, D.Y.; Kim, T.; Chung, H.; Jang, H.; Lee, J.; Kim, D.; Jang, E. Highly efficient and stable InP/ZnSe/ZnS quantum dot light-emitting diodes. Nature 2019, 575, 634–638. [Google Scholar] [CrossRef]
- Kim, K.; Suh, Y.-H.; Kim, D.; Choi, Y.; Bang, E.; Kim, B.H.; Park, J. Zinc oxo clusters improve the optoelectronic properties on indium phosphide quantum dots. Chem. Mater. 2020, 32, 2795–2802. [Google Scholar] [CrossRef]
- Pietra, F.; De Trizio, L.; Hoekstra, A.W.; Renaud, N.; Prato, M.; Grozema, F.C.; Baesjou, P.J.; Koole, R.; Manna, L.; Houtepen, A.J. Tuning the lattice parameter of InxZnyP for highly luminescent lattice-matched core/shell quantum dots. ACS Nano 2016, 10, 4754–4762. [Google Scholar] [CrossRef]
- Yin, J.B.; Zhao, X.P. Temperature effect of rare earth-doped TiO2 electrorheological fluids. J. Phys. D Appl. Phys. 2001, 34, 2063–2067. [Google Scholar] [CrossRef]
- Richards, D.; Zemlyanov, D.; Ivanisevic, A. Assessment of the passivation capabilities of two different covalent chemical modifications on GaP(100). Langmuir 2010, 26, 8141–8146. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.; Lee, P.-C.; Kuo, C.-H.; McLeod, A.J.; Zhang, Z.; Wang, V.; Huang, J.; Kashyap, H.; Winter, C.H.; Kummel, A.C. Gallium phosphide conformal film growth on in-situ tri-TBP dry-cleaned InGaP/GaAs using atomic hydrogen ALD. Vacuum 2024, 220, 112806. [Google Scholar] [CrossRef]
- Duan, X.; Ma, J.; Zhang, W.; Liu, P.; Liu, H.; Hao, J.; Wang, K.; Samuelson, L.; Sun, X.W. Study of the interfacial oxidation of InP quantum dots synthesized from tris(dimethylamino)phosphine. ACS Appl. Mater. Interfaces 2023, 15, 1619–1628. [Google Scholar] [CrossRef] [PubMed]
- Virieux, H.; Le Troedec, M.; Cros-Gagneux, A.; Ojo, W.S.; Delpech, F.; Nayral, C.; Martinez, H.; Chaudret, B. InP/ZnS nanocrystals: Coupling NMR and XPS for fine surface and interface description. J. Am. Chem. Soc. 2012, 134, 19701–19708. [Google Scholar] [CrossRef]
- Kim, K.; Yoo, D.; Choi, H.; Tamang, S.; Ko, J.H.; Kim, S.; Kim, Y.H.; Jeong, S. Halide-amine co-passivated indium phosphide colloidal quantum dots in tetrahedral shape. Angew. Chem. Int. Ed. Engl. 2016, 55, 3714–3718. [Google Scholar] [CrossRef]
- Chen, Q.; Chen, Y.; Wang, J.; Liu, M.; Chen, Z. The growth of high-quality hexagonal GaTe nanosheets induced by ZnO nanocrystals. Crystals 2022, 12, 627. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, S.U.; So, S.J.; Cho, Y.D.; Kim, Y.J.; Ahn, S.C.; Lee, C.H. Reduction of surface defects on the GaP window layer of 630 nm AlGaInP LED using post-Zn diffusion process. Curr. Appl. Phys. 2013, 13, 1032–1036. [Google Scholar] [CrossRef]
- Oh, H.S.; Ryu, H.S.; Park, J.M.; Lee, H.J.; Kim, Y.J.; Jang, I.K.; Park, J.H.; Kwak, J.S.; Baek, J.H. Investigation of Mg doping profile in the p-cladding layer for high-brightness AlGaInP-based light emitting diodes. J. Nanosci. Nanotechnol. 2014, 14, 6124–6127. [Google Scholar] [CrossRef]
- Feenstra, R.M.; McGill, T.C. Defect reactions in GaP-(Zn,O). Phys. Rev. Lett. 1981, 47, 925–927. [Google Scholar] [CrossRef]
- Hackett, W.H.; Rosenzwe, W.; Jayson, J.S. Saturation of Zn-O complexes in GaP diodes. Proc. IEEE 1969, 57, 2072. [Google Scholar] [CrossRef]
- Lin, J.F.; Wu, M.C.; Jou, M.J.; Chang, C.M.; Lee, B.J. Effects of substrate misorientation and Zn doping characteristics on the performance of algainp visible light-emitting-diodes. Jpn. J. Appl. Phys. Part 2 Lett. 1994, 33, L857–L859. [Google Scholar] [CrossRef]
- Oh, C.-H.; Shim, J.-I.; Shin, D.-S. Current- and temperature-dependent efficiency droops in InGaN-based blue and AlGaInP-based red light-emitting diodes. Jpn. J. Appl. Phys. 2019, 58, SCCC08. [Google Scholar] [CrossRef]
- Abdollahi, A.; Golzan, M.M.; Aghayar, K. Electronic properties of GaxIn1−xP ternary alloy from first-principles. Comput. Mater. Sci. 2016, 120, 70–76. [Google Scholar] [CrossRef]
- Degheidy, A.R.; Elwakil, S.A.A.; Elkenany, E.B. Energy band structure calculations of GaxIn1−xP alloys under the influence of temperature and pressure. J. Alloys Compd. 2013, 574, 580–590. [Google Scholar] [CrossRef]
- Chen, H.S.; Chen, C.Y.; Wu, Y.C. High-performance giant InP quantum dots with stress-released morphological ZnSe-ZnSeS-ZnS Shell. Adv. Mater. 2024, e2407026. [Google Scholar] [CrossRef]
- Xie, L.; Harris, D.K.; Bawendi, M.G.; Jensen, K.F. Effect of trace water on the growth of indium phosphide quantum dots. Chem. Mater. 2015, 27, 5058–5063. [Google Scholar] [CrossRef]
- Oh, H.S.; Park, J.M.; Jung, S.H.; Lee, D.W.; Lee, K.S.; Kwon, S.H.; Park, Y.T. Investigation of chirped well structures for broad-spectrum AlGaInP-Based light emitting diodes. J. Nanosci. Nanotechnol. 2019, 19, 2219–2223. [Google Scholar] [CrossRef]
| Materials | Chemical Formula or Abbreviation | Manufacturer | Purity |
|---|---|---|---|
| Gallium acetylacetonate | Ga(acac)3 | Aladdin Biochemical Technology Co., Ltd., Shanghai, China | 99.99% |
| Zinc acetylacetonate | Zn(acac)3 | Aladdin Biochemical Technology Co., Ltd. | 99.99% |
| Indium chloride | InCl3 | Aladdin Biochemical Technology Co., Ltd. | 99.99% |
| Tellurium powder | Te | Tianjin Damao Chemical Reagent Factory, Tianjin, China | 99.99% |
| Oleic acid | OA | Tianjin Damao Chemical Reagent Factory | AR |
| 1-Octadecene | ODE | Aladdin Biochemical Technology Co., Ltd. | GC |
| Tris(trimethylsilyl)phosphine | (TMS)3P | Macklin Biochemical Technology Co., Ltd., Shanghai, China | 10 wt% |
| Trioctylphosphine | TOP | Aladdin Biochemical Technology Co., Ltd. | 85% |
| Ethanol | CH3CH2OH | Guangdong Guanghua Technology Co., Ltd., Guangzhou, China | AR |
| N-hexane | C6H14 | Sinopharm Chemical Reagent Co., Ltd., Shanghai, China | AR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, D.; Bi, R.; Xun, L.; Li, X.; Hai, Q.; Qi, Y.; Zhao, X. Core–Shell Composite GaP Nanoparticles with Efficient Electroluminescent Properties. Materials 2025, 18, 487. https://doi.org/10.3390/ma18030487
Chen D, Bi R, Xun L, Li X, Hai Q, Qi Y, Zhao X. Core–Shell Composite GaP Nanoparticles with Efficient Electroluminescent Properties. Materials. 2025; 18(3):487. https://doi.org/10.3390/ma18030487
Chicago/Turabian StyleChen, Duo, Ruiyuan Bi, Lifeng Xun, Xiaoyan Li, Qingyu Hai, Yao Qi, and Xiaopeng Zhao. 2025. "Core–Shell Composite GaP Nanoparticles with Efficient Electroluminescent Properties" Materials 18, no. 3: 487. https://doi.org/10.3390/ma18030487
APA StyleChen, D., Bi, R., Xun, L., Li, X., Hai, Q., Qi, Y., & Zhao, X. (2025). Core–Shell Composite GaP Nanoparticles with Efficient Electroluminescent Properties. Materials, 18(3), 487. https://doi.org/10.3390/ma18030487







