Recent Advances in Barnacle-Inspired Biomaterials in the Field of Biomedical Research
Abstract
1. Introduction
2. The Basic Properties of Barnacle Cement
2.1. Specific Types and Functions of Proteins in Barnacle Cement
2.2. Self-Assembly of Barnacle Cement Proteins
2.2.1. The Self-Assembly Process of Barnacle Cement Proteins
2.2.2. Methods for Observing and Analyzing the Self-Assembly of Barnacle Cement Proteins
2.2.3. Characteristics of Fiber Structures Formed by the Self-Assembly of Barnacle Cement Proteins
2.3. The Adhesion Mechanism and Solidification Mechanism of Barnacle Cement Proteins
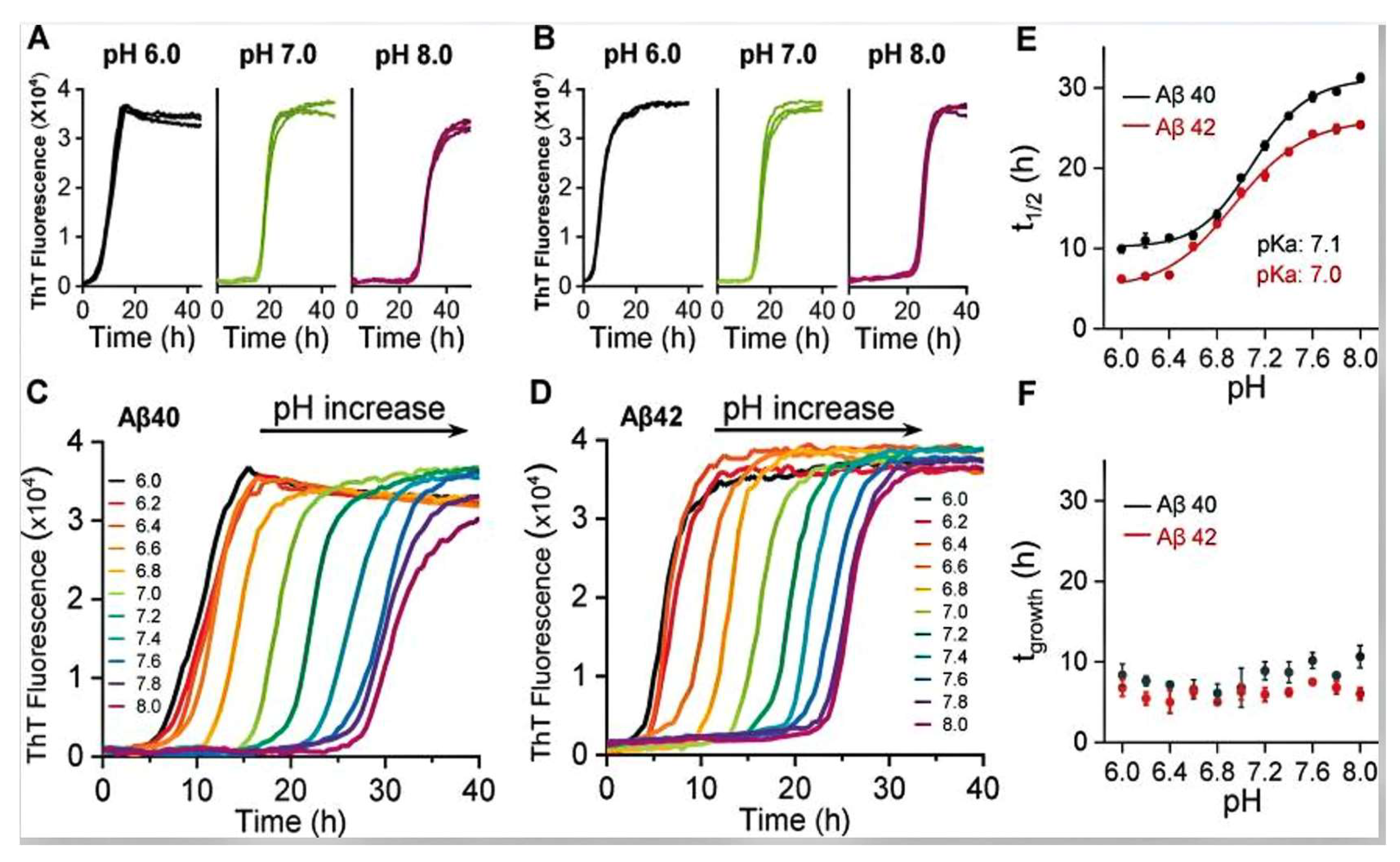
2.4. The Influence of pH on the Self-Assembly Behavior and Adhesive Properties of Barnacle Cement Proteins
2.5. The Differential Adsorption of Barnacle Cement on Various Substrate Materials
3. The Development of Barnacle Biomimetic Materials
3.1. Status Analysis
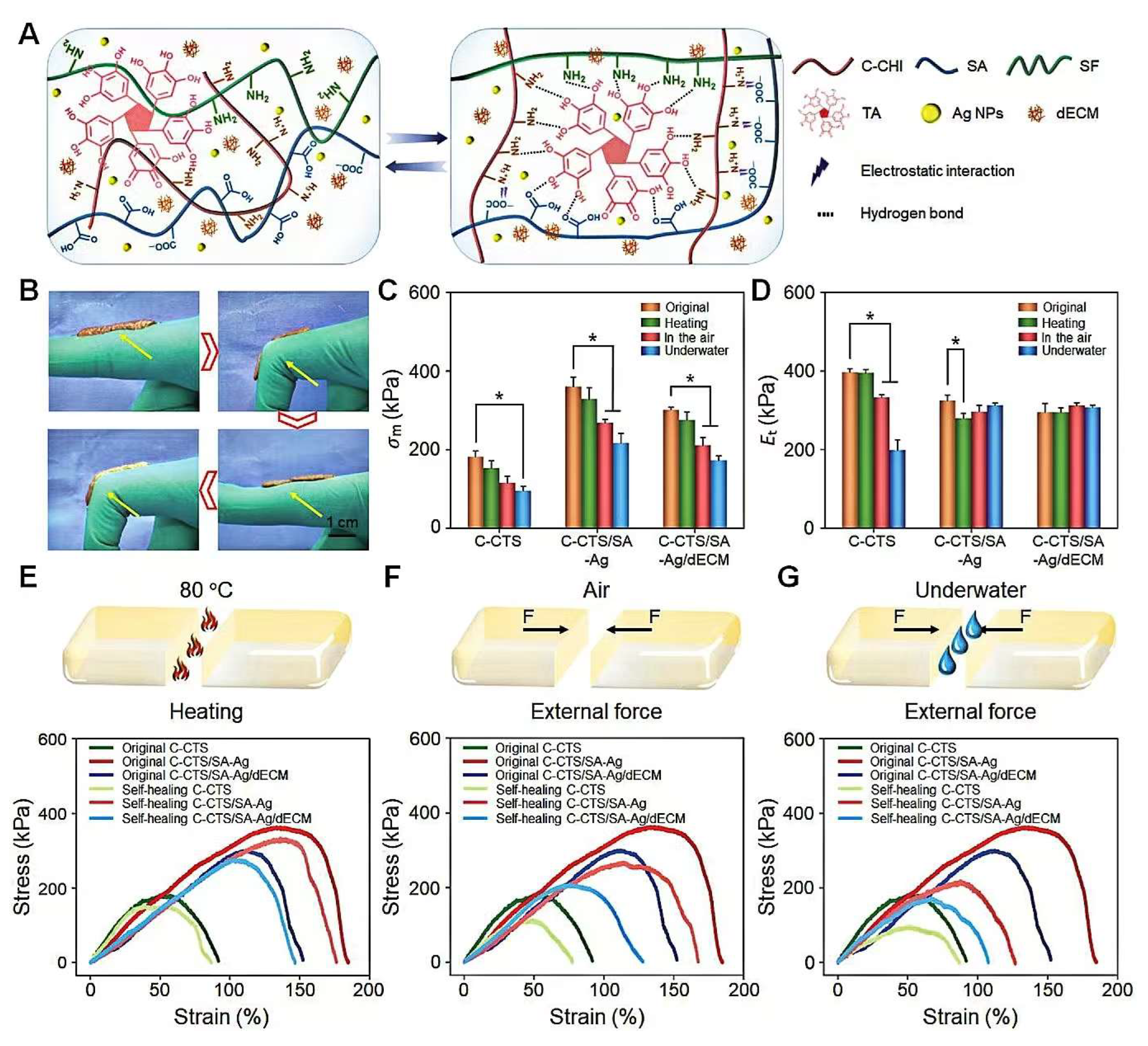
3.1.1. High-Strength Medical Adhesives
3.1.2. Tissue Engineering
3.1.3. Drug Delivery Systems
3.1.4. Antimicrobial Medical Materials
3.2. Trend Forecasting
3.2.1. Further Functionalization of Barnacle Biomimetic Materials
3.2.2. Multiscale Design and Fabrication of Barnacle Biomimetic Materials
3.2.3. The Clinical Translation and Industrialization of Barnacle Biomimetic Materials
4. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Youhei, U.; Masahiro, N.; Satoru, M.; Naoko, I.; Satoru, K.; Naho, K.; Takashi, N.; Kei, K. Identification and functional characterization of a novel barnacle cement protein. FEBS J. 2007, 274, 4336–4346. [Google Scholar]
- Li, J.; Celiz, A.D.; Yang, J.; Yang, Q.; Wamala, I.; Whyte, W.; Seo, B.R.; Vasilyev, N.V.; Vlassak, J.J.; Suo, Z.; et al. Tough adhesives for diverse wet surfaces. Science 2017, 357, 378–381. [Google Scholar] [CrossRef]
- Kamino, K. Barnacle Underwater Attachment. In Biological Adhesives; Springer International Publishing: Cham, Switzerland, 2016; pp. 153–176. [Google Scholar] [CrossRef]
- So, C.R.; Scancella, J.M.; Fears, K.P.; Essock-Burns, T.; Haynes, S.E.; Leary, D.H.; Diana, Z.; Wang, C.; North, S.; Oh, C.S.; et al. Oxidase Activity of the Barnacle Adhesive Interface Involves Peroxide-Dependent Catechol Oxidase and Lysyl Oxidase Enzymes. ACS Appl. Mater. Interfaces 2017, 9, 11493–11505. [Google Scholar] [CrossRef] [PubMed]
- Kamino, K.; Inoue, K.; Maruyama, T.; Takamatsu, N.; Harayama, S.; Shizuri, Y. Barnacle Cement Proteins: Importance of disulfide bonds in their insolubility. J. Biol. Chem. 2000, 275, 27360–27365. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Li, Y.; Zhang, X.; Zhang, R.; Hu, Y.; Boyer, C.; Xu, F.-J. Photo-responsive supramolecular hyaluronic acid hydrogels for accelerated wound healing. J. Control. Release 2020, 323, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Liu, Y.; Tang, L.; Xiao, H.; Yang, Z.; Wang, S. Preparation of Recombinant Human Collagen III Protein Hydrogels with Sustained Release of Extracellular Vesicles for Skin Wound Healing. Int. J. Mol. Sci. 2022, 23, 6289. [Google Scholar] [CrossRef]
- Li, H.; Wang, F.; Sun, X.-J.; Qi, L.-J.; Li, J.-A.; Iqbal, M.; Guan, S.-K. Bio-inspired barnacle cement coating of biodegradable magnesium alloy for cerebrovascular application. Rare Met. 2024, 43, 5164–5185. [Google Scholar] [CrossRef]
- Murray, R.Z.; West, Z.E.; Cowin, A.J.; Farrugia, B.L. Development and use of biomaterials as wound healing therapies. Burn. Trauma 2019, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Yuk, H.; Wu, J.; Sarrafian, T.; Mao, X.; Varela, C.E. Rapid and coagulation-independent haemostatic sealing by a paste inspired by barnacle glue. Nat. Biomed. Eng. 2021, 5, 1131–1142. [Google Scholar] [CrossRef]
- Ghobril, C.; Charoen, K.; Rodriguez, E.K.; Nazarian, A.; Grinstaff, M.W. A dendritic thioester hydrogel based on thiol-thioester exchange as a dissolvable sealant system for wound closure. Angew. Chem. Int. Ed. Engl. 2013, 52, 14070–14074. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Fermanian, S.; Gibson, M.; Unterman, S.; Herzka, D.A.; Cascio, B.; Coburn, J.; Hui, A.Y.; Marcus, N.; Gold, G.E.; et al. Human cartilage repair with a photoreactive adhesive-hydrogel composite. Sci. Transl. Med. 2013, 5, 167ra166. [Google Scholar] [CrossRef]
- Liu, Y.; Li, W. Nature-Inspired Multifunctional Bilayer Architecture Advances Bone Defect Repair. Chem 2019, 5, 2515–2517. [Google Scholar] [CrossRef]
- Yao, S.; Jin, B.; Liu, Z.; Shao, C.; Zhao, R.; Wang, X.; Tang, R. Biomineralization: From Material Tactics to Biological Strategy. Adv. Mater. 2017, 29, 1605903. [Google Scholar] [CrossRef]
- Rashid, M.; Roni, M.A.; Rahman, M. Chapter Fourteen—Clinical status of bioinspired and biomimetic materials. In Bioinspired and Biomimetic Materials for Drug Delivery; Woodhead Publishing: Sawston, UK, 2021; pp. 277–294. [Google Scholar] [CrossRef]
- Prausnitz, M.R.; Langer, R. Transdermal drug delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Priem, C.; Geyer, A. Reversible Covalent End-Capping of Collagen Model Peptides. Chemistry 2019, 25, 14278–14283. [Google Scholar] [CrossRef]
- Ye, Z. Effects and Mechanisms of rBalcpl9k on Mouse Skin Wound Healing. Master’s Thesis, Central South University, Changsha, China, 2023; pp. 23–27. [Google Scholar] [CrossRef]
- Buffet, J.P.; Corre, E.; Duvernois, B.E.; Fournier, J.; Lopez, P.J. Adhesive gland transcriptomics uncovers a diversity of genes involved in glue formation in marine tube-building polychaetes. Acta Biomater. 2018, 72, 316–328. [Google Scholar] [CrossRef]
- Jonker, J.L.; Abram, F.; Pires, E.; Varela Coelho, A.; Grunwald, I.; Power, A.M. Adhesive proteins of stalked and acorn barnacles display homology with low sequence similarities. PLoS ONE 2014, 9, e108902. [Google Scholar] [CrossRef] [PubMed]
- Kamino, K. Novel barnacle underwater adhesive protein is a charged amino acid-rich protein constituted by a Cys-rich repetitive sequence. Biochem. J. 2001, 356, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Mondarte, E.A.Q.; Wang, J.; Yu, J. Adaptive Adhesions of Barnacle-Inspired Adhesive Peptides. ACS Biomater. Sci. Eng. 2023, 9, 5679–5686. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liang, C.; Zhang, X.; Li, J.; Huang, J.; Zeng, L.; Ye, Z.; Hu, B.; Wu, W. Amyloid fibril aggregation: An insight into the underwater adhesion of barnacle cement. Biochem. Biophys. Res. Commun. 2017, 493, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Song, J.; Mao, T.; Zeng, L.; Ye, Z.; Hu, B. An essential role of disulfide bonds for the hierarchical self-assembly and underwater affinity of CP20-derived peptides. Front. Bioeng. Biotechnol. 2022, 10, 998194. [Google Scholar] [CrossRef]
- Xu, Y. Construction of Barnacle-Derived Adhesive Proteins Based on Functional Module Fusion. Master’s Thesis, Huazhong University of Science and Technology, Wuhan, China, 2021; pp. 73–75. [Google Scholar]
- Tilbury, M.A.; Tran, T.Q.; Shingare, D.; Lefevre, M.; Power, A.M.; Leclère, P.; Wall, J.G. Self-assembly of a barnacle cement protein into intertwined amyloid fibres and determination of their adhesive and viscoelastic properties. J. R. Soc. Interface 2023, 20, 20230332. [Google Scholar] [CrossRef]
- Ahmad, M.; Hirz, M.; Pichler, H.; Schwab, H. Protein expression in Pichia pastoris: Recent achievements and perspectives for heterologous protein production. Appl. Microbiol. Biotechnol. 2014, 98, 5301–5317. [Google Scholar] [CrossRef]
- Li, J. High-Level Expression of Barnacle Adhensive Protein Mrcp20K in Pichia Pastoris. Master’s Thesis, Huazhong University of Science and Technology, Wuhan, China, 2021; pp. 64–65. [Google Scholar]
- Kumar, A.; Mohanram, H.; Li, J.; Le Ferrand, H.; Verma, C.S.; Miserez, A. Disorder–Order Interplay of a Barnacle Cement Protein Triggered by Interactions with Calcium and Carbonate Ions: A Molecular Dynamics Study. Chem. Mater. 2020, 32, 8845–8859. [Google Scholar] [CrossRef]
- Liang, C.; Ye, Z.; Xue, B.; Zeng, L.; Wu, W.; Zhong, C.; Cao, Y.; Hu, B.; Messersmith, P.B. Self-Assembled Nanofibers for Strong Underwater Adhesion: The Trick of Barnacles. ACS Appl. Mater. Interfaces 2018, 10, 25017–25025. [Google Scholar] [CrossRef] [PubMed]
- Moretti, M.; Wendt, D.; Schaefer, D.; Jakob, M.; Hunziker, E.B.; Heberer, M.; Martin, I. Structural characterization and reliable biomechanical assessment of integrative cartilage repair. J. Biomech. 2005, 38, 1846–1854. [Google Scholar] [CrossRef]
- Power, A.M.; Klepal, W.; Zheden, V.; Jonker, J.; McEvilly, P.; von Byern, J. Mechanisms of Adhesion in Adult Barnacles. In Biological Adhesive Systems; Springer: Vienna, Austria, 2010; pp. 153–168. [Google Scholar] [CrossRef]
- Liang, C.; Strickland, J.; Ye, Z.; Wu, W.; Hu, B.; Rittschof, D. Biochemistry of Barnacle Adhesion: An Updated Review. Front. Mar. Sci. 2019, 6, 565. [Google Scholar] [CrossRef]
- Nakano, M.; Shen, J.R.; Kamino, K. Self-assembling peptide inspired by a barnacle underwater adhesive protein. Biomacromolecules 2007, 8, 1830–1835. [Google Scholar] [CrossRef] [PubMed]
- Wiegemann, M.; Watermann, B. Peculiarities of barnacle adhesive cured on non-stick surfaces. J. Adhes. Sci. Technol. 2003, 17, 1957–1977. [Google Scholar] [CrossRef]
- Bechtel, T.J.; Weerapana, E. From structure to redox: The diverse functional roles of disulfides and implications in disease. Proteomics 2017, 17, 1600391. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Li, W. Analysis of Sequence Features of Disulfide-Bonds in Proteins. J. Wuxi Univ. Light Ind. 2002, 21, 464–467. [Google Scholar] [CrossRef]
- Kim, Y.E.; Hipp, M.S.; Bracher, A.; Hayer-Hartl, M.; Ulrich Hartl, F. Molecular Chaperone Functions in Protein Folding and Proteostasis. Annu. Rev. Biochem. 2013, 82, 323–355. [Google Scholar] [CrossRef] [PubMed]
- Soares Moretti, A.I.; Martins Laurindo, F.R. Protein disulfide isomerases: Redox connections in and out of the endoplasmic reticulum. Arch. Biochem. Biophys. 2017, 617, 106–119. [Google Scholar] [CrossRef]
- Guan, D.; Ramirez, M.; Shao, L.; Jacobsen, D.; Barrera, I.; Lutkenhaus, J.; Chen, Z. Two-Component Protein Hydrogels Assembled Using an Engineered Disulfide-Forming Protein–Ligand Pair. Biomacromolecules 2013, 14, 2909–2916. [Google Scholar] [CrossRef] [PubMed]
- Patil, N.A.; Tailhades, J.; Hughes, R.A.; Separovic, F.; Wade, J.D.; Hossain, M.A. Cellular disulfide bond formation in bioactive peptides and proteins. Int. J. Mol. Sci. 2015, 16, 1791–1805. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Urushida, Y.; Nakano, M.; Uchiyama, S.; Kamino, K. Calcite-specific coupling protein in barnacle underwater cement. FEBS J. 2007, 274, 6436–6446. [Google Scholar] [CrossRef] [PubMed]
- Hur, S.; Méthivier, C.; Wilson, A.; Salmain, M.; Boujday, S.; Miserez, A. Biomineralization in Barnacle Base Plate in Association with Adhesive Cement Protein. ACS Appl. Bio Mater. 2023, 6, 3423–3432. [Google Scholar] [CrossRef] [PubMed]
- Nakano, M.; Kamino, K. Amyloid-like conformation and interaction for the self-assembly in barnacle underwater cement. Biochemistry 2015, 54, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, D.D.; Glazebrook, J.F. Chapter Eight—Quantum transport and utilization of free energy in protein α-helices. In Advances in Quantum Chemistry; Poznański, R.R., Brändas, E.J., Eds.; Academic Press: Cambridge, MA, USA, 2020; Volume 82, pp. 253–300. [Google Scholar]
- Ran, Y.; Su, W.; Ma, L.; Tan, Y.; Yi, Z.; Li, X. Developing exquisite collagen fibrillar assemblies in the presence of keratin nanoparticles for improved cellular affinity. Int. J. Biol. Macromol. 2021, 189, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Zhou, X.; Ye, C.; Yu, W.; Tang, Y. Using FTIR Imaging to Investigate Silk Fibroin-Based Materials. In Fibrous Proteins: Design, Synthesis, and Assembly; Ling, S., Ed.; Springer: New York, NY, USA, 2021; pp. 207–219. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, X.; Li, M.; Liu, Y.; Zhang, J.; Gao, X.; Sun, L.; Zhao, G. Study on secondary structure of meat protein by FTIR. Coll. Food Sci. Technol. 2015, 41, 247–251. [Google Scholar] [CrossRef]
- Kumar, M.R.; Merschrod, E.F.; Poduska, K.M. Correlating mechanical properties with aggregation processes in electrochemically fabricated collagen membranes. Biomacromolecules 2009, 10, 1970–1975. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Li, B.; Zhao, X.; Qin, S. Effect of concentration, pH and ionic strength on the kinetic self-assembly of acid-soluble collagen from walleye pollock (Theragra chalcogramma) skin. Food Hydrocoll. 2012, 29, 199–204. [Google Scholar] [CrossRef]
- Shen, L.; Bu, H.; Yang, H.; Liu, W.; Li, G. Investigation on the behavior of collagen self-assembly in vitro via adding sodium silicate. Int. J. Biol. Macromol. 2018, 115, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Liu, W.; Li, G. The aggregation behavior of native collagen in dilute solution studied by intrinsic fluorescence and external probing. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 102, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Ma, W.; Sun, C.; Wu, S. The aggregation behavior of collagen in aqueous solution and its property of stabilizing liposomes in vitro. Biomaterials 2001, 22, 1627–1634. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Wang, X. Study on the kinetic self-assembly of type I collagen from tilapia (Oreochromis niloticus) skin using the fluorescence probe thioflavin T. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 203, 342–347. [Google Scholar] [CrossRef]
- Zhu, L.; Li, J.; Wang, Y.; Sun, X.; Li, B.; Poungchawanwong, S.; Hou, H. Structural feature and self-assembly properties of type II collagens from the cartilages of skate and sturgeon. Food Chem. 2020, 331, 127340. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, N.J. Using circular dichroism spectra to estimate protein secondary structure. Nat. Protoc. 2006, 1, 2876–2890. [Google Scholar] [CrossRef]
- De Mesquita, J.; Patricio, P.; Donnici, C.; Petri, D.; Oliveira, L.; Pereira, F. Hybrid layer-by-layer assembly based on animal and vegetable structural materials: Multilayered films of collagen and cellulose nanowhiskers. Soft Matter 2011, 7, 4405–4413. [Google Scholar] [CrossRef]
- Stamov, D.R.; Stock, E.; Franz, C.M.; Jähnke, T.; Haschke, H. Imaging collagen type I fibrillogenesis with high spatiotemporal resolution. Ultramicroscopy 2015, 149, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Nudelman, F.; Pieterse, K.; George, A.; Bomans, P.H.; Friedrich, H.; Brylka, L.J.; Hilbers, P.A.; de With, G.; Sommerdijk, N.A. The role of collagen in bone apatite formation in the presence of hydroxyapatite nucleation inhibitors. Nat. Mater. 2010, 9, 1004–1009. [Google Scholar] [CrossRef]
- Lamprecht, A.; Schäfer, U.F.; Lehr, C. Characterization of microcapsules by confocal laser scanning microscopy: Structure, capsule wall composition and encapsulation rate. Eur. J. Pharm. Biopharm. 2000, 49, 1–9. [Google Scholar] [CrossRef]
- Zandomeneghi, G.; Krebs, M.R.H.; McCammon, M.G.; Fändrich, M. FTIR reveals structural differences between native β-sheet proteins and amyloid fibrils. Protein Sci. 2004, 13, 3314–3321. [Google Scholar] [CrossRef] [PubMed]
- Sullan, R.M.; Gunari, N.; Tanur, A.E.; Chan, Y.; Dickinson, G.H.; Orihuela, B.; Rittschof, D.; Walker, G.C. Nanoscale structures and mechanics of barnacle cement. Biofouling 2009, 25, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Barlow, D.E.; Dickinson, G.H.; Orihuela, B.; Kulp, J.L., 3rd; Rittschof, D.; Wahl, K.J. Characterization of the adhesive plaque of the barnacle Balanus amphitrite: Amyloid-like nanofibrils are a major component. Langmuir 2010, 26, 6549–6556. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Williams, R.J.; Tang, C.; Coppo, P.; Collins, R.F.; Turner, M.L.; Saiani, A.; Ulijn, R.V. Fmoc-Diphenylalanine Self Assembles to a Hydrogel via a Novel Architecture Based on π–π Interlocked β-Sheets. Adv. Mater. 2008, 20, 37–41. [Google Scholar] [CrossRef]
- Gan, K. Preparation and Performance Studies of Fusion Protein Adhesives Based on Marine Adhesive Proteins and Elastin-Like Polypeptides. Master’s Thesis, National University of Defense Technology, Changsha, China, 2022; pp. 64–65. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, X.; Zeng, L.; Ye, Z.; Hu, B.; Wu, W. Barnacle adhesion: From substrate detection to cement curing. Prog. Biochem. Biophys. 2017, 44, 204–214. [Google Scholar] [CrossRef]
- Zeng, L. Expression and Functional Characterization of the 52kDa Cement Protein in Megabalanus rosa. Master’s Thesis, National University of Defense Technology, Changsha, China, 2016; pp. 6–21. [Google Scholar]
- Wang, X.; Zhang, L.; Wang, L.; Yan, Y. The adhesion mechanism of barnacle and its cement proteins: A review. Sheng Wu Gong Cheng Xue Bao 2022, 38, 4449–4461. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, S.; Huang, X.; Chen, Y.; Cheng, J.; Zhan, A. Protein-mediated bioadhesion in marine organisms: A review. Mar. Environ. Res. 2021, 170, 105409. [Google Scholar] [CrossRef]
- Clare, A.S.; McClary, M. On the antennular secretion of the cyprid of balanus amphitrite amphitrite, and its role as a settlement pheromone. J. Mar. Biol. Assoc. UK 1994, 74, 243–250. [Google Scholar] [CrossRef]
- Jingliang, H.; Huan, L.; Weihua, L. Mechanism underlying barnacle larval adhesion to material surface: Transcriptome and proteome. J. Fish. China 2022, 46, 1743–1756. [Google Scholar] [CrossRef]
- Peng, X.; Ma, C.; Ji, J. Underwater Adhesion Mechanisms and Biomimetic Study of Marine Life. Tribology 2020, 40, 816–830. [Google Scholar] [CrossRef]
- Gohad, N.V.; Aldred, N.; Hartshorn, C.M.; Jong Lee, Y.; Cicerone, M.T.; Orihuela, B.; Clare, A.S.; Rittschof, D.; Mount, A.S. Synergistic roles for lipids and proteins in the permanent adhesive of barnacle larvae. Nat. Commun. 2014, 5, 4414. [Google Scholar] [CrossRef] [PubMed]
- Dreanno, C.; Kirby, R.R.; Clare, A.S. Involvement of the barnacle settlement-inducing protein complex (SIPC) in species recognition at settlement. J. Exp. Mar. Biol. Ecol. 2007, 351, 276–282. [Google Scholar] [CrossRef]
- Huang, J. A Study on Construction, Nature and Function of the Recombinant Cement Protein cp20k in Balanus Albicostatus. Master’s Thesis, National University of Defense Technology, Changsha, China, 2019. [Google Scholar]
- Waite, J.H. Nature’s underwater adhesive specialist. Int. J. Adhes. Adhes. 1987, 7, 9–14. [Google Scholar] [CrossRef]
- Fears, K.P.; Orihuela, B.; Rittschof, D.; Wahl, K.J. Acorn Barnacles Secrete Phase-Separating Fluid to Clear Surfaces Ahead of Cement Deposition. Adv. Sci. 2018, 5, 1700762. [Google Scholar] [CrossRef] [PubMed]
- Liu, X. Study on the Protein Components and Curing Mechanisms of the Balanus reticulatus Cement. Master’s Thesis, National University of Defense Technology, Changsha, China, 2018; pp. 58–59. [Google Scholar]
- So, C.R.; Yates, E.A.; Estrella, L.A.; Fears, K.P.; Schenck, A.M.; Yip, C.M.; Wahl, K.J. Molecular Recognition of Structures Is Key in the Polymerization of Patterned Barnacle Adhesive Sequences. ACS Nano 2019, 13, 5172–5183. [Google Scholar] [CrossRef]
- Zhigang, D.; Zichao, G.; Yaan, L.; Yuan, X.; Bingjie, L.; Rudong, Z.; Muli, W.; Dun, W. The Fouling Process of Typical Marine Organisms and the Influence of Coating Surface Characteristics on Their Attachment Behaviors. Paint. Coat. Ind. 2024, 54, 77–82. [Google Scholar] [CrossRef]
- Kotsiri, M.; Protopapa, M.; Mouratidis, S.; Zachariadis, M.; Vassilakos, D.; Kleidas, I.; Samiotaki, M.; Dedos, S.G. Should I stay or should I go? The settlement-inducing protein complex guides barnacle settlement decisions. J. Exp. Biol. 2018, 221, jeb185348. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liang, C.; Song, J.; Ye, Z.; Wu, W.; Hu, B. Transcriptome analyses suggest a molecular mechanism for the SIPC response of Amphibalanus amphitrite. Biochem. Biophys. Res. Commun. 2020, 525, 823–829. [Google Scholar] [CrossRef] [PubMed]
- So, C.R.; Fears, K.P.; Leary, D.H.; Scancella, J.M.; Wang, Z.; Liu, J.L.; Orihuela, B.; Rittschof, D.; Spillmann, C.M.; Wahl, K.J. Sequence basis of Barnacle Cement Nanostructure is Defined by Proteins with Silk Homology. Sci. Rep. 2016, 6, 36219. [Google Scholar] [CrossRef]
- Zhang, J. Design and Expression of Fibrous Fusion Protein and Study of Ultrasonic Treatment Effects Based on Barnacle Cement Proteincp19k. Master’s Thesis, National University of Defense Technology, Changsha, China, 2020; pp. 71–72. [Google Scholar]
- Mason, M.L.; Lalisse, R.F.; Finnegan, T.J.; Hadad, C.M.; Modarelli, D.A.; Parquette, J.R. pH-Controlled Chiral Packing and Self-Assembly of a Coumarin Tetrapeptide. Langmuir ACS J. Surf. Colloids 2019, 35, 12460–12468. [Google Scholar] [CrossRef]
- Dehsorkhi, A.; Castelletto, V.; Hamley, I.W.; Adamcik, J.; Mezzenga, R. The effect of pH on the self-assembly of a collagen derived peptide amphiphile. Soft Matter 2013, 9, 6033–6036. [Google Scholar] [CrossRef]
- Golinska, M.D.; Włodarczyk-Biegun, M.K.; Werten, M.W.T.; Stuart, M.A.C.; de Wolf, F.A.; de Vries, R. Dilute Self-Healing Hydrogels of Silk-Collagen-Like Block Copolypeptides at Neutral pH. Biomacromolecules 2014, 15, 699–706. [Google Scholar] [CrossRef]
- Shi, L.; Tian, H.; Wang, Y.; Hao, G.; Chen, J.; Weng, W. Effect of pH on properties of golden pompano skin collagen-based fibril gels by self-assembly in vitro. J. Sci. Food Agric. 2020, 100, 4801–4807. [Google Scholar] [CrossRef]
- Uesaka, A.; Ueda, M.; Makino, A.; Imai, T.; Sugiyama, J.; Kimura, S. Morphology Control between Twisted Ribbon, Helical Ribbon, and Nanotube Self-Assemblies with His-Containing Helical Peptides in Response to pH Change. Langmuir 2014, 30, 1022–1028. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.; Bolisetty, S.; Handschin, S.; Mezzenga, R. Self-assembly and fibrillization of a Fmoc-functionalized polyphenolic amino acid. Soft Matter 2013, 9, 10239–10242. [Google Scholar] [CrossRef]
- Tian, Y.; Viles, J.H. pH Dependence of Amyloid-β Fibril Assembly Kinetics: Unravelling the Microscopic Molecular Processes. Angew. Chem. Int. Ed. Engl. 2022, 61, e202210675. [Google Scholar] [CrossRef]
- Liang, C.; Bi, X.; Gan, K.; Wu, J.; He, G.; Xue, B.; Ye, Z.; Cao, Y.; Hu, B. Short Peptides Derived from a Block Copolymer-like Barnacle Cement Protein Self-Assembled into Diverse Supramolecular Structures. Biomacromolecules 2022, 23, 2019–2030. [Google Scholar] [CrossRef]
- Yan, T.; Li, Z.; Hu, Y. A review on the balanomorph barnacles in the coastal waters of China. Acta EcoIogica Sin. 2012, 32, 5230–5241. [Google Scholar] [CrossRef]
- Tilbury, M.A.; McCarthy, S.; Domagalska, M.; Ederth, T.; Power, A.M.; Wall, J.G. The expression and characterization of recombinant cp19k barnacle cement protein from Pollicipes pollicipes. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2019, 374, 20190205. [Google Scholar] [CrossRef]
- Andrews, H.G.; Badyal, J.P.S. Bioinspired hook surfaces based upon a ubiquitous weed (Galium aparine) for dry adhesion. J. Adhes. Sci. Technol. 2014, 28, 1243–1255. [Google Scholar] [CrossRef]
- Wang, X.; Wang, C.; Xu, B.; Wei, J.; Xiao, Y.; Huang, F. Adsorption of intrinsically disordered barnacle adhesive proteins on silica surface. Appl. Surf. Sci. 2018, 427, 942–949. [Google Scholar] [CrossRef]
- Crisp, D.J.; Walker, G.; Young, G.A.; Yule, A.B. Adhesion and substrate choice in mussels and barnacles. J. Colloid Interface Sci. 1985, 104, 40–50. [Google Scholar] [CrossRef]
- Mederos Gómez, M.M.; Garcia, I.M.; Leitune, V.C.B.; Collares, F.M. Surface and mechanical properties of adhesives with calcium phosphates challenged to different storage media. Braz. J. Oral Sci. 2020, 19, 5–12. [Google Scholar] [CrossRef]
- Li, J.; Huang, D.; Xiang, Y.; Liu, J.; Han, J. Research Progress of Bionic Medical Adhesives. Green Packag. 2024, 29–33. [Google Scholar] [CrossRef]
- Jakobsen, R.J.; Brown, L.L.; Hutson, T.B.; Fink, D.J.; Veis, A. Intermolecular Interactions in Collagen Self-Assembly as Revealed by Fourier Transform Infrared Spectroscopy. Science 1983, 220, 1288–1290. [Google Scholar] [CrossRef]
- Gan, K.; Liang, C.; Bi, X.; Wu, J.; Ye, Z.; Wu, W.; Hu, B. Adhesive Materials Inspired by Barnacle Underwater Adhesion: Biological Principles and Biomimetic Designs. Front. Bioeng. Biotechnol. 2022, 10, 870445. [Google Scholar] [CrossRef]
- Fan, H.; Wang, J.; Gong, J.P. Barnacle Cement Proteins-Inspired Tough Hydrogels with Robust, Long-Lasting, and Repeatable Underwater Adhesion. Adv. Funct. Mater. 2021, 31, 2009334. [Google Scholar] [CrossRef]
- Pan, G.; Li, F.; He, S.; Li, W.; Wu, Q.; He, J.; Ruan, R.; Xiao, Z.; Zhang, J.; Yang, H. Mussel- and Barnacle Cement Proteins-Inspired Dual-Bionic Bioadhesive with Repeatable Wet-Tissue Adhesion, Multimodal Self-Healing, and Antibacterial Capability for Nonpressing Hemostasis and Promoted Wound Healing. Adv. Funct. Mater. 2022, 32, 2200908. [Google Scholar] [CrossRef]
- Sun, L.; Xu, Y.; Han, Y.; Cui, J.; Jing, Z.; Li, D.; Liu, J.; Xiao, C.; Li, D.; Cai, B. Collagen-Based Hydrogels for Cartilage Regeneration. Orthop. Surg. 2023, 15, 3026–3045. [Google Scholar] [CrossRef]
- Fujii, D.; Takase, K.; Takagi, A.; Kamino, K.; Hirano, Y. Design of RGDS Peptide-Immobilized Self-Assembling β-Strand Peptide from Barnacle Protein. Int. J. Mol. Sci. 2021, 22, 1240. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Zhang, A.; Chen, X.; Xiang, G.; Jiang, T.; Zhao, X. Barnacle inspired strategy combined with solvent exchange for enhancing wet adhesion of hydrogels to promote seawater-immersed wound healing. Bioact. Mater. 2024, 41, 46–60. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, G.; Wu, J.; Deng, P.; Zhang, F.; Huang, J. Study on the growth process and attachment strength of marine-fouling barnacles. Mar. Sci. 2023, 47, 60–67. [Google Scholar]
- Xu, X.; Cheung, S.; Jia, X.; Fan, G.; Ai, Y.; Zhang, Y.; Liang, Q. Trends in organ-on-a-chip for pharmacological analysis. TrAC Trends Anal. Chem. 2024, 180, 117905. [Google Scholar] [CrossRef]
- Matai, I.; Kaur, G.; Seyedsalehi, A.; McClinton, A.; Laurencin, C.T. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials 2020, 226, 119536. [Google Scholar] [CrossRef]
- Chae, S.; Cho, D.-W. Biomaterial-based 3D bioprinting strategy for orthopedic tissue engineering. Acta Biomater. 2023, 156, 4–20. [Google Scholar] [CrossRef]
- Li, T.; Hou, J.; Wang, L.; Zeng, G.; Wang, Z.; Yu, L.; Yang, Q.; Yin, J.; Long, M.; Chen, L.; et al. Bioprinted anisotropic scaffolds with fast stress relaxation bioink for engineering 3D skeletal muscle and repairing volumetric muscle loss. Acta Biomater. 2023, 156, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.-J.; Gwak, S.-J.; Kang, N.-U.; Hong, M.W.; Kim, Y.Y.; Cho, Y.-S.; Lee, S.-J. Bioreactor mimicking knee-joint movement for the regeneration of tissue-engineered cartilage. J. Mech. Sci. Technol. 2019, 33, 1841–1850. [Google Scholar] [CrossRef]
- Selden, C.; Fuller, B. Role of Bioreactor Technology in Tissue Engineering for Clinical Use and Therapeutic Target Design. Bioengineering 2018, 5, 32. [Google Scholar] [CrossRef] [PubMed]
- Mason, J.; Öhlund, D. Key aspects for conception and construction of co-culture models of tumor-stroma interactions. Front. Bioeng. Biotechnol. 2023, 11, 1150764. [Google Scholar] [CrossRef]
- Correia, C.D.; Ferreira, A.; Fernandes, M.T.; Silva, B.M.; Esteves, F.; Leitão, H.S.; Bragança, J.; Calado, S.M. Human Stem Cells for Cardiac Disease Modeling and Preclinical and Clinical Applications—Are We on the Road to Success? Cells 2023, 12, 1727. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, M.H.; Mustaffa, Z.; Saari, M.M.; Daniyal, H. Barnacles Mating Optimizer: A new bio-inspired algorithm for solving engineering optimization problems. Eng. Appl. Artif. Intell. 2020, 87, 103330. [Google Scholar] [CrossRef]
- Tsukanov, A.A.; Turk, B.; Vasiljeva, O.; Psakhie, S.G. Computational Indicator Approach for Assessment of Nanotoxicity of Two-Dimensional Nanomaterials. Nanomaterials 2022, 12, 650. [Google Scholar] [CrossRef] [PubMed]
- Horbert, V.; Xin, L.; Foehr, P.; Brinkmann, O.; Bungartz, M.; Burgkart, R.H.; Graeve, T.; Kinne, R.W. In Vitro Analysis of Cartilage Regeneration Using a Collagen Type I Hydrogel (CaReS) in the Bovine Cartilage Punch Model. Cartilage 2019, 10, 346–363. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Zhou, Y.; Lee, M.S.; Zhang, Y.; Li, W.J. A newly identified mechanism involved in regulation of human mesenchymal stem cells by fibrous substrate stiffness. Acta Biomater. 2016, 42, 247–257. [Google Scholar] [CrossRef]
- Niu, L.N.; Jee, S.E.; Jiao, K.; Tonggu, L.; Li, M.; Wang, L.; Yang, Y.D.; Bian, J.H.; Breschi, L.; Jang, S.S.; et al. Collagen intrafibrillar mineralization as a result of the balance between osmotic equilibrium and electroneutrality. Nat. Mater. 2017, 16, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Carderelli, N.F. Barnacle Cement as a Dental Restorative Adhesive: Background and Summary of Study at the University of Akron; National Institutes of Health Publication: Bethesda, MD, USA, 1968. [Google Scholar]
- Khandeparker, L.; Anil, A.C. Underwater adhesion: The barnacle way. Int. J. Adhes. Adhes. 2007, 27, 165–172. [Google Scholar] [CrossRef]
- Hu, B.; Wu, J.; Liang, C.; Song, J.; Ye, Z.; Zeng, L.; Tang, J. Biomimetic Peptides Based on Barnacle Bioadhesive Proteins, Self-Assembled Hydrogels, and Their Preparation Process and Applications. CN116903719A, 31 May 2023. [Google Scholar]
- Bang, J.H.; Ryu, Y.C.; Kim, K.A.; Hwang, B.H. Targeted delivery of self-assembled nanocomplex between fusion peptides and siRNAs for breast cancer treatment. Biochem. Eng. J. 2022, 186, 108564. [Google Scholar] [CrossRef]
- Danielsen, M.; Hempel, C.; Andresen, T.L.; Urquhart, A.J. Biopharmaceutical nanoclusters: Towards the self-delivery of protein and peptide therapeutics. J. Control. Release 2022, 347, 282–307. [Google Scholar] [CrossRef] [PubMed]
- Luan, K.; Ban, Y.; Shi, D.; Shi, H. Construction Strategy and Research Progress of Anticoagulant Surface of Medical Polymeric Materials. J. Funct. Polym. 2021, 34, 172–181. [Google Scholar] [CrossRef]
- Cao, Y.; Samy, K.E.; Bernards, D.A.; Desai, T.A. Recent advances in intraocular sustained-release drug delivery devices. Drug Discov. Today 2019, 24, 1694–1700. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Scherer, N.; Messersmith, P. Single-Molecule Mechanics of Mussel Adhesion. Proc. Natl. Acad. Sci. USA 2006, 103, 12999–13003. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, Y.; Yin, Y.; Liu, J.; Li, P.; Zhao, Y.; Bai, D.; Zhao, H.; Han, X.; Chen, Q. High-Strength and Injectable Supramolecular Hydrogel Self-Assembled by Monomeric Nucleoside for Tooth-Extraction Wound Healing. Adv. Mater. 2022, 34, 2108300. [Google Scholar] [CrossRef] [PubMed]


| Experimental Methods | Analytical Approaches |
|---|---|
| IR | Collagen molecules undergo spontaneous self-assembly to form native collagen fibrils. Fourier Transform Infrared Spectroscopy (FTIR) can be used to monitor the heat-induced fibril formation process in aqueous media in real-time. |
| UV–Vis | Based on the measurement of protein absorption in the ultraviolet and visible light regions, molecular events during protein self-assembly can be inferred, and the rate of self-assembly can be studied. |
| TM | Measure the changes in solution turbidity, thereby indirectly reflecting the extent of protein self-assembly. |
| FS | Select appropriate fluorescent molecules as probes to interact with proteins and provide information about changes in the protein environment, thereby inferring the behavior of protein self-assembly. |
| CD | Based on the difference in absorption of left-handed and right-handed circularly polarized light by substances, detect the changes in the secondary structure of proteins during the self-assembly process. |
| NMR | Through methods such as three-dimensional structure determination, probing conformational changes, monitoring the self-assembly process, and identifying self-assembled products. |
| SEM | By comparing SEM images at different time points and analyzing morphological characteristics, infer the molecular interactions and assembly mechanisms during the protein self-assembly process. |
| AFM | By detecting the interactive forces between the sample surface and the probe, it is possible to directly observe the morphology, structure, and dynamic behavior of protein self-assemblies, as well as measure their mechanical properties. |
| TEM | Directly observing the morphology and structure of protein self-assemblies can also be used to verify the self-assembly conditions obtained through protein crystallization screening methods. |
| CLSM | Through fluorescent labeling and high-resolution imaging techniques, it is possible to precisely observe and analyze the morphology, structure, and dynamic processes of protein self-assemblies. |
| Phase | Event | References |
|---|---|---|
| Substrate detection | Barnacles use the action of the antennal suckers during the cyprid larval stage to detect and select suitable substrates for simple mechanical attachment. | [17] |
| Signal transduction | Through signal transduction mechanisms, barnacles activate the expression of genes related to cement secretion. | [5,70,71] |
| Cement secretion | The secretory gland connects to a duct network system that secretes a glue containing a variety of proteins, which is a key step in ensuring the even distribution of barnacle cement proteins and achieving effective adhesion. | [72] |
| Cement solidification | Adhesive proteins form cross-links with the tissue surface, generating strong adhesion, thereby achieving solidification. | [2,43] |
| Plan | Method | References |
|---|---|---|
| Multifactorial dynamic culture systems | Developing a multifactorial dynamic culture system to simulate the physical, chemical, and biological factors within the body, such as temperature variations, pH levels, oxygen gradients, extracellular matrix components, and the dynamic changes in biomolecules. | [107,108] |
| Three-dimensional printing technology | Utilizing three-dimensional printing technology to construct scaffolds with complex structures that mimic the architecture and function of in vivo tissues. These scaffolds can incorporate porous structures to facilitate cell infiltration and the transport of nutrients. | [109,110,111] |
| Bioreactor technology | Employing bioreactors to simulate mechanical stimuli within the body, such as pressure, tension, and vibration, which are crucial for cell behavior and tissue development. | [112,113] |
| Co-culture systems | Designing co-culture systems to cultivate different types of cells with biomaterials to simulate the interactions and signal transduction between different cell types within the body. | [114,115] |
| Computer simulations | Utilizing computer simulation technology to predict the behavior of materials within the body, and combining tissue engineering techniques to construct bioactive tissue models for drug screening and material testing. | [116,117] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Min, T.; Zhang, Z.; Chen, L.; Li, J. Recent Advances in Barnacle-Inspired Biomaterials in the Field of Biomedical Research. Materials 2025, 18, 502. https://doi.org/10.3390/ma18030502
Min T, Zhang Z, Chen L, Li J. Recent Advances in Barnacle-Inspired Biomaterials in the Field of Biomedical Research. Materials. 2025; 18(3):502. https://doi.org/10.3390/ma18030502
Chicago/Turabian StyleMin, Tiantian, Zhongna Zhang, Lan Chen, and Jingan Li. 2025. "Recent Advances in Barnacle-Inspired Biomaterials in the Field of Biomedical Research" Materials 18, no. 3: 502. https://doi.org/10.3390/ma18030502
APA StyleMin, T., Zhang, Z., Chen, L., & Li, J. (2025). Recent Advances in Barnacle-Inspired Biomaterials in the Field of Biomedical Research. Materials, 18(3), 502. https://doi.org/10.3390/ma18030502








