Hierarchical CoMn-LDH and Heterostructured Composites for Advanced Supercapacitors and Electrocatalysis Applications
Abstract
:1. Introduction
2. Materials and Methods
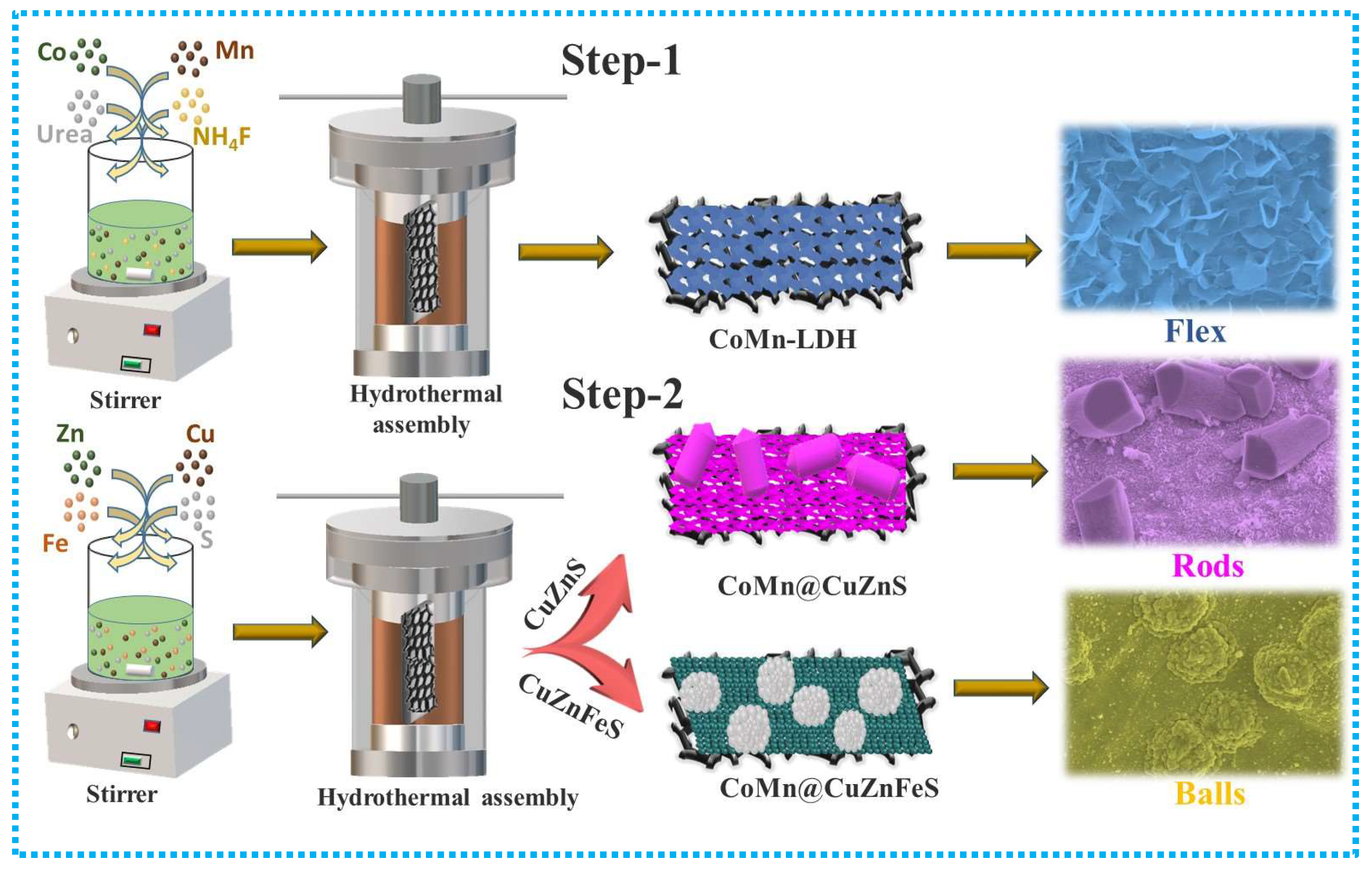
2.1. Synthesis of CoMn-LDH
2.2. Synthesis of CoMn@CuZnS and CoMn@CuZnFeS Heterostructures
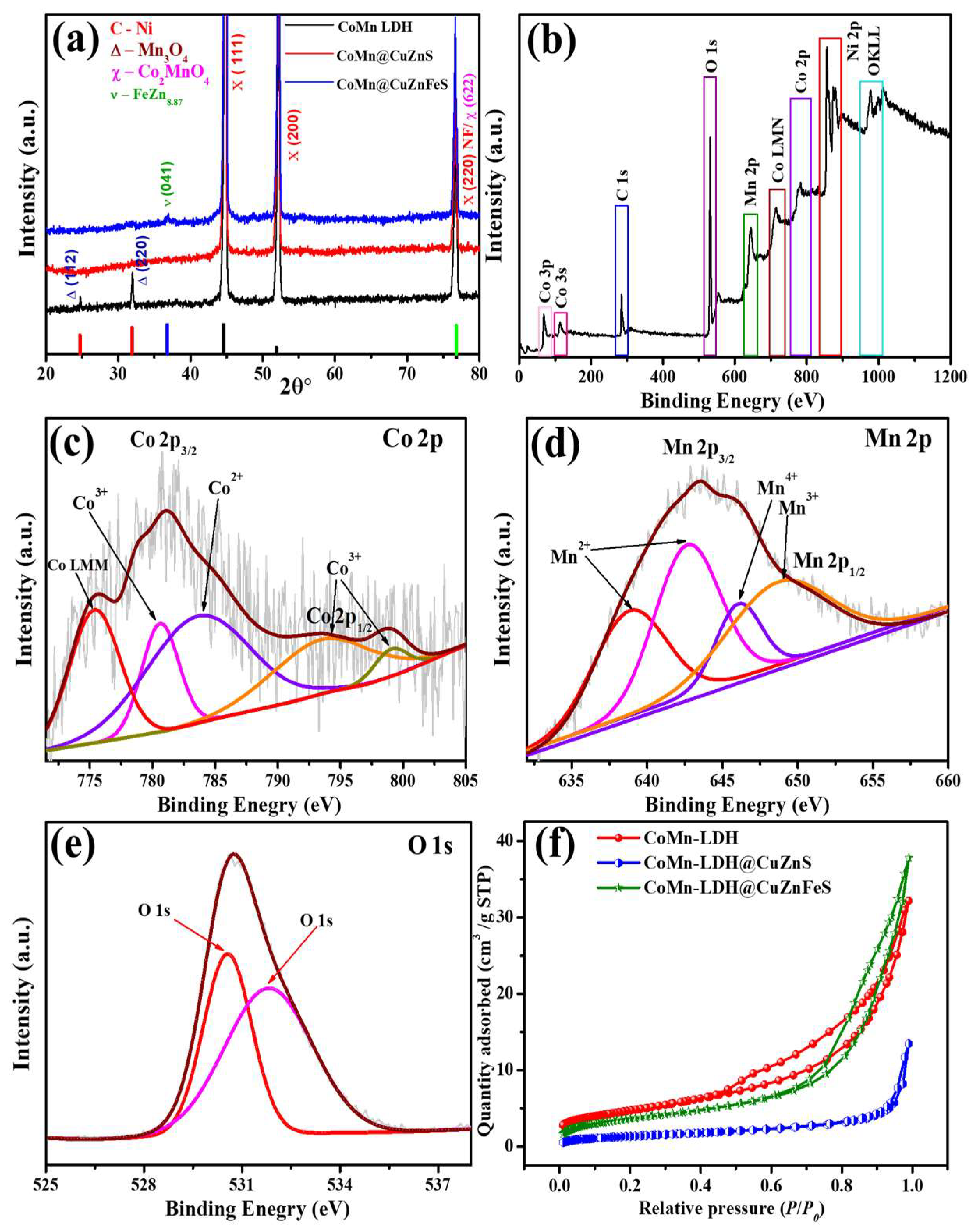
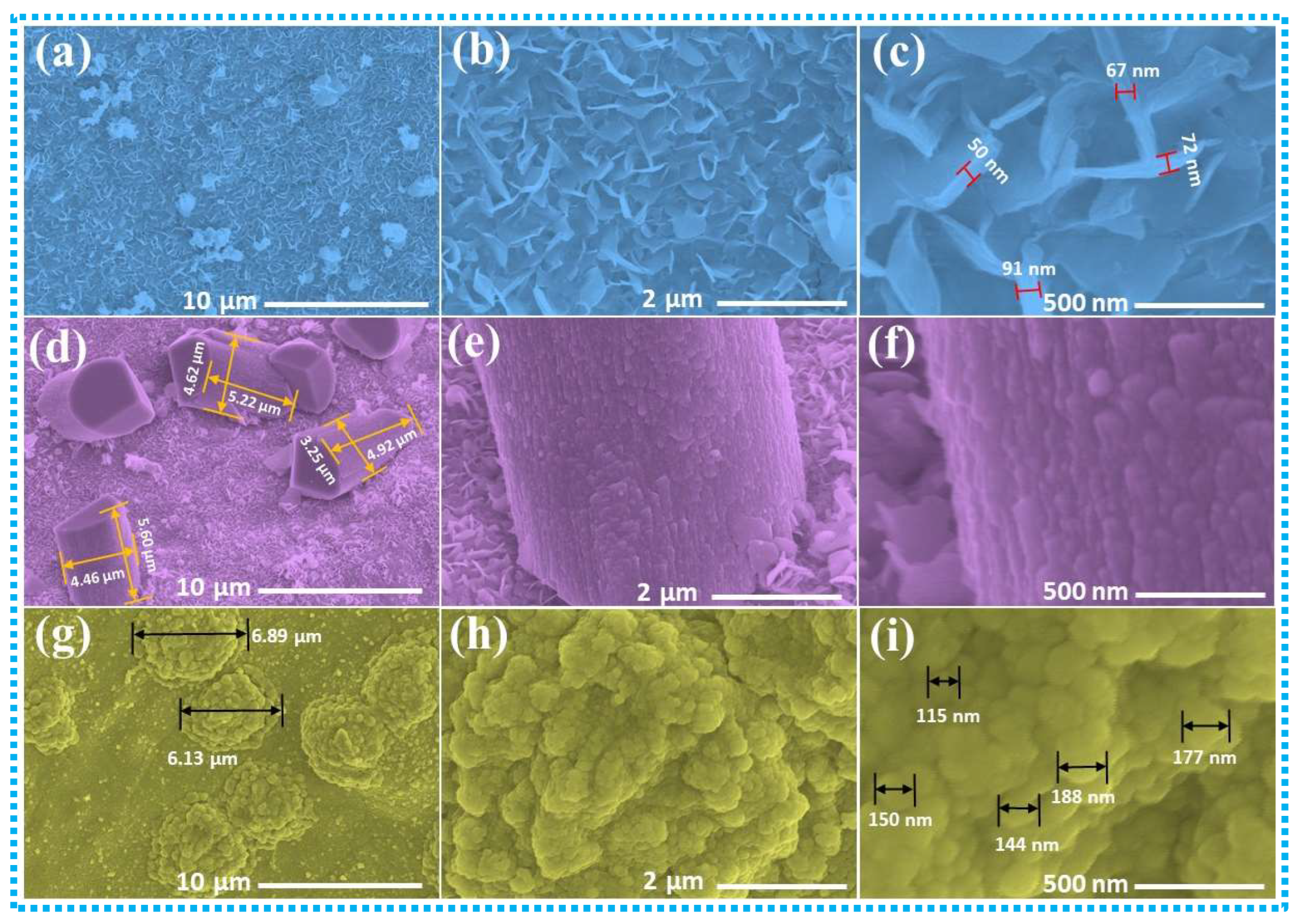
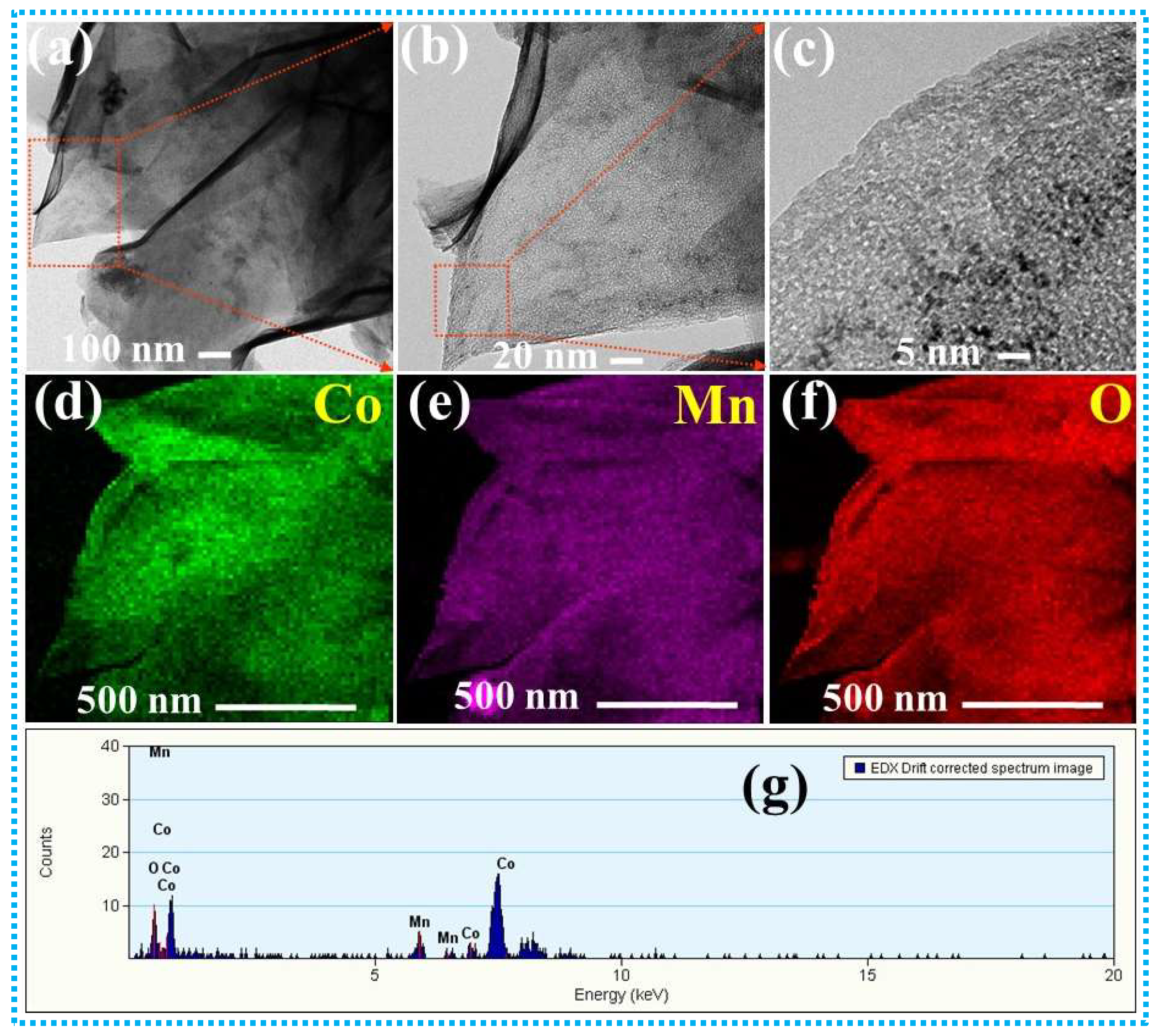
3. Results and Discussion
3.1. Supercapacitive Properties of CoMn-LDH, CoMn@CuZnS, and CoMn@CuZnFeS Heterostructures
3.2. Fabrication and Testing of AC//CoMn-LDH HSC
3.3. Electrocatalytic Performance of CoMn-LDH
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wei, L.; Ang, E.H.; Yang, Y.; Qin, Y.; Zhang, Y.; Ye, M.; Liu, Q.; Li, C.C. Recent advances of transition metal based bifunctional electrocatalysts for rechargeable zinc-air batteries. J. Power Sources 2020, 477, 228696. [Google Scholar] [CrossRef]
- Wang, P.; Qi, J.; Li, C.; Li, W.; Wang, T.; Liang, C. Hierarchical CoNi2S4@NiMn-layered double hydroxide heterostructure nanoarrays on superhydrophilic carbon cloth for enhanced overall water splitting. Electrochim. Acta 2020, 345, 136247. [Google Scholar] [CrossRef]
- Chavan, G.T.; Shinde, N.M.; Sabah, F.A.; Patil, S.S.; Sikora, A.; Prakshale, V.M.; Kamble, S.S.; Chaure, N.B.; Deshmukh, L.P.; Kim, A.; et al. Chemical synthesis of Cd1−x−yZnxCuySzSe1−z composite thin films for photoelectrochemical solar cell. Appl. Surf. Sci. 2020, 574, 151581. [Google Scholar] [CrossRef]
- Dong, Y.; Oloman, C.W.; Gyenge, E.L.; Su, J.; Chen, L. Transition metal based heterogeneous electrocatalysts for the oxygen evolution reaction at near-neutral pH. Nanoscale 2020, 12, 9924–9934. [Google Scholar] [CrossRef] [PubMed]
- Chavan, G.T.; Amate, R.U.; Lee, H.; Syed, A.; Bahkali, A.H.; Elgorban, A.M.; Jeon, C.W. Rational design of 3D hollow cube architecture for next-generation efficient aqueous asymmetric supercapacitors. J. Energy Storage 2023, 61, 106757. [Google Scholar] [CrossRef]
- Chavan, G.T.; Yadav, A.; Fugare, B.Y.; Shinde, N.M.; Tamboli, M.S.; Kamble, S.S.; Sikora, A.; Warycha, J.; Lokhande, B.J.; Kang, S.W.; et al. Three dimensional hierarchical flower-like CoCuS/Co1−xCuxS electrodes for electrochemical supercapacitors. J. Alloys Compd. 2022, 901, 162822. [Google Scholar] [CrossRef]
- Chavan, G.T.; Sikora, A.; Pawar, R.C.; Warycha, J.; Morankar, P.J.; Jeon, C.W. Hierarchical framework of CoZnS as a high-performance electrode material for supercapacitors. Ceram. Int. 2023, 49, 282–293. [Google Scholar] [CrossRef]
- Chavan, G.T.; Yadav, A.A.; Kamble, S.S.; Sabah, F.A.; Prakshale, V.M.; Sikora, A.; Warycha, J.; Bulakhe, R.N.; In, I.; Cho, E.C.; et al. Electrochemical supercapacitive studies of chemically deposited Co1-xNixS thin films. Mater. Sci. Semicond. Process. 2020, 107, 104799. [Google Scholar] [CrossRef]
- Tong, Q.; Wang, Q.; Li, H.; Li, J.; Yang, W. Polymer Cross-Linking on Highly Continuous Carbon for High-power Supercapacitor. Adv. Sustain. Syst. 2023, 7, 2300189. [Google Scholar] [CrossRef]
- Xuan, X.; Qian, M.; Han, L.; Wan, L.; Li, Y.; Lu, T.; Pan, L.; Niu, Y.; Gong, S. In-situ growth of hollow NiCo layered double hydroxide on carbon substrate for flexible supercapacitor. Electrochim. Acta 2019, 321, 134710. [Google Scholar] [CrossRef]
- Hao, C.; Wang, X.; Wu, X.; Guo, Y.; Zhu, L.; Wang, X. Composite material CCO/Co-Ni-Mn LDH made from sacrifice template CCO/ZIF-67 for high-performance supercapacitor. Appl. Surf. Sci. 2022, 572, 151373. [Google Scholar] [CrossRef]
- Wang, T.; Yu, F.; Wang, X.; Xi, S.; Chen, K.J.; Wang, H. Enhancing cycling stability of transition metal-based layered double hydroxides through a self-sacrificial strategy for hybrid supercapacitors. Electrochim. Acta 2020, 334, 135586. [Google Scholar] [CrossRef]
- Pang, X.; Xue, S.; Zhou, T.; Qiao, M.; Li, H.; Liu, X.; Xu, Q.; Liu, G.; Lei, W. Noble Metal-Free Heterojunction of Ultrathin Ti3C2 MXene/WO3 for Boosted Visible-Light-Driven Photoreactivity. Adv. Sustain. Syst. 2023, 7, 2100507. [Google Scholar] [CrossRef]
- Na, R.; Min, K.; Kim, M.; Min, S.; Baeck, S.H. Enhancing Electrocatalytic Performance for Overall Water Splitting using Hollow Structured Fe-doped CoP with Phosphorus Vacancies. Adv. Sustain. Syst. 2023, 7, 2300130. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, T.; Zhou, G.; Liu, P.; Yan, X.; Xu, B.; Guo, J. Cu nanoclusters on N-doped carbon nanotubes as efficient electrocatalyst for oxygen reduction reaction. Appl. Surf. Sci. 2022, 589, 153022. [Google Scholar] [CrossRef]
- Ma, C.; Li, W.; Xu, D.; Wu, Q.; Li, C.; Tu, J.; Zhang, K. Confined Growth of MoS2 by using a Two-Dimensional Metal-Organic Framework for Efficient Hydrogen Evolution. Adv. Sustain. Syst. 2023, 7, 2300017. [Google Scholar] [CrossRef]
- Jeghan, S.M.N.; Kim, N.; Lee, G. Mo-incorporated three-dimensional hierarchical ternary nickel-cobalt-molybdenum layer double hydroxide for high-efficiency water splitting. Int. J. Hydrog. Energy 2021, 46, 22463–22477. [Google Scholar] [CrossRef]
- Chen, Q.; Ding, R.; Liu, H.; Zhou, L.; Wang, Y.; Zhang, Y.; Fan, G. Flexible Active-Site Engineering of Monometallic Co-Layered Double Hydroxides for Achieving High-Performance Bifunctional Electrocatalyst toward Oxygen Evolution and H2O2 Reduction. ACS Appl. Mater. Interfaces 2020, 12, 12919–12929. [Google Scholar] [CrossRef]
- Xuan, H.; Guan, Y.; Han, X.; Liang, X.; Xie, Z.; Han, P.; Wu, Y. Hierarchical MnCo-LDH/rGO@NiCo2S4 heterostructures on Ni foam with enhanced electrochemical properties for battery-supercapacitors. Electrochim. Acta 2020, 335, 135691. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Z.; Pan, Z.; Liu, L.; Xi, J.; Luo, X.; Shen, Y. Exceptional Performance of Hierarchical Ni–Fe hydroxide@NiCu Electrocatalysts for Water Splitting. Adv. Mater. 2019, 31, e1806769. [Google Scholar] [CrossRef]
- Emin, A.; Song, X.; Du, Y.; Chen, Y.; Yang, M.; Zou, S.; Fu, Y.; Li, J.; Li, Y.; He, D. One-step electrodeposited Co and Mn layered double hydroxides on Ni foam for high-performance aqueous asymmetric supercapacitors. J. Energy Storage 2022, 50, 104667. [Google Scholar] [CrossRef]
- Sirisomboonchai, S.; Kitiphatpiboon, N.; Chen, M.; Li, S.; Li, X.; Kongparakul, S.; Samart, C.; Zhang, L.; Abudula, A.; Guan, G. Multi-Hierarchical Porous Mn-Doped CoP Catalyst on Nickel Phosphide Foam for Hydrogen Evolution Reaction. ACS Appl. Energy Mater. 2022, 5, 149–158. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, Y.; Liu, W.; Xu, L.; Guan, M.; Zhao, Y.; Bao, J.; Li, H. Manganese-Modulated Cobalt-Based Layered Double Hydroxide Grown on Nickel Foam with 1D–2D–3D Heterostructure for Highly Efficient Oxygen Evolution Reaction and Urea Oxidation Reaction. Chem. A Eur. J. 2020, 26, 9382–9388. [Google Scholar] [CrossRef]
- Jagadale, A.D.; Guan, G.; Li, X.; Du, X.; Ma, X.; Hao, X.; Abudula, A. Ultrathin nanoflakes of cobalt-manganese layered double hydroxide with high reversibility for asymmetric supercapacitor. J. Power Sources 2016, 306, 526–534. [Google Scholar] [CrossRef]
- Ochai-Ejeh, F.O.; Madito, M.J.; Momodu, D.Y.; Khaleed, A.A.; Olaniyan, O.; Manyala, N. High performance hybrid supercapacitor device based on cobalt manganese layered double hydroxide and activated carbon derived from cork (Quercus suber). Electrochim. Acta 2017, 252, 41–54. [Google Scholar] [CrossRef]
- Chen, D.; Chen, H.; Chang, X.; Liu, P.; Zhao, Z.; Zhou, J.; Xu, G.; Lin, H.; Han, S. Hierarchical CoMn-layered double hydroxide nanowires on nickel foam as electrode material for high-capacitance supercapacitor. J. Alloys Compd. 2017, 729, 866–873. [Google Scholar] [CrossRef]
- Yan, F.; Guo, D.; Kang, J.; Liu, L.; Zhu, C.; Gao, P.; Zhang, X.; Chen, Y. Fast fabrication of ultrathin CoMn LDH nanoarray as flexible electrode for water oxidation. Electrochim. Acta 2018, 283, 755–763. [Google Scholar] [CrossRef]
- Pan, S.; Li, B.; Yu, J.; Zhao, L.; Zhang, Y. Composition controllable fabrication of ultrathin 2D CoMn layered double hydroxides for highly efficient electrocatalytic oxygen evolution. Appl. Surf. Sci. 2021, 539, 148305. [Google Scholar] [CrossRef]
- Bao, J.; Wang, Z.; Xie, J.; Xu, L.; Lei, F.; Guan, M.; Huang, Y.; Zhao, Y.; Xia, J.; Li, H. The CoMo-LDH ultrathin nanosheet as a highly active and bifunctional electrocatalyst for overall water splitting. Inorg. Chem. Front. 2018, 5, 2964–2970. [Google Scholar] [CrossRef]
- Song, F.; Hu, X. Ultrathin Cobalt − Manganese Layered Double Hydroxide Is an. J. Am. Chem. Soc. 2014, 136, 16481–16484. [Google Scholar] [CrossRef]
- Chavan, G.T.; Ahir, N.A.; Ingole, R.S.; Jeon, C.W.; An, J. Morphological engineering of quaternary mixed metal sulfide CuMnZnS electrodes for high-performance hybrid energy storage device. J. Energy Storage 2024, 92, 112262. [Google Scholar] [CrossRef]
- Saghafi, M.; Hosseini, S.A.; Zangeneh, S.; Moghanian, A.H.; Salarvand, V.; Vahedi, S.; Mohajerzadeh, S. Charge storage properties of mixed ternary transition metal ferrites MZnFe oxides (M = Al, Mg, Cu, Fe, Ni) prepared by hydrothermal method. SN Appl. Sci. 2019, 1, 1303. [Google Scholar] [CrossRef]
- Anjana, P.M.; Rupa Ranjani, P.; Rakhi, R.B. Cu-Fe based oxides and selenides as advanced electrode materials for high performance symmetric supercapacitors. Mat. Lett. 2021, 296, 129827. [Google Scholar] [CrossRef]
- Chavan, G.T.; Sabah, F.A.; Kamble, S.S.; Prakshale, V.M.; Pawar, S.T.; Patil, S.; Lee, S.; Sikora, A.; Deshmukh, L.P.; Cho, Y.; et al. Novel synthesis method for quaternary Cd(Cu, Zn)Se thin films and its characterizations. Ceram. Int. 2020, 46, 74–80. [Google Scholar] [CrossRef]
- Ahmed, N.; Ali, B.A.; Allam, N.K. Optimized electrosynthesis approach of Manganese-Nickel-Cobalt chalcogenide nanosheet arrays as binder-free battery materials for asymmetric electrochemical supercapacitors. Electrochim. Acta 2021, 396, 139191. [Google Scholar] [CrossRef]
- Raju, T.D.; Gopalakrishnan, A.; Badhulika, S. Facile synthesis of 3D/2D Cu2Se cauliflower/CuS nanosheets composite as a binder-free electrode for high-performance asymmetric solid-state supercapacitors. J. Alloys Compd. 2020, 845, 156241. [Google Scholar] [CrossRef]
- Conway, B. Electrochemical Supercapacitors Scientific Fundamentals and Technological Applications; Kluwer Acadamic/Plenum Publisher: Dordrecht, The Netherlands, 1999. [Google Scholar]
- Liu, H.; Li, Z.; Yao, Z.; Liu, Y.; Zhang, Q.; Sun, Y.; Li, Z. Designed MnS/Co9S8 micro-flowers composites with serrate edges as high-performance electrodes for asymmetric supercapacitor. J. Colloid Interface Sci. 2019, 551, 119–129. [Google Scholar] [CrossRef]
- Yu, Q.; Gong, J.; Kong, W.; Long, Y.; Chen, J.; Pu, L.; Zhang, H.; Dai, Y. Preparation of NiAl LDH@Mn3O4@Co-MOF ternary composites using MOFs as a framework for high-performance asymmetric supercapacitors. Electrochim. Acta 2022, 428, 140913. [Google Scholar] [CrossRef]
- Ying, Y.; Godínez Salomón, J.F.; Lartundo-Rojas, L.; Moreno, A.; Meyer, R.; Damin, C.A.; Rhodes, C.P. Hydrous cobalt-iridium oxide two-dimensional nanoframes: Insights into activity and stability of bimetallic acidic oxygen evolution electrocatalysts. Nanoscale Adv. 2021, 3, 1976–1996. [Google Scholar] [CrossRef]
- Wang, X.; Hao, C.; Zhang, J.; Ni, C.; Wang, X.; Shen, Y. Reasonable design and synthesis of nickel manganese sulfide nanoparticles derived from metal organic frameworks as electrode materials for supercapacitors. J. Power Sources 2022, 539, 231594. [Google Scholar] [CrossRef]
- Li, M.; Wang, J.; Wang, F.; Zhai, Y.; Zhang, X.; Lv, H.; Yu, T.; Lv, G. Construction of internal and external defect electrode materials based on hollow manganese-cobalt-nickel sulfide nanotube arrays. Appl. Surf. Sci. 2021, 568, 150900. [Google Scholar] [CrossRef]
- Raj, C.J.; Manikandan, R.; Cho, W.J.; Yu, K.H.; Kim, B.C. High-performance flexible and wearable planar supercapacitor of manganese dioxide nanoflowers on carbon fiber cloth. Ceram. Int. 2020, 46, 21736–21743. [Google Scholar] [CrossRef]
- Elkholy, A.E.; El-Taib Heakal, F.; Allam, N.K. A facile electrosynthesis approach of amorphous Mn-Co-Fe ternary hydroxides as binder-free active electrode materials for high-performance supercapacitors. Electrochim. Acta 2019, 296, 59–68. [Google Scholar] [CrossRef]
- Su, D.; Tang, Z.; Xie, J.; Bian, Z.; Zhang, J.; Yang, D.; Zhang, D.; Wang, J.; Liu, Y.; Yuan, A.; et al. Co, Mn-LDH nanoneedle arrays grown on Ni foam for high performance supercapacitors. Appl. Surf. Sci. 2019, 469, 487–494. [Google Scholar] [CrossRef]
- Chowdhury, A.; Biswas, S.; Sharma, V.; Halder, J.; Dhar, A.; Sundaram, B.; Dubey, B.; Burada, P.S.; Chandra, A. High performance magnetic pseudocapacitors—Direct correlation between specific capacitance and diffusion coefficients. Electrochim. Acta 2021, 397, 139252. [Google Scholar] [CrossRef]
- Huang, D.; Lu, Z.; Liu, X.; Gao, J.; Chen, Z.; Wang, X.; Fu, X. High-performance flexible supercapacitors with hierarchical structured cathode (NiCo2O4/Au/MnO2) and anode (NiCo2S4/PPy). Appl. Surf. Sci. 2022, 605, 154707. [Google Scholar] [CrossRef]
- Patil, A.M.; Wang, J.; Li, S.; Hao, X.; Du, X.; Wang, Z.; Hao, X.; Abudula, A.; Guan, G. Bilateral growth of monoclinic WO3 and 2D Ti3C2Tx on 3D free-standing hollow graphene foam for all-solid-state supercapacitor. Chem. Eng. J. 2021, 421, 127883. [Google Scholar] [CrossRef]
- Moradi, M.; Afkhami, A.; Madrakian, T.; Moazami, H.R. Electrosynthesis of Co-Mn layered-double-hydroxide as a precursor for Co-Mn-MOFs and subsequent electrochemical sulfurization for supercapacitor application. J. Energy Storage 2023, 71, 108177. [Google Scholar] [CrossRef]
- Wang, F.; Wang, T.; Sun, S.; Xu, Y.; Yu, R.; Li, H. One-step synthesis of Nickle Iron-layered double hydroxide/reduced graphene oxide/carbon nanofibres composite as electrode materials for asymmetric supercapacitor. Sci. Rep. 2018, 8, 8908. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, S.; Wang, J.; Liu, Z.; Liu, Y. Facile preparation of high-performance cobalt–manganese layered double hydroxide/polypyrrole composite for battery-type asymmetric supercapacitors. J. Alloys Compd. 2020, 832, 154899. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, J.; Xu, S.; Shao, M.; Yan, D.; Wei, M.; Evans, D.G.; Duan, X. CoMn-layered double hydroxide nanowalls supported on carbon fibers for high-performance flexible energy storage devices. J. Mater. Chem. A 2023, 1, 8836–8843. [Google Scholar] [CrossRef]
- Meng, S.; Wang, Y.; Zhang, Y.; Xu, Q.; Jiang, D.; Chen, M. Designing positive electrodes based on 3D hierarchical CoMn2O4@NiMn-LDH nanoarray composites for high energy and power density supercapacitors. CrystEngComm 2020, 22, 6864–6875. [Google Scholar] [CrossRef]
- Zou, J.; Xie, D.; Xu, J.; Song, X.; Zeng, X.; Wang, H.; Zhao, F. Rational design of honeycomb Ni-Co LDH/graphene composite for remarkable supercapacitor via ultrafast microwave synthesis. Appl. Surf. Sci. 2022, 571, 151322. [Google Scholar] [CrossRef]
- Zhao, C.; Tian, S.; Nie, P.; Deng, T.; Ren, F.; Chang, L. Electrodeposited binder-free CoMn LDH/CFP electrode with high electrochemical performance for asymmetric supercapacitor. Ionics 2020, 26, 1389–1396. [Google Scholar] [CrossRef]
- Meng, X.; Feng, M.; Zhang, H.; Ma, Z.; Zhang, C. Solvothermal synthesis of cobalt/nickel layered double hydroxides for energy storage devices. J. Alloys Compd. 2017, 695, 3522–3529. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, J.; Yang, Y.; Luo, B.; Zhang, X.; Fong, E.; Chu, W.; Huang, K. Unique 3D flower-on-sheet nanostructure of NiCo LDHs: Controllable microwave-assisted synthesis and its application for advanced supercapacitors. J. Alloys Compd. 2019, 788, 1029–1036. [Google Scholar] [CrossRef]
- Huang, C.; Ni, C.; Yang, L.; Zhou, T.; Hao, C.; Wang, X.; Ge, C.; Zhu, L. High-performance supercapacitor based on graphene oxide through in-situ polymerization and co-precipitation method. J. Alloys Compd. 2020, 829, 154536. [Google Scholar] [CrossRef]
- Hu, W.; Chen, L.; Du, M.; Song, Y.; Wu, Z.; Zheng, Q. Hierarchical NiCo-layered double hydroxide nanoscroll@PANI nanocomposite for high performance battery-type supercapacitor. Electrochim. Acta 2020, 338, 135869. [Google Scholar] [CrossRef]
- Ouyang, L.; Hsiao, C.H.; Chen, Y.C.; Lee, C.Y.; Tai, N.H. Fabrication of Ni-Mn LDH/Co3O4 on carbon paper for the application in supercapacitors. Surf. Interfaces 2022, 28, 101574. [Google Scholar] [CrossRef]
- Quan, W.; Xu, Y.; Wang, Y.; Meng, S.; Jiang, D.; Chen, M. Hierarchically structured Co3O4@glucose-modified LDH architectures for high-performance supercapacitors. Appl. Surf. Sci. 2019, 488, 639–647. [Google Scholar] [CrossRef]
- Chatterjee, A.; Chakraborty, P.; Kumar, B.; Mandal, S.; Dey, S.K. Fe-Based Materials for Electrocatalytic Water Splitting: A Mini Review. ChemCatChem 2024, 16, e202400622. [Google Scholar] [CrossRef]
- Li, X.; Patil, K.; Agarwal, A.; Babar, P.; Jang, J.S.; Chen, X.; Yoo, Y.T.; Kim, J.H. Ni(OH)2Coated CoMn-layered double hydroxide nanowires as efficient water oxidation electrocatalysts. N. J. Chem. 2022, 46, 2044–2052. [Google Scholar] [CrossRef]
- Jia, G.; Hu, Y.; Qian, Q.; Yao, Y.; Zhang, S.; Li, Z.; Zou, Z. Formation of Hierarchical Structure Composed of (Co/Ni)Mn-LDH Nanosheets on MWCNT Backbones for Efficient Electrocatalytic Water Oxidation. ACS Appl. Mater. Interfaces 2016, 8, 14527–14534. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, F.M.; Meng, X.Y.; Li, S.N.; Zeng, J.H.; Chen, Y. Ultrathin Co3O4 Nanomeshes for the Oxygen Evolution Reaction. ACS Catal. 2018, 8, 1913–1920. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, C.; Han, X.; Yang, J.; Zhao, C.; Huang, H.; Qiu, J. CoMn Layered Double Hydroxides/Carbon Nanotubes Architectures as High-Performance Electrocatalysts for the Oxygen Evolution Reaction. ChemElectroChem 2016, 3, 906–912. [Google Scholar] [CrossRef]
- Wang, T.; He, J.; Zhu, Z.; Cheng, X.B.; Zhu, J.; Lu, B.; Wu, Y. Heterostructures Regulating Lithium Polysulfides for Advanced Lithium-Sulfur Batteries. Adv. Mat. 2023, 35, 2303520. [Google Scholar] [CrossRef]
- Gaur, A.; Sharma, J.; Lim, D.H.; Lee, H.I.; Han, H. Recent Advances in Electronic Structure Modifications of Layered Double Hydroxide (LDH) for the Water Splitting Application. ChemCatChem 2024, 17, e202401584. [Google Scholar] [CrossRef]
- Khaladkar, S.R.; Maurya, O.; Gund, G.; Sinha, B.; Dubal, D.; Deshmukh, R.R.; Kalekar, A. Improving the charge kinetics through in-situ growth of NiSe nanoparticles on g-C3N4 nanosheets for efficient hybrid supercapacitors. J. Energy Chem. 2023, 87, 304–313. [Google Scholar] [CrossRef]
- Vidales, A.G.; Kim, J.; Omanovic, S. Ni0.6−xMo0.4−xIrx-oxide as an electrode material for supercapacitors: Investigation of the influence of iridium content on the charge storage/delivery. J. Solid State Electrochem. 2019, 23, 2129–2139. [Google Scholar] [CrossRef]
- Kumar, M.; Kar, K.K.; Paik, P. Supercapacitor electrodes based on Ru/RuO2 decorated on N,S-doped few-layer graphene. Chem. Eng. J. 2024, 499, 156414. [Google Scholar] [CrossRef]
- Ming, Z.; Yan, C.; Dingyu, Y.; Jitao, L. High performance MnO2 supercapacitor material prepared by modified electrodeposition method with different electrodeposition voltages. J. Energy Storage 2020, 29, 101363. [Google Scholar] [CrossRef]
- Dhas, S.D.; Maldar, P.S.; Patil, M.D.; Nagare, A.B.; Waikar, M.R.; Sonkawade, R.G.; Moholkar, A.V. Synthesis of NiO nanoparticles for supercapacitor application as an efficient electrode material. Vacuum 2020, 181, 109646. [Google Scholar] [CrossRef]
- Beknalkar, S.A.; Teli, A.M.; Harale, N.S.; Patil, D.S.; Pawar, S.A.; Shin, J.C.; Patil, P.S. Fabrication of high energy density supercapacitor device based on hollow iridium oxide nanofibers by single nozzle electrospinning. Appl. Surf. Sci. 2021, 546, 149102. [Google Scholar] [CrossRef]
- Wang, J.; Yu, H.; Wang, X.; Chen, C.; Li, S.; Cai, N.; Chen, W.; Xue, Y.; Li, H.; Yu, F. Tri-metal-based hollow nanorods-on-microrod arrays as efficient water splitting electrocatalysts. J. Indust. Eng. Chem. 2022, 105, 427–434. [Google Scholar] [CrossRef]
- Bao, J.; Wang, Z.; Xie, J.; Xu, L.; Lei, F.; Guan, M.; Zhao, Y.; Huang, Y.; Li, H. A ternary cobalt–molybdenum–vanadium layered double hydroxide nanosheet array as an efficient bifunctional electrocatalyst for overall water splitting. Chem. Commun. 2019, 55, 3521–3524. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Wang, Z.; Long, X.; An, Y.; Lin, H.; Xing, Z.; Ma, M.; Yang, S. One-pot synthesis of manganese oxides and cobalt phosphides nanohybrids with abundant heterointerfaces in an amorphous matrix for efficient hydrogen evolution in alkaline solution. J. Mater. Chem. A 2019, 7, 22530–22538. [Google Scholar] [CrossRef]
- Zhai, P.; Xia, M.; Wu, Y.; Zhang, G.; Gao, J.; Zhnag, B.; Cao, S.; Zhang, Y.; Li, Z.; Fan, Z.; et al. Engineering single-atomic ruthenium catalytic sites on defective nickel-iron layered double hydroxide for overall water splitting. Nat. Commun. 2021, 12, 4587. [Google Scholar] [CrossRef]
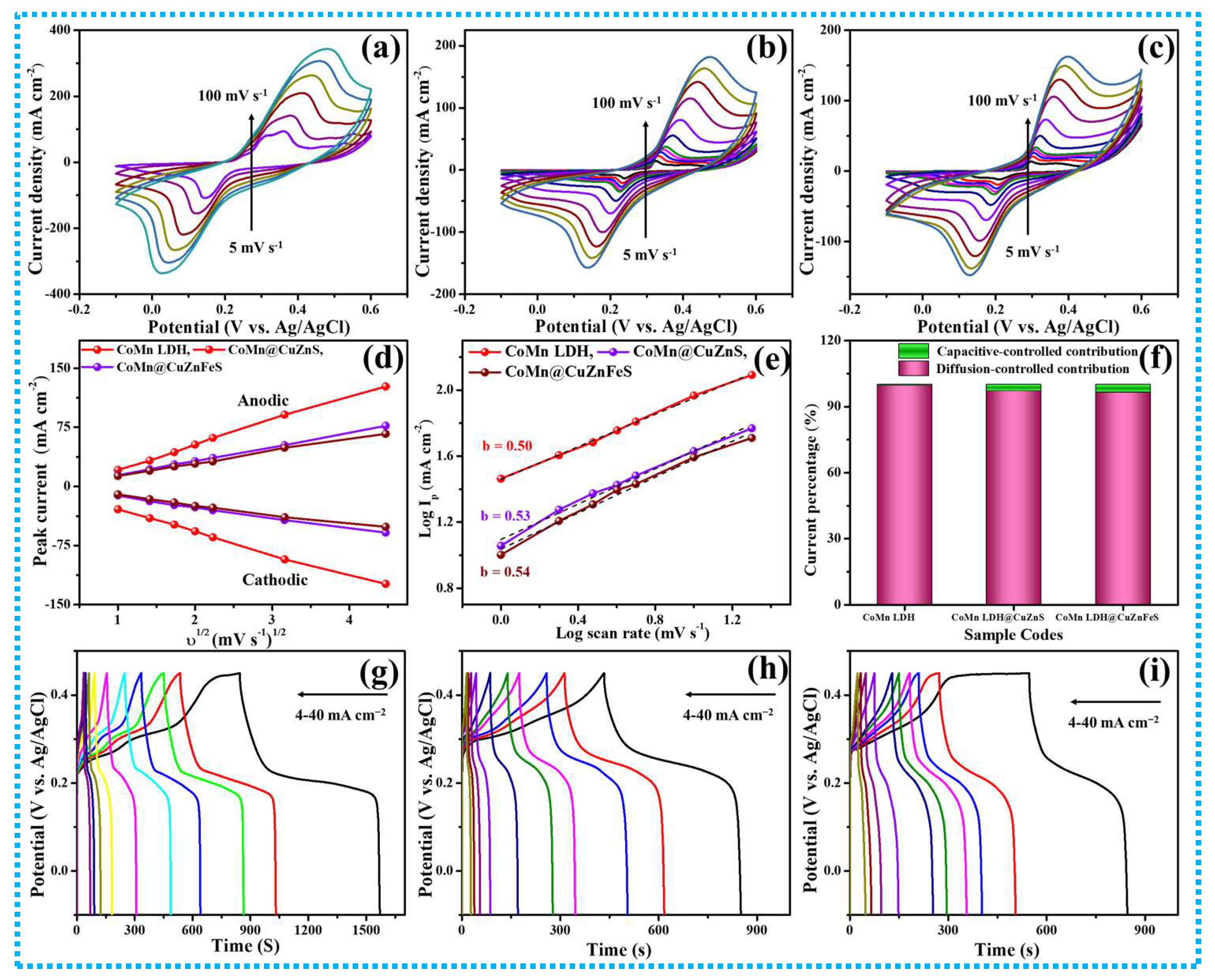
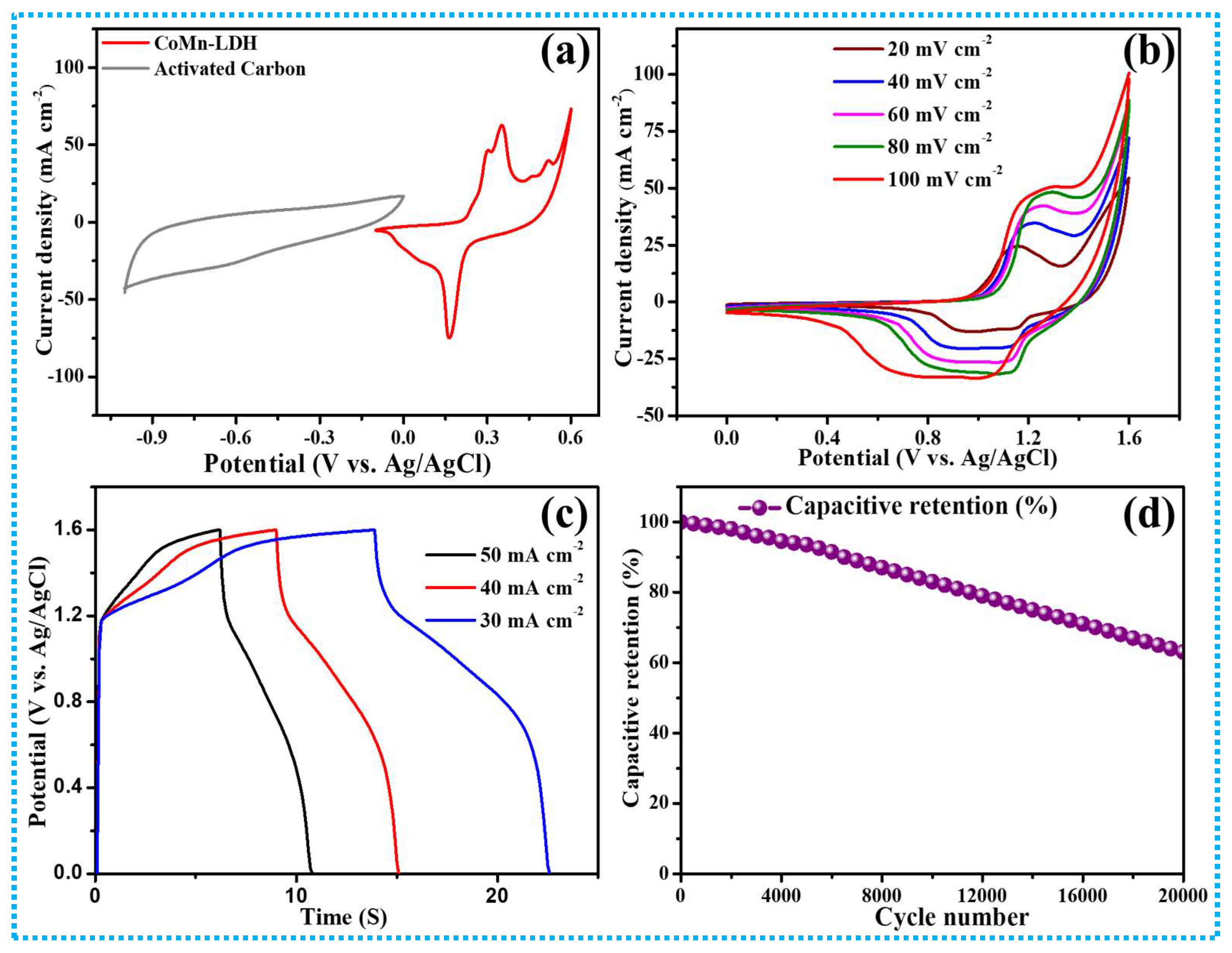

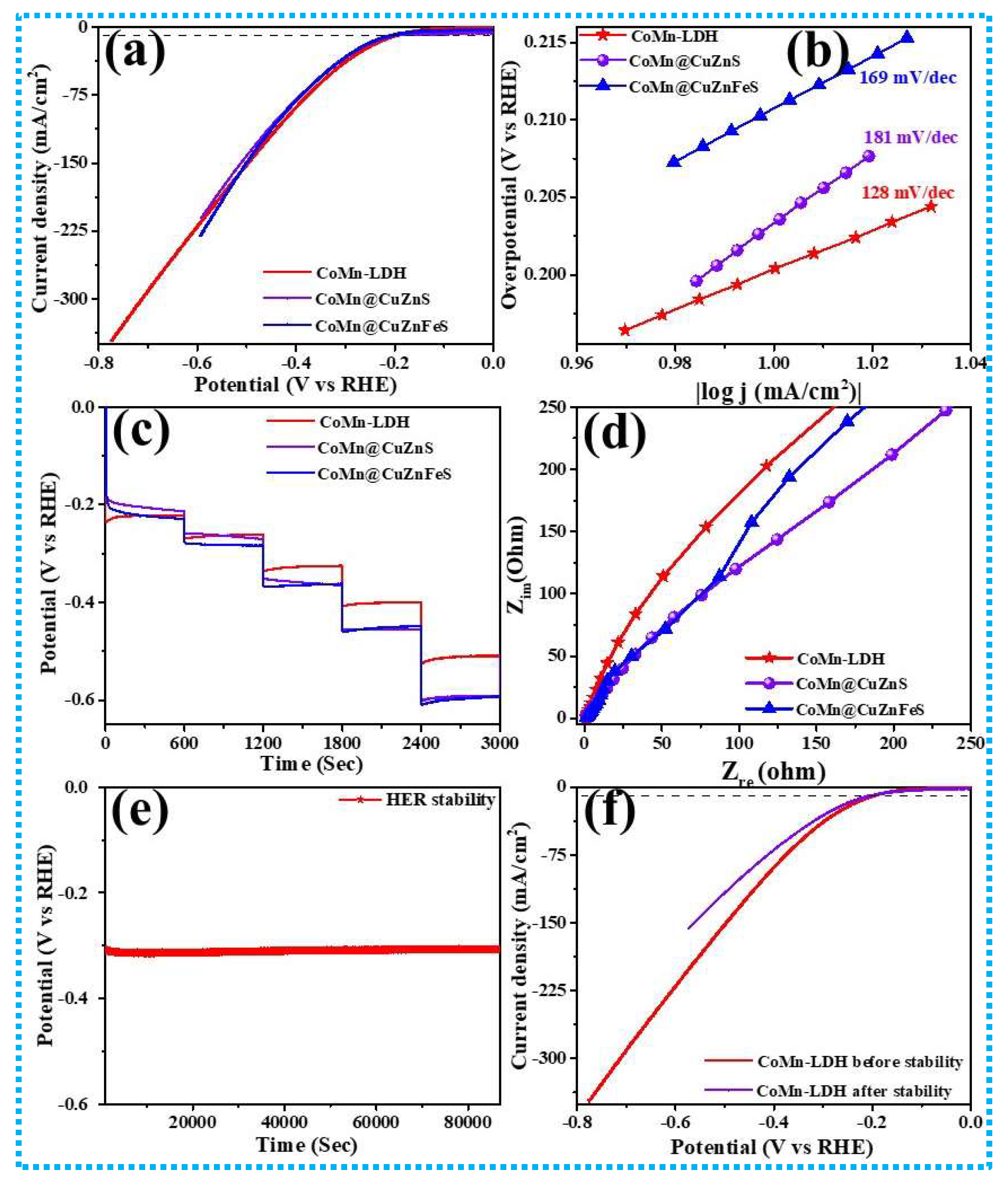
| Sr. No. | Current Density (mA cm−2) | Areal Capacitance (CA) mFcm−2 | Specific Capacitance (CS) F g−1 | Specific Capacity mAh/g | Energy Density Wh cm−2 | Power Density W cm−2 |
|---|---|---|---|---|---|---|
| 1 | 30 | 853.12 | 293.90 | 130.62 | 0.347 | 250 |
| 2 | 40 | 846.25 | 291.53 | 129.57 | 0.376 | 200 |
| 3 | 50 | 781.25 | 269.14 | 119.62 | 0.379 | 150 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chavan, G.T.; Dubal, D.P.; Morankar, P.J.; Jeon, C.-W.; An, J.; Song, K.-H. Hierarchical CoMn-LDH and Heterostructured Composites for Advanced Supercapacitors and Electrocatalysis Applications. Materials 2025, 18, 604. https://doi.org/10.3390/ma18030604
Chavan GT, Dubal DP, Morankar PJ, Jeon C-W, An J, Song K-H. Hierarchical CoMn-LDH and Heterostructured Composites for Advanced Supercapacitors and Electrocatalysis Applications. Materials. 2025; 18(3):604. https://doi.org/10.3390/ma18030604
Chicago/Turabian StyleChavan, Ganesh T., Deepak P. Dubal, Pritam J. Morankar, Chan-Wook Jeon, Jinsung An, and Ki-Han Song. 2025. "Hierarchical CoMn-LDH and Heterostructured Composites for Advanced Supercapacitors and Electrocatalysis Applications" Materials 18, no. 3: 604. https://doi.org/10.3390/ma18030604
APA StyleChavan, G. T., Dubal, D. P., Morankar, P. J., Jeon, C.-W., An, J., & Song, K.-H. (2025). Hierarchical CoMn-LDH and Heterostructured Composites for Advanced Supercapacitors and Electrocatalysis Applications. Materials, 18(3), 604. https://doi.org/10.3390/ma18030604








