Boosting Electrooxidation of Ethanol by Nickel Addition to Metallic Glass Ribbon Precursors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of MG Ribbons and Dealloyed Electrocatalysts
2.3. Structure Characterization
2.4. Electrochemical Test
3. Results
3.1. EOR Performance
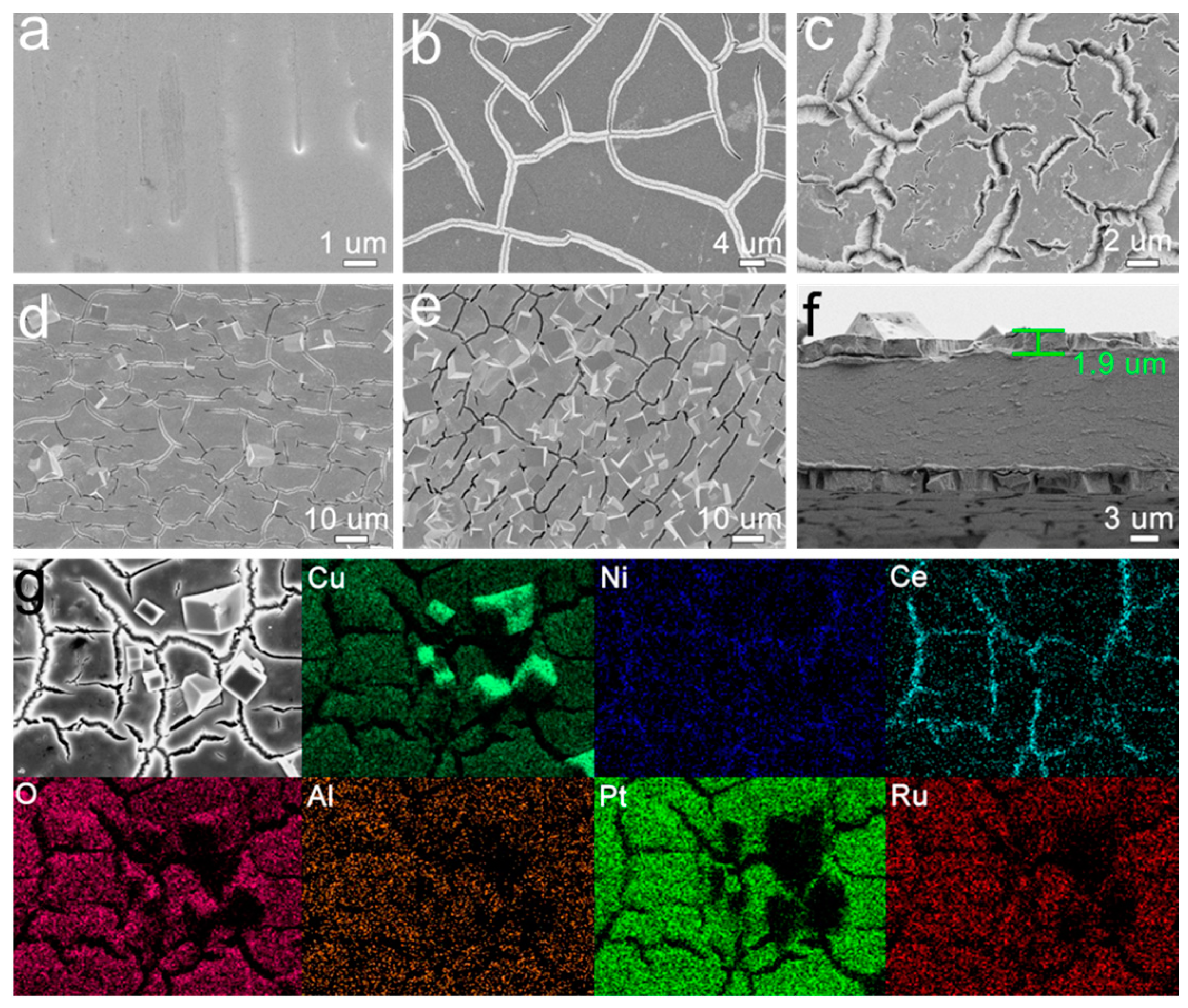
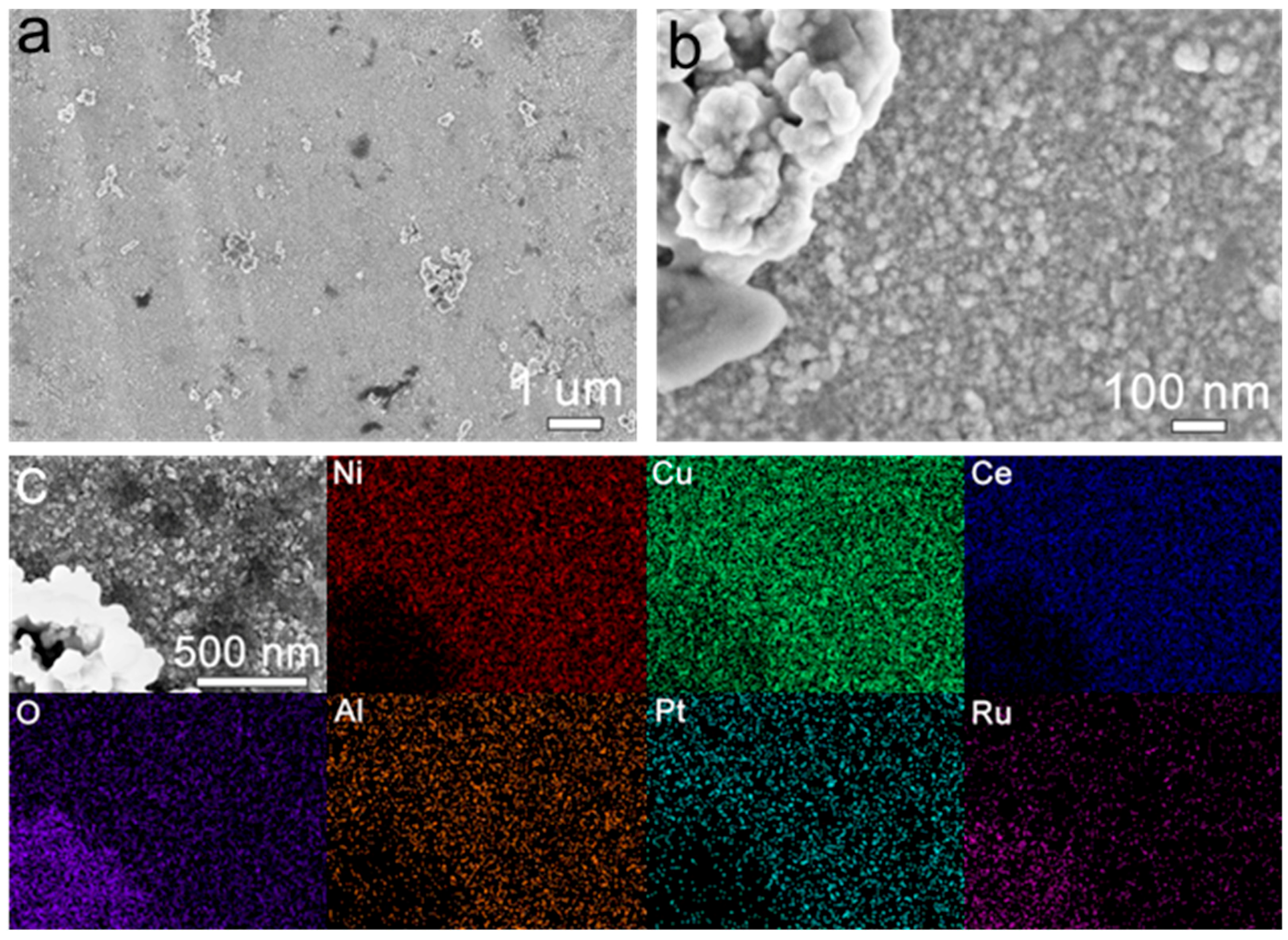
3.2. Morphological and Structural Characterization
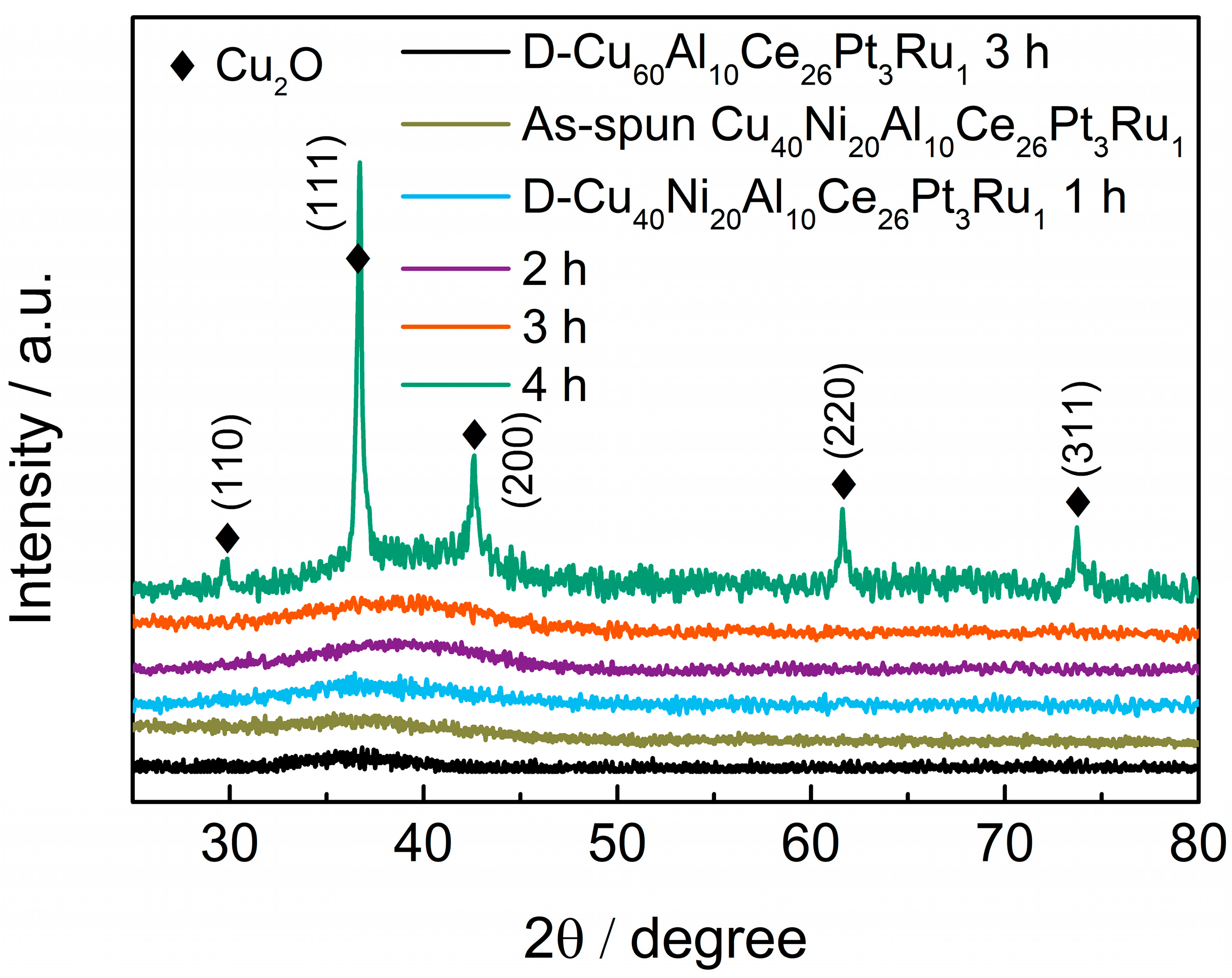
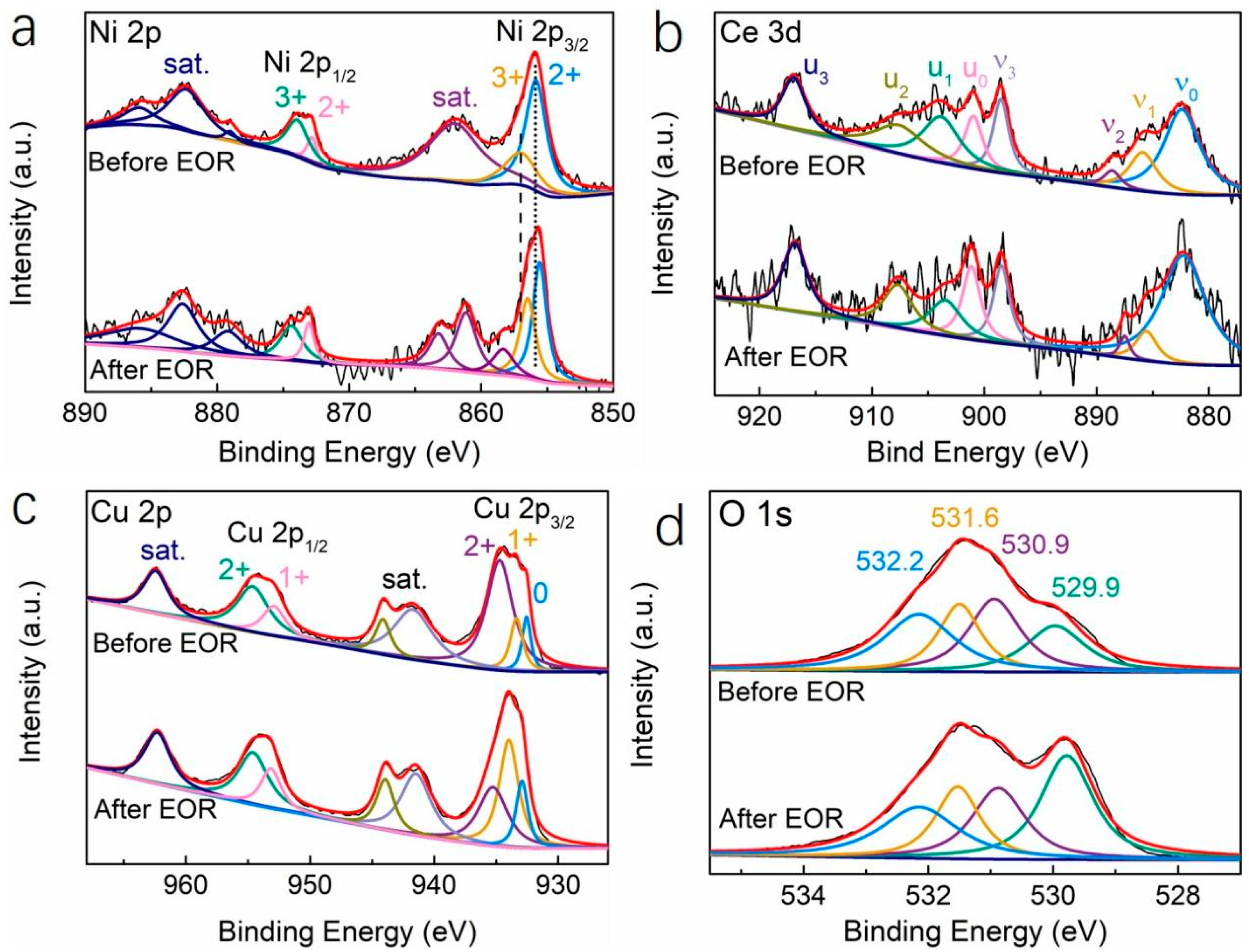
3.3. Dealloying Reaction and EOR Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shaari, N.; Kamarudin, S.K.; Bahru, R.; Osman, S.H.; Ishak, N.A.I.M. Progress and challenges: Review for direct liquid fuelcell. Int. J. Energy Res. 2021, 45, 6644–6688. [Google Scholar] [CrossRef]
- Tan, X.; Chen, S.; Yan, D.; Du, R.; Zhong, Q.; Liao, L.; Tang, Z.; Zeng, F. Recent advances in Ni-based catalysts for the electrochemical oxidation of ethanol. J. Energy Chem. 2024, 98, 588–614. [Google Scholar] [CrossRef]
- Kang, Z.; Zhao, M.; Wu, Y.; Xia, T.; Cao, J.P.; Cai, W.; Chen, L. Facial fabrication of yolk-shell Pd-Ni-P alloy with mesoporous structure as an advanced catalyst for methanol electro-oxidation. Appl. Surf. Sci. 2019, 484, 441–445. [Google Scholar] [CrossRef]
- Cheng, T.; Tian, J.; Du, J.; Wang, Z.; Ye, J.; Liu, A.; Chen, Q.; Zhu, Y. Self-Interface in Rh Nanosheets-Supported Tetrahedral Rh Nanocrystals for Promoting Electrocatalytic Oxidation of Ethanol. Small 2024, 20, 2306221. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, C.; Shang, H.; Wang, Y.; You, H.; Xu, H.; Du, Y. Metal-modified PtTe2 nanorods: Surface reconstruction for efficient methanol oxidation electrocatalysis. Chem. Eng. J. 2021, 424, 130319. [Google Scholar] [CrossRef]
- Ren, X.; Lv, Q.; Liu, L.; Liu, B.; Wang, Y.; Liu, A.; Wu, G. Current progress of Pt and Pt-based electrocatalysts used for fuel cells. Sustain. Energy Fuels 2020, 4, 15–30. [Google Scholar] [CrossRef]
- Wang, H.; Lee, H.W.; Deng, Y.; Lu, Z.; Hsu, P.C.; Liu, Y.; Lin, D.; Cui, Y. Bifunctional non-noble metal oxide nanoparticle electrocatalysts through lithium-induced conversion for overall water splitting. Nat. Commun. 2015, 6, 7261. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Bagchi, D.; Dheer, L.; Sarma, S.; Rajaji, V.; Narayana, C.; Waghmare, U.; Peter, S. Mechanistic insights into the promotional effect of Ni substitution in non-noble metal carbides for highly enhanced water splitting. Appl. Catal. B Environ. 2021, 298, 120560. [Google Scholar] [CrossRef]
- She, H.; Sun, Y.; Li, S.; Huang, J.; Wang, L.; Zhu, G.; Wang, Q. Synthesis of non-noble metal nickel doped sulfide solid solution for improved photocatalytic performance. Appl. Catal. B Environ. 2019, 245, 439–447. [Google Scholar] [CrossRef]
- Yu, L.; Zhu, Q.; Song, S.; McElhenny, B.; Wang, D.; Wu, C.; Qin, Z.; Bao, J.; Yu, Y.; Chen, S.; et al. Non-noble metal-nitride based electrocatalysts for high-performance alkaline seawater electrolysis. Nat. Commun. 2019, 10, 5106. [Google Scholar] [CrossRef]
- Shekhawat, A.; Samanta, R.; Panigrahy, S.; Barman, S. Electrocatalytic oxidation of urea and ethanol on two-dimensional amorphous nickel oxide encapsulated on N-doped carbon nanosheets. ACS Appl. Energy Mater. 2023, 6, 3135–3146. [Google Scholar] [CrossRef]
- Zhou, X.C.; Yang, X.Y.; Fu, Z.B.; Yang, Q.; Yang, X.; Tang, Y.J.; Wang, C.Y.; Yi, Y. Single-crystalline ultrathin nanofilms of Ni aerogel with Ni(OH)2 hybrid nanoparticles towards enhanced catalytic performance for ethanol electro-oxidation. Appl. Surf. Sci. 2019, 492, 756–764. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, W.; Fang, J.; Xu, Z.; Xue, Y.; Liu, D.; Sui, R.; Lv, Q.; Liu, X.; Wang, Y.; et al. Defective Ni3S2 nanowires as highly active electrocatalysts for ethanol oxidative upgrading. Nano Res. 2022, 15, 2987–2993. [Google Scholar] [CrossRef]
- Shi, W.; Wang, Q.; Qin, F.; Yu, J.; Jia, M.; Gao, H.; Zhang, Y.; Zhao, Y.; Li, G. N-doped carbon encapsulated nickel nanoparticles: Rational fabrication and ultra-high performance for ethanol oxidation. Electrochim. Acta 2017, 232, 332–338. [Google Scholar] [CrossRef]
- Sharma, P.; Radhakrishnan, S.; Khil, M.S.; Kim, H.Y.; Kim, B.S. Simple room temperature synthesis of porous nickel phosphate foams for electrocatalytic ethanol oxidation. J. Electroanal. Chem. 2018, 808, 236–244. [Google Scholar] [CrossRef]
- Zhang, K.; Han, Y.; Qiu, J.; Ding, X.; Deng, Y.; Wu, Y.; Zhang, G.; Yan, L. Interface engineering of Ni/NiO heterostructures with abundant catalytic active sites for enhanced methanol oxidation electrocatalysis. J. Colloid Interface Sci. 2023, 630, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Pei, C.; Wu, Y.; Chen, S.; Zhao, Z.J.; Gong, J. Tunable metal-oxide interaction with balanced Ni0/Ni2+ sites of NixMg1−xO for ethanol steam reforming. Appl. Catal. B Environ. 2021, 293, 120178. [Google Scholar] [CrossRef]
- Chen, W.; Shi, J.; Wu, Y.; Jiang, Y.; Huang, Y.C.; Zhou, W.; Liu, J.; Dong, C.; Zou, Y.; Wang, S. Vacancy-induced catalytic mechanism for alcohol electrooxidation on nickel-based electrocatalyst. Angew. Chem. Int. Ed. 2024, 63, e202316449. [Google Scholar] [CrossRef] [PubMed]
- Sheng, S.; Ye, K.; Sha, L.; Zhu, K.; Gao, Y.; Yan, J.; Wang, G.; Cao, D. Rational design of Co-SP nanosheet arrays as bifunctional electrocatalysts for both ethanol oxidation reaction and hydrogen evolution reaction. Inorg. Chem. Front. 2020, 7, 4498–4506. [Google Scholar] [CrossRef]
- Ghalkhani, M.; Mirzaie, R.A.; Banimostafa, A.; Sohouli, E.; Hashemi, E. Electrosynthesis of ternary nonprecious Ni, Cu, Fe oxide nanostructure as efficient electrocatalyst for ethanol electro-oxidation: Design strategy and electrochemical performance. Int. J. Hydrogen Energy 2023, 48, 21214–21223. [Google Scholar] [CrossRef]
- Baruah, S.; Kumar, A.; Peela, N.R. Role of ZSM-5/AC hybrid support on the catalytic activity of Pd-Ag electrocatalysts towards ethanol oxidation: An experimental and kinetic study. Electrochim. Acta 2023, 453, 142357. [Google Scholar] [CrossRef]
- Tang, X.D.; Wang, Y.; Wang, C.Y.; Yuan, L.; Yi, Y. Pt-Bi on carbon aerogels as efficient electrocatalysts for ethanol oxidation reaction in alkaline medium. J. Alloys Compd. 2023, 938, 168398. [Google Scholar] [CrossRef]
- Liu, D.; Zhu, Z.; Li, J.; Chen, L.W.; Huang, H.Z.; Jing, X.T.; Yin, A.X. Rh–Cu alloy nano-dendrites with enhanced electrocatalytic ethanol oxidation activity. J. Energy Chem. 2023, 82, 343–349. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, A.; Bai, Y.; Chu, M.; Li, H.; Liu, Y.; Zhu, P.; Chen, X.; Deng, C.; Yuan, X. One stone two birds: Unlocking the synergy between amorphous Ni(OH)2 and Pd nanocrystals toward ethanol and formic acid oxidation. Inorg. Chem. 2022, 61, 14419–14427. [Google Scholar] [CrossRef]
- Baruah, S.; Kumar, A.; Peela, N.R. Activated Carbon Supported Ni-Co Layered Double Hydroxides Nanowires: An Effective and Low-Cost Electrocatalyst for Ethanol Electro-Oxidation in Alkaline Media. J. Electrochem. Soc. 2023, 170, 034509. [Google Scholar] [CrossRef]
- Zhao, Y.; Björk, E.M.; Yan, Y.; Schaaf, P.; Wang, D. Recent progress in transition metal based catalysts and mechanism analysis for alcohol electrooxidation reactions. Green Chem. 2024, 22, 100913. [Google Scholar] [CrossRef]
- Vassileva, E.; Mihaylov, L.; Lyubenova, L.; Spassov, T.; Scaglione, F.; Rizzi, P. Porous metallic structures by dealloying amorphous alloys. J. Alloys Compd. 2023, 969, 172417. [Google Scholar] [CrossRef]
- Yu, J.; Ding, Y.; Xu, C.; Inoue, A.; Sakurai, T.; Chen, M. Nanoporous metals by dealloying multicomponent metallic glasses. Chem. Mater. 2008, 20, 4548–4550. [Google Scholar] [CrossRef]
- Nozaki, A.; Deguchi, R.; Ichiwara, H.; Kameo, R.; Morishita, M. Pd-Dispersed CeO2 Catalyst Prepared from Dealloying the Pd–Ce–Al Ternary Amorphous Alloy Used for Oxidation Reaction. Mater. Trans. 2020, 61, 1848–1852. [Google Scholar] [CrossRef]
- Nozaki, A.; Fujiwara, R.; Ueda, C.; Yamashita, A.; Yamamoto, H.; Morishita, M. Preparation of nanoporous CeO2 Catalyst supports by chemical treatment of amorphous alloys and investigation of Ni/CeO2 catalytic activity. Mater. Trans. 2019, 60, 1964–1967. [Google Scholar] [CrossRef]
- Li, M.; Liu, J.; Wang, C.; Liu, Y.; Sun, Y.; Qin, C.; Wang, Z.; Li, Y.; Liu, L.; Liu, S. Controllable nanoporous copper synthesized by dealloying metallic glasses: New insights into the tuning pore structure and applications. Chem. Eng. J. 2022, 427, 130861. [Google Scholar] [CrossRef]
- Li, S.; Guan, A.; Wang, H.; Yan, Y.; Huang, H.; Jing, C.; Zhang, L.; Zheng, G. Hybrid palladium nanoparticles and nickel single atom catalysts for efficient electrocatalytic ethanol oxidation. J. Mater. Chem. A 2022, 10, 6129–6133. [Google Scholar] [CrossRef]
- Song, J.; Zhang, F.; Hu, Q.; Lu, D.; Lu, Z.; Zhang, B. Synthesis of Pt-CuO/RuO2 composites by dealloying from metallic glass and their ethanol electrooxidation performance. Appl. Surf. Sci. 2022, 590, 153123. [Google Scholar] [CrossRef]
- He, D.; Cao, L.; Huang, J.; Zhang, X.; Wang, C.; Zhao, K.; Kajiyoshi, K.; Feng, L. Nanosheets-assembled hollow N-doped carbon nanospheres encapsulated with ultrasmall NiSe nanoparticles for electrocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2024, 64, 733–743. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, Q.; Li, Q.; Chen, L.; Mao, L.; Wang, H.; Chen, S. Two-dimensional electrocatalysts for alcohol oxidation: Acritical review. Chem. Eng. J. 2020, 400, 125744. [Google Scholar] [CrossRef]
- Liu, B.; Yao, X.; Zhang, Z.; Li, C.; Zhang, J.; Wang, P.; Zhao, J.; Guo, Y.; Sun, J.; Zhao, C. Synthesis of Cu2O nanostructures with tunable crystal facets for electrochemical CO2 reduction to alcohols. ACS Appl. Mater. Interfaces 2021, 13, 39165–39177. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Liu, X.; Wu, R.; Wang, J.; Li, Z.; Chan, K.C.; Wang, H.; Wu, Y.; Lu, Z. Flexible honeycombed nanoporous/glassy hybrid for efficient electrocatalytic hydrogen generation. Adv. Mater. 2019, 31, 1904989. [Google Scholar] [CrossRef]
- Sun, H.; Li, L.; Chen, Y.; Kim, H.; Xu, X.; Guan, D.; Hu, Z.; Zhang, L.; Shao, Z.; Jung, W. Boosting ethanol oxidation by NiOOH-CuO nano-heterostructure for energy-saving hydrogen production and biomass upgrading. Appl. Catal. B Environ. 2023, 325, 122388. [Google Scholar] [CrossRef]
- Song, J.; Zhang, F.; Hu, Q.; Lu, Z.; Zhang, B. Dealloying fabrication of nickel Oxyhydroxide/Oxide-based composites boosting ethanol electrooxidation. Appl. Surf. Sci. 2023, 637, 157915. [Google Scholar] [CrossRef]
- Wang, N.; Pan, Y.; Lu, T.; Li, X.; Wu, S.; Wu, J. A new ribbon-ignition method for fabricating p-CuO/n-CeO2 heterojunction with enhanced photocatalytic activity. Appl. Surf. Sci. 2017, 403, 699–706. [Google Scholar] [CrossRef]
- Putanenko, P.K.; Dorofeeva, N.V.; Kharlamova, T.S.; Grabchenko, M.V.; Kulinich, S.A.; Vodyankina, O.V. La2O3-CeO2-Supported Bimetallic Cu-Ni DRM Catalysts. Materials 2023, 16, 7701. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Fan, L.; Liao, W.; Zhao, F.; Tang, C.; Zhang, J.; Feng, M.; Lu, J.Q. Structure sensitivity of CuO in CO oxidation over CeO2-CuO/Cu2O catalysts. J. Catal. 2022, 405, 333–345. [Google Scholar] [CrossRef]
- Chang, F.; Zhang, Z.; Zhang, Y.; Liu, Y.; Yang, L.; Wang, X.; Bai, Z.; Zhang, Q. Synergistic modulation of valence state and oxygen vacancy induced by surface reconstruction of the CeO2/CuO catalyst toward enhanced electrochemical CO2 reduction. Carbon Energy 2024, 6, e588. [Google Scholar] [CrossRef]
- Mohana, P.; Yuvakkumar, R.; Ravi, G.; Arunmetha, S. Enhanced electrochemical performance of CuO/NiO/rGO for oxygen evolution reaction. Electrochim. Acta 2024, 473, 143464. [Google Scholar] [CrossRef]
- Gu, F.; Chen, K.; Du, Y.; Song, Y.; Wang, L. CeO2-NiO/N,O-rich porous carbon derived from covalent-organic framework for enhanced Li-storage. Chem. Eng. J. 2022, 442, 136298. [Google Scholar] [CrossRef]
- Li, W.; Song, Z.; Deng, X.; Fu, X.; Luo, J. Decoration of NiO hollow spheres composed of stacked nanosheets with CeO2 nanoparticles: Enhancement effect of CeO2 for electrocatalytic methanol oxidation. Electrochim. Acta 2020, 337, 135684. [Google Scholar] [CrossRef]
- Sekar, K.; Chuaicham, C.; Vellaichamy, B.; Li, W.; Zhuang, W.; Lu, X.; Ohtani, B.; Sasaki, K. Cubic Cu2O nanoparticles decorated on TiO2 nanofiber heterostructure as an excellent synergistic photocatalyst for H2 production and sulfamethoxazole degradation. Appl. Catal. B Environ. 2021, 294, 120221. [Google Scholar] [CrossRef]
- Park, S.; Khan, Z.; Shin, T.J.; Kim, Y.; Ko, H. Rechargeable Na/Ni batteries based on the Ni(OH)2/NiOOH redox couple with high energy density and good cycling performance. J. Mater. Chem. A 2019, 7, 1564–1573. [Google Scholar] [CrossRef]
- Wang, S.; Wang, W.; Zuo, J.; Qian, Y. Study of the Raman spectrum of CeO2 nanometer thin films. Mater. Chem. Phys. 2001, 68, 246–248. [Google Scholar] [CrossRef]
- Ma, X.C.; Yu, Z.; Zhang, Y.; Jin, J.; Li, J.; Fan, X.; Wan, Q.; Zheng, J.; Zhou, X.; Wang, Y. In situ Raman Monitoring of Surface Structures and Oxygen Species on Ni(OH)2/Au/Nickel Foam During Oxygen Evolution Reaction. J. Phys. Chem. C 2024, 128, 19736–19742. [Google Scholar] [CrossRef]
- Tang, C.; Sun, B.; Sun, J.; Hong, X.; Deng, Y.; Gao, F.; Dong, L. Solid state preparation of NiO-CeO2 catalyst for NO reduction. Catal. Today 2017, 281, 575–582. [Google Scholar] [CrossRef]
- AbdRahman, N.; Wong, K.T.; Choong, C.E.; Nah, I.; Park, C.; Kim, J.; Oh, S.; Yoon, Y.; Choi, E.; Jang, M. Revealing the roles of oxygen vacancies in NiO-CeO2 redox catalysts for electrocatalytic ozonation: Mechanistic study via in situ Raman spectroscopy. Appl. Surf. Sci. 2025, 679, 161174. [Google Scholar] [CrossRef]
- Ascencio, F.; Rangel-Gamboa, L.; Maqueda-Cabrera, B.; Zorrilla, C.; Herrera, R.; Cruz, R. Structural and vibrational study of porous CeO2 nanoparticles. Mater. Chem. Phys. 2024, 311, 128492. [Google Scholar] [CrossRef]
- Wang, H.; Guan, A.; Zhang, J.; Mi, Y.; Li, S.; Yuan, T.; Jing, C.; Zhang, L.; Zheng, G. Copper-doped nickel oxyhydroxide for efficient electrocatalytic ethanol oxidation. Chin. J. Catal. 2022, 43, 1478–1484. [Google Scholar] [CrossRef]
- Miao, Z.; Xu, C.; Zhan, J.; Xu, Z. Morphology-control and template-free fabrication of bimetallic Cu–Ni alloy rods for ethanol electro-oxidation in alkaline media. J. Alloys Compd. 2021, 855, 157438. [Google Scholar] [CrossRef]
- An, Y.; Ijaz, H.; Huang, M.; Qu, J.; Hu, S. The one-pot synthesis of CuNi nanoparticles with a Ni-rich surface for the electrocatalytic methanol oxidation reaction. Dalton Trans. 2020, 49, 1646–1651. [Google Scholar] [CrossRef]
- Ismail, K.M.; Hassan, S.S.; Medany, S.S.; Hefnawy, M. A novel Ni-complex nanocatalyst for efficient electrochemical oxidation of alcohol in alkaline medium: Mechanism and DFT study. Electrochim. Acta 2024, 504, 144954. [Google Scholar] [CrossRef]
- Raveendran, A.; Chandran, M.; Wabaidur, S.M.; Islam, M.; Dhanusuraman, R.; Ponnusamy, V. Facile electrochemical fabrication of Nickel-Coated Polydiphenylamine (Ni/PDPA) nanocomposite material as efficient anode catalyst for direct alcohol fuel cell application. Fuel 2022, 324, 124424. [Google Scholar] [CrossRef]
- Sieben, J.M.; Alvarez, A.E.; Sanchez, M.D. Glycerol electrooxidation on carbon-supported Pt-CuO and PtCu-CuO catalysts. Electrochim. Acta 2023, 439, 141672. [Google Scholar] [CrossRef]

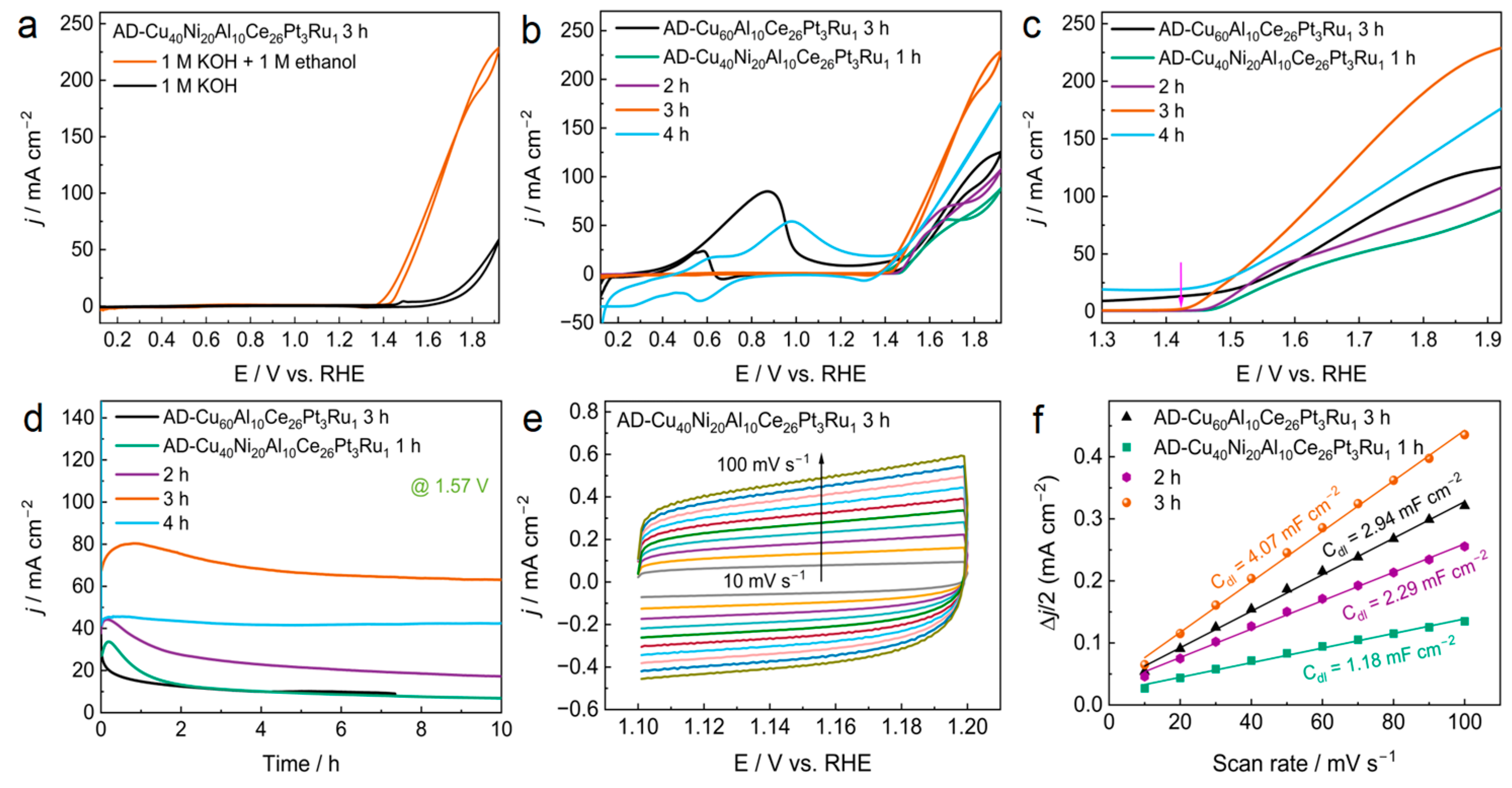

| Dealloyed MG Ribbons After Activated (AD-MG Ribbons) | Initial Potentials (V) |
|---|---|
| AD-Cu60Al10Ce26Pt3Ru1 | 1.511 |
| AD-Cu40Ni20Al10Ce26Pt3Ru1-1 h | 1.475 |
| AD-Cu40Ni20Al10Ce26Pt3Ru1-2 h | 1.469 |
| AD-Cu40Ni20Al10Ce26Pt3Ru1-3 h | 1.437 |
| AD-Cu40Ni20Al10Ce26Pt3Ru1-4 h | 1.473 |
| Ion conc. */ppb | Ni | Ce | Cu | Al | Pt | Ru |
|---|---|---|---|---|---|---|
| Cu40Ni20Al10Ce26Pt3Ru1 0.05 M HNO3, 3 h | 42,101.2 | 123,896 | 84,455.7 | 10,750 | 79.278 | 263.962 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, J.; Zhang, B.; Chen, Y.; Hu, Q.; Zhang, F.; Zhong, L. Boosting Electrooxidation of Ethanol by Nickel Addition to Metallic Glass Ribbon Precursors. Materials 2025, 18, 701. https://doi.org/10.3390/ma18030701
Song J, Zhang B, Chen Y, Hu Q, Zhang F, Zhong L. Boosting Electrooxidation of Ethanol by Nickel Addition to Metallic Glass Ribbon Precursors. Materials. 2025; 18(3):701. https://doi.org/10.3390/ma18030701
Chicago/Turabian StyleSong, Jingjing, Bo Zhang, Yu Chen, Qingzhuo Hu, Fabao Zhang, and Langxiang Zhong. 2025. "Boosting Electrooxidation of Ethanol by Nickel Addition to Metallic Glass Ribbon Precursors" Materials 18, no. 3: 701. https://doi.org/10.3390/ma18030701
APA StyleSong, J., Zhang, B., Chen, Y., Hu, Q., Zhang, F., & Zhong, L. (2025). Boosting Electrooxidation of Ethanol by Nickel Addition to Metallic Glass Ribbon Precursors. Materials, 18(3), 701. https://doi.org/10.3390/ma18030701






