A Study of Redox Properties of Ceria and Fe-Ceria Solid Materials Through Small Molecules Catalytic Oxidation
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Material Characterization
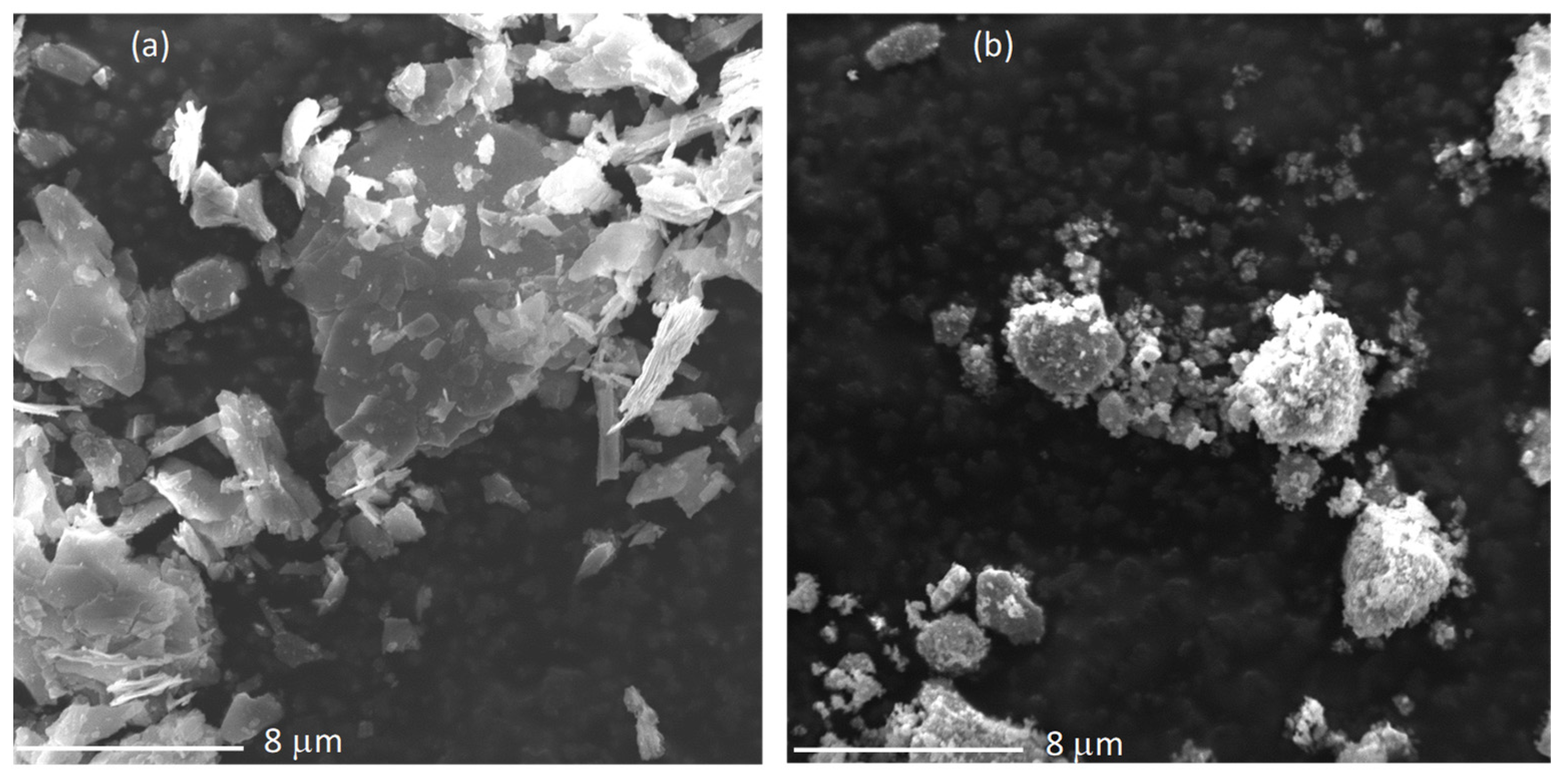
3.1.1. Textural Properties and Phase Composition
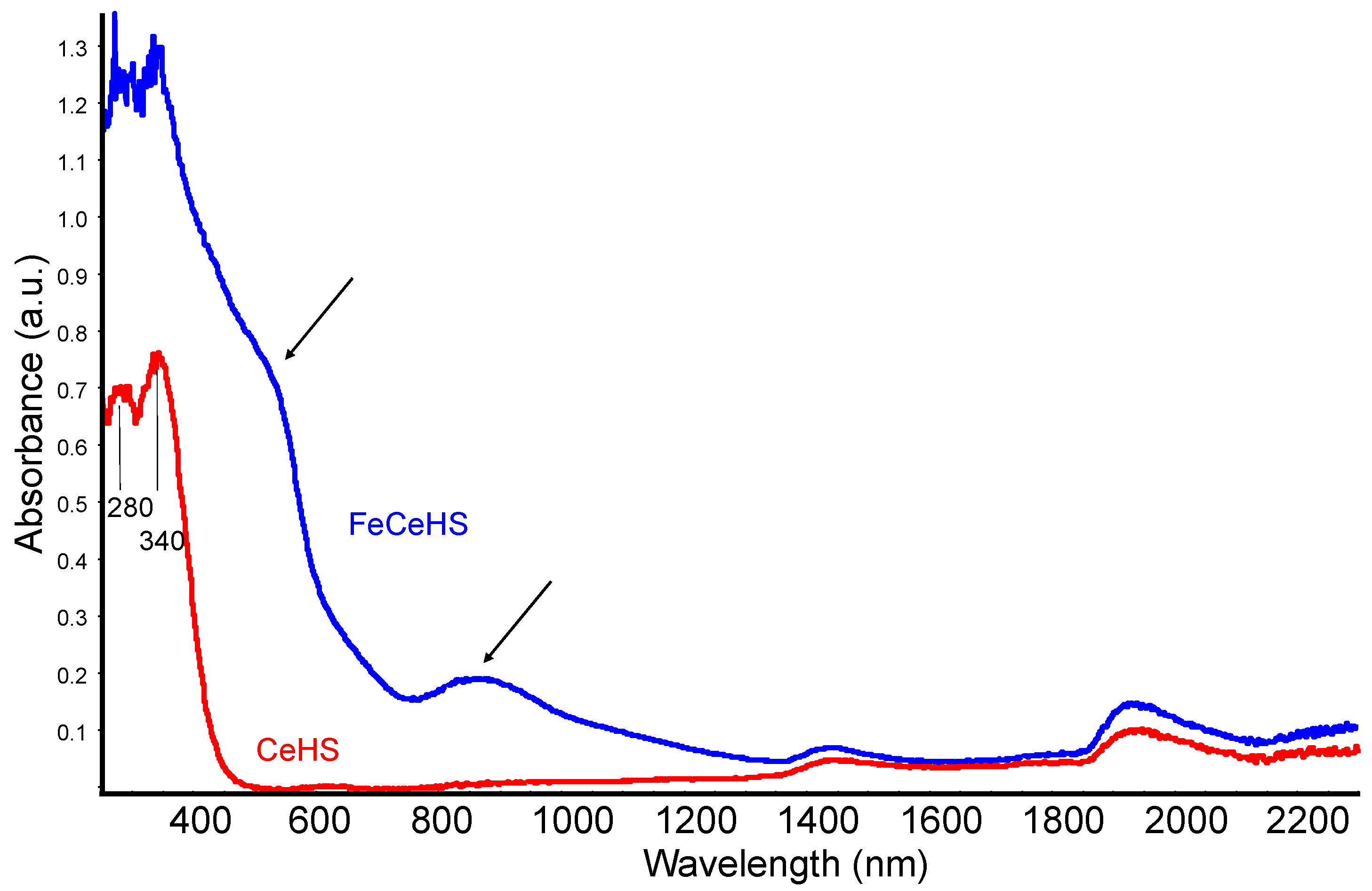
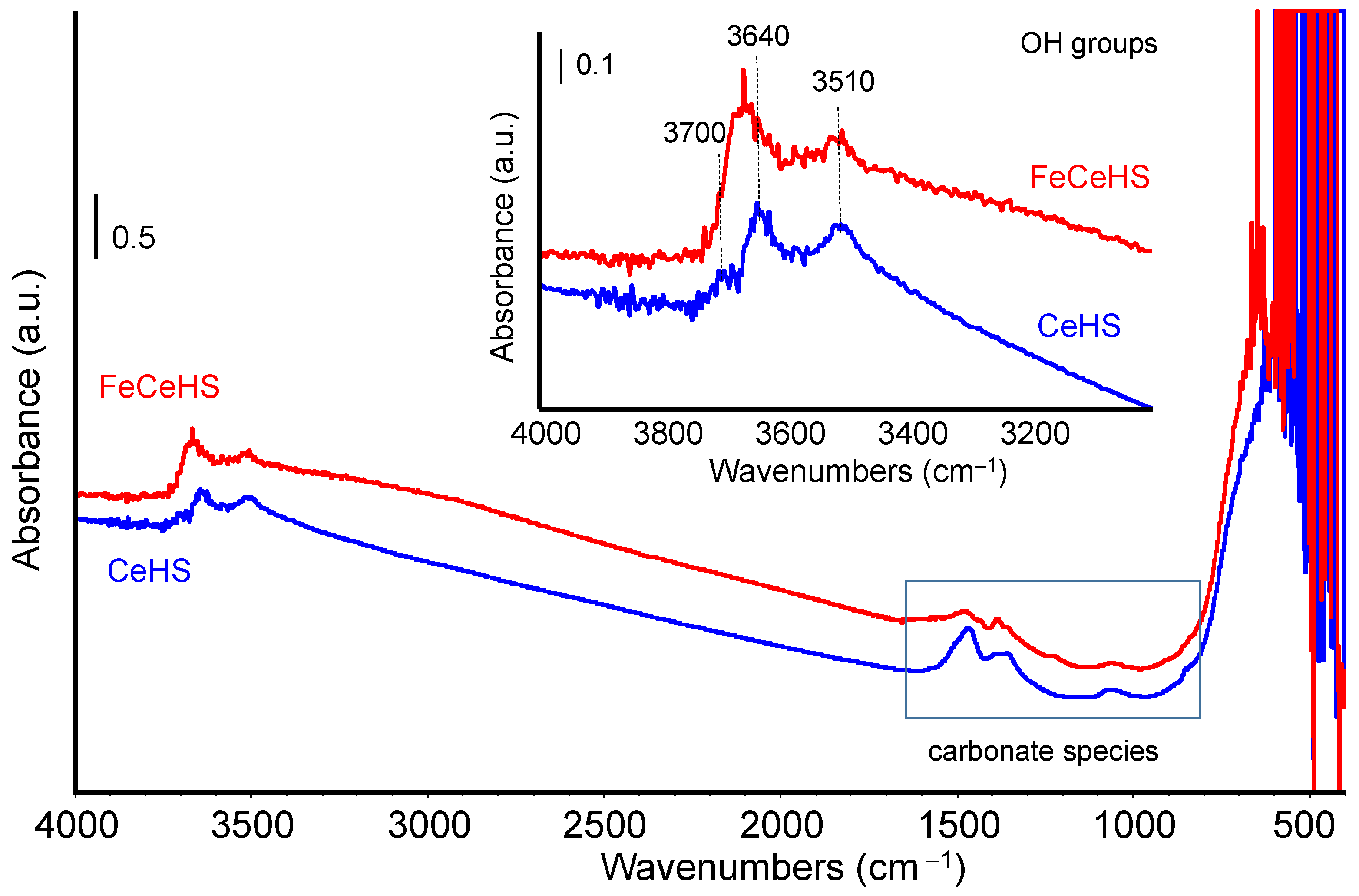
3.1.2. Surface Characterization
3.2. Surface Reactivity: Alcohol Adsorption and Conversion
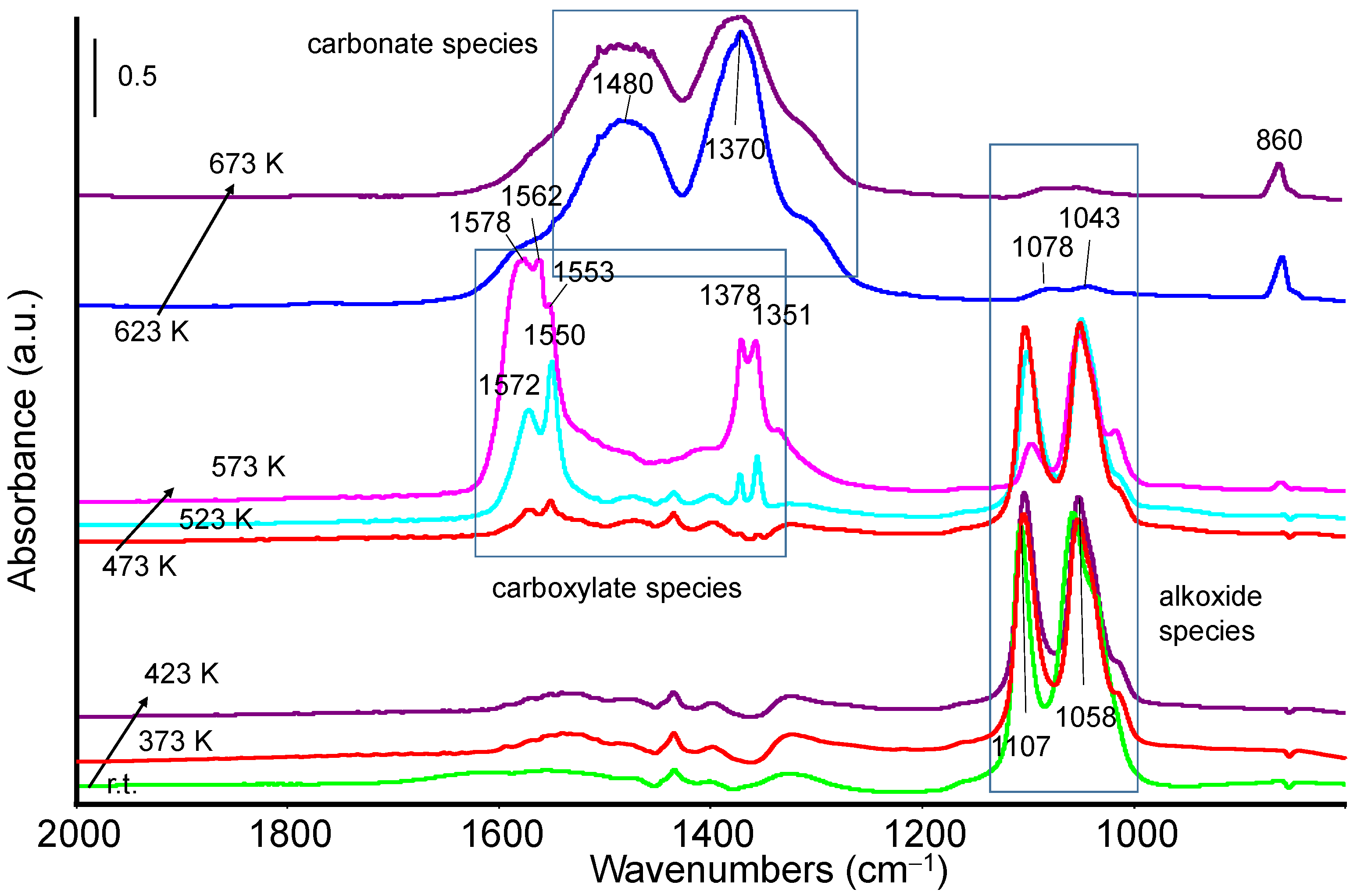
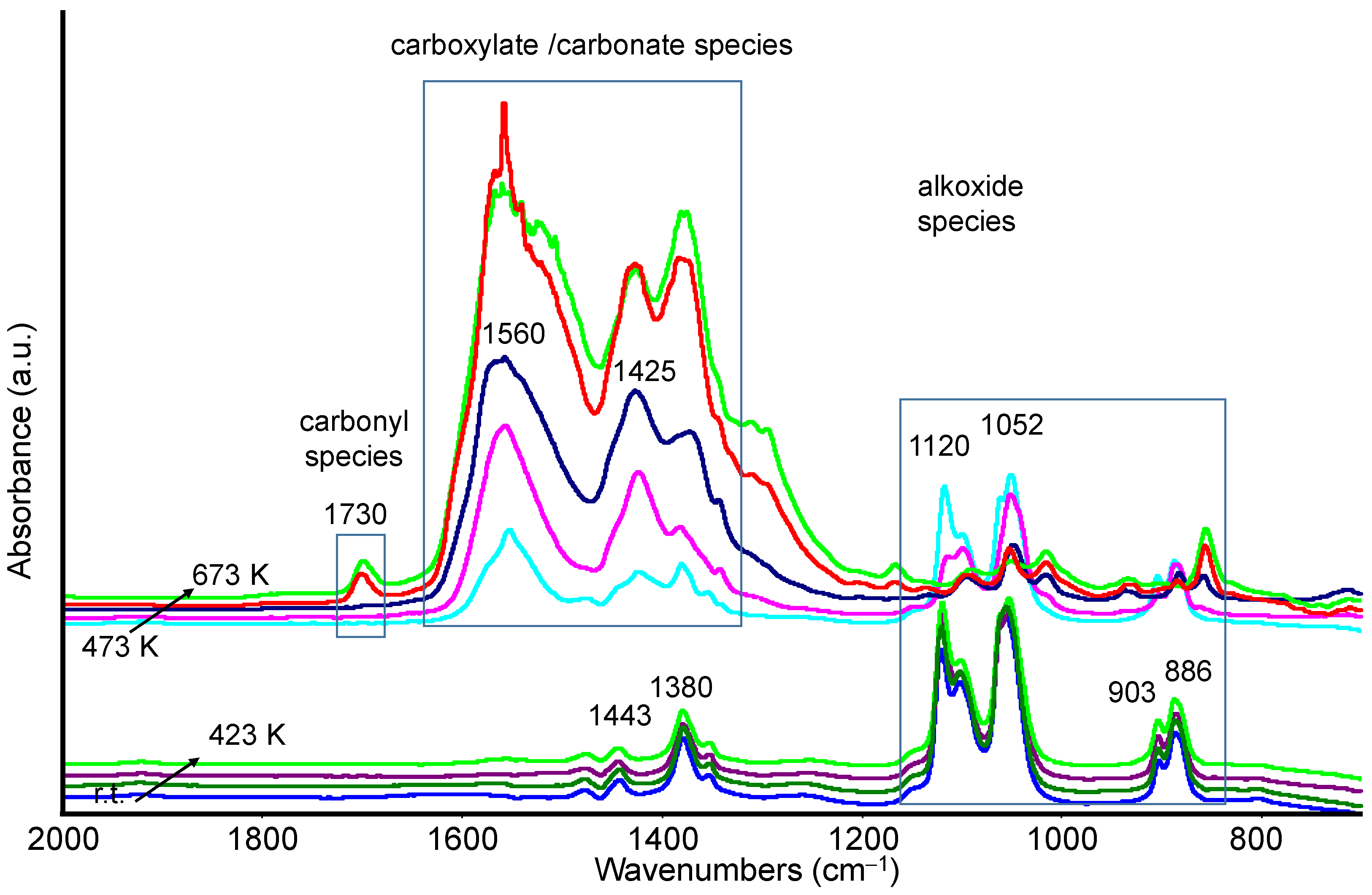
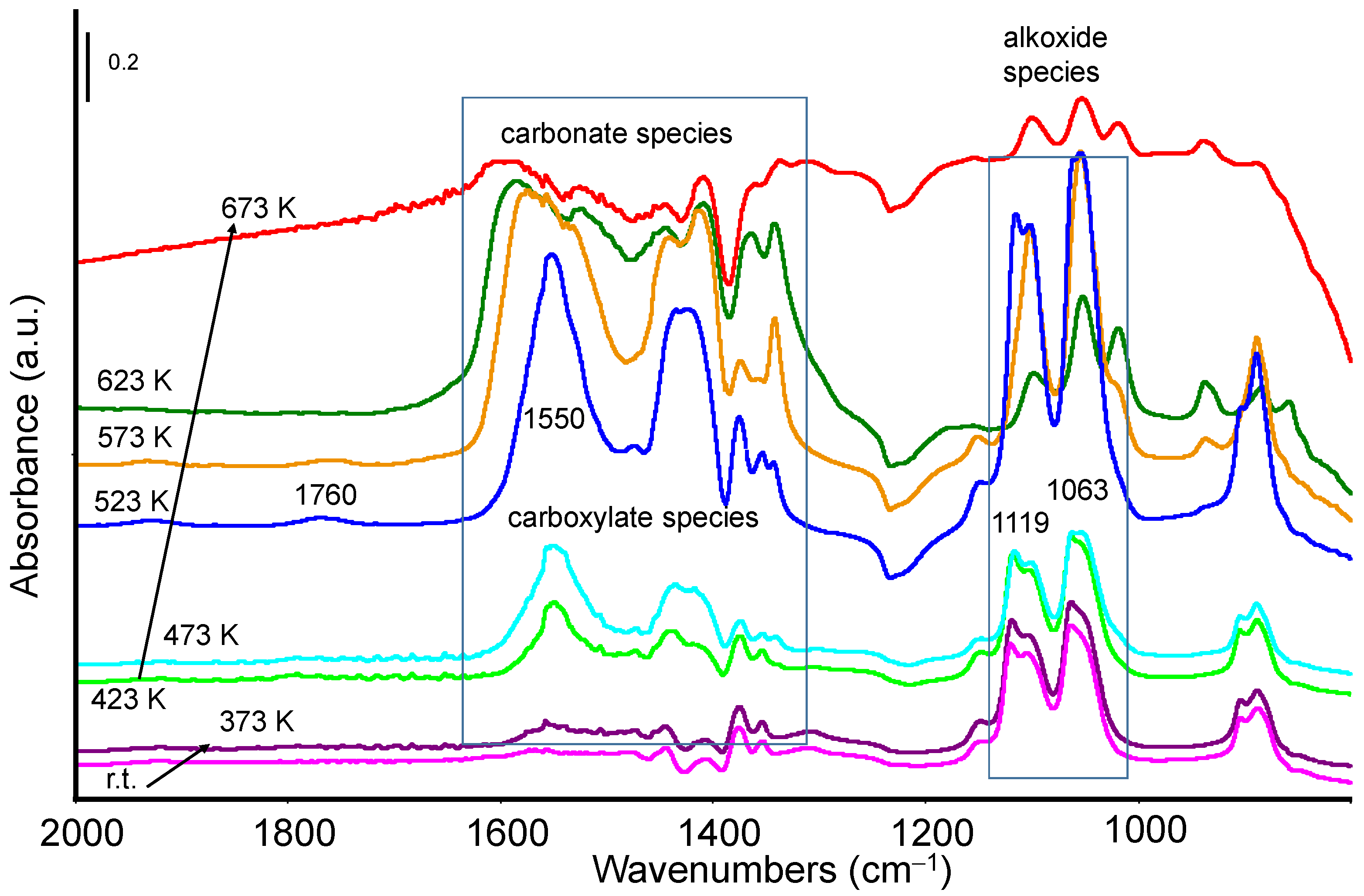
3.2.1. Methanol Adsorption and Conversion
3.2.2. Ethanol Adsorption and Conversion
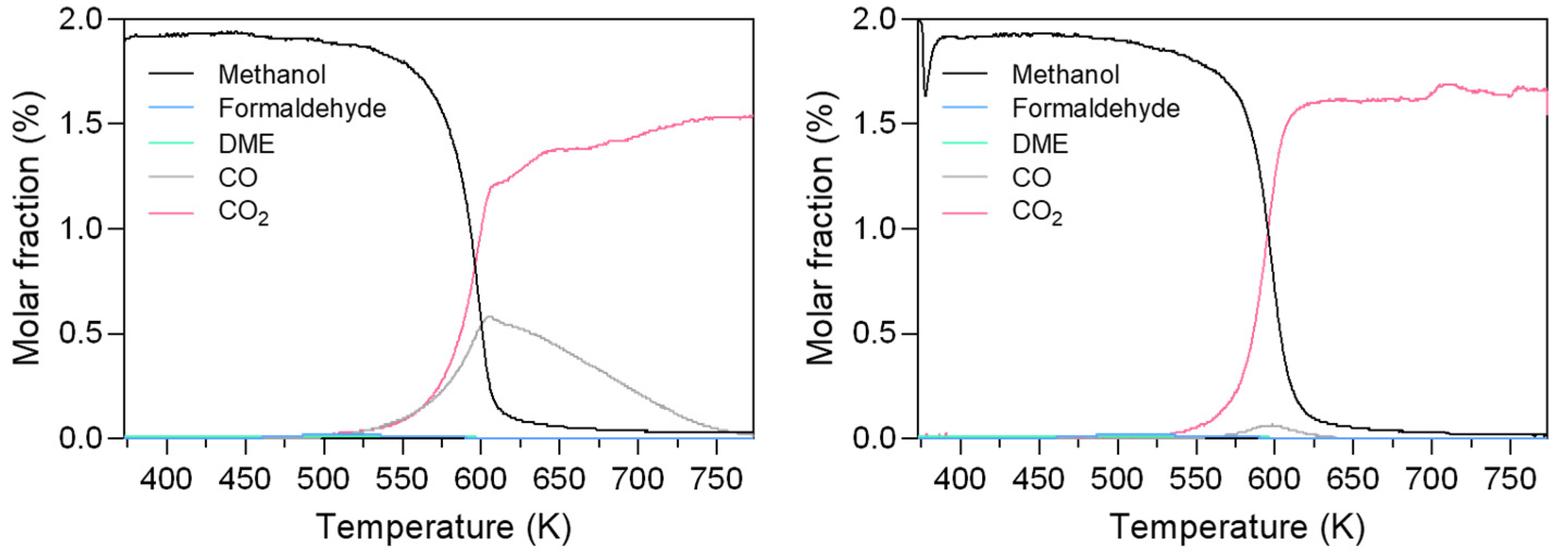
3.3. Catalytic Activity: Methanol and Ethanol TPSR (MeOH-TPSR and EtOH-TPSR)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aneggi, E.; Boaro, M.; de Leitenburg, C.; Dolcetti, G.; Trovarelli, A. Insights into the redox properties of ceria-based oxides and their implications in catalysis. J. Alloys Compd. 2006, 408–412, 1096–1102. [Google Scholar] [CrossRef]
- Montini, T.; Melchionna, M.; Monai, M.; Fornasiero, P. Fundamentals and catalytic applications of CeO2-based materials. Chem. Rev. 2016, 116, 5987–6041. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yeung, K.L.; Bañares, M.A. Ceria and its related materials for VOC catalytic combustion: A review. Catal. Today 2020, 356, 141–154. [Google Scholar] [CrossRef]
- Gaálová, J.; Topka, P. Gold and ceria as catalysts for VOC abatement: A review. Catalysts 2021, 11, 789. [Google Scholar] [CrossRef]
- Stoian, M.; Rogé, V.; Lazar, L.; Maurer, T.; Védrine, J.C.; Marcu, I.-C.; Fechete, I. Total oxidation of methane on oxide and mixed oxide ceria-containing catalysts. Catalysts 2021, 11, 427. [Google Scholar] [CrossRef]
- Piumetti, M.; Andana, T.; Bensaid, S.; Russo, N.; Fino, D.; Pirone, R. Study on the CO oxidation over ceria-based nanocatalysts. Nanoscale Res. Lett. 2016, 11, 165. [Google Scholar] [CrossRef]
- Moretti, E.; Storaro, L.; Talon, A.; Chitsazan, S.; Garbarino, G.; Busca, G.; Finocchio, E. Ceria-zirconia based catalysts for ethanol steam reforming. Fuel 2015, 153, 166–175. [Google Scholar] [CrossRef]
- Graciani, J.; Mudiyanselage, K.; Xu, F.; Baber, A.E.; Evans, J.; Senanayake, S.D.; Stacchiola, D.J.; Liu, P.; Hrbek, J.; Sanz, J.F.; et al. Highly active copper-ceria and copper-ceria-titania catalysts for methanol synthesis from CO2. Science 2014, 345, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Zenou, V.Y.; Kennouche, D.; Marks, L.; Barnett, S.A. Solid oxide cells with zirconia/ceria bi-layer electrolytes fabricated by reduced temperature firing. J. Mater. Chem. A 2015, 3, 9955–9964. [Google Scholar] [CrossRef]
- Trovarelli, A.; Llorca, J. Ceria catalysts at nanoscale: How do crystal shapes shape catalysis? ACS Catal. 2017, 7, 4716–4735. [Google Scholar] [CrossRef]
- Liu, M.; Min, K.; Han, B.; Lee, L.Y.S. Interfacing or doping? Role of Ce in highly promoted water oxidation of NiFe-layered double hydroxide. Adv. Energy Mater. 2021, 11, 2101281. [Google Scholar] [CrossRef]
- Zhang, C.; Yu, Y.; Grass, M.E.; Dejoie, C.; Ding, W.; Gaskell, K.; Jabeen, N.; Hong, Y.P.; Shavorskiy, A.; Bluhm, H.; et al. Mechanistic studies of water electrolysis and hydrogen electro-oxidation on high temperature ceria-based solid oxide electrochemical cells. J. Am. Chem. Soc. 2013, 135, 11572–11579. [Google Scholar] [CrossRef]
- Konsolakis, M.; Lykaki, M. Facet-dependent reactivity of ceria nanoparticles exemplified by CeO2-based transition metal catalysts: A critical review. Catalysts 2021, 11, 452. [Google Scholar] [CrossRef]
- Deganello, F.; Joshi, M.; Liotta, L.F.; La Parola, V.; Marcì, G.; Pantaleo, G. Sustainable recycling of insoluble rust waste for the synthesis of iron-containing perovskite-type catalysts. ACS Omega 2019, 4, 6994–7004. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wang, H.; Wei, Y.; Yan, D. Direct conversion of methane to synthesis gas using lattice oxygen of CeO2–Fe2O3 complex oxides. Chem. Eng. J. 2010, 156, 512–518. [Google Scholar] [CrossRef]
- Tang, L.; Yamaguchi, D.; Burke, N.; Trimm, D.; Chiang, K. Methane decomposition over ceria modified iron catalysts. Catal. Commun. 2010, 11, 1215–1219. [Google Scholar] [CrossRef]
- Li, G.; Wang, Q.; Zhao, B.; Shen, M.; Zhou, R. Effect of iron doping into CeO2–ZrO2 on the properties and catalytic behaviour of Pd-only three-way catalyst for automotive emission control. J. Hazard. Mater. 2011, 186, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Laguna, O.H.; Centeno, M.A.; Boutonnet, M.; Odriozola, J.A. Fe-doped ceria solids synthesized by the microemulsion method for CO oxidation reactions. Appl. Catal. B Environ. 2011, 106, 621–629. [Google Scholar] [CrossRef]
- Reina, T.R.; Ivanova, S.; Idakiev, V.; Tabakova, T.; Centeno, M.A.; Deng, Q.-F.; Yuan, Z.-Y.; Odriozola, J.A. Nanogold mesoporous iron promoted ceria catalysts for total and preferential CO oxidation reactions. J. Mol. Catal. A Chem. 2016, 414, 62–71. [Google Scholar] [CrossRef]
- Landi, G.; Di Benedetto, A.; Colussi, S.; Barbato, P.S.; Lisi, L. Effect of carbon dioxide and water on the performances of an iron-promoted copper/ceria catalyst for CO preferential oxidation in H2-rich streams. Int. J. Hydrogen Energy 2016, 41, 7332–7341. [Google Scholar] [CrossRef]
- Lykaki, M.; Stefa, S.; Carabineiro, S.; Pandis, P.; Stathopoulos, V.; Konsolakis, M. Facet-dependent reactivity of Fe2O3/CeO2 nanocomposites: Effect of ceria morphology on CO oxidation. Catalysts 2019, 9, 371. [Google Scholar] [CrossRef]
- Li, B.; Raj, A.; Croiset, E.; Wen, J.Z. Reactive Fe-O-Ce sites in ceria catalysts for soot oxidation. Catalysts 2019, 9, 815. [Google Scholar] [CrossRef]
- Gao, Y.; Teng, S.; Wang, Z.; Wang, B.; Liu, W.; Liu, W.; Wang, L. Enhanced catalytic performance of cobalt and iron co-doped ceria catalysts for soot combustion. J. Mater. Sci. 2020, 55, 283–297. [Google Scholar] [CrossRef]
- Perez-Alonso, F.J.; Melián-Cabrera, I.; López Granados, M.; Kapteijn, F.; Fierro, J.L.G. Synergy of FexCe1−xO2 mixed oxides for N2O decomposition. J. Catal. 2006, 239, 340–346. [Google Scholar] [CrossRef]
- Reddy, A.S.; Chen, C.-Y.; Chen, C.-C.; Chien, S.-H.; Lin, C.-J.; Lin, K.-H.; Chen, C.-L.; Chang, S.-C. Synthesis and characterization of Fe/CeO2 catalysts: Epoxidation of cyclohexene. J. Mol. Catal. A Chem. 2010, 318, 60–67. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, Q.; Dai, Q.; Wang, X. Fe doped CeO2 nanosheets for catalytic oxidation of 1,2-dichloroethane: Effect of preparation method. Chem. Eng. J. 2017, 307, 1037–1046. [Google Scholar] [CrossRef]
- Sudhakaran, M.S.; Sultana, L.; Hossain, M.M.; Pawlat, J.; Diatczyk, J.; Brüser, V.; Reuter, S.; Mok, Y.S. Iron-ceria spinel (FeCe2O4) catalyst for dry reforming of propane to inhibit carbon formation. J. Ind. Eng. Chem. 2018, 61, 142–151. [Google Scholar] [CrossRef]
- Li, C.; Nakagawa, Y.; Tamura, M.; Nakayama, A.; Tomishige, K. Hydrodeoxygenation of guaiacol to phenol over ceria-supported iron catalysts. ACS Catal. 2020, 10, 14624–14639. [Google Scholar] [CrossRef]
- Tsoncheva, T.; Rosmini, C.; Dimitrov, M.; Issa, G.; Henych, J.; Němečková, Z.; Kovacheva, D.; Velinov, N.; Atanasova, G.; Spassova, I. Formation of catalytic active sites in hydrothermally obtained binary ceria-iron oxides: Composition and preparation effects. ACS Appl. Mater. Interfaces 2021, 13, 1838–1852. [Google Scholar] [CrossRef]
- Li, C.; Nakagawa, Y.; Yabushita, M.; Nakayama, A.; Tomishige, K. Guaiacol hydrodeoxygenation over iron-ceria catalysts with platinum single-atom alloy clusters as a promoter. ACS Catal. 2021, 11, 12794–12814. [Google Scholar] [CrossRef]
- Galvita, V.V.; Poelman, H.; Bliznuk, V.; Detavernier, C.; Marin, G.B. CeO2-modified Fe2O3 for CO2 utilization via chemical looping. Ind. Eng. Chem. Res. 2013, 52, 8416–8426. [Google Scholar] [CrossRef]
- Lai, H.; Zeng, X.; Song, T.; Yin, S.; Long, B.; Ali, A.; Deng, G.-J. Fast synthesis of porous iron doped CeO2 with oxygen vacancy for effective CO2 photoreduction. J. Colloid Interface Sci. 2022, 608, 1792–1801. [Google Scholar] [CrossRef]
- Gili, A.; Bekheet, M.F.; Thimm, F.; Bischoff, B.; Geske, M.; Konrad, M.; Praetz, S.; Schlesiger, C.; Selve, S.; Gurlo, A.; et al. One-pot synthesis of iron-doped ceria catalysts for tandem carbon dioxide hydrogenation. Catal. Sci. Technol. 2024, 14, 4174–4186. [Google Scholar] [CrossRef]
- Liu, H.; Wu, Y.; Liu, L.; Chu, B.; Qin, Z.; Jin, G.; Tong, Z.; Dong, L.; Li, B. Three-dimensionally ordered macroporous Fe-doped ceria catalyst with enhanced activity at a wide operating temperature window for selective catalytic reduction of NOx. Appl. Surf. Sci. 2019, 498, 143780. [Google Scholar] [CrossRef]
- Zhang, S.; Kim, T. Effects of iron precursor and loading on the catalytic performance of FeOx/CeO2 catalysts for NO reduction by CO. Mol. Catal. 2020, 494, 111123. [Google Scholar] [CrossRef]
- Zhang, J.; Qiu, S.; Feng, H.; Hu, T.; Wu, Y.; Luo, T.; Tang, W.; Wang, D. Efficient degradation of tetracycline using core–shell Fe@Fe2O3-CeO2 composite as novel heterogeneous electro-Fenton catalyst. Chem. Eng. J. 2022, 428, 131403. [Google Scholar] [CrossRef]
- Zhang, F.; Ke, R.; Liu, M.; Zhang, X.; Wang, Y.; Wang, Y. Improved electrocatalytic performance of Fe/CeO2 bifunctional electrocatalyst by simultaneous H2O2 in-situ generation and activation. Chem. Eng. J. Adv. 2022, 9, 100231. [Google Scholar] [CrossRef]
- Tammaro, O.; Paparo, R.; Chianese, M.; Ritacco, I.; Caporaso, L.; Camellone, M.F.; Masenelli, B.; Lamirand, A.D.; Bluet, J.-M.; Fontana, M.; et al. Reverse micelle strategy for effective substitutional Fe-doping in small-sized CeO2 nanocrystals: Assessment of adsorption and photodegradation efficiency of ibuprofen under visible light. Chem. Eng. J. 2024, 479, 147909. [Google Scholar] [CrossRef]
- Sun, Q.; Liu, Y.; Li, X.; Guo, X.; Huang, W.-H.; Zhu, Y.; Wang, Z.; Chueh, C.-C.; Chen, C.-L.; Peng, Y.-K.; et al. Highly disordered Fe-doped CeO2 with oxygen vacancies facilitates electrocatalytic water oxidation. Energy Fuels 2023, 37, 9434–9443. [Google Scholar] [CrossRef]
- Govender, S.; Friedrich, H. Monoliths: A review of the basics, preparation methods and their relevance to oxidation. Catalysts 2017, 7, 62. [Google Scholar] [CrossRef]
- Balzarotti, R.; Cristiani, C.; Francis, L.F. Combined dip-coating/spin-coating depositions on ceramic honeycomb monoliths for structured catalysts preparation. Catal. Today 2019, 334, 90–95. [Google Scholar] [CrossRef]
- Balzarotti, R.; Italiano, C.; Pino, L.; Cristiani, C.; Vita, A. Ni/CeO2-thin ceramic layer depositions on ceramic monoliths for syngas production by oxy steam reforming of biogas. Fuel Process. Technol. 2016, 149, 40–48. [Google Scholar] [CrossRef]
- Chapter 9 Preparation of supported catalysts. In Studies in Surface Science and Catalysis; Moulijin, J.A., van Leeuwen, P.W.N.M., van Santen, R.A., Eds.; Elsevier B.V.: Amsterdam, The Netherlands, 1993; Volume 79, pp. 335–360. ISBN 978-04-44892-29-4_9. [Google Scholar]
- Munnik, P.; de Jongh, P.E.; de Jong, K.P. Recent developments in the synthesis of supported catalysts. Chem. Rev. 2015, 115, 6687–6718. [Google Scholar] [CrossRef] [PubMed]
- Peck, T.C.; Reddy, G.K.; Jones, M.; Roberts, C.A. Monolayer detection of supported Fe and Co oxides on ceria to establish structure-activity relationships for reduction of NO by CO. J. Phys. Chem. C 2017, 121, 8435–8443. [Google Scholar] [CrossRef]
- Wieczorek-Ciurowa, K.; Kozak, A.J. The thermal decomposition of Fe(NO3)3·9H2O. J. Therm. Anal. Calorim. 1999, 58, 647–651. [Google Scholar] [CrossRef]
- Melnikov, P.; Nascimento, V.A.; Arkhangelsky, I.V.; Zanoni Consolo, L.Z.; de Oliveira, L.C.S. Thermal decomposition mechanism of iron(III) nitrate and characterization of intermediate products by the technique of computerized modeling. J. Therm. Anal. Calorim. 2014, 115, 145–151. [Google Scholar] [CrossRef]
- Dietmar, K.; Goverde, T.; Carsten, B. Particle World—Technical Papers of QUANTACHROME. Available online: https://www.3p-instruments.com/wp-content/uploads/PDF/particleworld/PW-02/Particle_World_2.pdf (accessed on 13 February 2023).
- Polakowski, C.; Ryżak, M.; Sochan, A.; Beczek, M.; Mazur, R.; Bieganowski, A. Particle size distribution of various soil materials measured by laser diffraction—The problem of reproducibility. Minerals 2021, 11, 465. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Phys. B Condens. Matter 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Pampararo, G.; Garbarino, G.; Ardoino, N.; Riani, P.; Busca, G. A study of molybdena catalysts in ethanol oxidation. Part 1. Unsupported and silica-supported MoO3. J. Chem. Technol. Biotechnol. 2021, 96, 3293–3303. [Google Scholar] [CrossRef]
- Farahmandjou, M.; Soflaee, F. Synthesis and characterization of α-Fe2O3 nanoparticles by simple co-precipitation method. Phys. Chem. Res. 2015, 3, 191–196. [Google Scholar] [CrossRef]
- Prieur, D.; Bonani, W.; Popa, K.; Walter, O.; Kriegsman, K.W.; Engelhard, M.H.; Guo, X.; Eloirdi, R.; Gouder, T.; Beck, A.; et al. Size dependence of lattice parameter and electronic structure in CeO2 nanoparticles. Inorg. Chem. 2020, 59, 5760–5767. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Li, K.; Qing, S.; Zhu, X.; Wei, Y.; Li, Y.; Wang, H. Enhanced reducibility and redox stability of Fe2O3 in the presence of CeO2 nanoparticles. RSC Adv. 2014, 4, 47191–47199. [Google Scholar] [CrossRef]
- Bao, H.; Qian, K.; Fang, J.; Huang, W. Fe-doped CeO2 solid solutions: Substituting-site doping versus interstitial-site doping, bulk doping versus surface doping. Appl. Surf. Sci. 2017, 414, 131–139. [Google Scholar] [CrossRef]
- Tianshu, Z.; Hing, P.; Huang, H.; Kilner, J. The effect of Fe doping on the sintering behavior of commercial CeO2 powder. J. Mater. Process. Technol. 2001, 113, 463–468. [Google Scholar] [CrossRef]
- Ozawa, M. Sintering behavior of nanocrystalline CeO2 by master sintering curve analysis. J. Jpn. Soc. Powder Powder Metall. 2018, 65, 311–315. [Google Scholar] [CrossRef]
- Martín-Martín, J.A.; González-Marcos, M.P.; Aranzabal, A.; González-Velasco, J.R.; Finocchio, E. Promotion of different active phases in MnOX-CeO2 catalysts for simultaneous NO reduction and o-DCB oxidation. Top. Catal. 2024, 68, 13–32. [Google Scholar] [CrossRef]
- Babitha, K.K.; Sreedevi, A.; Priyanka, K.P.; Sabu, B.; Varghese, T. Structural characterization and optical studies of CeO2 nanoparticles synthesized by chemical precipitation. Indian J. Pure Appl. Phys. 2015, 53, 596–603. [Google Scholar]
- Wang, Y.; Wang, F.; Chen, Y.; Zhang, D.; Li, B.; Kang, S.; Li, X.; Cui, L. Enhanced photocatalytic performance of ordered mesoporous Fe-doped CeO2 catalysts for the reduction of CO2 with H2O under simulated solar irradiation. Appl. Catal. B Environ. 2014, 147, 602–609. [Google Scholar] [CrossRef]
- Gálvez, N.; Barrón, V.; Torrent, J. Preparation and properties of hematite with structural phosphorus. Clays Clay Miner. 1999, 47, 375–385. [Google Scholar] [CrossRef]
- Torrent, J.; Barrón, V. The visible diffuse reflectance spectrum in relation to the color and crystal properties of hematite. Clays Clay Miner. 2003, 51, 309–317. [Google Scholar] [CrossRef]
- Manning, P.G. The optical absorption spectra of the garnets almandine-pyrope, pyrope, and spessartine and some structural interpretations of mineralogical significance. Can. Mineral. 1967, 9, 237–251. [Google Scholar]
- Parkinson, G.S.; Diebold, U.; Tang, J.; Malkinski, L. Tailoring the interface properties of magnetite for spintronics. In Advanced Magnetic Materials; Malkinski, L., Ed.; InTech: Houston TX, USA, 2012; pp. 1–30. ISBN 978-953-51-0637-1. [Google Scholar]
- Goldman, D.S.; Rossman, G.R. The identification of Fe (super 2+) in the M(4) site of calcic amphiboles. Am. Mineral. 1977, 62, 205–216. [Google Scholar]
- Makuła, P.; Pacia, M.; Macyk, W. How to correctly determine the band gap energy of modified semiconductor photocatalysts based on UV–Vis spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef]
- Dai, J.; Zhu, Y.; Tahini, H.A.; Lin, Q.; Chen, Y.; Guan, D.; Zhou, C.; Hu, Z.; Lin, H.-J.; Chan, T.-S.; et al. Single-phase perovskite oxide with super-exchange induced atomic-scale synergistic active centers enables ultrafast hydrogen evolution. Nat. Commun. 2020, 11, 5657. [Google Scholar] [CrossRef] [PubMed]
- Puerto-Jiménez, M.; Goma, D.; Aguinaco, A.; López-Maya, E.; Álvarez, M.G.; María Pintado, J.; Blanco, G.; Bogeat-Barroso, A. Band gap engineering of ceria nanostructures by incorporating nitrogen-containing heterocyclic ligands. Inorg. Chem. Commun. 2024, 164, 112399. [Google Scholar] [CrossRef]
- Abdulwahab, K.O.; Khan, M.M.; Jennings, J.R. Doped ceria nanomaterials: Preparation, properties, and uses. ACS Omega 2023, 8, 30802–30823. [Google Scholar] [CrossRef]
- Venkataswamy, P.; Jampaiah, D.; Kandjani, A.E.; Sabri, Y.M.; Reddy, B.M.; Vithal, M. Transition (Mn, Fe) and rare earth (La, Pr) metal doped ceria solid solutions for high performance photocatalysis: Effect of metal doping on catalytic activity. Res. Chem. Intermed. 2018, 44, 2523–2543. [Google Scholar] [CrossRef]
- Ning, J.; Shi, P.; Jiang, M.; Liu, C.; Li, X. Effect of Ce doping on the structure and chemical stability of nano-α-Fe2O3. Nanomaterials 2019, 9, 1039. [Google Scholar] [CrossRef]
- Badri, A.; Binet, C.; Lavalley, J.-C. An FTIR study of surface ceria hydroxy groups during a redox process with H2. J. Chem. Soc. Faraday Trans. 1996, 92, 4669–4673. [Google Scholar] [CrossRef]
- Finocchio, E.; Daturi, M.; Binet, C.; Lavalley, J.C.; Blanchard, G. Thermal evolution of the adsorbed methoxy species on CexZr1−xO2 solid solution samples: A FT-IR study. Catal. Today 1999, 52, 53–63. [Google Scholar] [CrossRef]
- Binet, C.; Daturi, M. Methanol as an IR probe to study the reduction process in ceria-zirconia mixed compounds. Catal. Today 2001, 70, 155–167. [Google Scholar] [CrossRef]
- Busca, G.; Lorenzelli, V. Infrared study of methanol, formaldehyde, and formic acid adsorbed on hematite. J. Catal. 1980, 66, 155–161. [Google Scholar] [CrossRef]
- Lorenzelli, V.; Busca, G. Infrared studies of the surface of α-Fe2O3. Mater. Chem. Phys. 1985, 13, 261–281. [Google Scholar] [CrossRef]
- Street, S.C.; Gellman, A.J. FT-IRAS of adsorbed alkoxides: Ethoxides on Cu(111). Colloids Surf. A Physicochem. Eng. Asp. 1995, 105, 27–34. [Google Scholar] [CrossRef]
- Garbarino, G.; Prasath Parameswari Vijayakumar, R.; Riani, P.; Finocchio, E.; Busca, G. Ethanol and diethyl ether catalytic conversion over commercial alumina and lanthanum-doped alumina: Reaction paths, catalyst structure and coking. Appl. Catal. B Environ. 2018, 236, 490–500. [Google Scholar] [CrossRef]
- Podobiński, J.; Zimowska, M.; Śliwa, M.; Datka, J. IR studies of ethoxy groups on CeO2. Molecules 2023, 28, 1251. [Google Scholar] [CrossRef] [PubMed]
- Phung, T.K.; Lagazzo, A.; Rivero Crespo, M.Á.; Sánchez Escribano, V.; Busca, G. A study of commercial transition aluminas and of their catalytic activity in the dehydration of ethanol. J. Catal. 2014, 311, 102–113. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, 4th ed.; Wiley: Hoboken, NJ, USA, 1986; ISBN 978-04-71010-66-1. [Google Scholar]
- Podobiński, J.; Zimowska, M.; Samson, K.; Śliwa, M.; Datka, J. Ethoxy groups on ZrO2, CuO, CuO/ZrO2 Al2O3, Ga2O3, SiO2 and NiO: Formation and reactivity. Molecules 2023, 28, 3463. [Google Scholar] [CrossRef] [PubMed]
- Busca, G.; Daturi, M.; Finocchio, E.; Lorenzelli, V.; Ramis, G.; Willey, R.J. Transition metal mixed oxides as combustion catalysts: Preparation, characterization and activity mechanisms. Catal. Today 1997, 33, 239–249. [Google Scholar] [CrossRef]
- Pistarino, C.; Finocchio, E.; Romezzano, G.; Brichese, F.; Di Felice, R.; Busca, G.; Baldi, M. A study of the catalytic dehydrochlorination of 2-chloropropane in oxidizing conditions. Ind. Eng. Chem. Res. 2000, 39, 2752–2760. [Google Scholar] [CrossRef]
- Rossi, P.F.; Busca, G.; Lorenzelli, V.; Saur, O.; Lavalley, J.C. Microcalorimetric and FT-IR spectroscopic study of the adsorption of isopropyl alcohol and hexafluoroisopropyl alcohol on titanium dioxide. Langmuir 1987, 3, 52–58. [Google Scholar] [CrossRef]




| Sample | Dp (μm) | Morphology | ||||
|---|---|---|---|---|---|---|
| 10% | 50% | 90% | SA (m2 g−1) | Vp (cm3 g−1) | dp (nm) | |
| CeHS | 2 | 9 | 19 | 90 | 0.05 | 4 |
| FeCeHS | 0.3 | 2 | 15 | 53 | 0.09 | 9 |
| Sample | Phase | Lattice Parameters (Å) | Crystallite Size (nm) | Microstrain (%) | Phase % w | χ2 (%) | |
|---|---|---|---|---|---|---|---|
| a0 | c0 | ||||||
| CeHS | CeO2 | 5.413 (1) | 9.1 (1) | 0.562 (1) | 100 | 3.59 | |
| FeCeHS | CeO2 | 5.409 (1) | 9.7 (1) | 0.582 (1) | 93.0 (3) | 3.02 | |
| Fe2O3 | 5.040 (1) | 13.768 (1) | * | * | 7.0 (3) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balzarotti, R.; Basso Peressut, A.; Garbarino, G.; Spennati, E.; Basbus, J.F.; Carpanese, M.P.; Latorrata, S.; Cristiani, C.; Finocchio, E. A Study of Redox Properties of Ceria and Fe-Ceria Solid Materials Through Small Molecules Catalytic Oxidation. Materials 2025, 18, 806. https://doi.org/10.3390/ma18040806
Balzarotti R, Basso Peressut A, Garbarino G, Spennati E, Basbus JF, Carpanese MP, Latorrata S, Cristiani C, Finocchio E. A Study of Redox Properties of Ceria and Fe-Ceria Solid Materials Through Small Molecules Catalytic Oxidation. Materials. 2025; 18(4):806. https://doi.org/10.3390/ma18040806
Chicago/Turabian StyleBalzarotti, Riccardo, Andrea Basso Peressut, Gabriella Garbarino, Elena Spennati, Juan Felipe Basbus, Maria Paola Carpanese, Saverio Latorrata, Cinzia Cristiani, and Elisabetta Finocchio. 2025. "A Study of Redox Properties of Ceria and Fe-Ceria Solid Materials Through Small Molecules Catalytic Oxidation" Materials 18, no. 4: 806. https://doi.org/10.3390/ma18040806
APA StyleBalzarotti, R., Basso Peressut, A., Garbarino, G., Spennati, E., Basbus, J. F., Carpanese, M. P., Latorrata, S., Cristiani, C., & Finocchio, E. (2025). A Study of Redox Properties of Ceria and Fe-Ceria Solid Materials Through Small Molecules Catalytic Oxidation. Materials, 18(4), 806. https://doi.org/10.3390/ma18040806












