Effect of Trace Sc Addition on Microstructure and Mechanical Properties of Al-Zn-Mg-Cu-Zr Alloy
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
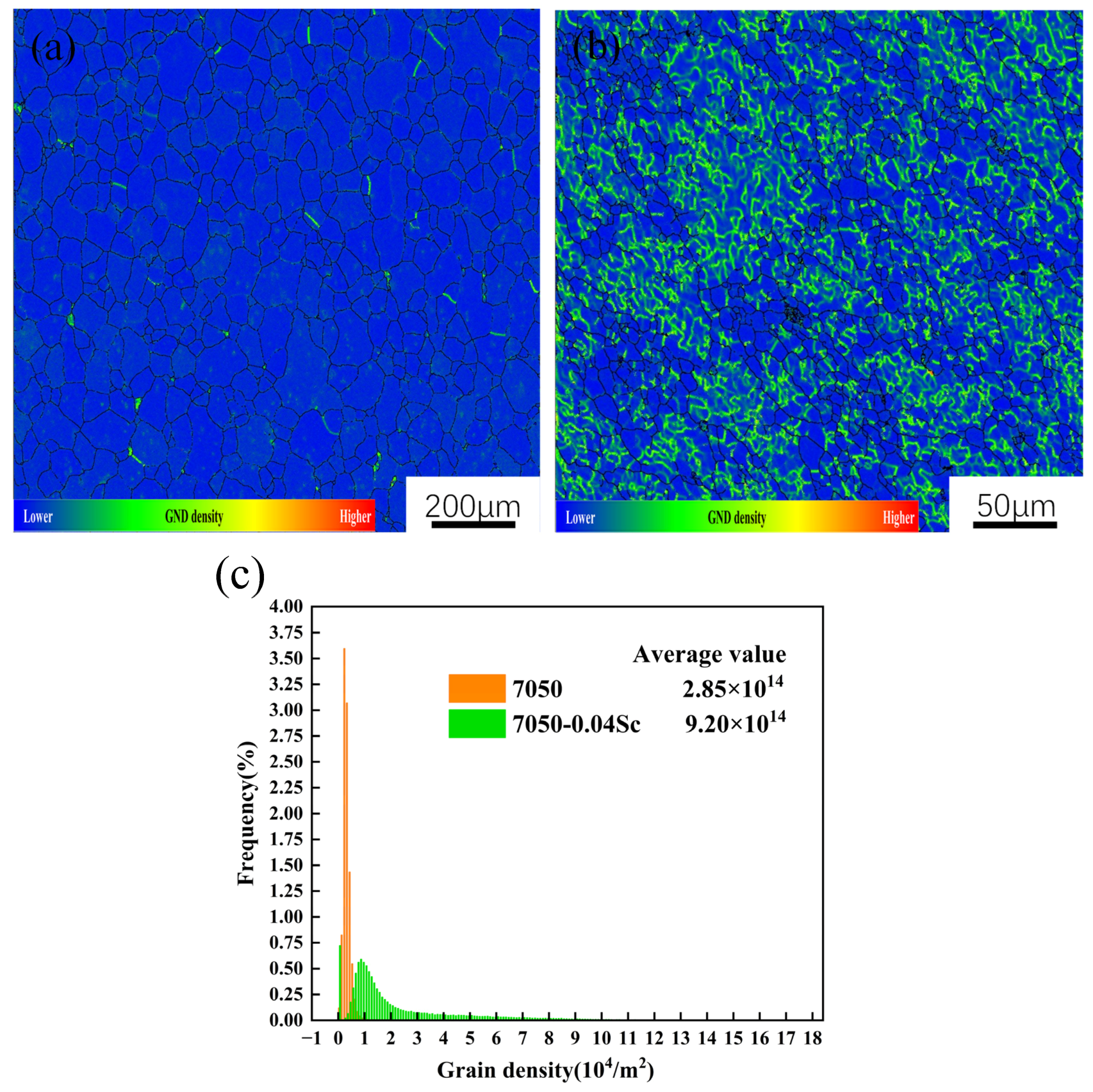
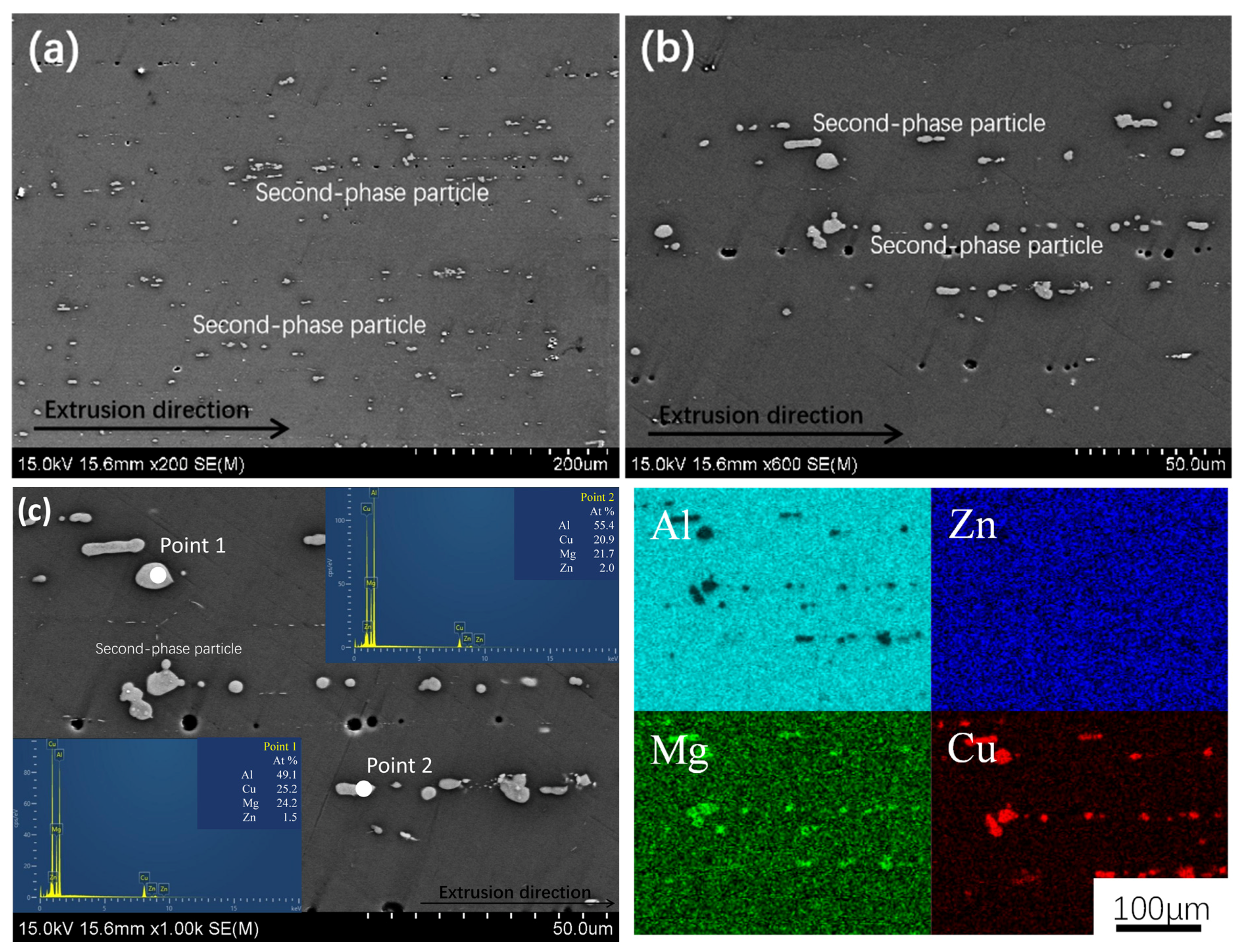
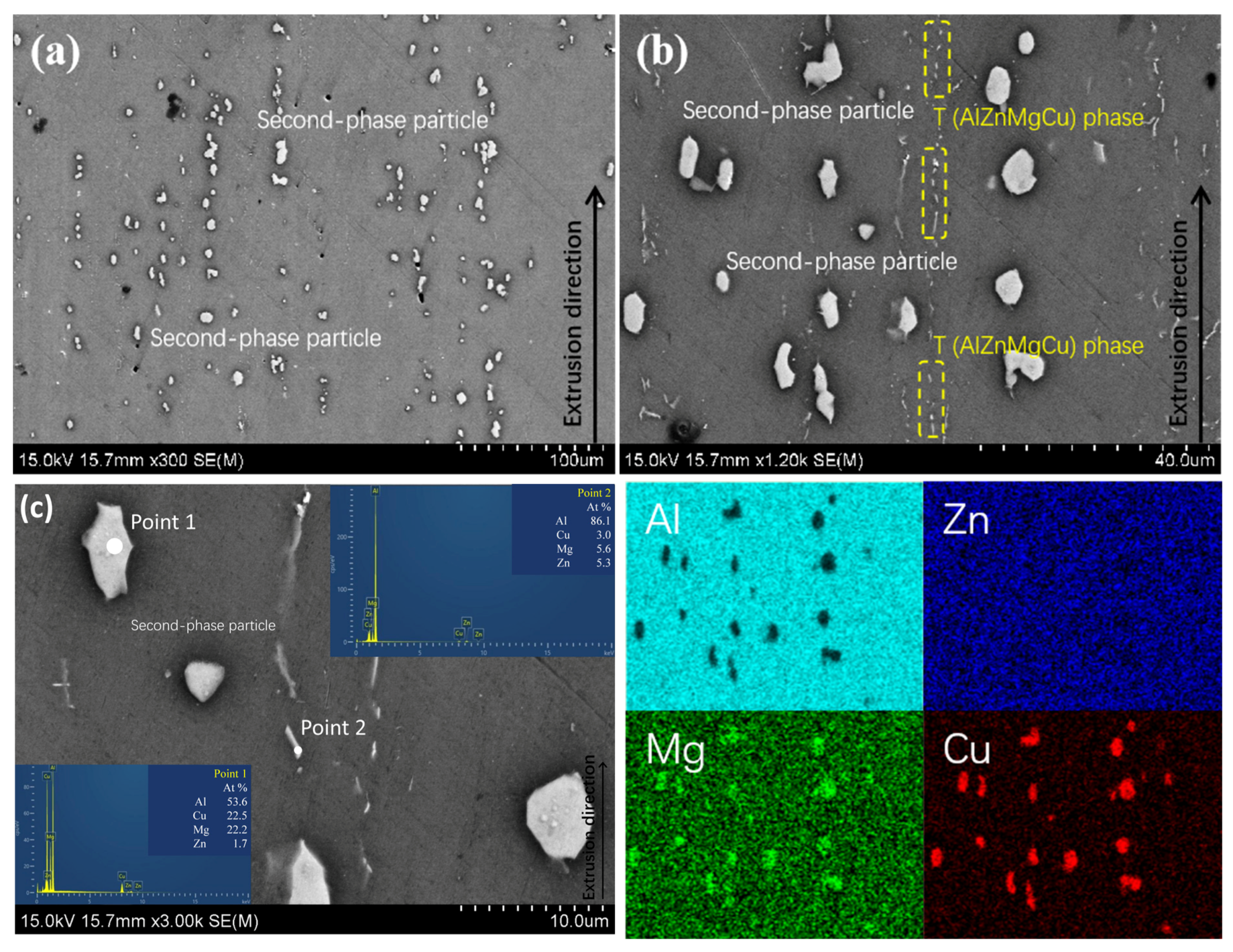
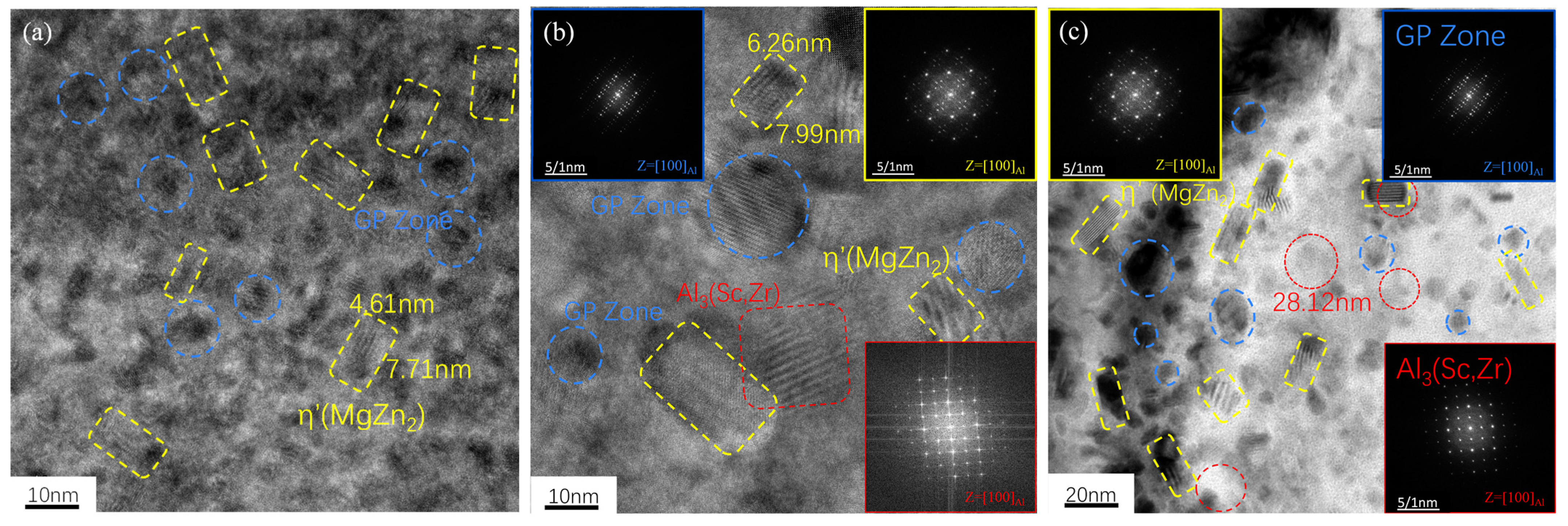
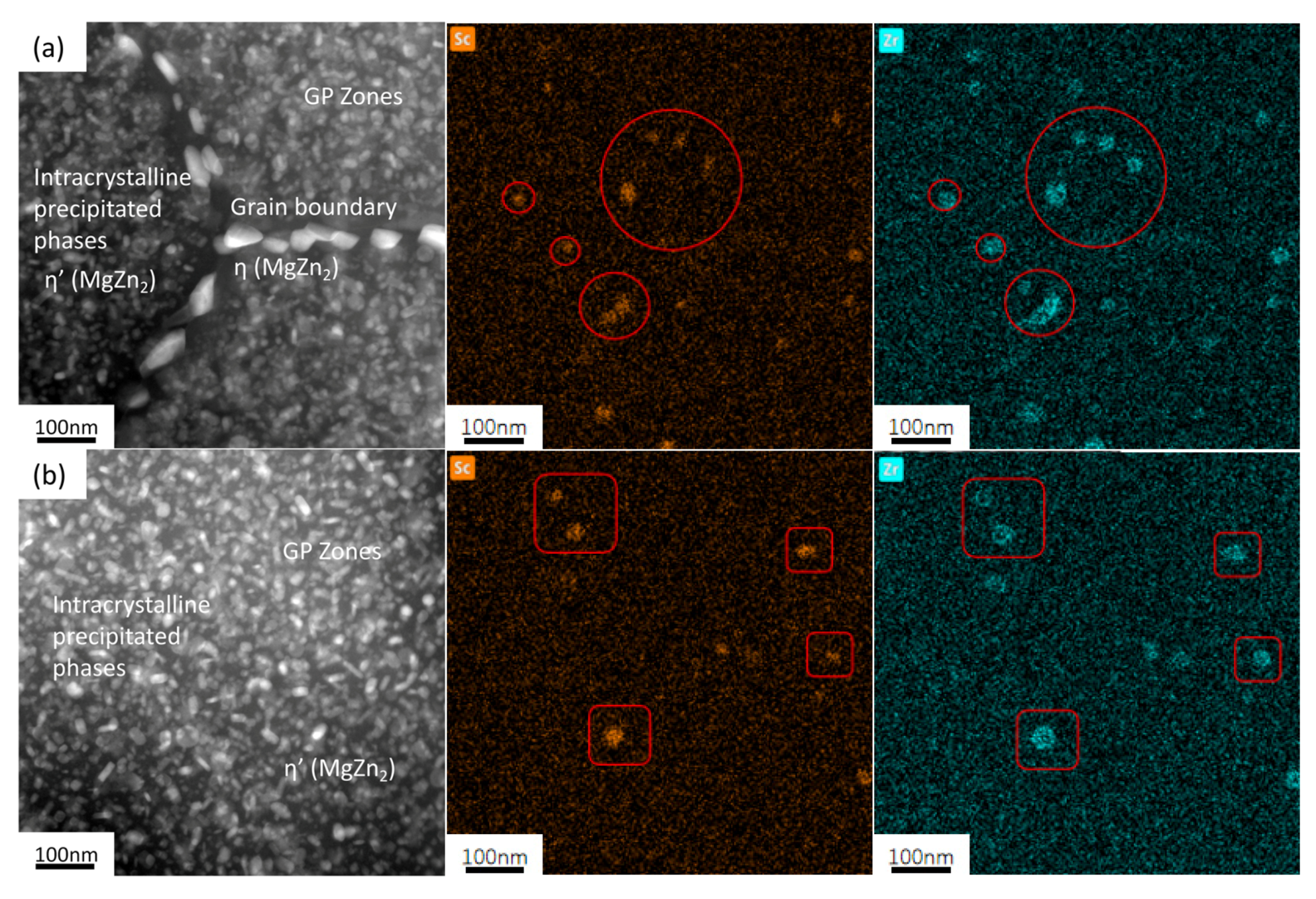
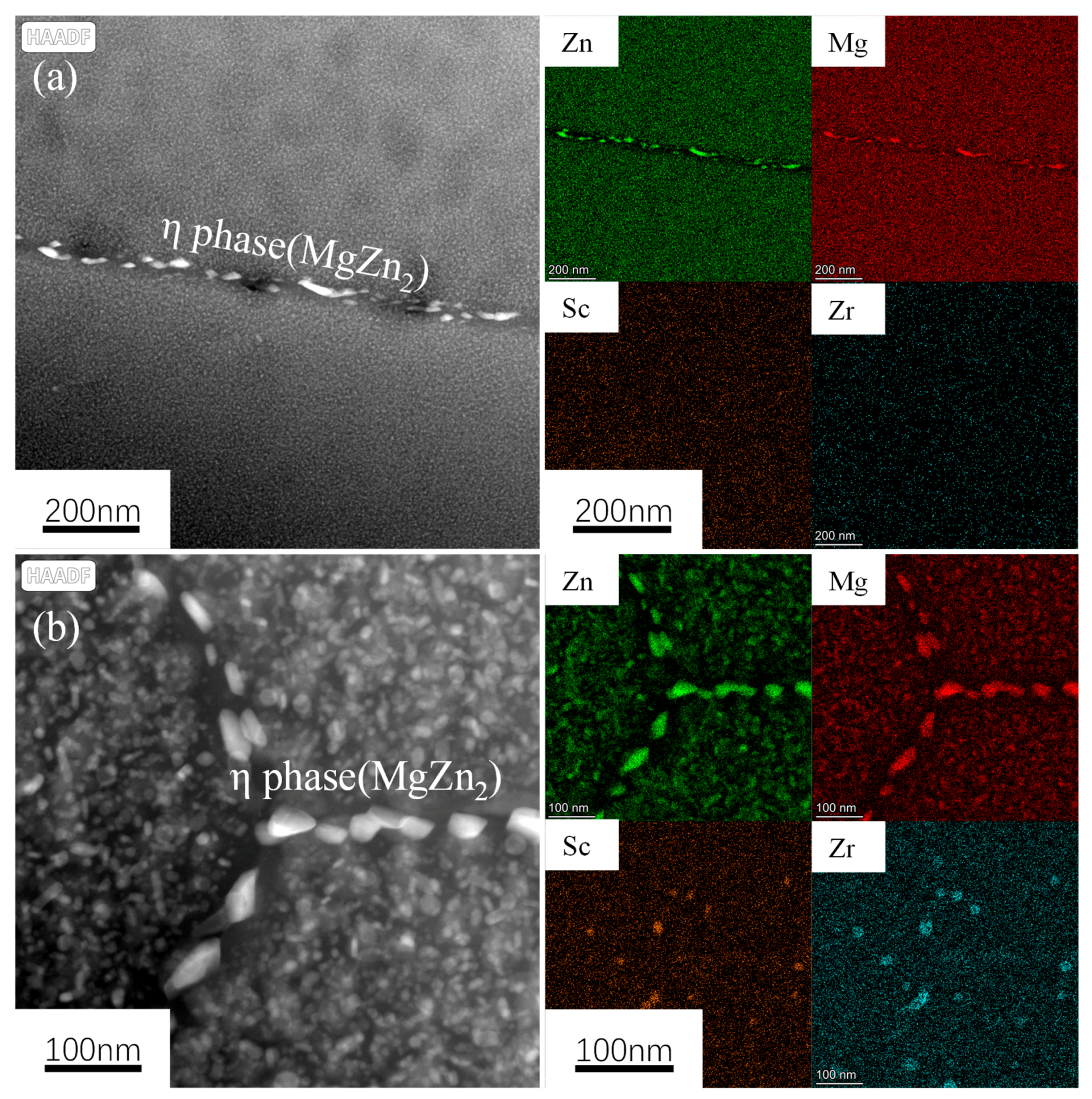
3.1. Alloy Microstructure Characterization
3.2. Alloy Mechanical Properties
4. Conclusions
- (1)
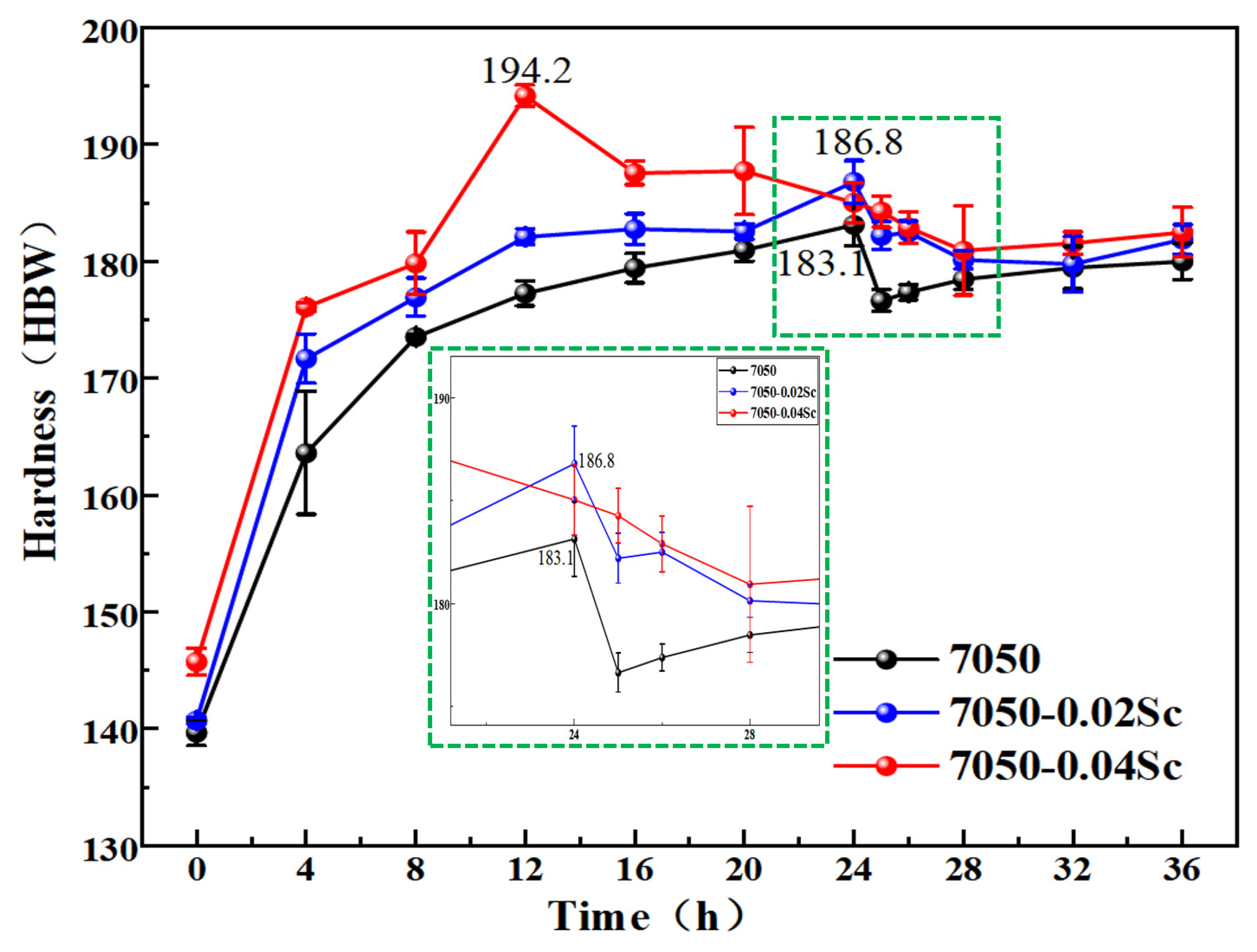
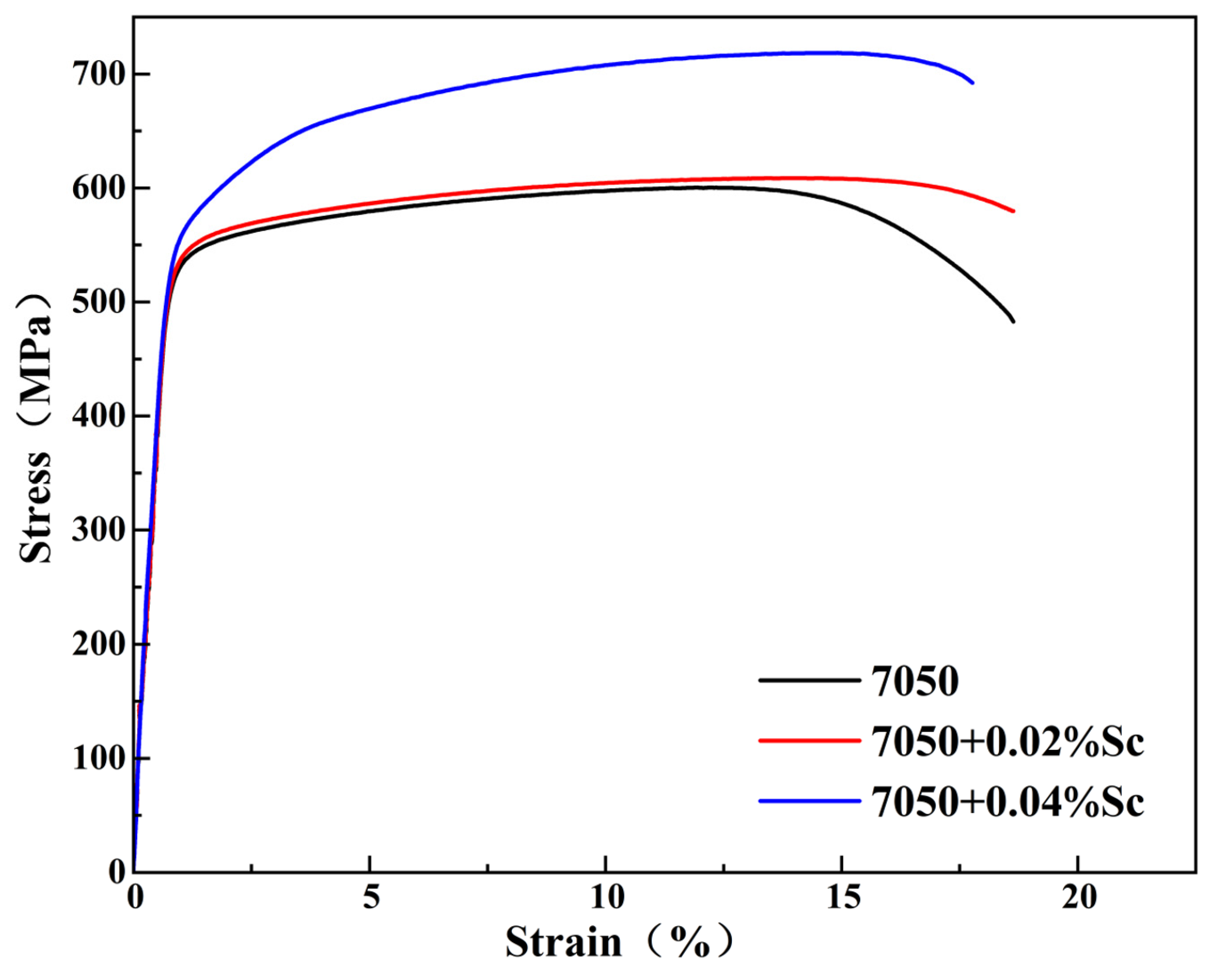
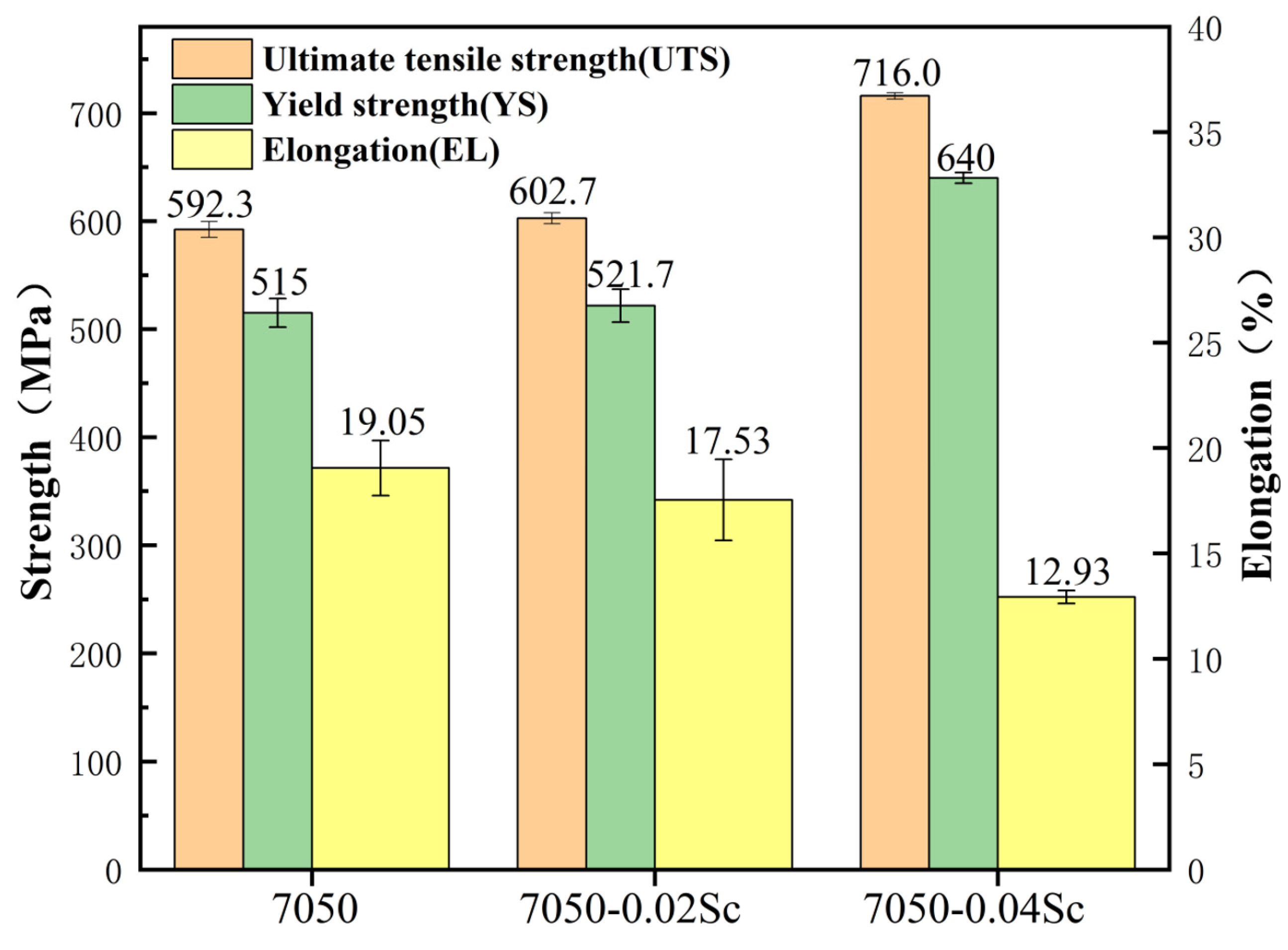
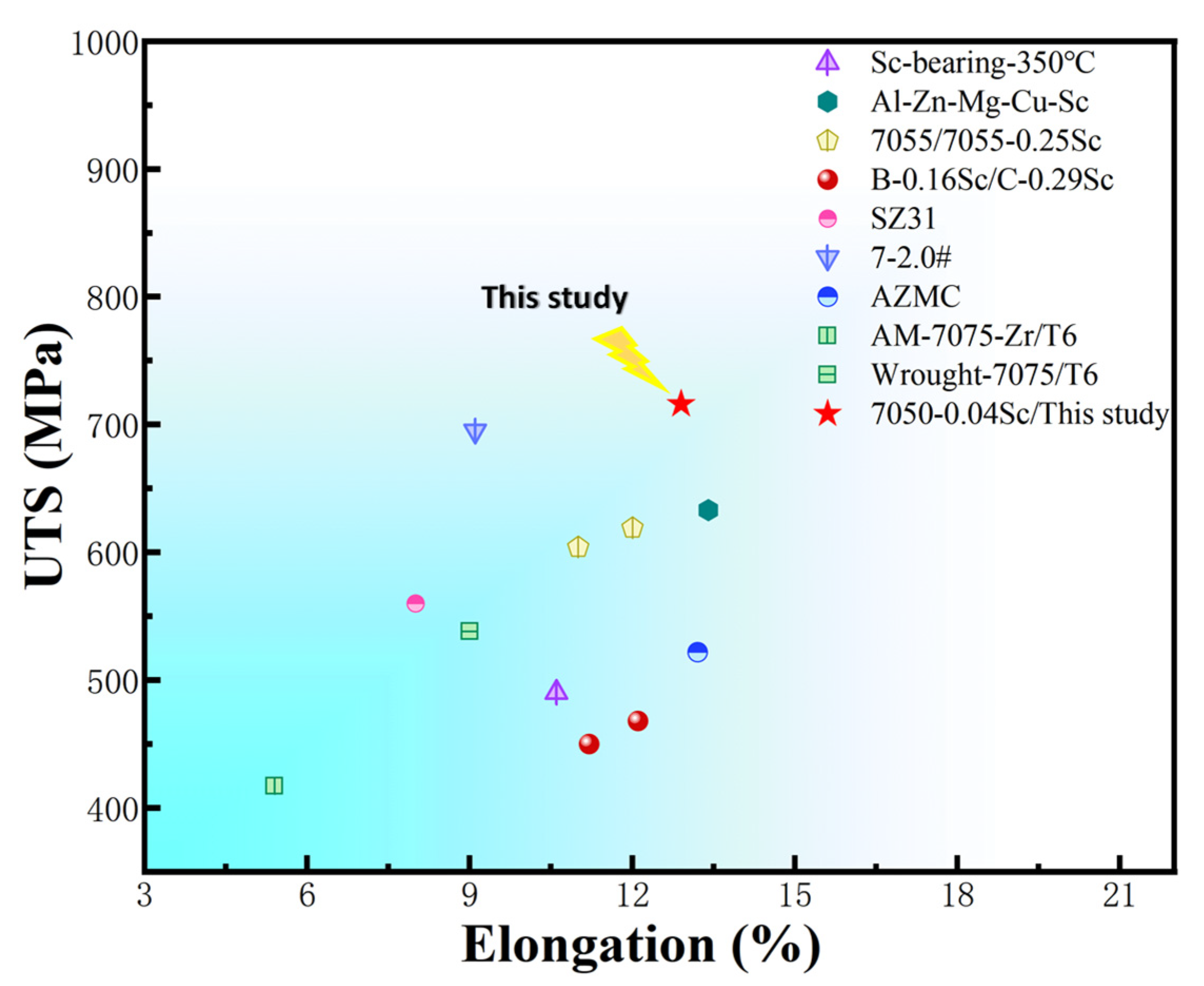
- During the aging process, the hardness of the alloys with added Sc is greater than that of the 7050 alloy at various time points. The peak hardness of the 7050-0.04Sc alloy reaches 194.2HBW, and its tensile strength, yield strength, and elongation are 716 MPa, 640 MPa, and 12.93%, respectively, which indicate strong plastic matching.
- (2)
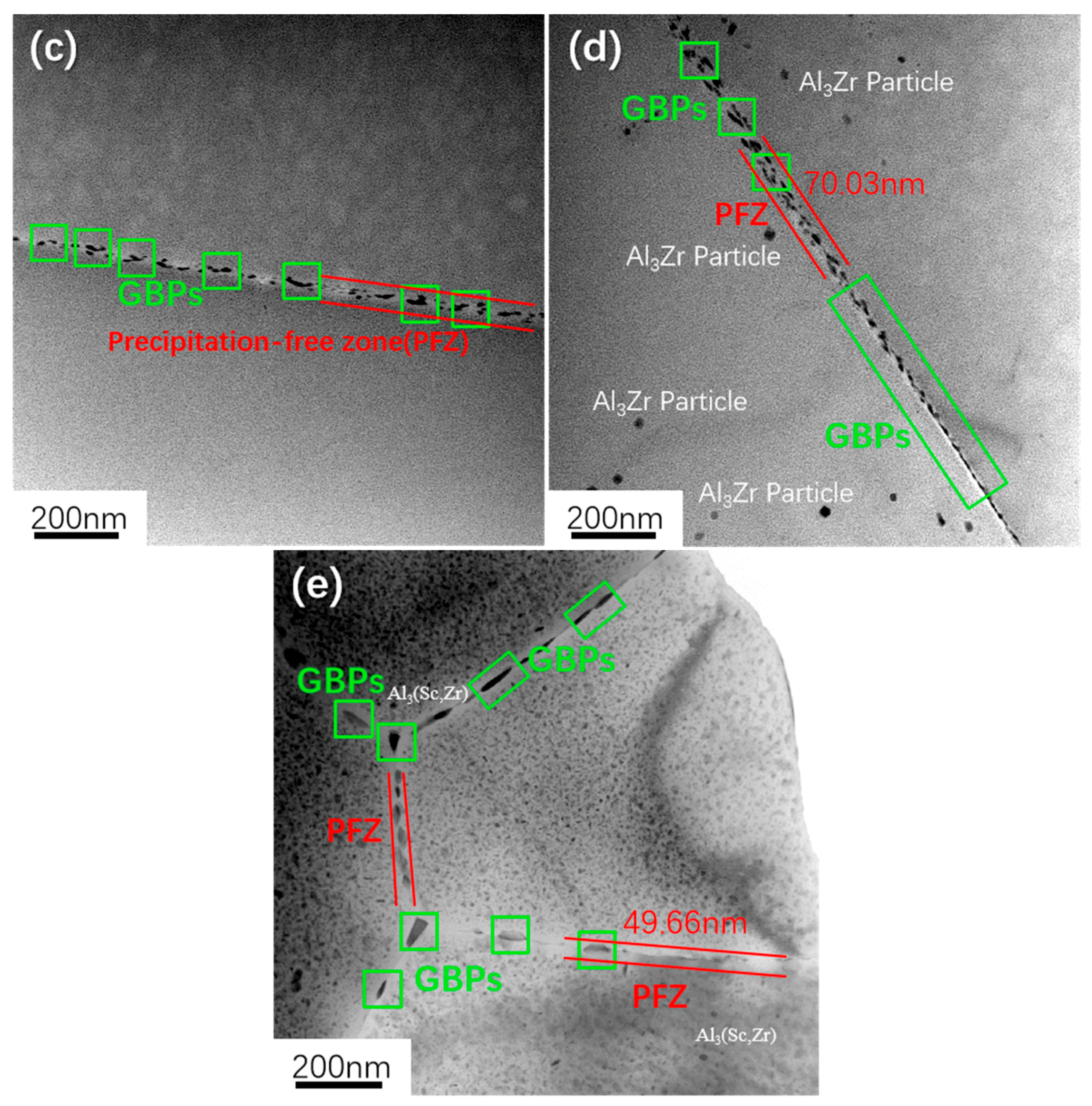
- The addition of Sc effectively inhibits the recrystallization of the 7050 alloy. After Sc is added, the original coarse recrystallized grains disappear and become fine recrystallized grains distributed around deformed grains, while the average grain size decreases from 50 μm to 20 μm. At the same time, Sc refines the precipitated phase at the grain boundary (GB) of the alloy, reduces the width of the PFZ at the grain boundary, and improves aging’s strengthening effect.
- (3)
- In the 7050 alloy, in addition to normal Al3Zr, there is an AlZr phase that is not coherent with the matrix, but after adding Sc, Al3(Sc, Zr) can form stably, and the strengthening effect is better. On the other hand, the distribution of the GP region and η phase in the grains is not affected by Sc.
- (4)
- The strengthening mechanisms include grain boundary strengthening, solid-solution strengthening, and dislocation strengthening. The main precipitated phases of the studied alloy after T6 heat treatment are the η’ phase, GP zone, and Al3Zr or Al3(Sc, Zr) phase, while the main phase on the grain boundary is the η phase. As for the Al3(Sc, Zr) particles, there is an η’ phase and a GP zone, and the strengthening effect is exerted through the Orowan bypass mechanism.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ying, T.; Gu, L.; Tang, X.; Wang, J.; Zeng, X. Effect of Sc microalloying on microstructure evolution and mechanical properties of extruded Al–Zn–Mg–Cu alloys. Mater. Sci. Eng. A 2022, 831, 142197. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, H.; Yu, S.; Ma, H.; Zheng, J.; Hu, Z. Effect of Sc on the recrystallization behavior and mechanical properties of Al-Zn-Mg-Cu-Zr alloys under isothermal multidirectional compression. Mater. Today Commun. 2024, 39, 108986. [Google Scholar] [CrossRef]
- Jiang, H.; Xing, H.; Xu, Z.; Yang, B.; Liang, E.; Zhang, J.; Sun, B. Effect of Zn content and Sc, Zr addition on microstructure and mechanical properties of Al-Zn-Mg-Cu alloys. J. Alloys Compd. 2023, 947, 169246. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, C.; Ma, Y.; Liu, Y. Effects of homogenization on the dissolution and precipitation behaviors of intermetallic phase for a Zr and Er containing Al–Zn–Mg–Cu alloy. Prog. Nat. Sci. Mater. Int. 2020, 30, 47–53. [Google Scholar] [CrossRef]
- Rometsch, P.A.; Zhang, Y.; Knight, S. Heat treatment of 7xxx series aluminium alloys—Some recent developments. Trans. Nonferrous Met. Soc. China 2014, 24, 2003–2017. [Google Scholar] [CrossRef]
- Yang, Y.; Tan, P.; Sui, Y.; Jiang, Y.; Zhou, R. Influence of Zr content on microstructure and mechanical properties of As-cast Al-Zn-Mg-Cu alloy. J. Alloys Compd. 2021, 867, 158920. [Google Scholar] [CrossRef]
- Teng, G.B.; Liu, C.Y.; Ma, Z.Y.; Zhou, W.B.; Wei, L.L.; Chen, Y.; Li, J.; Mo, Y.F. Effects of minor Sc addition on the microstructure and mechanical properties of 7055 Al alloy during aging. Mater. Sci. Eng. A 2018, 713, 61–66. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, W.; Liu, S.; Zhou, M. Effect of processing parameters on quench sensitivity of an AA7050 sheet. Mater. Sci. Eng. A 2011, 528, 795–802. [Google Scholar] [CrossRef]
- Fang, H.C.; Chao, H.; Chen, K.H. Effect of Zr, Er and Cr additions on microstructures and properties of Al–Zn–Mg–Cu alloys. Mater. Sci. Eng. A 2014, 610, 10–16. [Google Scholar] [CrossRef]
- Buranova, Y.; Kulitskiy, V.; Peterlechner, M.; Mogucheva, A.; Kaibyshev, R.; Divinski, S.V.; Wilde, G. Al3(Sc,Zr)-based precipitates in Al–Mg alloy: Effect of severe deformation. Acta Mater. 2017, 124, 210–224. [Google Scholar] [CrossRef]
- Xiao, Q.-F.; Huang, J.-W.; Jiang, Y.-G.; Jiang, F.-Q.; Wu, Y.-F.; Xu, G.-F. Effects of minor Sc and Zr additions on mechanical properties and microstructure evolution of Al−Zn−Mg−Cu alloys. Trans. Nonferrous Met. Soc. China 2020, 30, 1429–1438. [Google Scholar] [CrossRef]
- Li, G.; Zhao, N.; Liu, T.; Li, J.; He, C.; Shi, C.; Liu, E.; Sha, J. Effect of Sc/Zr ratio on the microstructure and mechanical properties of new type of Al–Zn–Mg–Sc–Zr alloys. Mater. Sci. Eng. A 2014, 617, 219–227. [Google Scholar] [CrossRef]
- Liu, C.Y.; Teng, G.B.; Ma, Z.Y.; Wei, L.L.; Zhang, B.; Chen, Y. Effects of Sc and Zr microalloying on the microstructure and mechanical properties of high Cu content 7xxx Al alloy. Int. J. Miner. Metall. Mater. 2019, 26, 1559–1569. [Google Scholar] [CrossRef]
- Zakharov, V.V. Special Features of Crystallization of Scandium-Alloyed Aluminum Alloys. Met. Sci. Heat Treat. 2012, 53, 414–419. [Google Scholar] [CrossRef]
- Xu, G.F.; Nie, Z.R.; Jin, T.N.; Rong, L.; Ruan, H.Q.; Yin, Z.M. Microstructure and properties of Al-4Cu alloy containing Sc. Trans. Nonferrous Met. Soc. China 2004, 14, 63–66. [Google Scholar]
- Zhao, K.; Gao, T.; Yang, H.; Hu, K.; Liu, G.; Nie, J.; Liu, X. Strengthening behavior of B doped TiC particles on an Al–Zn–Mg alloy. Compos. Commun. 2021, 24, 100649. [Google Scholar] [CrossRef]
- Zhao, K.; Gao, T.; Yang, H.; Liu, G.; Sun, Q.; Wu, C.; Nie, J.; Liu, X. Influence of a new AlTiC–B master alloy on the casting and extruding behaviors of 7050 alloys. J. Alloys Compd. 2020, 820, 153089. [Google Scholar] [CrossRef]
- Lee, S.-H.; Ahn, T.-Y.; Baik, S.-I.; Seidman, D.N.; Lee, S.-J.; Lee, Y.-K.; Euh, K.; Jung, J.-G. Unravelling precipitation behavior and mechanical properties of Al–Zn–Mg–Cu alloy. J. Mater. Sci. Technol. 2025, 204, 177–189. [Google Scholar] [CrossRef]
- Senkov, O.N.; Shagiev, M.R.; Senkova, S.V.; Miracle, D.B. Precipitation of Al3(Sc,Zr) particles in an Al–Zn–Mg–Cu–Sc–Zr alloy during conventional solution heat treatment and its effect on tensile properties. Acta Mater. 2008, 56, 3723–3738. [Google Scholar] [CrossRef]
- Fan, X.; Jiang, D.; Meng, Q.; Zhong, L. The microstructural evolution of an Al–Zn–Mg–Cu alloy during homogenization. Mater. Lett. 2006, 60, 1475–1479. [Google Scholar] [CrossRef]
- Yang, H.; Li, B.; Xu, L.; Huang, Z.; Chen, W.; Du, Y. Effect of homogenization treatment on microstructure and properties of novel Al-Mg-Zn-Sc-Zr alloys with different Mg/Zn ratios. J. Alloys Compd. 2025, 1010, 177355. [Google Scholar] [CrossRef]
- Yan, G.-Y.; Mao, F.; Chen, F.; Cao, Z.-Q.; Wang, T.-M. Effect of homogenization annealing on microstructure, composition and mechanical properties of 7050/6009 bimetal slab. Trans. Nonferrous Met. Soc. China 2016, 26, 2532–2541. [Google Scholar] [CrossRef]
- GB/T 24196-2009; Corrosion of Metals and Alloys—Electrochemical Test Methods—Guidelines for Conducting Potentiostatic and Potentiodynamic Polarization Measurements. China National Standardization Administration Committee: Beijing, China, 2009.
- Li, M.-G.; Sun, M.-Y.; Meng, L.-H.; Guo, X.-B. Quasi-in situ immersion characterization of grain structures evolution revealing the corrosion resistance of Al-Zn-Mg alloys with various Sc additions. J. Cent. South Univ. 2024, 30, 3964–3974. [Google Scholar] [CrossRef]
- Chen, J.; Rong, L.; Huang, H.; Ma, C.; Wei, W.; Wen, S.; Wang, Z.; Nie, Z. The effects of η phases and Al3(Er, Zr) particles pre-precipitation on the hot workability and recrystallization of Al–Zn–Mg–Er–Zr alloy. J. Mater. Res. Technol. 2024, 29, 2414–2424. [Google Scholar] [CrossRef]
- Leng, J.-F.; Ren, B.-H.; Zhou, Q.-B.; Zhao, J.-W. Effect of Sc and Zr on recrystallization behavior of 7075 aluminum alloy. Trans. Nonferrous Met. Soc. China 2021, 31, 2545–2557. [Google Scholar] [CrossRef]
- Hou, X.; Zhao, L.; Ren, S.; Peng, Y.; Ma, C.; Tian, Z.; Qu, X. Effect of Sc and Zr addition on microstructure and mechanical properties of high strength 7A52 aluminum alloy welded joints fabricated by double-wire gas metal arc welding. Mater. Today Commun. 2024, 38, 108341. [Google Scholar] [CrossRef]
- Huang, R.; Yang, H.; Zheng, S.; Li, M.; Wang, H.; Duan, Y.; Yue, C.; Yang, C. The evolution mechanism of the second phase during homogenization of Al-Zn-Mg-Cu aluminum alloy. Mater. Des. 2023, 235, 112395. [Google Scholar] [CrossRef]
- Wu, H.; Wen, S.-P.; Lu, J.-T.; Mi, Z.-P.; Zeng, X.-L.; Huang, H.; Nie, Z.-R. Microstructural evolution of new type Al–Zn–Mg–Cu alloy with Er and Zr additions during homogenization. Trans. Nonferrous Met. Soc. China 2017, 27, 1476–1482. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, T.; He, C.; Ding, J.; Liu, E.; Shi, C.; Li, J.; Zhao, N. Evolution of microstructure and properties of Al–Zn–Mg–Cu–Sc–Zr alloy during aging treatment. J. Alloys Compd. 2016, 658, 946–951. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, H.; Yan, Z.; Liaw, P.K.; Dong, P. Cyclic deformation and fatigue behavior of 7075-T651 Al alloy with a gradient structure. Mater. Sci. Eng. A 2021, 822, 141669. [Google Scholar] [CrossRef]
- Kim, Y.-Y.; Euh, K.; Kim, S.-H.; Son, H.-W. Effects of Ag/Sc microadditions on the precipitation of over-aged Al–Zn–Mg–Cu alloys. J. Mater. Sci. Technol. 2025, 209, 219–229. [Google Scholar] [CrossRef]
- Chung, T.-F.; Yang, Y.-L.; Huang, B.-M.; Shi, Z.; Lin, J.; Ohmura, T.; Yang, J.-R. Transmission electron microscopy investigation of separated nucleation and in-situ nucleation in AA7050 aluminium alloy. Acta Mater. 2018, 149, 377–387. [Google Scholar] [CrossRef]
- Chung, T.-F.; Yang, Y.-L.; Shiojiri, M.; Hsiao, C.-N.; Li, W.-C.; Tsao, C.-S.; Shi, Z.; Lin, J.; Yang, J.-R. An atomic scale structural investigation of nanometre-sized η precipitates in the 7050 aluminium alloy. Acta Mater. 2019, 174, 351–368. [Google Scholar] [CrossRef]
- Shu, W.X.; Hou, L.G.; Zhang, C.; Zhang, F.; Liu, J.C.; Liu, J.T.; Zhuang, L.Z.; Zhang, J.S. Tailored Mg and Cu contents affecting the microstructures and mechanical properties of high-strength Al–Zn–Mg–Cu alloys. Mater. Sci. Eng. A 2016, 657, 269–283. [Google Scholar] [CrossRef]
- Zhu, X.-W.; Deng, Y.; Lai, Y.; Guo, Y.-F.; Yang, Z.-A.; Fu, L.; Xu, G.-F.; Huang, J.-W. Effects of Al3(Sc1-Zr) nano-particles on microstructure and mechanical properties of friction-stir-welded Al-Mg-Mn alloys. Trans. Nonferrous Met. Soc. China 2023, 33, 25–35. [Google Scholar] [CrossRef]
- Shi, Y.; Pan, Q.; Li, M.; Huang, X.; Li, B. Influence of alloyed Sc and Zr, and heat treatment on microstructures and stress corrosion cracking of Al–Zn–Mg–Cu alloys. Mater. Sci. Eng. A 2015, 621, 173–181. [Google Scholar] [CrossRef]
- Deng, Y.; Yin, Z.; Zhao, K.; Duan, J.; Hu, J.; He, Z. Effects of Sc and Zr microalloying additions and aging time at 120 °C on the corrosion behaviour of an Al–Zn–Mg alloy. Corros. Sci. 2012, 65, 288–298. [Google Scholar] [CrossRef]
- Shi, Y.; Pan, Q.; Li, M.; Huang, X.; Li, B. Effect of Sc and Zr additions on corrosion behaviour of Al–Zn–Mg–Cu alloys. J. Alloys Compd. 2014, 612, 42–50. [Google Scholar] [CrossRef]
- Cassell, A.M.; Robson, J.D.; Race, C.P.; Eggeman, A.; Hashimoto, T.; Besel, M. Dispersoid composition in zirconium containing Al-Zn-Mg-Cu (AA7010) aluminium alloy. Acta Mater. 2019, 169, 135–146. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, Y.; Gault, B.; Makineni, S.K.; Ponge, D.; Raabe, D. (Al, Zn)3Zr dispersoids assisted η′ precipitation in anAl-Zn-Mg-Cu-Zr alloy. Materialia 2020, 10, 100641. [Google Scholar] [CrossRef]
- Hu, T.; Ma, K.; Topping, T.D.; Schoenung, J.M.; Lavernia, E.J. Precipitation phenomena in an ultrafine-grained Al alloy. Acta Mater. 2013, 61, 2163–2178. [Google Scholar] [CrossRef]
- Vafaeenezhad, H.; Shahverdi, H.R. Synergic effects of Si and V micro-additions on microstructural and mechanical properties of a dilute Al–Sc–Zr alloy containing L12 Al3(Sc, Zr, V) nanoprecipitates. J. Alloys Compd. 2023, 967, 171747. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, D.; Li, B.; Ying, T.; Hu, J. Heating aging behavior of Al–8.35Zn–2.5Mg–2.25Cu alloy. Mater. Des. 2014, 60, 116–124. [Google Scholar] [CrossRef]
- Yang, M.; Lei, L.; Jiang, Y.; Xu, F.; Yin, C. Simultaneously improving tensile properties and stress corrosion cracking resistance of 7075-T6 aluminum alloy by USRP treatment. Corros. Sci. 2023, 218, 111211. [Google Scholar] [CrossRef]
- Ekaputra, C.N.; Mogonye, J.-E.; Dunand, D.C. Microstructure and mechanical properties of cast, eutectic Al-Ce-Ni-Mn-Sc-Zr alloys with multiple strengthening mechanisms. Acta Mater. 2024, 274, 120006. [Google Scholar] [CrossRef]
- Ekaputra, C.N.; Rakhmonov, J.U.; Weiss, D.; Mogonye, J.-E.; Dunand, D.C. Microstructure and mechanical properties of cast Al-Ce-Sc-Zr-(Er) alloys strengthened by Al11Ce3 micro-platelets and L12 Al3(Sc,Zr,Er) nano-precipitates. Acta Mater. 2022, 240, 118354. [Google Scholar] [CrossRef]
- Martin, J.H.; Yahata, B.D.; Hundley, J.M.; Mayer, J.A.; Schaedler, T.A.; Pollock, T.M. 3D printing of high-strength aluminium alloys. Nature 2017, 549, 365–369. [Google Scholar] [CrossRef]
- Chen, Z.; Mo, Y.; Nie, Z. Effect of Zn Content on the Microstructure and Properties of Super-High Strength Al-Zn-Mg-Cu Alloys. Metall. Mater. Trans. A 2013, 44, 3910–3920. [Google Scholar] [CrossRef]
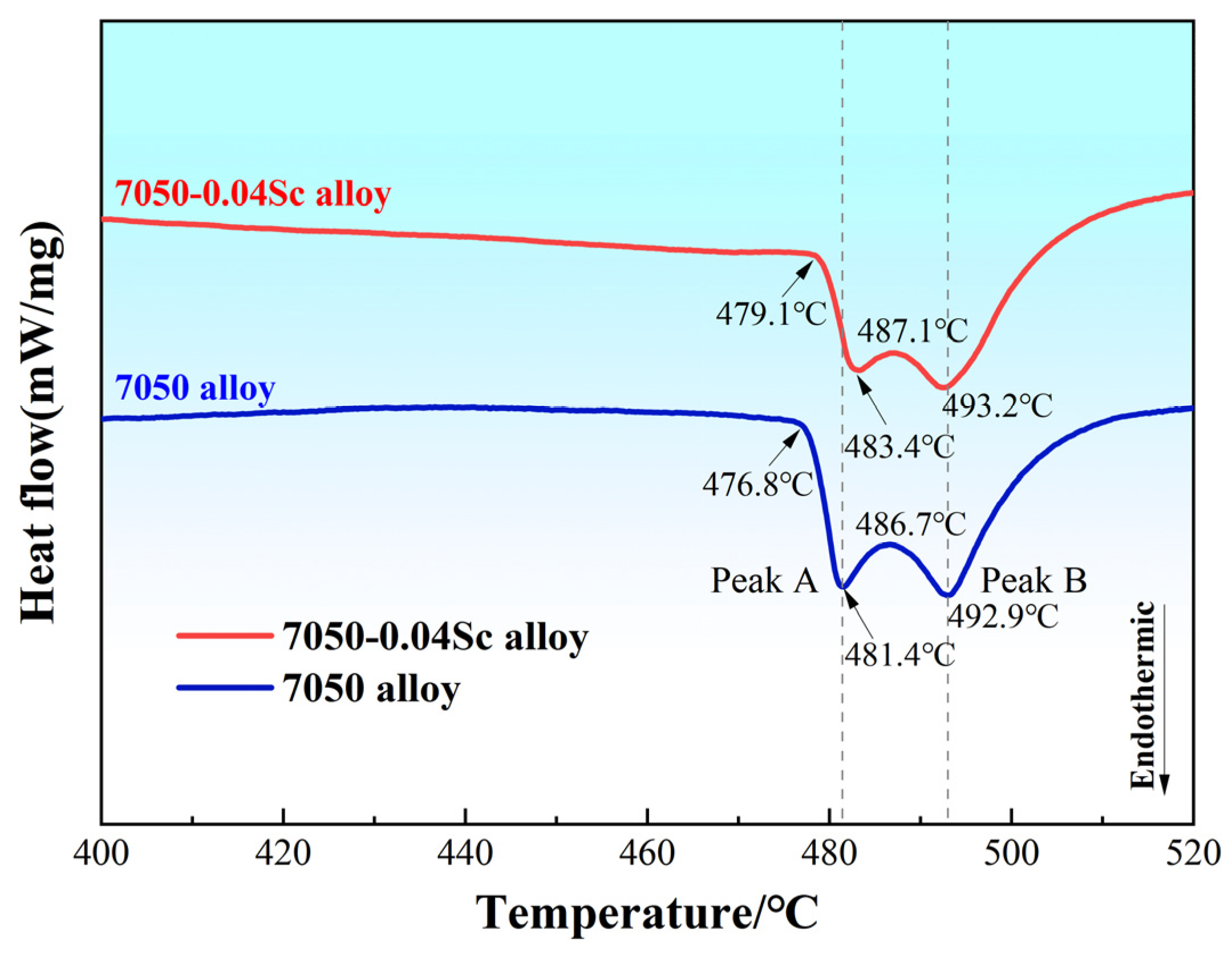
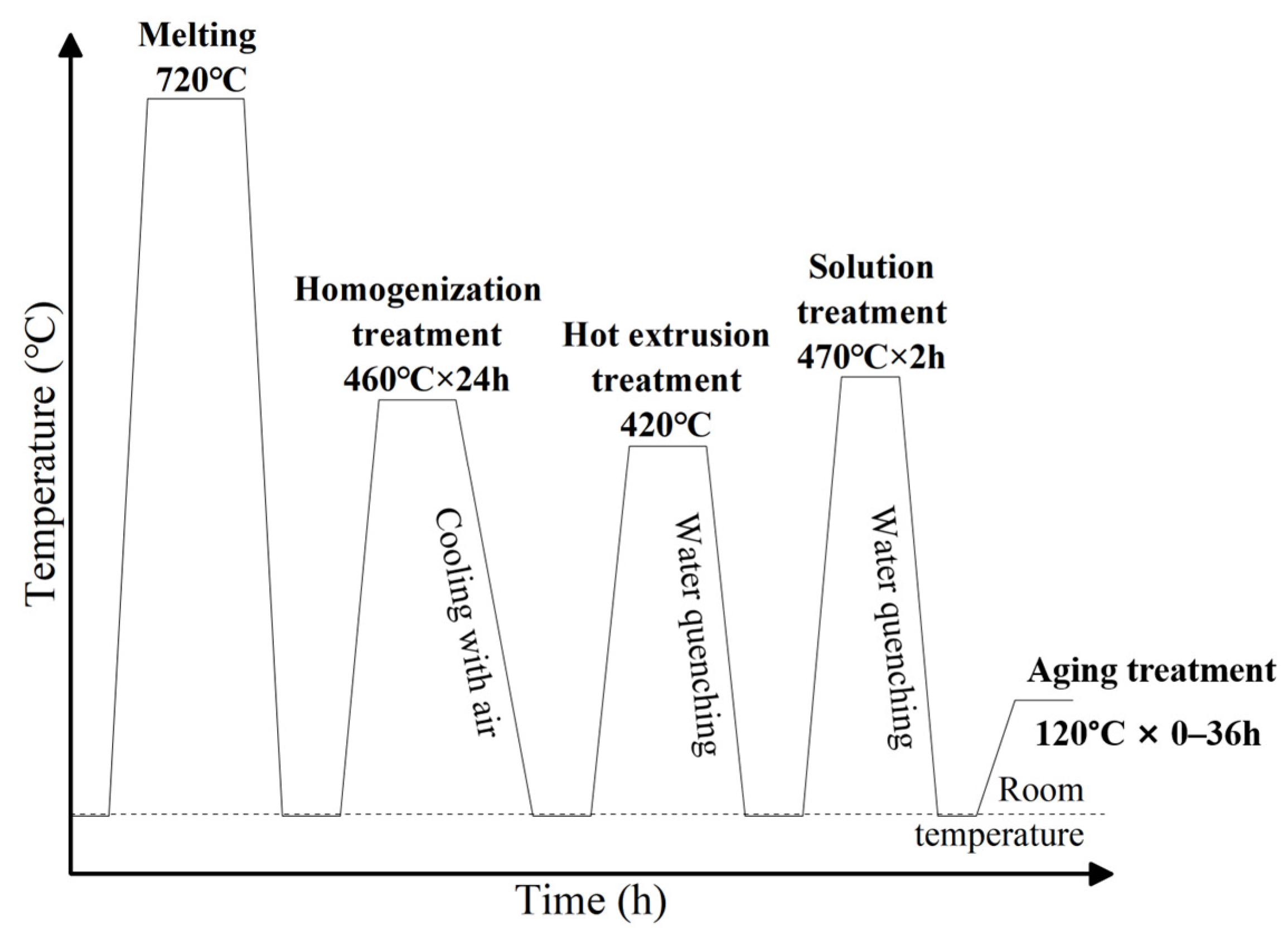

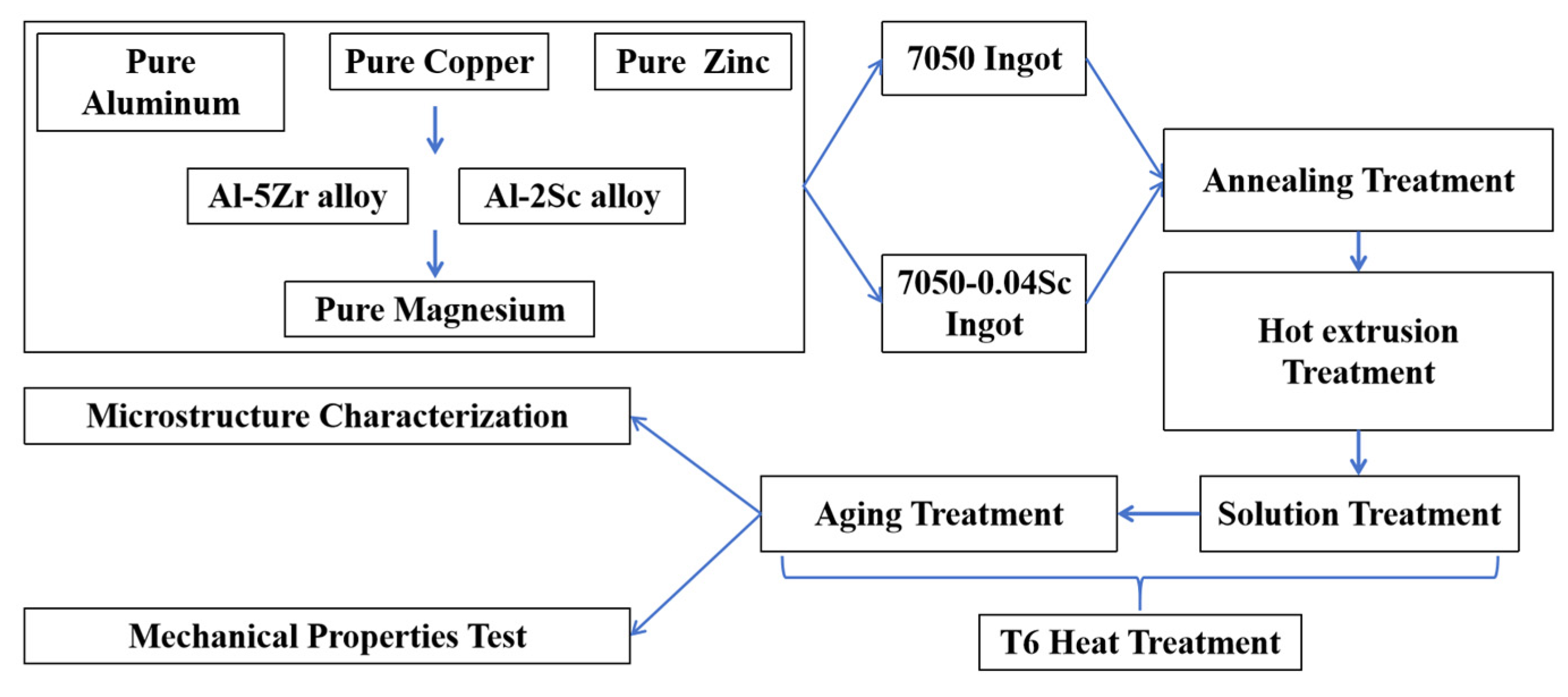

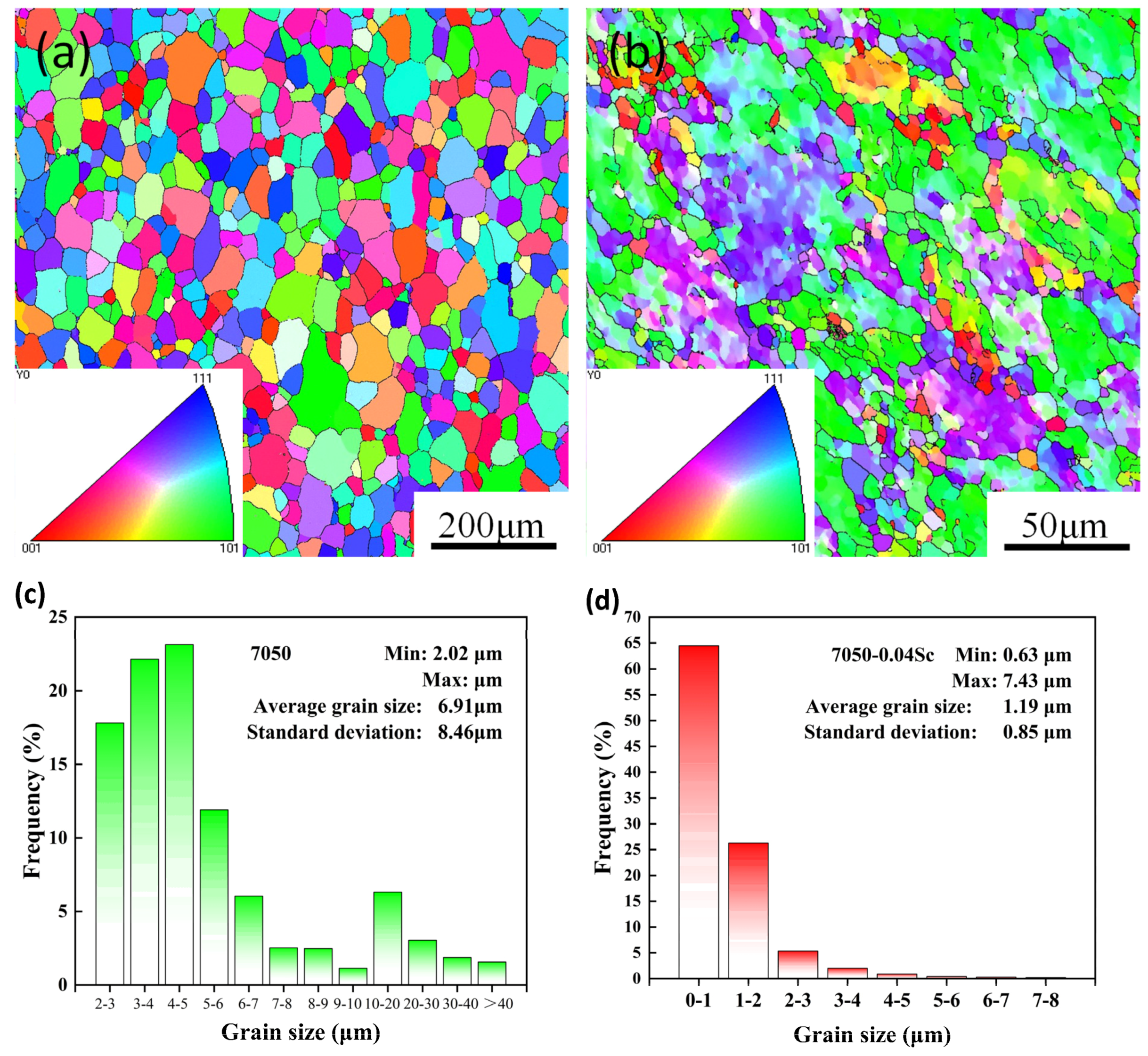


| Alloy No. | Mass Fraction/% | |||||
|---|---|---|---|---|---|---|
| Zn | Mg | Cu | Zr | Sc | Al | |
| 7050 | 6.2 (5.90, 0.022) | 2.3 (2.37, 0.021) | 2.3 (1.92, 0.047) | 0.08 (0.072, 0.001) | 0 | Bal. |
| 7050-0.02Sc | 6.2 (5.89, 0.022) | 2.3 (2.33, 0.022) | 2.3 (1.90, 0.017) | 0.08 (0.072, 0.002) | 0.02 (0.018, 0.001) | Bal. |
| 7050-0.04Sc | 6.2 (5.57, 0.021) | 2.3 (2.19, 0.024) | 2.3 (1.79, 0.029) | 0.08 (0.084, 0.001) | 0.04 (0.033, 0.001) | Bal. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Xia, L.; Yang, H.; Wang, C.; Wu, Y.; Liu, X. Effect of Trace Sc Addition on Microstructure and Mechanical Properties of Al-Zn-Mg-Cu-Zr Alloy. Materials 2025, 18, 648. https://doi.org/10.3390/ma18030648
Huang Y, Xia L, Yang H, Wang C, Wu Y, Liu X. Effect of Trace Sc Addition on Microstructure and Mechanical Properties of Al-Zn-Mg-Cu-Zr Alloy. Materials. 2025; 18(3):648. https://doi.org/10.3390/ma18030648
Chicago/Turabian StyleHuang, Yuchen, Linfei Xia, Huabing Yang, Chengguo Wang, Yuying Wu, and Xiangfa Liu. 2025. "Effect of Trace Sc Addition on Microstructure and Mechanical Properties of Al-Zn-Mg-Cu-Zr Alloy" Materials 18, no. 3: 648. https://doi.org/10.3390/ma18030648
APA StyleHuang, Y., Xia, L., Yang, H., Wang, C., Wu, Y., & Liu, X. (2025). Effect of Trace Sc Addition on Microstructure and Mechanical Properties of Al-Zn-Mg-Cu-Zr Alloy. Materials, 18(3), 648. https://doi.org/10.3390/ma18030648







