Advanced Strategies for Enhancing the Biocompatibility and Antibacterial Properties of Implantable Structures
Abstract
1. Introduction
2. Osseointegration and Surface Quality Management
Reconciling Conflicting Findings on Surface Roughness and Coating Compositions
3. PEO, Aspects of Morphology
4. Effect of Adding Particles on Coating Composition, Microstructure, and Morphology
5. Coating Thickness
6. Crystallinity Features
- The morphological characteristics of the surface during PEO are significantly influenced by the spark’s size and shape, as well as the chemical composition of the anodizing solution.
- Anodic coatings produced in the P-Si solution exhibited lower porosity than those formed in other solutions, enhanced corrosion resistance, and increased hardness. Conversely, coatings created in the P-S solution demonstrated high surface porosity, with a morphology resembling bone structures. In the P solution, circular pore structures were predominantly observed.
- The anatase crystalline phase was the dominant structure in anodic coatings developed with the P and P-Si electrodes, with only a tiny amount of the rutile phase present. In contrast, the primary crystalline phase in coatings formed in the P-S solution was distinctly different.
- Potentiostatic coatings outperformed those produced under galvanostatic control in terms of tribological properties. This was particularly evident in the anodic coatings obtained in the P solution at 250 V and in the P-Si solution at 400 V, both of which exhibited the lowest wear rates [387].
7. PEO Using Microparticles and Elements
8. PEO—Aspects of Surface Strength
9. Plasma Electrolytic Oxidation Method Calcium-Phosphate-Base Composite Layer via PEO
10. PEO—Antibacterial Effect
11. PEO with the Inclusion of Nanocomponents
12. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, L.; Liao, X.; Fok, A.; Ning, C.; Ng, P.; Wang, Y. Effect of Crystalline Phase Changes in Titania (TiO2) Nanotube Coatings on Platelet Adhesion and Activation. Mater. Sci. Eng. C 2018, 82, 91–101. [Google Scholar] [CrossRef]
- Mishchenko, O.; Solodovnik, O.; Oleshko, O. Osteointegration of Dental Implants with Different Surface Types. Bukovinian Med. Bull. 2020, 24, 79–89. [Google Scholar] [CrossRef]
- Mouhyi, J.; Dohan Ehrenfest, D.M.; Albrektsson, T. The Peri-Implantitis: Implant Surfaces, Microstructure, and Physicochemical Aspects. Clin. Implant Dent. Relat. Res. 2012, 14, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Carmona, G.; Rodriguez, A.; Juarez, D.; Corzo, G.; Villegas, E. Improved Protease Stability of the Antimicrobial Peptide Pin2 Substituted with D-Amino Acids. Protein J. 2013, 32, 456–466. [Google Scholar] [CrossRef] [PubMed]
- Smeets, R.; Henningsen, A.; Jung, O.; Heiland, M.; Hammächer, C.; Stein, J.M. Definition, Etiology, Prevention and Treatment of Peri-Implantitis—A Review. Head Face Med. 2014, 10, 34. [Google Scholar] [CrossRef] [PubMed]
- Arciola, C.R.; Campoccia, D.; Ehrlich, G.D.; Montanaro, L. Biofilm-Based Implant Infections in Orthopaedics. Adv. Exp. Med. Biol. 2015, 830, 29–46. [Google Scholar] [CrossRef]
- Prathapachandran, J.; Suresh, N. Management of Peri-Implantitis. Dent. Res. J. 2012, 9, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Katsikogianni, M.; Missirlis, Y.F.; Harris, L.; Douglas, J. Concise Review of Mechanisms of Bacterial Adhesion to Biomaterials and of Techniques Used in Estimating Bacteria-Material Interactions. Eur. Cells Mater. 2004, 8, 37–57. [Google Scholar] [CrossRef]
- Orapiriyakul, W.; Young, P.S.; Damiati, L.; Tsimbouri, P.M. Antibacterial Surface Modification of Titanium Implants in Orthopaedics. J. Tissue Eng. 2018, 9, 2041731418789838. [Google Scholar] [CrossRef]
- Romanò, C.L.; Scarponi, S.; Gallazzi, E.; Romanò, D.; Drago, L. Antibacterial Coating of Implants in Orthopaedics and Trauma: A Classification Proposal in an Evolving Panorama. J. Orthop. Surg. Res. 2015, 10, 157. [Google Scholar] [CrossRef] [PubMed]
- Hasan, J.; Crawford, R.J.; Ivanova, E.P. Antibacterial Surfaces: The Quest for a New Generation of Biomaterials. Trends Biotechnol. 2013, 31, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Tsimbouri, P.M.; Fisher, L.; Holloway, N.; Sjostrom, T.; Nobbs, A.H.; Meek, R.M.D.; Su, B.; Dalby, M.J. Osteogenic and Bactericidal Surfaces from Hydrothermal Titania Nanowires on Titanium Substrates. Sci. Rep. 2016, 6, 36857. [Google Scholar] [CrossRef] [PubMed]
- Colon, G.; Ward, B.C.; Webster, T.J. Increased Osteoblast and Decreased Staphylococcus epidermidis Functions on Nanophase ZnO and TiO2. J. Biomed. Mater. Res. Part A 2006, 78A, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Xiong, W.; Fang, Z.; Guan, H.; Wu, W.; Li, Y.; Zhang, Y.; Alvarez, M.M.; Gao, B.; Huo, K.; et al. Strontium (Sr) and Silver (Ag) Loaded Nanotubular Structures with Combined Osteoinductive and Antimicrobial Activities. Acta Biomater. 2016, 31, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Huo, K.; Zhang, X.; Wang, H.; Zhao, L.; Liu, X.; Chu, P.K. Osteogenic Activity and Antibacterial Effects on Titanium Surfaces Modified with Zn-Incorporated Nanotube Arrays. Biomaterials 2013, 34, 3467–3478. [Google Scholar] [CrossRef]
- Ferraris, S.; Spriano, S.; Miola, M.; Bertone, E.; Allizond, V.; Cuffini, A.M.; Banche, G. Surface Modification of Titanium Surfaces through a Modified Oxide Layer and Embedded Silver Nanoparticles: Effect of Reducing/Stabilizing Agents on Precipitation and Properties of the Nanoparticles. Surf. Coat. Technol. 2018, 344, 177–189. [Google Scholar] [CrossRef]
- Mas-Moruno, C.; Espanol, M.; Montufar, E.B.; Mestres, G.; Aparicio, C.; Gil, F.J.; Ginebra, M.P. Bioactive Ceramic and Metallic Surfaces for Bone Engineering. Biomater. Surf. Sci. 2013, 12, 337–374. [Google Scholar] [CrossRef]
- Surmeneva, M.A.; Sharonova, A.A.; Chernousova, S.; Prymak, O.; Loza, K.; Tkachev, M.S.; Shulepov, I.A.; Epple, M.; Surmenev, R.A. Incorporation of Silver Nanoparticles into Magnetron-Sputtered Calcium Phosphate Layers on Titanium as an Antibacterial Coating. Colloids Surf. B Biointerfaces 2017, 156, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Besinis, A.; Hadi, S.D.; Le, H.R.; Tredwin, C.; Handy, R.D. Antibacterial Activity and Biofilm Inhibition by Surface Modified Titanium Alloy Medical Implants Following Application of Silver, Titanium Dioxide and Hydroxyapatite Nanocoatings. Nanotoxicology 2017, 11, 327–338. [Google Scholar] [CrossRef]
- Harrison, R.G. On the Stereotropism of Embryonic Cells. Science 1911, 34, 279–281. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, C.D.W.; Dalby, M.; Curtis, A.S.G. Making Structures for Cell Engineering. Eur. Cells Mater. 2004, 8, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Denis, F.A.; Hanarp, P.; Sutherland, D.S.; Gold, J.; Mustin, C.; Rouxhet, P.G.; Dufrêne, Y.F. Protein Adsorption on Model Surfaces with Controlled Nanotopography and Chemistry. Langmuir 2002, 18, 819–828. [Google Scholar] [CrossRef]
- Meyer, E.; Hegner, M.; Gerber, C.; Güntherodt, H.-J. Proceedings of the International Conference on Nanoscience and Technology (ICN&T 2006) (30 July to 4 August 2006, Basel, Switzerland). J. Phys. Conf. Ser. 2007, 61, E01. [Google Scholar] [CrossRef]
- Yim, E.K.F.; Reano, R.M.; Pang, S.W.; Yee, A.F.; Chen, C.S.; Leong, K.W. Nanopattern-Induced Changes in Morphology and Motility of Smooth Muscle Cells. Biomaterials 2005, 26, 5405–5413. [Google Scholar] [CrossRef] [PubMed]
- Rottmar, M.; Müller, E.; Guimond-Lischer, S.; Stephan, M.; Berner, S.; Maniura-Weber, K. Assessing the Osteogenic Potential of Zirconia and Titanium Surfaces with an Advanced in Vitro Model. Dent. Mater. 2019, 35, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Patelli, A.; Mussano, F.; Brun, P.; Genova, T.; Ambrosi, E.; Michieli, N.; Mattei, G.; Scopece, P.; Moroni, L. Nanoroughness, Surface Chemistry, and Drug Delivery Control by Atmospheric Plasma Jet on Implantable Devices. ACS Appl. Mater. Interfaces 2018, 10, 39512–39523. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Nie, Z.; Liu, Z.; Hou, S.; Ji, J. Developments of Nano-TiO2 Incorporated Hydroxyapatite/PEEK Composite Strut for Cervical Reconstruction and Interbody Fusion after Corpectomy with Anterior Plate Fixation. J. Photochem. Photobiol. B Biol. 2018, 187, 120–125. [Google Scholar] [CrossRef]
- Xu, R.; Hu, X.; Yu, X.; Wan, S.; Wu, F.; Ouyang, J.; Deng, F. Micro-/Nano-Topography of Selective Laser Melting Titanium Enhances Adhesion and Proliferation and Regulates Adhesion-Related Gene Expressions of Human Gingival Fibroblasts and Human Gingival Epithelial Cells. Int. J. Nanomed. 2018, 13, 5045–5057. [Google Scholar] [CrossRef] [PubMed]
- Smeets, R.; Stadlinger, B.; Schwarz, F.; Beck-Broichsitter, B.; Jung, O.; Precht, C.; Kloss, F.; Gröbe, A.; Heiland, M.; Ebker, T. Impact of Dental Implant Surface Modifications on Osseointegration. Biomed. Res. Int. 2016, 2016, 6285620. [Google Scholar] [CrossRef]
- Jiang, X.; Yao, Y.; Tang, W.; Han, D.; Zhang, L.; Zhao, K.; Wang, S.; Meng, Y. Design of Dental Implants at Materials Level: An Overview. J. Biomed. Mater. Res. Part A 2020, 108, 1634–1661. [Google Scholar] [CrossRef]
- Liu, X.Y.; Sawant, P.D. Mechanism of the Formation of Self-Organized Microstructures in Soft Functional Materials. Adv. Mater. 2002, 14, 421–426. [Google Scholar] [CrossRef]
- Vercaigne, S.; Wolke, J.G.C.; Naert, I.; Jansen, J.A. Histomorphometrical and Mechanical Evaluation of Titanium Plasma-Spray-Coated Implants Placed in the Cortical Bone of Goats. J. Biomed. Mater. Res. 1998, 41, 41–48. [Google Scholar] [CrossRef]
- Mattox, D.M. Handbook of Physical Vapor Deposition (PVD) Processing. In Handbook of Physical Vapor Deposition (PVD) Processing; Elsevier Inc.: Amsterdam, The Netherlands, 2010; pp. 301–331. ISBN 9780815520375. [Google Scholar]
- LeClair, P.; Berera, G.P.; Moodera, J.S. Titanium Nitride Thin Films Obtained by a Modified Physical Vapor Deposition Process. Thin Solid Films 2000, 1-2, 9–15. [Google Scholar] [CrossRef]
- Arregui, M.; Latour, F.; Gil, F.J.; Pérez, R.A.; Giner-Tarrida, L.; Delgado, L.M. Ion Release from Dental Implants, Prosthetic Abutments and Crowns under Physiological and Acidic Conditions. Coatings 2021, 11, 98. [Google Scholar] [CrossRef]
- Mitamura, Y.; Hosooka, K.; Matsumoto, T.; Otaki, K.; Sakai, K.; Tanabe, T.; Yuta, T.; Mikami, T. Development of a Ceramic Heart Valve. J. Biomater. Appl. 1989, 4, 33–55. [Google Scholar] [CrossRef]
- Ghadai, R.K.; Logesh, K.; Čep, R.; Chohan, J.S.; Kalita, K. Influence of Deposition Time on Titanium Nitride (TiN) Thin Film Coating Synthesis Using Chemical Vapour Deposition. Materials 2023, 16, 4611. [Google Scholar] [CrossRef]
- Ballo, A.M.; Bjöörn, D.; Åstrand, M.; Palmquist, A.; Lausmaa, J.; Thomsen, P. Bone Response to Physical-Vapour-Deposited Titanium Dioxide Coatings on Titanium Implants. Clin. Oral Implants Res. 2013, 24, 1009–1017. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, Z.; Ren, X.; Wang, B.; Cai, Y.; Song, Q.; Wan, Y. Coating-Thickness-Dependent Physical Properties and Cutting Temperature for Cutting Inconel 718 with TiAlN Coated Tools. J. Adv. Res. 2021, 38, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Jinno, Y.; Jimbo, R.; Hjalmarsson, J.; Johansson, K.; Stavropoulos, A.; Becktor, J.P. Impact of Surface Contamination of Implants with Saliva during Placement in Augmented Bone Defects in Sheep Calvaria. Br. J. Oral Maxillofac. Surg. 2019, 57, 41–46. [Google Scholar] [CrossRef]
- Korzec, D.; Andres, T.; Brandes, E.; Nettesheim, S. Visualization of Activated Area on Polymers for Evaluation of Atmospheric Pressure Plasma Jets. Polymers 2021, 13, 2711. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Gong, H.; Li, D.; Dong, L.; Zhao, M.; Wan, R.; Gu, H. Ag+ Implantation Induces Mechanical Properties, Cell Adhesion and Antibacterial Effects of TiN/Ag Multilayers In Vitro. Nanomedicine 2017, 12, 2257–2268. [Google Scholar] [CrossRef] [PubMed]
- Kasemo, B.; Lausmaa, J. Biomaterial and Implant Surfaces: On the Role of Cleanliness, Contamination, and Preparation Procedures. J. Biomed. Mater. Res. 1988, 22, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.C.; Pilliar, R.M.; Metson, J.B.; McIntyre, N.S. Dental Implant Materials. II. Preparative Procedures and Surface Spectroscopic Studies. J. Biomed. Mater. Res. 1991, 25, 1069–1084. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.C.; Pilliar, R.M.; Chernecky, R. Dental Implant Materials. I. Some Effects of Preparative Procedures on Surface Topography. J. Biomed. Mater. Res. 1991, 25, 1045–1068. [Google Scholar] [CrossRef] [PubMed]
- Aronsson, B.-O.; Lausmaa, J.; Kasemo, B. Glow Discharge Plasma Treatment for Surface Cleaning and Modification of Metallic Biomaterials. J. Biomed. Mater. Res. 1997, 35, 49–73. [Google Scholar] [CrossRef]
- Sobiecki, J.R.; Wierzchoń, T.; Rudnicki, J. The Influence of Glow Discharge Nitriding, Oxynitriding and Carbonitriding on Surface Modification of Ti-1Al-1Mn Titanium Alloy. Vacuum 2001, 64, 41–46. [Google Scholar] [CrossRef]
- Jagielski, J.; Piatkowska, A.; Aubert, P.; Thomé, L.; Turos, A.; Abdul Kader, A. Ion Implantation for Surface Modification of Biomaterials. Surf. Coat. Technol. 2006, 22-23, 6355–6361. [Google Scholar] [CrossRef]
- Shi, M.; Mo, W.; Qi, H.; Ni, Y.; Wang, R.; Shen, K.; Zhang, F.; Jiang, S.; Zhang, X.; Chen, L.; et al. Oxygen Ion Implantation Improving Cell Adhesion on Titanium Surfaces through Increased Attraction of Fibronectin PHSRN Domain. Adv. Healthc. Mater. 2022, 11, 2101983. [Google Scholar] [CrossRef] [PubMed]
- Chen, K. Bonding Characteristics of TiC and TiN. Model. Numer. Simul. Mater. Sci. 2013, 3, 7–11. [Google Scholar] [CrossRef]
- Hanawa, T.; Ukai, H.; Murakami, K. X-Ray Photoelectron Spectroscopy of Calcium-Ion-Implanted Titanium. J. Electron Spectros. Relat. Phenom. 1993, 63, 347–354. [Google Scholar] [CrossRef]
- Yang, P.; Huang, N.; Leng, Y.X.; Chen, J.Y.; Sun, H.; Wang, J.; Chen, F.; Chu, P.K. In Vivo Study of Ti–O Thin Film Fabricated by PIII. Surf. Coat. Technol. 2002, 156, 284–288. [Google Scholar] [CrossRef]
- Berberich, F.; Matz, W.; Kreissig, U.; Richter, E.; Schell, N.; Möller, W. Structural Characterisation of Hardening of Ti–Al–V Alloys after Nitridation by Plasma Immersion Ion Implantation. Appl. Surf. Sci. 2001, 179, 13–19. [Google Scholar] [CrossRef]
- Wen, F.; Dai, H.; Huang, N.; Sun, H.; Leng, Y.X.; Chu, P.K. Controlling Synthesis of Ti–O/Ti–N Gradient Films by PIII. Surf. Coat. Technol. 2002, 156, 208–213. [Google Scholar] [CrossRef]
- Yatsuzuka, M.; Miki, S.; Morita, R.; Azuma, K.; Fujiwara, E.; Uchida, H. Enhanced Corrosion Resistance of TiN Prepared by Plasma-Based Ion Implantation. Vacuum 2000, 59, 330–337. [Google Scholar] [CrossRef]
- Krupa, D.; Jezierska, E.; Baszkiewicz, J.; Wierzchoń, T.; Barcz, A.; Gawlik, G.; Jagielski, J.; Sobczak, J.W.; Biliński, A.; Larisch, B. Effect of Carbon Ion Implantation on the Structure and Corrosion Resistance of OT-4-0 Titanium Alloy. Surf. Coat. Technol. 1999, 114, 250–259. [Google Scholar] [CrossRef]
- Hanawa, T.; Kamiura, Y.; Yamamoto, S.; Kohgo, T.; Amemiya, A.; Ukai, H.; Murakami, K.; Asaoka, K. Early Bone Formation around Calcium-Ion-Implanted Titanium Inserted into Rat Tibia. J. Biomed. Mater. Res. 1997, 36, 131–136. [Google Scholar] [CrossRef]
- Hanawa, T. In Vivo Metallic Biomaterials and Surface Modification. Mater. Sci. Eng. A 1999, 267, 260–266. [Google Scholar] [CrossRef]
- Yoshinari, M.; Oda, Y.; Ueki, H.; Yokose, S. Immobilization of Bisphosphonates on Surface Modified Titanium. Biomaterials 2001, 22, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Krupa, D.; Baszkiewicz, J.; Kozubowski, J.A.; Barcz, A.; Sobczak, J.W.; Biliński, A.; Lewandowska-Szumieł, M.; Rajchel, B. Effect of Phosphorus-Ion Implantation on the Corrosion Resistance and Biocompatibility of Titanium. Biomaterials 2002, 23, 3329–3340. [Google Scholar] [CrossRef]
- Tsyganov, I.; Wieser, E.; Matz, W.; Reuther, H.; Richter, E. Modification of the Ti–6Al–4V Alloy by Ion Implantation of Calcium and/or Phosphorus. Surf. Coat. Technol. 2002, 158-159, 318–323. [Google Scholar] [CrossRef]
- Wieser, E.; Tsyganov, I.; Matz, W.; Reuther, H.; Oswald, S.; Pham, T.; Richter, E. Modification of Titanium by Ion Implantation of Calcium and/or Phosphorus. Surf. Coat. Technol. 1999, 111, 103–109. [Google Scholar] [CrossRef]
- Baumann, H.; Bethge, K.; Bilger, G.; Jones, D.; Symietz, I. Thin Hydroxyapatite Surface Layers on Titanium Produced by Ion Implantation. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2002, 196, 286–292. [Google Scholar] [CrossRef]
- Pham, M.T.; Maitz, M.F.; Matz, W.; Reuther, H.; Richter, E.; Steiner, G. Promoted Hydroxyapatite Nucleation on Titanium Ion-Implanted with Sodium. Thin Solid Films 2000, 379, 50–56. [Google Scholar] [CrossRef]
- Maitz, M.F.; Pham, M.T.; Matz, W.; Reuther, H.; Steiner, G. Promoted Calcium-Phosphate Precipitation from Solution on Titanium for Improved Biocompatibility by Ion Implantation. Surf. Coat. Technol. 2002, 158-159, 151–156. [Google Scholar] [CrossRef]
- Maitz, M.F.; Pham, M.T.; Matz, W.; Reuther, H.; Steiner, G.; Richter, E. Ion Beam Treatment of Titanium Surfaces for Enhancing Deposition of Hydroxyapatite from Solution. Biomol. Eng. 2002, 19, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Yoshinari, M.; Oda, Y.; Kato, T.; Okuda, K. Influence of Surface Modifications to Titanium on Antibacterial Activity In Vitro. Biomaterials 2001, 22, 2043–2048. [Google Scholar] [CrossRef]
- Balasundaram, G.; Webster, T.J. A Perspective on Nanophase Materials for Orthopedic Implant Applications. J. Mater. Chem. 2006, 16, 3737–3745. [Google Scholar] [CrossRef]
- Dobrzański, L.A.; Dobrzańska-Danikiewicz, A.D.; Dobrzański, L.B. Effect of Biomedical Materials in the Implementation of a Long and Healthy Life Policy. Processes 2021, 9, 865. [Google Scholar] [CrossRef]
- Zhang, L.; Webster, T.J. Nanotechnology and Nanomaterials: Promises for Improved Tissue Regeneration. Nano Today 2009, 4, 66–80. [Google Scholar] [CrossRef]
- Stevens, M.M.; George, J.H. Exploring and Engineering the Cell Surface Interface. Science 2005, 310, 1135–1138. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.J.; Clegg, R.E.; Leavesley, D.I.; Pearcy, M.J. Mediation of Biomaterial-Cell Interactions by Adsorbed Proteins: A Review. Tissue Eng. 2005, 11, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Webster, T.J.; Ejiofor, J.U. Increased Osteoblast Adhesion on Nanophase Metals: Ti, Ti6Al4V, and CoCrMo. Biomaterials 2004, 25, 4731–4739. [Google Scholar] [CrossRef] [PubMed]
- Price, R.L.; Waid, M.C.; Haberstroh, K.M.; Webster, T.J. Selective Bone Cell Adhesion on Formulations Containing Carbon Nanofibers. Biomaterials 2003, 24, 1877–1887. [Google Scholar] [CrossRef] [PubMed]
- Webster, T.J.; Smith, T.A. Increased Osteoblast Function on PLGA Composites Containing Nanophase Titania. J. Biomed. Mater. Res. A 2005, 74, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Manjubala, I.; Scheler, S.; Bössert, J.; Jandt, K.D. Mineralisation of Chitosan Scaffolds with Nano-Apatite Formation by Double Diffusion Technique. Acta Biomater. 2006, 2, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Desai, T.A. Micro- and Nanoscale Structures for Tissue Engineering Constructs. Med. Eng. Phys. 2000, 22, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Webster, T.J.; Ergun, C.; Doremus, R.H.; Siegel, R.W.; Bizios, R. Enhanced Osteoclast-like Cell Functions on Nanophase Ceramics. Biomaterials 2001, 22, 1327–1333. [Google Scholar] [CrossRef] [PubMed]
- Elias, K.L.; Price, R.L.; Webster, T.J. Enhanced Functions of Osteoblasts on Nanometer Diameter Carbon Fibers. Biomaterials 2002, 23, 3279–3287. [Google Scholar] [CrossRef] [PubMed]
- Webster, T.J.; Hellenmeyer, E.L.; Price, R.L. Increased Osteoblast Functions on Theta+delta Nanofiber Alumina. Biomaterials 2005, 26, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Gutwein, L.G.; Webster, T.J. Increased Viable Osteoblast Density in the Presence of Nanophase Compared to Conventional Alumina and Titania Particles. Biomaterials 2004, 25, 4175–4183. [Google Scholar] [CrossRef] [PubMed]
- Balasundaram, G.; Sato, M.; Webster, T.J. Using Hydroxyapatite Nanoparticles and Decreased Crystallinity to Promote Osteoblast Adhesion Similar to Functionalizing with RGD. Biomaterials 2006, 27, 2798–2805. [Google Scholar] [CrossRef] [PubMed]
- Ergun, C.; Liu, H.; Webster, T.J.; Olcay, E.; Yilmaz, Ş.; Sahin, F.C. Increased Osteoblast Adhesion on Nanoparticulate Calcium Phosphates with Higher Ca/P Ratios. J. Biomed. Mater. Res. A 2008, 85, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Brammer, K.S.; Oh, S.; Cobb, C.J.; Bjursten, L.M.; van der Heyde, H.; Jin, S. Improved Bone-Forming Functionality on Diameter-Controlled TiO2 Nanotube Surface. Acta Biomater. 2009, 5, 3215–3223. [Google Scholar] [CrossRef]
- Oh, S.; Daraio, C.; Chen, L.H.; Pisanic, T.R.; Fiñones, R.R.; Jin, S. Significantly Accelerated Osteoblast Cell Growth on Aligned TiO2 Nanotubes. J. Biomed. Mater. Res. Part A 2006, 78A, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Popat, K.C.; Daniels, R.H.; Dubrow, R.S.; Hardev, V.; Desai, T.A. Nanostructured Surfaces for Bone Biotemplating Applications. J. Orthop. Res. 2006, 24, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Popat, K.C.; Leoni, L.; Grimes, C.A.; Desai, T.A. Influence of Engineered Titania Nanotubular Surfaces on Bone Cells. Biomaterials 2007, 28, 3188–3197. [Google Scholar] [CrossRef] [PubMed]
- Swan, E.E.L.; Popat, K.C.; Grimes, C.A.; Desai, T.A. Fabrication and Evaluation of Nanoporous Alumina Membranes for Osteoblast Culture. J. Biomed. Mater. Res. A 2005, 72, 288–295. [Google Scholar] [CrossRef] [PubMed]
- McNamara, L.E.; McMurray, R.J.; Biggs, M.J.P.; Kantawong, F.; Oreffo, R.O.C.; Dalby, M.J. Nanotopographical Control of Stem Cell Differentiation. J. Tissue Eng. 2010, 2010, 120623. [Google Scholar] [CrossRef] [PubMed]
- Li, W.J.; Laurencin, C.T.; Caterson, E.J.; Tuan, R.S.; Ko, F.K. Electrospun Nanofibrous Structure: A Novel Scaffold for Tissue Engineering. J. Biomed. Mater. Res. 2002, 60, 613–621. [Google Scholar] [CrossRef]
- Khanna, A.; Zamani, M.; Huang, N.F. Extracellular Matrix-Based Biomaterials for Cardiovascular Tissue Engineering. J. Cardiovasc. Dev. Dis. 2021, 8, 137. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Slamovich, E.B.; Webster, T.J. Enhanced Osteoblast Adhesion on Hydrothermally Treated Hydroxyapatite/Titania/Poly(Lactide-Co-Glycolide) Sol–Gel Titanium Coatings. Biomaterials 2005, 26, 1349–1357. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Sambito, M.A.; Aslani, A.; Kalkhoran, N.M.; Slamovich, E.B.; Webster, T.J. Increased Osteoblast Functions on Undoped and Yttrium-Doped Nanocrystalline Hydroxyapatite Coatings on Titanium. Biomaterials 2006, 27, 2358–2369. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.J.; Chen, J.; Karageorgiou, V.; Altman, G.H.; Kaplan, D.L. Human Bone Marrow Stromal Cell Responses on Electrospun Silk Fibroin Mats. Biomaterials 2004, 25, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.J.; Park, J.; Valluzzi, R.; Cebe, P.; Kaplan, D.L. Biomaterial Films of Bombyx Mori Silk Fibroin with Poly(Ethylene Oxide). Biomacromolecules 2004, 5, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Webster, T.J. Anodization: A Promising Nano-Modification Technique of Titanium Implants for Orthopedic Applications. J. Nanosci. Nanotechnol. 2006, 6, 2682–2692. [Google Scholar] [CrossRef]
- Yao, C.; Slamovich, E.B.; Webster, T.J. Enhanced Osteoblast Functions on Anodized Titanium with Nanotube-like Structures. J. Biomed. Mater. Res. A 2008, 85, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Khang, D.; Lu, J.; Yao, C.; Haberstroh, K.M.; Webster, T.J. The Role of Nanometer and Sub-Micron Surface Features on Vascular and Bone Cell Adhesion on Titanium. Biomaterials 2008, 29, 970–983. [Google Scholar] [CrossRef] [PubMed]
- Gimble, J.M.; Guilak, F.; Nuttall, M.E.; Sathishkumar, S.; Vidal, M.; Bunnell, B.A. In Vitro Differentiation Potential of Mesenchymal Stem Cells. Transfus. Med. Hemother. 2008, 35, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Usmaniya, N.; Shishir, R.; Ponnilavan, V.; Rameshbabu, N. Development of hydroxyapatite/bioactive glass incorporated chitosan layer on plasma electrolytic oxidised ZM21 alloy for temporary implant applications. J. Alloys Compd. 2024, 1004, 175723. [Google Scholar] [CrossRef]
- Dolatshahi-Pirouz, A.; Jensen, T.; Kraft, D.C.; Foss, M.; Kingshott, P.; Hansen, J.L.; Larsen, A.N.; Chevallier, J.; Besenbacher, F. Fibronectin Adsorption, Cell Adhesion, and Proliferation on Nanostructured Tantalum Surfaces. ACS Nano 2010, 4, 2874–2882. [Google Scholar] [CrossRef]
- Yin, Z.; Chen, X.; Chen, J.L.; Shen, W.L.; Hieu Nguyen, T.M.; Gao, L.; Ouyang, H.W. The Regulation of Tendon Stem Cell Differentiation by the Alignment of Nanofibers. Biomaterials 2010, 31, 2163–2175. [Google Scholar] [CrossRef] [PubMed]
- Dalby, M.J.; Gadegaard, N.; Tare, R.; Andar, A.; Riehle, M.O.; Herzyk, P.; Wilkinson, C.D.W.; Oreffo, R.O.C. The Control of Human Mesenchymal Cell Differentiation Using Nanoscale Symmetry and Disorder. Nat. Mater. 2007, 6, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Yim, E.K.F.; Darling, E.M.; Kulangara, K.; Guilak, F.; Leong, K.W. Nanotopography-Induced Changes in Focal Adhesions, Cytoskeletal Organization, and Mechanical Properties of Human Mesenchymal Stem Cells. Biomaterials 2010, 31, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Bauer, S.; Von Der Mark, K.; Schmuki, P. Nanosize and Vitality: TiO2 Nanotube Diameter Directs Cell Fate. Nano Lett. 2007, 7, 1686–1691. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.Q.; Jiang, X.Q.; Zhang, F.Q.; Xu, L. The Effect of Anatase TiO2 Nanotube Layers on MC3T3-E1 Preosteoblast Adhesion, Proliferation, and Differentiation. J. Biomed. Mater. Res. Part A 2010, 94A, 1012–1022. [Google Scholar] [CrossRef]
- Oh, S.; Brammer, K.S.; Li, Y.S.J.; Teng, D.; Engler, A.J.; Chien, S.; Jin, S. Stem Cell Fate Dictated Solely by Altered Nanotube Dimension. Proc. Natl. Acad. Sci. USA 2009, 106, 2130–2135. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Ogura, Y.; Enomoto, K.; Hara, M.; Maurstad, G.; Stokke, B.T.; Kitamura, S. Dense Carbon-Nanotube Coating Scaffolds Stimulate Osteogenic Differentiation of Mesenchymal Stem Cells. PLoS ONE 2020, 15, e0225589. [Google Scholar] [CrossRef]
- Chen, C.S. Mechanotransduction—A Field Pulling Together? J. Cell Sci. 2008, 121, 3285–3292. [Google Scholar] [CrossRef]
- Orr, A.W.; Helmke, B.P.; Blackman, B.R.; Schwartz, M.A. Mechanisms of Mechanotransduction. Dev. Cell 2006, 10, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Han, M.K.L.; de Rooij, J. Converging and Unique Mechanisms of Mechanotransduction at Adhesion Sites. Trends Cell Biol. 2016, 26, 612–623. [Google Scholar] [CrossRef]
- Vitkov, L.; Hartl, D.; Hannig, M. Is Osseointegration Inflammation-Triggered? Med. Hypotheses 2016, 93, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Luu, T.U.; Gott, S.C.; Woo, B.W.K.; Rao, M.P.; Liu, W.F. Micro- and Nanopatterned Topographical Cues for Regulating Macrophage Cell Shape and Phenotype. ACS Appl. Mater. Interfaces 2015, 7, 28665–28672. [Google Scholar] [CrossRef] [PubMed]
- Östberg, A.K.; Dahlgren, U.; Sul, Y.T.; Johansson, C.B. Inflammatory Cytokine Release Is Affected by Surface Morphology and Chemistry of Titanium Implants. J. Mater. Sci. Mater. Med. 2015, 26, 155. [Google Scholar] [CrossRef] [PubMed]
- Neacsu, P.; Mazare, A.; Schmuki, P.; Cimpean, A. Attenuation of the Macrophage Inflammatory Activity by TiO2 Nanotubes via Inhibition of MAPK and NF-ΚB Pathways. Int. J. Nanomed. 2015, 10, 6455–6467. [Google Scholar] [CrossRef]
- Ma, Q.L.; Fang, L.; Jiang, N.; Zhang, L.; Wang, Y.; Zhang, Y.M.; Chen, L.H. Bone Mesenchymal Stem Cell Secretion of SRANKL/OPG/M-CSF in Response to Macrophage-Mediated Inflammatory Response Influences Osteogenesis on Nanostructured Ti Surfaces. Biomaterials 2018, 154, 234–247. [Google Scholar] [CrossRef]
- Yao, S.; Feng, X.; Li, W.; Wang, L.N.; Wang, X. Regulation of RAW 264.7 Macrophages Behavior on Anodic TiO2 Nanotubular Arrays. Front. Mater. Sci. 2017, 11, 318–327. [Google Scholar] [CrossRef]
- Hotchkiss, K.M.; Reddy, G.B.; Hyzy, S.L.; Schwartz, Z.; Boyan, B.D.; Olivares-Navarrete, R. Titanium Surface Characteristics, Including Topography and Wettability, Alter Macrophage Activation. Acta Biomater. 2016, 31, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Prasopthum, A.; Cooper, M.; Shakesheff, K.M.; Yang, J. Three-Dimensional Printed Scaffolds with Controlled Micro-/Nanoporous Surface Topography Direct Chondrogenic and Osteogenic Differentiation of Mesenchymal Stem Cells. ACS Appl. Mater. Interfaces 2019, 11, 18896–18906. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Bauer, S.; Schlegel, K.A.; Neukam, F.W.; der Von Mark, K.; Schmuki, P. TiO2 Nanotube Surfaces: 15 Nm—An Optimal Length Scale of Surface Topography for Cell Adhesion and Differentiation. Small 2009, 5, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Dulgar-Tulloch, A.J.; Bizios, R.; Siegel, R.W. Human Mesenchymal Stem Cell Adhesion and Proliferation in Response to Ceramic Chemistry and Nanoscale Topography. J. Biomed. Mater. Res. Part A 2009, 90A, 586–594. [Google Scholar] [CrossRef]
- Bauer, S.; Park, J.; von der Mark, K.; Schmuki, P. Improved Attachment of Mesenchymal Stem Cells on Super-Hydrophobic TiO2 Nanotubes. Acta Biomater. 2008, 4, 1576–1582. [Google Scholar] [CrossRef]
- Bauer, S.; Park, J.; Pittrof, A.; Song, Y.Y.; Von Der Mark, K.; Schmuki, P. Covalent Functionalization of TiO2 Nanotube Arrays with EGF and BMP-2 for Modified Behavior towards Mesenchymal Stem Cells. Integr. Biol. 2011, 3, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Kodama, A.; Bauer, S.; Komatsu, A.; Asoh, H.; Ono, S.; Schmuki, P. Bioactivation of Titanium Surfaces Using Coatings of TiO2 Nanotubes Rapidly Pre-Loaded with Synthetic Hydroxyapatite. Acta Biomater. 2009, 5, 2322–2330. [Google Scholar] [CrossRef] [PubMed]
- Brammer, K.S.; Choi, C.; Frandsen, C.J.; Oh, S.; Johnston, G.; Jin, S. Comparative Cell Behavior on Carbon-Coated TiO2 Nanotube Surfaces for Osteoblasts vs. Osteo-Progenitor Cells. Acta Biomater. 2011, 7, 2697–2703. [Google Scholar] [CrossRef]
- Bauer, S.; Park, J.; Faltenbacher, J.; Berger, S.; Von Der Mark, K.; Schmuki, P. Size Selective Behavior of Mesenchymal Stem Cells on ZrO2 and TiO2 Nanotube Arrays. Integr. Biol. 2009, 1, 525–532. [Google Scholar] [CrossRef]
- Berger, S.; Faltenbacher, J.; Bauer, S.; Schmuki, P. Enhanced Self-Ordering of Anodic ZrO2 Nanotubes in Inorganic and Organic Electrolytes Using Two-Step Anodization. Phys. Status Solidi–Rapid Res. Lett. 2008, 2, 102–104. [Google Scholar] [CrossRef]
- Oliveira, N.T.C.; Biaggio, S.R.; Rocha-Filho, R.C.; Bocchi, N. Electrochemical Studies on Zirconium and Its Biocompatible Alloys Ti-50Zr at.% and Zr-2.5Nb Wt.% in Simulated Physiologic Media. J. Biomed. Mater. Res. Part A 2005, 74A, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Lischer, S.; Körner, E.; Balazs, D.J.; Shen, D.; Wick, P.; Grieder, K.; Haas, D.; Heuberger, M.; Hegemann, D. Antibacterial Burst-Release from Minimal Ag-Containing Plasma Polymer Coatings. J. R. Soc. Interface 2011, 8, 1019–1030. [Google Scholar] [CrossRef]
- Li, X.; Qi, M.; Sun, X.; Weir, M.D.; Tay, F.R.; Oates, T.W.; Dong, B.; Zhou, Y.; Wang, L.; Xu, H.H.K. Surface Treatments on Titanium Implants via Nanostructured Ceria for Antibacterial and Anti-Inflammatory Capabilities. Acta Biomater. 2019, 94, 627–643. [Google Scholar] [CrossRef]
- Wang, M.; Tang, T. Surface Treatment Strategies to Combat Implant-Related Infection from the Beginning. J. Orthop. Transl. 2019, 17, 42. [Google Scholar] [CrossRef]
- Wang, Y.; Subbiahdoss, G.; Swartjes, J.; Van Der Mei, H.C.; Busscher, H.J.; Libera, M. Length-Scale Mediated Differential Adhesion of Mammalian Cells and Microbes. Adv. Funct. Mater. 2011, 21, 3916–3923. [Google Scholar] [CrossRef]
- Narendrakumar, K.; Kulkarni, M.; Addison, O.; Mazare, A.; Junkar, I.; Schmuki, P.; Sammons, R.; Iglič, A. Adherence of Oral Streptococci to Nanostructured Titanium Surfaces. Dent. Mater. 2015, 31, 1460–1468. [Google Scholar] [CrossRef] [PubMed]
- Ercan, B.; Taylor, E.; Alpaslan, E.; Webster, T.J. Diameter of Titanium Nanotubes Influences Anti-Bacterial Efficacy. Nanotechnology 2011, 22, 295102. [Google Scholar] [CrossRef]
- Kavathekar, R.S.; English, N.J.; MacElroy, J.M.D. Spatial Distribution of Adsorbed Water Layers at the TiO2 Rutile and Anatase Interfaces. Chem. Phys. Lett. 2012, 554, 102–106. [Google Scholar] [CrossRef]
- Xu, Q.; Li, X.; Jin, Y.; Sun, L.; Ding, X.; Liang, L.; Wang, L.; Nan, K.; Ji, J.; Chen, H.; et al. Bacterial self-defense antibiotics release from organic–inorganic hybrid multilayer films for long-term anti-adhesion and biofilm inhibition properties. Nanoscale 2017, 9, 19245–19254. [Google Scholar] [CrossRef]
- Bakhshandeh, S.; Gorgin Karaji, Z.; Lietaert, K.; Fluit, A.C.; Boel, C.H.E.; Vogely, H.C.; Vermonden, T.; Hennink, W.E.; Weinans, H.; Zadpoor, A.A.; et al. Simultaneous Delivery of Multiple Antibacterial Agents from Additively Manufactured Porous Biomaterials to Fully Eradicate Planktonic and Adherent Staphylococcus aureus. ACS Appl. Mater. Interfaces 2017, 9, 25691–25699. [Google Scholar] [CrossRef] [PubMed]
- Necula, B.S.; Van Leeuwen, J.P.T.M.; Fratila-Apachitei, L.E.; Zaat, S.A.J.; Apachitei, I.; Duszczyk, J. In Vitro Cytotoxicity Evaluation of Porous TiO2-Ag Antibacterial Coatings for Human Fetal Osteoblasts. Acta Biomater. 2012, 8, 4191–4197. [Google Scholar] [CrossRef]
- Jia, Z.; Xiu, P.; Xiong, P.; Zhou, W.; Cheng, Y.; Wei, S.; Zheng, Y.; Xi, T.; Cai, H.; Liu, Z.; et al. Additively Manufactured Macroporous Titanium with Silver-Releasing Micro-/Nanoporous Surface for Multipurpose Infection Control and Bone Repair—A Proof of Concept. ACS Appl. Mater. Interfaces 2016, 8, 28495–28510. [Google Scholar] [CrossRef] [PubMed]
- van Hengel, I.A.J.; Riool, M.; Fratila-Apachitei, L.E.; Witte-Bouma, J.; Farrell, E.; Zadpoor, A.A.; Zaat, S.A.J.; Apachitei, I. Selective Laser Melting Porous Metallic Implants with Immobilized Silver Nanoparticles Kill and Prevent Biofilm Formation by Methicillin-Resistant Staphylococcus aureus. Biomaterials 2017, 140, 1–15. [Google Scholar] [CrossRef]
- Amin Yavari, S.; Loozen, L.; Paganelli, F.L.; Bakhshandeh, S.; Lietaert, K.; Groot, J.A.; Fluit, A.C.; Boel, C.H.E.; Alblas, J.; Vogely, H.C.; et al. Antibacterial Behavior of Additively Manufactured Porous Titanium with Nanotubular Surfaces Releasing Silver Ions. ACS Appl. Mater. Interfaces 2016, 8, 17080–17089. [Google Scholar] [CrossRef] [PubMed]
- Gallardo-Godoy, A.; Muldoon, C.; Becker, B.; Elliott, A.G.; Lash, L.H.; Huang, J.X.; Butler, M.S.; Pelingon, R.; Kavanagh, A.M.; Ramu, S.; et al. Activity and Predicted Nephrotoxicity of Synthetic Antibiotics Based on Polymyxin B. J. Med. Chem. 2016, 59, 1068–1077. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Q.; Fang, L.; Ling, J.; Ding, C.Z.; Kang, B.; Huang, C.Z. Nanotoxicity of Silver Nanoparticles to Red Blood Cells: Size Dependent Adsorption, Uptake, and Hemolytic Activity. Chem. Res. Toxicol. 2015, 28, 501–509. [Google Scholar] [CrossRef]
- Singh, R.P.; Ramarao, P. Cellular Uptake, Intracellular Trafficking and Cytotoxicity of Silver Nanoparticles. Toxicol. Lett. 2012, 213, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Panáček, A.; Kvítek, L.; Smékalová, M.; Večeřová, R.; Kolář, M.; Röderová, M.; Dyčka, F.; Šebela, M.; Prucek, R.; Tomanec, O.; et al. Bacterial Resistance to Silver Nanoparticles and How to Overcome It. Nat. Nanotechnol. 2017, 13, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Elbourne, A.; Crawford, R.J.; Ivanova, E.P. Nano-Structured Antimicrobial Surfaces: From Nature to Synthetic Analogues. J. Colloid Interface Sci. 2017, 508, 603–616. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Chen, A.; Song, X.; Brasch, M.E.; Henderson, J.H.; Ren, D. How Escherichia coli Lands and Forms Cell Clusters on a Surface: A New Role of Surface Topography. Sci. Rep. 2016, 6, 29516. [Google Scholar] [CrossRef]
- Crawford, R.J.; Webb, H.K.; Truong, V.K.; Hasan, J.; Ivanova, E.P. Surface Topographical Factors Influencing Bacterial Attachment. Adv. Colloid Interface Sci. 2012, 179–182, 142–149. [Google Scholar] [CrossRef]
- Song, F.; Brasch, M.E.; Wang, H.; Henderson, J.H.; Sauer, K.; Ren, D. How Bacteria Respond to Material Stiffness during Attachment: A Role of Escherichia coli Flagellar Motility. ACS Appl. Mater. Interfaces 2017, 9, 22176–22184. [Google Scholar] [CrossRef]
- Anselme, K.; Davidson, P.; Popa, A.M.; Giazzon, M.; Liley, M.; Ploux, L. The Interaction of Cells and Bacteria with Surfaces Structured at the Nanometre Scale. Acta Biomater. 2010, 6, 3824–3846. [Google Scholar] [CrossRef]
- Dobbenga, S.; Fratila-Apachitei, L.E.; Zadpoor, A.A. Nanopattern-Induced Osteogenic Differentiation of Stem Cells—A Systematic Review. Acta Biomater. 2016, 46, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Busscher, H.J.; Van Der Mei, H.C.; Subbiahdoss, G.; Jutte, P.C.; Van Den Dungen, J.J.A.M.; Zaat, S.A.J.; Schultz, M.J.; Grainger, D.W. Biomaterial-Associated Infection: Locating the Finish Line in the Race for the Surface. Sci. Transl. Med. 2012, 4, 153rv10. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.T.H.; Truong, V.K.; Orlowska, A.; Ghanaati, S.; Barbeck, M.; Booms, P.; Fulcher, A.J.; Bhadra, C.M.; Buividas, R.; Baulin, V.; et al. Race for the Surface: Eukaryotic Cells Can Win. ACS Appl. Mater. Interfaces 2016, 8, 22025–22031. [Google Scholar] [CrossRef]
- Persat, A. Bacterial Mechanotransduction. Curr. Opin. Microbiol. 2017, 36, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Vorobyev, A.Y.; Guo, C. Femtosecond Laser Blackening of Platinum. J. Appl. Phys. 2008, 104, 053516. [Google Scholar] [CrossRef]
- Ardron, M.; Weston, N.; Hand, D. A Practical Technique for the Generation of Highly Uniform LIPSS. Appl. Surf. Sci. 2014, 313, 123–131. [Google Scholar] [CrossRef]
- Birnbaum, M. Semiconductor Surface Damage Produced by Ruby Lasers. J. Appl. Phys. 1965, 36, 3688–3689. [Google Scholar] [CrossRef]
- Baudach, S.; Bonse, J.; Kautek, W. Ablation Experiments on Polyimide with Femtosecond Laser Pulses. Appl. Phys. A Mater. Sci. Process. 1999, 69, S395–S398. [Google Scholar] [CrossRef]
- Bonse, J.; Sturm, H.; Schmidt, D.; Kautek, W. Chemical, Morphological and Accumulation Phenomena in Ultrashort-Pulse Laser Ablation of TiN in Air. Appl. Phys. A Mater. Sci. Process. 2000, 71, 657–665. [Google Scholar] [CrossRef]
- Simova, E.; Hnatovsky, C.; Taylor, R.S.; Rayner, D.M.; Corkum, P.B. Femtosecond Laser-Induced Long-Range Self-Organized Periodic Planar Nanocracks for Applications in Biophotonics. Photon Process. Microelectron. Photonics VI 2007, 6458, 317–330. [Google Scholar] [CrossRef]
- Shen, M.; Carey, J.E.; Crouch, C.H.; Kandyla, M.; Stone, H.A.; Mazur, E. High-Density Regular Arrays of Nanometer-Scale Rods Formed on Silicon Surfaces via Femtosecond Laser Irradiation in Water. Nano Lett. 2008, 8, 2087–2091. [Google Scholar] [CrossRef] [PubMed]
- Vorobyev, A.Y.; Makin, V.S.; Guo, C. Brighter Light Sources from Black Metal: Significant Increase in Emission Efficiency of Incandescent Light Sources. Phys. Rev. Lett. 2009, 102, 234301. [Google Scholar] [CrossRef]
- Wong, M.; Eulenberger, J.; Schenk, R.; Hunziker, E. Effect of Surface Topology on the Osseointegration of Implant Materials in Trabecular Bone. J. Biomed. Mater. Res. 1995, 29, 1567–1575. [Google Scholar] [CrossRef] [PubMed]
- Gotfredson, K.; Wennerberg, A.; Johansson, C.; Skovgaard, L.T.; Hjørting-Hansen, E. Anchorage of TiO2-Blasted, HA-Coated, and Machined Implants: An Experimental Study with Rabbits. J. Biomed. Mater. Res. 1995, 29, 1223–1231. [Google Scholar] [CrossRef]
- Gottlander, M.; Johansson, C.B.; Wennerberg, A.; Albrektsson, T.; Radin, S.; Ducheyne, P. Bone Tissue Reactions to an Electrophoretically Applied Calcium Phosphate Coating. Biomaterials 1997, 18, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Vercaigne, S.; Wolke, J.G.C.; Naert, I.; Jansen, J.A. Bone Healing Capacity of Titanium Plasma-Sprayed and Hydroxylapatite-Coated Oral Implants. Clin. Oral Implants Res. 1998, 9, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Li, J. Behaviour of Titanium and Titania-Based Ceramics In Vitro and In Vivo. Biomaterials 1993, 14, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Yeo, I.S.; Han, J.S.; Yang, J.H. Biomechanical and Histomorphometric Study of Dental Implants with Different Surface Characteristics. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 87B, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Vercaigne, S.; Wolke, J.G.C.; Naert, I.; Jansen, J.A. A Histological Evaluation of TiO2-Gritblasted and Ca-P Magnetron Sputter Coated Implants Placed into the Trabecular Bone of the Goat: Part 2. Clin. Oral Implants Res. 2000, 11, 314–324. [Google Scholar] [CrossRef]
- Borsari, V.; Fini, M.; Giavaresi, G.; Rimodini, L.; Consolo, U.; Chiusoli, L.; Salito, A.; Volpert, A.; Chiesa, R.; Giardino, R. Osteointegration of Titanium and Hydroxyapatite Rough Surfaces in Healthy and Compromised Cortical and Trabecular Bone: In Vivo Comparative Study on Young, Aged, and Estrogen-Deficient Sheep. J. Orthop. Res. 2007, 25, 1250–1260. [Google Scholar] [CrossRef]
- Savarino, L.; Fini, M.; Ciapetti, G.; Cenni, E.; Granchi, D.; Baldini, N.; Greco, M.; Rizzi, G.; Giardino, R.; Giunti, A. Biologic Effects of Surface Roughness and Fluorhydroxyapatite Coating on Osteointegration in External Fixation Systems: An In Vivo Experimental Study. J. Biomed. Mater. Res. A 2003, 66, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Mendes, V.C.; Moineddin, R.; Davies, J.E. The Effect of Discrete Calcium Phosphate Nanocrystals on Bone-Bonding to Titanium Surfaces. Biomaterials 2007, 28, 4748–4755. [Google Scholar] [CrossRef] [PubMed]
- Meirelles, L.; Currie, F.; Jacobsson, C.M.; Albrektsson, T.; Wennerberg, A. The Effect of Chemical and Nano Topographical Modifications on Early Stage of Osseointegration. Int. J. Oral Maxillofac. Implant. 2008, 23, 641–647. [Google Scholar]
- Schliephake, H.; Aref, A.; Scharnweber, D.; Rößler, S.; Sewing, A. Effect of Modifications of Dual Acid-Etched Implant Surfaces on Periimplant Bone Formation. Part II: Calcium Phosphate Coatings. Clin. Oral Implants Res. 2009, 20, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Wennerberg, A.; Albrektsson, T. Effects of Titanium Surface Topography on Bone Integration: A Systematic Review. Clin. Oral Implants Res. 2009, 20 (Suppl. S4), 172–184. [Google Scholar] [CrossRef]
- Cardoso, G.C.; Grandini, C.R.; Rau, J. V Comprehensive review of PEO coatings on titanium alloys for biomedical implants. J. Mater. Res. Technol. 2024, 31, 311–328. [Google Scholar] [CrossRef]
- Grizon, F.; Aguado, E.; Huré, G.; Baslé, M.F.; Chappard, D. Enhanced Bone Integration of Implants with Increased Surface Roughness: A Long Term Study in the Sheep. J. Dent. 2002, 30, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Becker, W.; Becker, B.E.; Ricci, A.; Bahat, O.; Rosenberg, E.; Rose, L.F.; Handelsman, M.; Israelson, H. A Prospective Multicenter Clinical Trial Comparing One- and Two-Stage Titanium Screw-Shaped Fixtures with One-Stage Plasma-Sprayed Solid-Screw Fixtures. Clin. Implant Dent. Relat. Res. 2000, 2, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Wennerberg, A.; Albrektsson, T.; Andersson, B.; Krol, J.J. A Histomorphometric and Removal Torque Study of Screw-Shaped Titanium Implants with Three Different Surface Topographies. Clin. Oral Implants Res. 1995, 6, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Roi, A.; Ardelean, L.C.; Roi, C.I.; Boia, E.R.; Boia, S.; Rusu, L.C. Oral Bone Tissue Engineering: Advanced Biomaterials for Cell Adhesion, Proliferation and Differentiation. Materials 2019, 12, 2296. [Google Scholar] [CrossRef] [PubMed]
- Cochran, D.L.; Schenk, R.K.; Lussi, A.; Higginbottom, F.L.; Buser, D. Bone Response to Unloaded and Loaded Titanium Implants with a Sandblasted and Acid-Etched Surface: A Histometric Study in the Canine Mandible. J. Biomed. Mater. Res. 1998, 40, 1–11. [Google Scholar] [CrossRef]
- López-valverde, N.; Flores-fraile, J.; Ramírez, J.M.; de Sousa, B.M.; Herrero-hernández, S.; López-valverde, A. Bioactive Surfaces vs. Conventional Surfaces in Titanium Dental Implants: A Comparative Systematic Review. J. Clin. Med. 2020, 9, 2047. [Google Scholar] [CrossRef] [PubMed]
- Beutner, R.; Michael, J.; Schwenzer, B.; Scharnweber, D. Biological nano-functionalization of titanium-based biomaterial surfaces: A flexible toolbox. J. R. Soc. Interface 2009, 7 (Suppl. S1), S93–S105. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Yadav, A.; Gade, A. Silver Nanoparticles as a New Generation of Antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Alla, R.K.; Ginjupalli, K.; Upadhya, N.; Shammas, M.; Ravi, R.K.; Sekhar, R. Surface Roughness of Implants: A Review. Trends Biomater. Artif. Organs 2011, 25, 112–118. [Google Scholar]
- Gittens, R.A.; McLachlan, T.; Olivares-Navarrete, R.; Cai, Y.; Berner, S.; Tannenbaum, R.; Schwartz, Z.; Sandhage, K.H.; Boyan, B.D. The Effects of Combined Micron-/Submicron-Scale Surface Roughness and Nanoscale Features on Cell Proliferation and Differentiation. Biomaterials 2011, 32, 3395–3403. [Google Scholar] [CrossRef]
- Gittens, R.A.; Olivares-Navarrete, R.; McLachlan, T.; Cai, Y.; Hyzy, S.L.; Schneider, J.M.; Schwartz, Z.; Sandhage, K.H.; Boyan, B.D. Differential Responses of Osteoblast Lineage Cells to Nanotopographically-Modified, Microroughened Titanium–Aluminum–Vanadium Alloy Surfaces. Biomaterials 2012, 33, 8986–8994. [Google Scholar] [CrossRef] [PubMed]
- Gittens, R.A.; Olivares-Navarrete, R.; Cheng, A.; Anderson, D.M.; McLachlan, T.; Stephan, I.; Geis-Gerstorfer, J.; Sandhage, K.H.; Fedorov, A.G.; Rupp, F.; et al. The Roles of Titanium Surface Micro/Nanotopography and Wettability on the Differential Response of Human Osteoblast Lineage Cells. Acta Biomater. 2013, 9, 6268–6277. [Google Scholar] [CrossRef]
- Browne, M.; Gregson, P.J. Effect of Mechanical Surface Pretreatment on Metal Ion Release. Biomaterials 2000, 21, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Song, W.W.; Heo, J.H.; Lee, J.H.; Park, Y.M.; Kim, Y.D. Osseointegration of Magnesium-Incorporated Sand-Blasted Acid-Etched Implant in the Dog Mandible: Resonance Frequency Measurements and Histomorphometric Analysis. Tissue Eng. Regen. Med. 2016, 13, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.; Bandyopadhyay, A.; Bose, S. Induction Plasma Sprayed Nano Hydroxyapatite Coatings on Titanium for Orthopaedic and Dental Implants. Surf. Coat. Technol. 2011, 205, 2785–2792. [Google Scholar] [CrossRef]
- Liao, H.; Fartash, B.; Li, J. Stability of Hydroxyapatite-Coatings on Titanium Oral Implants (IMZ). Clin. Oral Implants Res. 1997, 8, 68–72. [Google Scholar] [CrossRef]
- Peltola, T.; Patsi, M.; Rahiala, H.; Kangasniemi, I.; Yli-Urpo, A. Calcium Phosphate Induction by Sol-Gel-Derived Titania Coatings on Titanium Substratesin Vitro. J. Biomed. Mater. Res. 1998, 41, 504–510. [Google Scholar] [CrossRef]
- Mustafa, K.; Wennerberg, A.; Wroblewski, J.; Hultenby, K.; Lopez, B.S.; Arvidson, K. Determining Optimal Surface Roughness of TiO2 Blasted Titanium Implant Material for Attachment, Proliferation and Differentiation of Cells Derived from Human Mandibular Alveolar Bone. Clin. Oral Implants Res. 2001, 12, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Hou, F.; Gorthy, R.; Mardon, I.; Tang, D.; Goode, C. Protecting Light Metal Alloys Using a Sustainable Plasma Electrolytic Oxidation Process. ACS Omega 2022, 7, 8570–8580. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liang, J.; Wang, Q. Plasma Electrolytic Oxidation Coatings on Lightweight Metals. Mod. Surf. Eng. Treat. 2013, 4, 75–99. [Google Scholar] [CrossRef]
- Wu, J.; Wu, L.; Yao, W.; Chen, Y.; Chen, Y.; Yuan, Y.; Wang, J.; Atrens, A.; Pan, F. Effect of Electrolyte Systems on Plasma Electrolytic Oxidation Coatings Characteristics on LPSO Mg-Gd-Y-Zn Alloy. Surf. Coat. Technol. 2023, 454, 129192. [Google Scholar] [CrossRef]
- Oh, G.H.; Yoon, J.K.; Huh, J.Y.; Doh, J.M. Effect of Frequency of Plasma Electrolytic Oxidation on the Microstructure and Corrosion Resistance of 6061 Aluminium Alloy. Surf. Coat. Technol. 2023, 471, 129861. [Google Scholar] [CrossRef]
- Nominé, A.; Nominé, A.V.; Braithwaite, N.S.J.; Belmonte, T.; Henrion, G. High-Frequency-Induced Cathodic Breakdown during Plasma Electrolytic Oxidation. Phys. Rev. Appl. 2017, 8, 031001. [Google Scholar] [CrossRef]
- Dehnavi, V.; Luan, B.L.; Shoesmith, D.W.; Liu, X.Y.; Rohani, S. Effect of Duty Cycle and Applied Current Frequency on Plasma Electrolytic Oxidation (PEO) Coating Growth Behavior. Surf. Coat. Technol. 2013, 226, 100–107. [Google Scholar] [CrossRef]
- Wang, S.; Liu, X.; Yin, X.; Du, N. Influence of Electrolyte Components on the Microstructure and Growth Mechanism of Plasma Electrolytic Oxidation Coatings on 1060 Aluminum Alloy. Surf. Coat. Technol. 2020, 381, 125214. [Google Scholar] [CrossRef]
- Luo, S.; Wang, Q.; Ye, R.; Ramachandran, C.S. Effects of Electrolyte Concentration on the Microstructure and Properties of Plasma Electrolytic Oxidation Coatings on Ti-6Al-4V Alloy. Surf. Coat. Technol. 2019, 375, 864–876. [Google Scholar] [CrossRef]
- Attarzadeh, N.; Ramana, C.V. Plasma Electrolytic Oxidation Ceramic Coatings on Zirconium (Zr) and ZrAlloys: Part I—Growth Mechanisms, Microstructure, and Chemical Composition. Coatings 2021, 11, 634. [Google Scholar] [CrossRef]
- Huang, Z.Q.; Wang, R.Q.; Zhang, H.; Shen, X.J.; Zhang, X.Z.; He, Y.; Huang, C.; Shen, D.J.; Li, D.L. Effect of Nanosized Silicon Dioxide Additive on Plasma Electrolytic Oxidation Coatings Fabricated on Aluminium. Int. J. Electrochem. Sci. 2020, 15, 11191–11202. [Google Scholar] [CrossRef]
- Arrabal, R.; Matykina, E.; Skeldon, P.; Thompson, G.E. Incorporation of Zirconia Particles into Coatings Formed on Magnesium by Plasma Electrolytic Oxidation. J. Mater. Sci. 2008, 43, 1532–1538. [Google Scholar] [CrossRef]
- Savushkina, S.; Gerasimov, M.; Apelfeld, A.; Suminov, I. Study of Coatings Formed on Zirconium Alloy by Plasma Electrolytic Oxidation in Electrolyte with Submicron Yttria Powder Additives. Metals 2021, 11, 1392. [Google Scholar] [CrossRef]
- Li, W.; Zhu, L.; Li, Y. Electrochemical oxidation characteristic of AZ91D magnesium alloy under the action of silica sol. Surf. Coat. Technol. 2006, 201, 1085–1092. [Google Scholar] [CrossRef]
- Li, W.; Zhu, L.; Liu, H. Preparation of Hydrophobic Anodic Film on AZ91D Magnesium Alloy in Silicate Solution Containing Silica Sol. Surf. Coat. Technol. 2006, 201, 2573–2577. [Google Scholar] [CrossRef]
- Tang, M.; Li, W.; Liu, H.; Zhu, L. Influence of Titania Sol in the Electrolyte on Characteristics of the Microarc Oxidation Coating Formed on 2A70 Aluminum Alloy. Surf. Coat. Technol. 2011, 205, 4135–4140. [Google Scholar] [CrossRef]
- Laleh, M.; Rouhaghdam, A.S.; Shahrabi, T.; Shanghi, A. Effect of Alumina Sol Addition to Micro-Arc Oxidation Electrolyte on the Properties of MAO Coatings Formed on Magnesium Alloy AZ91D. J. Alloys Compd. 2010, 496, 548–552. [Google Scholar] [CrossRef]
- Lu, X.; Blawert, C.; Huang, Y.; Ovri, H.; Zheludkevich, M.L.; Kainer, K.U. Plasma Electrolytic Oxidation Coatings on Mg Alloy with Addition of SiO2 Particles. Electrochim. Acta 2016, 187, 20–33. [Google Scholar] [CrossRef]
- Lu, X.; Mohedano, M.; Blawert, C.; Matykina, E.; Arrabal, R.; Kainer, K.U.; Zheludkevich, M.L. Plasma electrolytic oxidation coatings with particle additions—A review. Surf. Coat. Technol. 2016, 307, 1165–1182. [Google Scholar] [CrossRef]
- Lederer, S.; Sankaran, S.; Smith, T.; Fürbeth, W. Formation of Bioactive Hydroxyapatite-Containing Titania Coatings on CP-Ti 4+ Alloy Generated by Plasma Electrolytic Oxidation. Surf. Coat. Technol. 2019, 363, 66–74. [Google Scholar] [CrossRef]
- Arrabal, R.; Matykina, E.; Viejo, F.; Skeldon, P.; Thompson, G.E.; Merino, M.C. AC Plasma Electrolytic Oxidation of Magnesium with Zirconia Nanoparticles. Appl. Surf. Sci. 2008, 254, 6937–6942. [Google Scholar] [CrossRef]
- Gowtham, S.; Hariprasad, S.; Arunnellaiappan, T.; Rameshbabu, N. An Investigation on ZrO2 Nano-Particle Incorporation, Surface Properties and Electrochemical Corrosion Behaviour of PEO Coating Formed on Cp-Ti. Surf. Coat. Technol. 2017, 313, 263–273. [Google Scholar] [CrossRef]
- Rapheal, G.; Kumar, S.; Scharnagl, N.; Blawert, C. Effect of Current Density on the Microstructure and Corrosion Properties of Plasma Electrolytic Oxidation (PEO) Coatings on AM50 Mg Alloy Produced in an Electrolyte Containing Clay Additives. Surf. Coat. Technol. 2016, 289, 150–164. [Google Scholar] [CrossRef]
- Blawert, C.; Sah, S.P.; Liang, J.; Huang, Y.; Höche, D. Role of Sintering and Clay Particle Additions on Coating Formation during PEO Processing of AM50 Magnesium Alloy. Surf. Coat. Technol. 2012, 213, 48–58. [Google Scholar] [CrossRef]
- Polunin, A.V.; Borgardt, E.D.; Shafeev, M.R.; Katsman, A.V.; Krishtal, M.M. Effects of Different Nanoparticles Additions on Composition and Properties of Oxide Layers Formed by Plasma Electrolytic Oxidation on Cast Al-Si Alloy. J. Phys. Conf. Ser. 2020, 1713, 012035. [Google Scholar] [CrossRef]
- Ceriani, F.; Casanova, L.; Massimini, L.; Brenna, A.; Ormellese, M. TiO2 Microparticles Incorporation in Coatings Produced by Plasma Electrolytic Oxidation (PEO) on Titanium. Coatings 2023, 13, 1718. [Google Scholar] [CrossRef]
- Rizwan, M.; Alias, R.; Zaidi, U.Z.; Mahmoodian, R.; Hamdi, M. Surface Modification of Valve Metals Using Plasma Electrolytic Oxidation for Antibacterial Applications: A Review. J. Biomed. Mater. Res. Part A 2018, 106, 590–605. [Google Scholar] [CrossRef] [PubMed]
- Pezzato, L.; Settimi, A.G.; Fanchin, D.; Moschin, E.; Moro, I.; Dabalà, M. Effect of Cu Addition on the Corrosion and Antifouling Properties of PEO Coated Zinc-Aluminized Steel. Materials 2022, 15, 7895. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Lee, B.U.; Yoon, S.I.; Lee, E.S.; Yoo, B.; Shin, D.H. Evaluation of Plasma Temperature during Plasma Oxidation Processing of AZ91 Mg Alloy through Analysis of the Melting Behavior of Incorporated Particles. Electrochim. Acta 2012, 67, 6–11. [Google Scholar] [CrossRef]
- Guo, J.; Wang, L.; Wang, S.C.; Liang, J.; Xue, Q.; Yan, F. Preparation and Performance of a Novel Multifunctional Plasma Electrolytic Oxidation Composite Coating Formed on Magnesium Alloy. J. Mater. Sci. 2009, 44, 1998–2006. [Google Scholar] [CrossRef]
- Balaji, R.; Pushpavanam, M.; Kumar, K.Y.; Subramanian, K. Electrodeposition of Bronze–PTFE Composite Coatings and Study on Their Tribological Characteristics. Surf. Coat. Technol. 2006, 201, 3205–3211. [Google Scholar] [CrossRef]
- Atiyeh, B.S.; Costagliola, M.; Hayek, S.N.; Dibo, S.A. Effect of Silver on Burn Wound Infection Control and Healing: Review of the Literature. Burns 2007, 33, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Burg, K.J.L.; Porter, S.; Kellam, J.F. Biomaterial Developments for Bone Tissue Engineering. Biomaterials 2000, 21, 2347–2359. [Google Scholar] [CrossRef] [PubMed]
- Mu, M.; Liang, J.; Zhou, X.; Xiao, Q. One-Step Preparation of TiO2/MoS2 Composite Coating on Ti6Al4V Alloy by Plasma Electrolytic Oxidation and Its Tribological Properties. Surf. Coat. Technol. 2013, 214, 124–130. [Google Scholar] [CrossRef]
- Hannink, R.H.J. Microstructural Development of Sub-Eutectoid Aged MgO-ZrO2 Alloys. J. Mater. Sci. 1983, 18, 457–470. [Google Scholar] [CrossRef]
- Fukumasa, O.; Tagashira, R.; Tachino, K.; Mukunoki, H. Spraying of MgO Films with a Well-Controlled Plasma Jet. Surf. Coat. Technol. 2003, 169–170, 579–582. [Google Scholar] [CrossRef]
- Galliano, P.; De Damborenea, J.J.; Pascual, M.J.; Durán, A. Sol-Gel Coatings on 316L Steel for Clinical Applications. J. Sol-Gel Sci. Technol. 1998, 13, 723–727. [Google Scholar] [CrossRef]
- Vasconcelos, D.C.L.; Carvalho, J.A.N.; Mantel, M.; Vasconcelos, W.L. Corrosion Resistance of Stainless Steel Coated with Sol–Gel Silica. J. Non. Cryst. Solids 2000, 273, 135–139. [Google Scholar] [CrossRef]
- Wang, D.; Bierwagen, G.P. Sol–Gel Coatings on Metals for Corrosion Protection. Prog. Org. Coat. 2009, 64, 327–338. [Google Scholar] [CrossRef]
- Khazrayie, M.A.; Aghdam, A.R.S. Si3N4/Ni Nanocomposite Formed by Electroplating: Effect of Average Size of Nanoparticulates. Trans. Nonferrous Met. Soc. China 2010, 20, 1017–1023. [Google Scholar] [CrossRef]
- Pepe, A.; Aparicio, M.; Durán, A.; Ceré, S. Cerium Hybrid Silica Coatings on Stainless Steel AISI 304 Substrate. J. Sol-Gel Sci. Technol. 2006, 39, 131–138. [Google Scholar] [CrossRef]
- Schem, M.; Schmidt, T.; Gerwann, J.; Wittmar, M.; Veith, M.; Thompson, G.E.; Molchan, I.S.; Hashimoto, T.; Skeldon, P.; Phani, A.R.; et al. CeO2-Filled Sol–Gel Coatings for Corrosion Protection of AA2024-T3 Aluminium Alloy. Corros. Sci. 2009, 51, 2304–2315. [Google Scholar] [CrossRef]
- Czosnek, C.; Bućko, M.M.; Janik, J.F.; Olejniczak, Z.; Bystrzejewski, M.; Łabędź, O.; Huczko, A. Preparation of Silicon Carbide SiC-Based Nanopowders by the Aerosol-Assisted Synthesis and the DC Thermal Plasma Synthesis Methods. Mater. Res. Bull. 2015, 63, 164–172. [Google Scholar] [CrossRef]
- Bhatt, R.T.; Choi, S.R.; Cosgriff, L.M.; Fox, D.S.; Lee, K.N. Impact Resistance of Environmental Barrier Coated SiC/SiC Composites. Mater. Sci. Eng. A 2008, 476, 8–19. [Google Scholar] [CrossRef]
- Hou, Y.Q.; Zhuang, D.M.; Zhang, G.; Wu, M.S.; Liu, J.J. Tribological Performances of Diamond Film and Graphite/Diamond Composite Film. Wear 2002, 253, 711–719. [Google Scholar] [CrossRef]
- Jones, D.W.; Smith, J.A.S. Hydrogen Bonding in Calcium Orthophosphates. Natute 1962, 195, 1090–1091. [Google Scholar] [CrossRef]
- Soejima, T.; Yagyu, H.; Ito, S. One-Pot Synthesis and Photocatalytic Activity of Fe-Doped TiO2 Films with Anatase-Rutile Nanojunction Prepared by Plasma Electrolytic Oxidation. J. Mater. Sci. 2011, 46, 5378–5384. [Google Scholar] [CrossRef]
- Rudnev, V.S.; Lukiyanchuk, I.V.; Adigamova, M.V.; Morozova, V.P.; Tkachenko, I.A. The effect of nanocrystallites in the pores of PEO coatings on their magnetic properties. Surf. Coat. Technol. 2015, 269, 23–29. [Google Scholar] [CrossRef]
- Hang, R.; Gao, A.; Huang, X.; Wang, X.; Zhang, X.; Qin, L.; Tang, B. Antibacterial Activity and Cytocompatibility of Cu–Ti–O Nanotubes. J. Biomed. Mater. Res. Part A 2014, 102, 1850–1858. [Google Scholar] [CrossRef]
- Vasilyeva, M.S.; Rudnev, V.S.; Korotenko, I.A.; Nedozorov, P.M. Producing and Studying Oxide Coatings Containing Manganese and Nickel Compounds on Titanium from Electrolyte Suspensions. Prot. Met. Phys. Chem. Surf. 2012, 48, 106–115. [Google Scholar] [CrossRef]
- Mohedano, M.; Matykina, E.; Arrabal, R.; Pardo, A.; Merino, M.C. Metal Release from Ceramic Coatings for Dental Implants. Dent. Mater. 2014, 30, e28–e40. [Google Scholar] [CrossRef]
- Matykina, E.; Montuori, M.; Gough, J.; Monfort, F.; Berkani, A.; Skeldon, P.; Thompson, G.E.; Habazaki, H. Spark Anodising of Titanium for Biomedical Applications. Trans. IMF 2006, 84, 125–133. [Google Scholar] [CrossRef]
- Whiteside, P.; Matykina, E.; Gough, J.E.; Skeldon, P.; Thompson, G.E. In Vitro Evaluation of Cell Proliferation and Collagen Synthesis on Titanium Following Plasma Electrolytic Oxidation. J. Biomed. Mater. Res. Part A 2010, 94A, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Mohedano, M.; Guzman, R.; Arrabal, R.; Lõpez Lacomba, J.L.; Matykina, E. Bioactive Plasma Electrolytic Oxidation Coatings—The Role of the Composition, Microstructure, and Electrochemical Stability. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 101, 1524–1537. [Google Scholar] [CrossRef]
- Cimenoglu, H.; Gunyuz, M.; Kose, G.T.; Baydogan, M.; Uǧurlu, F.; Sener, C. Micro-Arc Oxidation of Ti6Al4V and Ti6Al7Nb Alloys for Biomedical Applications. Mater. Charact. 2011, 62, 304–311. [Google Scholar] [CrossRef]
- Li, W.; Tang, M.; Zhu, L.; Liu, H. Formation of Microarc Oxidation Coatings on Magnesium Alloy with Photocatalytic Performance. Appl. Surf. Sci. 2012, 258, 10017–10021. [Google Scholar] [CrossRef]
- Lim, T.S.; Ryu, H.S.; Hong, S.H. Electrochemical Corrosion Properties of CeO2-Containing Coatings on AZ31 Magnesium Alloys Prepared by Plasma Electrolytic Oxidation. Corros. Sci. 2012, 62, 104–111. [Google Scholar] [CrossRef]
- Liang, J.; Hu, L.; Hao, J. Preparation and Characterization of Oxide Films Containing Crystalline TiO2 on Magnesium Alloy by Plasma Electrolytic Oxidation. Electrochim. Acta 2007, 52, 4836–4840. [Google Scholar] [CrossRef]
- Tang, M.; Liu, H.; Li, W.; Zhu, L. Effect of Zirconia Sol in Electrolyte on the Characteristics of Microarc Oxidation Coating on AZ91D Magnesium. Mater. Lett. 2011, 65, 413–415. [Google Scholar] [CrossRef]
- Liu, J.; Lu, Y.; Jing, X.; Yuan, Y.; Zhang, M. Characterization of Plasma Electrolytic Oxidation Coatings Formed on Mg–Li Alloy in an Alkaline Silicate Electrolyte Containing Silica Sol. Mater. Corros. 2009, 60, 865–870. [Google Scholar] [CrossRef]
- Mohedano, M.; Blawert, C.; Zheludkevich, M.L. Silicate-Based Plasma Electrolytic Oxidation (PEO) Coatings with Incorporated CeO2 Particles on AM50 Magnesium Alloy. Mater. Des. 2015, 86, 735–744. [Google Scholar] [CrossRef]
- Mingo, B.; Guo, Y.; Němcová, A.; Gholinia, A.; Mohedano, M.; Sun, M.; Matthews, A.; Yerokhin, A. Incorporation of Halloysite Nanotubes into Forsterite Surface Layer during Plasma Electrolytic Oxidation of AM50 Mg Alloy. Electrochim. Acta 2019, 299, 772–788. [Google Scholar] [CrossRef]
- Seyfoori, A.; Mirdamadi, S.; Seyedraoufi, Z.S.; Khavandi, A.; Aliofkhazraei, M. Synthesis of Biphasic Calcium Phosphate Containing Nanostructured Films by Micro Arc Oxidation on Magnesium Alloy. Mater. Chem. Phys. 2013, 142, 87–94. [Google Scholar] [CrossRef]
- Lu, X.; Blawert, C.; Scharnagl, N.; Kainer, K.U. Influence of Incorporating Si3N4 Particles into the Oxide Layer Produced by Plasma Electrolytic Oxidation on AM50 Mg Alloy on Coating Morphology and Corrosion Properties. J. Magnes. Alloy. 2013, 1, 267–274. [Google Scholar] [CrossRef]
- Zoubi, W.A.; Kamil, M.P.; Ko, Y.G. Synergistic Influence of Inorganic Oxides (ZrO2 and SiO2) with N2H4 to Protect Composite Coatings Obtained via Plasma Electrolyte Oxidation on Mg Alloy. Phys. Chem. Chem. Phys. 2017, 19, 2372–2382. [Google Scholar] [CrossRef]
- Wang, Y.M.; Wang, F.H.; Xu, M.J.; Zhao, B.; Guo, L.X.; Ouyang, J.H. Microstructure and Corrosion Behavior of Coated AZ91 Alloy by Microarc Oxidation for Biomedical Application. Appl. Surf. Sci. 2009, 255, 9124–9131. [Google Scholar] [CrossRef]
- Peng, Z.X.; Wang, L.; Du, L.; Guo, S.R.; Wang, X.Q.; Tang, T.T. Adjustment of the Antibacterial Activity and Biocompatibility of Hydroxypropyltrimethyl Ammonium Chloride Chitosan by Varying the Degree of Substitution of Quaternary Ammonium. Carbohydr. Polym. 2010, 81, 275–283. [Google Scholar] [CrossRef]
- Shishir, R.; Lokeshkumar, E.; Manojkumar, P.; Nasiruddin, U.; Premchand, C.; Ponnilavan, V.; Rameshbabu, N. Development of Biocompatible and Corrosion-Resistant Plasma Electrolytic Oxidation Coating over Zinc for Orthopedic Implant Applications. Surf. Coat. Technol. 2022, 450, 128990. [Google Scholar] [CrossRef]
- Francisca, F.G.S.; Vitoriano, J.D.O.; Alves-Junior, C. Controlling Plasma Electrolytic Oxidation of Titanium Using Current Pulses Compatible with the Duration of Microdischarges. Results Mater. 2022, 15, 100310. [Google Scholar] [CrossRef]
- Gorgin Karaji, Z.; Hedayati, R.; Pouran, B.; Apachitei, I.; Zadpoor, A.A. Effects of Plasma Electrolytic Oxidation Process on the Mechanical Properties of Additively Manufactured Porous Biomaterials. Mater. Sci. Eng. C 2017, 76, 406–416. [Google Scholar] [CrossRef]
- van Hengel, I.A.J.; Tierolf, M.W.A.M.; Fratila-apachitei, L.E.; Apachitei, I.; Zadpoor, A.A. Antibacterial Titanium Implants Biofunctionalized by Plasma Electrolytic Oxidation with Silver, Zinc, and Copper: A Systematic Review. Int. J. Mol. Sci. 2021, 22, 3800. [Google Scholar] [CrossRef]
- Hou, F.; Gorthy, R.; Mardon, I.; Tang, D.; Goode, C. Low Voltage Environmentally Friendly Plasma Electrolytic Oxidation Process for Titanium Alloys. Sci. Rep. 2022, 12, 6037. [Google Scholar] [CrossRef]
- Polizzi, G.; Gualini, F.; Friberg, B. A Two-Center Retrospective Analysis of Long-Term Clinical and Radiologic Data of TiUnite and Turned Implants Placed in the Same Mouth. Int. J. Prosthodont. 2013, 26, 350–358. [Google Scholar] [CrossRef][Green Version]
- Szymonowicz, M.; Kazek-Kęsik, A.; Sowa, M.; Żywicka, B.; Rybak, Z.; Simka, W. On Influence of Anodic Oxidation on Thrombogenicity and Bioactivity of the Ti-13Nb- 13Zr Alloy. Acta Bioeng. Biomech. 2017, 19, 41–50. [Google Scholar]
- Imburgia, M.; Fabbro, M. Del Long-Term Retrospective Clinical and Radiographic Follow-up of 205 Brånemark System Mk III TiUnite Implants Submitted to Either Immediate or Delayed Loading. Implant Dent. 2015, 24, 533–540. [Google Scholar] [CrossRef]
- Sowa, M.; Simka, W. Effect of DC Plasma Electrolytic Oxidation on Surface Characteristics and Corrosion Resistance of Zirconium. Materials 2018, 11, 723. [Google Scholar] [CrossRef]
- Duarte, L.T.; Biaggio, S.R.; Rocha-Filho, R.C.; Bocchi, N. Preparation and Characterization of Biomimetically and Electrochemically Deposited Hydroxyapatite Coatings on Micro-Arc Oxidized Ti-13Nb-13Zr. J. Mater. Sci. Mater. Med. 2011, 22, 1663–1670. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Y.S.; Zeng, X.T.; Khor, K.A.; Weng, W.; Sun, D.E. Evaluation of Adhesion Strength and Toughness of Fluoridated Hydroxyapatite Coatings. Thin Solid Films 2008, 516, 5162–5167. [Google Scholar] [CrossRef]
- Lugovskoy, A.; Lugovskoy, S. Production of Hydroxyapatite Layers on the Plasma Electrolytically Oxidized Surface of Titanium Alloys. Mater. Sci. Eng. C 2014, 43, 527–532. [Google Scholar] [CrossRef]
- Santos-Coquillat, A.; Mohedano, M.; Martinez-Campos, E.; Arrabal, R.; Pardo, A.; Matykina, E. Bioactive Multi-Elemental PEO-Coatings on Titanium for Dental Implant Applications. Mater. Sci. Eng. C 2019, 97, 738–752. [Google Scholar] [CrossRef]
- Zhou, K.; Xie, F.; Wu, X.; Wang, S. Fabrication of High Temperature Oxidation Resistance Nanocomposite Coatings on PEO Treated TC21 Alloy. Materials 2019, 13, 11. [Google Scholar] [CrossRef]
- Sandhyarani, M.; Ashfaq, M.; Arunnellaiappan, T.; Selvan, M.P.; Subramanian, S.; Rameshbabu, N. Effect of Electrical Parameters on Morphology and In-Vitro Corrosion Resistance of Plasma Electrolytic Oxidized Films Formed on Zirconium. Surf. Coat. Technol. 2015, 269, 286–294. [Google Scholar] [CrossRef]
- Nikoomanzari, E.; Fattah-alhosseini, A.; Pajohi Alamoti, M.R.; Keshavarz, M.K. Effect of ZrO2 Nanoparticles Addition to PEO Coatings on Ti–6Al–4V Substrate: Microstructural Analysis, Corrosion Behavior and Antibacterial Effect of Coatings in Hank’s Physiological Solution. Ceram. Int. 2020, 46, 13114–13124. [Google Scholar] [CrossRef]
- Junker, R.; Manders, P.J.D.; Wolke, J.; Borisov, Y.; Braceras, I.; Jansen, J.A. Bone Reaction Adjacent to Microplasma-Sprayed Calcium Phosphate-Coated Oral Implants Subjected to an Occlusal Load, an Experimental Study in the Dog. Clin. Oral Implants Res. 2011, 22, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, A.; Kossenko, A.; Zinigrad, M.; Gofer, Y.; Borodianskiy, K.; Sobolev, A. Hydroxyapatite Coating on Ti-6Al-7Nb Alloy by Plasma Electrolytic Oxidation in Salt-Based Electrolyte. Materials 2022, 15, 7374. [Google Scholar] [CrossRef] [PubMed]
- Momesso, G.A.C.; de Souza Santos, A.M.; Fonseca e Santos, J.M.; da Cruz, N.C.; Okamoto, R.; Ricardo Barão, V.A.; Siroma, R.S.; Shibli, J.A.; Faverani, L.P. Comparison between Plasma Electrolytic Oxidation Coating and Sandblasted Acid-Etched Surface Treatment: Histometric, Tomographic, and Expression Levels of Osteoclastogenic Factors in Osteoporotic Rats. Materials 2020, 13, 1604. [Google Scholar] [CrossRef] [PubMed]
- Ishizawa, H.; Ogino, M. Formation and Characterization of Anodic Titanium Oxide Films Containing Ca and P. J. Biomed. Mater. Res. 1995, 29, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Blawert, C.; Serdechnova, M.; Karlova, P.; Dovzhenko, G.; Florian Wieland, D.C.; Stojadinovic, S.; Vasilic, R.; Mojsilovic, K.; Zheludkevich, M.L. Formation of Plasma Electrolytic Oxidation Coatings on Pure Niobium in Different Electrolytes. Appl. Surf. Sci. 2022, 573, 151629. [Google Scholar] [CrossRef]
- Stevens, H.Y.; Meays, D.R.; Yeh, J.; Bjursten, L.M.; Frangos, J.A. COX-2 Is Necessary for Venous Ligation-Mediated Bone Adaptation in Mice. Bone 2006, 38, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zheng, W.; Li, X.; Li, A.; Ye, N.; Zhang, L.; Liu, Y.; Liu, X.; Zhang, R.; Wang, M.; et al. Investigating the Effect of Ti3C2 (MXene) Nanosheet on Human Umbilical Vein Endothelial Cells via a Combined Untargeted and Targeted Metabolomics Approach. Carbon 2021, 178, 810–821. [Google Scholar] [CrossRef]
- Liu, Q.; Meng, Q.; Guo, S.; Zhao, X. A′ Type Ti–Nb–Zr Alloys with Ultra-Low Young’s Modulus and High Strength. Prog. Nat. Sci. Mater. Int. 2013, 23, 562–565. [Google Scholar] [CrossRef]
- Nie, L.; Zhan, Y.; Liu, H.; Tang, C. Novel β-Type Zr–Mo–Ti Alloys for Biological Hard Tissue Replacements. Mater. Des. 2014, 53, 8–12. [Google Scholar] [CrossRef]
- Shin, K.R.; Ko, Y.G.; Shin, D.H. Surface Characteristics of ZrO2-Containing Oxide Layer in Titanium by Plasma Electrolytic Oxidation in K4P2O7 Electrolyte. J. Alloys Compd. 2012, 536, S226–S230. [Google Scholar] [CrossRef]
- Komasa, S.; Nishizaki, M.; Zhang, H.; Takao, S.; Yin, D.; Terada, C.; Kobayashi, Y.; Kusumoto, T.; Yoshimine, S.; Nishizaki, H.; et al. Osseointegration of Alkali-Modified NANOZR Implants: An In Vivo Study. Int. J. Mol. Sci. 2019, 20, 842. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, Z.; Li, C.; Yin, K.; Hao, D.; Lan, J. Application of Plasma-Sprayed Zirconia Coating in Dental Implants: Study in Implants. J. Oral Implantol. 2018, 53, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Gomez Sanchez, A.; Ballarre, J.; Orellano, J.C.; Duffó, G.; Ceré, S. Surface Modification of Zirconium by Anodisation as Material for Permanent Implants: In Vitro and In Vivo Study. J. Mater. Sci. Mater. Med. 2013, 24, 81. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kang, I.G.; Cheon, K.H.; Lee, S.; Park, S.; Kim, H.E.; Han, C.M. Stable Sol-Gel Hydroxyapatite Coating on Zirconia Dental Implant for Improved Osseointegration. J. Mater. Sci. Mater. Med. 2021, 32, 161–169. [Google Scholar] [CrossRef]
- Attarzadeh, N.; Ramana, C.V. Plasma Electrolytic Oxidation Ceramic Coatings on Zirconium (Zr) and Zr-Alloys: Part-II: Properties and Applications. Coatings 2021, 11, 620. [Google Scholar] [CrossRef]
- Peng, F.; Wang, D.; Tian, Y.; Cao, H.; Qiao, Y.; Liu, X. Sealing the Pores of PEO Coating with Mg-Al Layered Double Hydroxide: Enhanced Corrosion Resistance, Cytocompatibility and Drug Delivery Ability. Sci. Rep. 2017, 7, 620. [Google Scholar] [CrossRef] [PubMed]
- Katić, J.; Šarić, A.; Despotović, I.; Matijaković, N.; Petković, M.; Petrović, Ž. Bioactive Coating on Titanium Dental Implants for Improved Anticorrosion Protection: A Combined Experimental and Theoretical Study. Coatings 2019, 9, 8167. [Google Scholar] [CrossRef]
- Bai, L.; Du, Z.; Du, J.; Yao, W.; Zhang, J.; Weng, Z.; Liu, S.; Zhao, Y.; Liu, Y.; Zhang, X.; et al. A Multifaceted Coating on Titanium Dictates Osteoimmunomodulation and Osteo/Angio-Genesis towards Ameliorative Osseointegration. Biomaterials 2018, 162, 154–169. [Google Scholar] [CrossRef]
- Ha, S.W.; Jang, H.L.; Nam, K.T.; Beck, G.R. Nano-Hydroxyapatite Modulates Osteoblast Lineage Commitment by Stimulation of DNA Methylation and Regulation of Gene Expression. Biomaterials 2015, 65, 32. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.H.; Hong, S.J.; Kim, C.H.; Kim, E.C.; Jang, J.H.; Shin, H.I.; Kim, H.W. Preparation of Hydroxyapatite Spheres with an Internal Cavity as a Scaffold for Hard Tissue Regeneration. J. Mater. Sci. Mater. Med. 2008, 19, 3029–3034. [Google Scholar] [CrossRef] [PubMed]
- Yeung, W.K.; Reilly, G.C.; Matthews, A.; Yerokhin, A. In Vitro Biological Response of Plasma Electrolytically Oxidized and Plasma-Sprayed Hydroxyapatite Coatings on Ti-6Al-4V Alloy. J. Biomed. Mater. Res. B. Appl. Biomater. 2013, 101, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Thomas, K.A. Hydroxyapatite Coatings. Orthopedics 1994, 17, 267–278. [Google Scholar] [CrossRef]
- Yazdani, J.; Ahmadian, E.; Sharifi, S.; Shahi, S.; Maleki Dizaj, S. A Short View on Nanohydroxyapatite as Coating of Dental Implants. Biomed. Pharmacother. 2018, 105, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Harun, W.S.W.; Asri, R.I.M.; Sulong, A.B.; Ghani, S.A.C.; Ghazalli, Z.; Harun, W.S.W.; Asri, R.I.M.; Sulong, A.B.; Ghani, S.A.C.; Ghazalli, Z. Hydroxyapatite-Based Coating on Biomedical Implant. Hydroxyapatite Adv. Compos. Nanomater. Biomed. Appl. Technol. Facets 2018. [Google Scholar] [CrossRef]
- Takahashi, N.; Maeda, K.; Ishihara, A.; Uehara, S.; Kobayashi, Y. Regulatory Mechanism of Osteoclastogenesis by RANKL and Wnt Signals. Front. Biosci. (Landmark Ed.) 2011, 16, 21–30. [Google Scholar] [CrossRef]
- Cao, J.; Lian, R.; Jiang, X. Magnesium and Fluoride Doped Hydroxyapatite Coatings Grown by Pulsed Laser Deposition for Promoting Titanium Implant Cytocompatibility. Appl. Surf. Sci. 2020, 515, 146069. [Google Scholar] [CrossRef]
- Zhao, Q.M.; Li, X.; Guo, S.; Wang, N.; Liu, W.W.; Shi, L.; Guo, Z. Osteogenic Activity of a Titanium Surface Modified with Silicon-Doped Titanium Dioxide. Mater. Sci. Eng. C 2020, 110, 110682. [Google Scholar] [CrossRef] [PubMed]
- Michalska, J.; Sowa, M.; Piotrowska, M.; Widziołek, M.; Tylko, G.; Dercz, G.; Socha, R.P.; Osyczka, A.M.; Simka, W. Incorporation of Ca Ions into Anodic Oxide Coatings on the Ti-13Nb-13Zr Alloy by Plasma Electrolytic Oxidation. Mater. Sci. Eng. C 2019, 104, 109957. [Google Scholar] [CrossRef] [PubMed]
- van Oirschot, B.A.J.A.; Bronkhorst, E.M.; van den Beucken, J.J.J.P.; Meijer, G.J.; Jansen, J.A.; Junker, R. Long-Term Survival of Calcium Phosphate-Coated Dental Implants: A Meta-Analytical Approach to the Clinical Literature. Clin. Oral Implants Res. 2013, 24, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Sandhyarani, M.; Rameshbabu, N.; Venkateswarlu, K.; Rama Krishna, L. Fabrication, Characterization and in-Vitro Evaluation of Nanostructured Zirconia/Hydroxyapatite Composite Film on Zirconium. Surf. Coat. Technol. 2014, 238, 58–67. [Google Scholar] [CrossRef]
- Abbasi, S.; Golestani-Fard, F.; Rezaie, H.R.; Mirhosseini, S.M.M. MAO-Derived Hydroxyapatite/TiO2 Nanostructured Multi-Layer Coatings on Titanium Substrate. Appl. Surf. Sci. 2012, 261, 37–42. [Google Scholar] [CrossRef]
- Durdu, S.; Bayramoglu, S.; Demirtaş, A.; Usta, M.; Üçşk, A.H. Characterization of AZ31 Mg Alloy Coated by Plasma Electrolytic Oxidation. Vacuum 2013, 88, 130–133. [Google Scholar] [CrossRef]
- Shi, W.; Dong, L.; Li, Q.; Wang, C.; Liang, T.; Tian, J. One-Step Approach for the Fabrication and Characterization of Hydroxyapatite/TiO2 Composite Ceramic Coatings by Micro-Arc Oxidation in Situ on the Surface of Pure Titanium. Key Eng. Mater. 2013, 602–603, 598–601. [Google Scholar] [CrossRef]
- Khan, R.H.U.; Yerokhin, A.L.; Li, X.; Dong, H.; Matthews, A. Influence of Current Density and Electrolyte Concentration on DC PEO Titania Coatings. Surf. Eng. 2014, 30, 102–108. [Google Scholar] [CrossRef]
- Aliasghari, S.; Skeleton, P.; Thompson, G.E. Plasma Electrolytic Oxidation of Titanium in a Phosphate/Silicate Electrolyte and Tribological Performance of the Coatings. Appl. Surf. Sci. 2014, 316, 463–476. [Google Scholar] [CrossRef]
- Durdu, S.; Deniz, Ö.F.; Kutbay, I.; Usta, M. Characterization and Formation of Hydroxyapatite on Ti6Al4V Coated by Plasma Electrolytic Oxidation. J. Alloys Compd. 2013, 551, 422–429. [Google Scholar] [CrossRef]
- Kim, M.S.; Ryu, J.J.; Sung, Y.M. One-Step Approach for Nano-Crystalline Hydroxyapatite Coating on Titanium via Micro-Arc Oxidation. Electrochem. Commun. 2007, 9, 1886–1891. [Google Scholar] [CrossRef]
- Langstaff, S.; Sayer, M.; Smith, T.J.N.; Pugh, S.M. Resorbable Bioceramics Based on Stabilized Calcium Phosphates. Part II: Evaluation of Biological Response. Biomaterials 2001, 22, 135–150. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.H.; Lee, D.H.; Kim, K.M.; Lee, Y.K. Study on Bioactivity and Bonding Strength between Ti Alloy Substrate and TiO2 Film by Micro-Arc Oxidation. Thin Solid Films 2011, 519, 7065–7070. [Google Scholar] [CrossRef]
- Jaspard-Mécuson, F.; Czerwiec, T.; Henrion, G.; Belmonte, T.; Dujardin, L.; Viola, A.; Beauvir, J. Tailored Aluminium Oxide Layers by Bipolar Current Adjustment in the Plasma Electrolytic Oxidation (PEO) Process. Surf. Coat. Technol. 2007, 201, 8677–8682. [Google Scholar] [CrossRef]
- Kaluđerović, M.R.; Schreckenbach, J.P.; Graf, H.L. Titanium Dental Implant Surfaces Obtained by Anodic Spark Deposition—From the Past to the Future. Mater. Sci. Eng. C 2016, 69, 1429–1441. [Google Scholar] [CrossRef]
- Huang, T.; Wang, H.; Zhang, Z.; Feng, K.; Xiang, L. Incorporation of Inorganic Elements onto Titanium-Based Implant Surfaces by One-Step Plasma Electrolytic Oxidation: An Efficient Method to Enhance Osteogenesis. Biomater. Sci. 2022, 10, 6656–6674. [Google Scholar] [CrossRef] [PubMed]
- Kaluderović, M.R.; Schreckenbach, J.P.; Graf, H.L. Plasma-Electrochemical Deposition of Porous Zirconia on Titanium-Based Dental Material and in Vitro Interactions with Primary Osteoblasts Cells. J. Biomater. Appl. 2016, 30, 711–721. [Google Scholar] [CrossRef]
- Schreckenbach, J.; Schlottig, F.; Marx, G.; Kriven, W.M.; Popoola, O.O.; Jilavi, M.H.; Brown, S.D. Preparation and Microstructure Characterization of Anodic Spark Deposited Barium Titanate Conversion Layers. J. Mater. Res. 1999, 14, 1437–1443. [Google Scholar] [CrossRef]
- Vizureanu, P.; Perju, M.-C.; Achiţei, D.-C.; Nejneru, C. Advanced Electro-Spark Deposition Process on Metallic Alloys. Adv. Surf. Eng. Res. 2018, 25, 45–68. [Google Scholar] [CrossRef]
- Wirtz, G.P.; Brown, S.D.; Kriven, W.M. Ceramic Coatings by Anodic Spark Deposition. Mater. Manuf. Process 1991, 6, 87–115. [Google Scholar] [CrossRef]
- Schreckenbach, J.P.; Rabending, K.; Turtle, T. Demonstration of the Plasma State. J. Chem. Educ. 1996, 73, 782. [Google Scholar] [CrossRef]
- Maxian, S.H.; Zawadsky, J.P.; Dunn, M.G. Effect of Ca/P Coating Resorption and Surgical Fit on the Bone/Implant Interface. J. Biomed. Mater. Res. 1994, 28, 1311–1319. [Google Scholar] [CrossRef] [PubMed]
- Schreckenbach, J.P.; Marx, G.; Schlottig, F.; Textor, M.; Spencer, N.D. Characterization of Anodic Spark-Converted Titanium Surfaces for Biomedical Applications. J. Mater. Sci. Mater. Med. 1999, 10, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Sul, Y.T.; Johansson, C.B.; Jeong, Y.; Albrektsson, T. The Electrochemical Oxide Growth Behaviour on Titanium in Acid and Alkaline Electrolytes. Med. Eng. Phys. 2001, 23, 329–346. [Google Scholar] [CrossRef]
- Sul, Y.T.; Johansson, C.B.; Petronis, S.; Krozer, A.; Jeong, Y.; Wennerberg, A.; Albrektsson, T. Characteristics of the Surface Oxides on Turned and Electrochemically Oxidized Pure Titanium Implants up to Dielectric Breakdown: The Oxide Thickness, Micropore Configurations, Surface Roughness, Crystal Structure and Chemical Composition. Biomaterials 2002, 23, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Frauchiger, V.M.; Schlottig, F.; Gasser, B.; Textor, M. Anodic Plasma-Chemical Treatment of CP Titanium Surfaces for Biomedical Applications. Biomaterials 2004, 25, 593–606. [Google Scholar] [CrossRef] [PubMed]
- Graf, W.L.; Stromberg, J.; Valentine, B. Rivers, Dams, and Willow Flycatchers: A Summary of Their Science and Policy Connections. Geomorphology 2002, 47, 169–188. [Google Scholar] [CrossRef]
- Ishizawa, H.; Fujino, M.; Ogino, M. Mechanical and Histological Investigation of Hydrothermally Treated and Untreated Anodic Titanium Oxide Films Containing Ca and P. J. Biomed. Mater. Res. 1995, 29, 1459–1468. [Google Scholar] [CrossRef]
- Sul, Y.T. The Significance of the Surface Properties of Oxidized Titanium to the Bone Response: Special Emphasis on Potential Biochemical Bonding of Oxidized Titanium Implant. Biomaterials 2003, 24, 3893–3907. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, T.S.N.S.; Lee, M.H. A Simple Strategy to Modify the Porous Structure of Plasma Electrolytic Oxidation Coatings on Magnesium. RSC Adv. 2016, 6, 16100–16114. [Google Scholar] [CrossRef]
- Leknes, K.N.; Yang, J.; Qahash, M.; Polimeni, G.; Susin, C.; Wikesjö, U.M.E. Alveolar Ridge Augmentation Using Implants Coated with Recombinant Human Bone Morphogenetic Protein-2: Radiographic Observations. Clin. Oral Implants Res. 2008, 19, 1027–1033. [Google Scholar] [CrossRef] [PubMed]
- Balasundaram, G.; Yao, C.; Webster, T.J. TiO2 Nanotubes Functionalized with Regions of Bone Morphogenetic Protein-2 Increases Osteoblast Adhesion. J. Biomed. Mater. Res. Part A 2008, 84A, 447–453. [Google Scholar] [CrossRef]
- Hilbig, H.; Kirsten, M.; Rupietta, R.; Graf, H.L.; Thalhammer, S.; Strasser, S.; Armbruster, F.P. Implant Surface Coatings with Bone Sialoprotein, Collagen, and Fibronectin and Their Effects on Cells Derived from Human Maxillar Bone. Eur. J. Med. Res. 2007, 12, 6–12. [Google Scholar]
- Graf, H.L.; Stoeva, S.; Armbruster, F.P.; Neuhaus, J.; Hilbig, H. Effect of Bone Sialoprotein and Collagen Coating on Cell Attachment to TICER and Pure Titanium Implant Surfaces. Int. J. Oral Maxillofac. Surg. 2008, 37, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Brammer, K.S.; Frandsen, C.J.; Jin, S. TiO2 Nanotubes for Bone Regeneration. Trends Biotechnol. 2012, 30, 315–322. [Google Scholar] [CrossRef]
- Zwilling, V.; Aucouturier, M.; Darque-Ceretti, E. Anodic Oxidation of Titanium and TA6V Alloy in Chromic Media. An Electrochemical Approach. Electrochim. Acta 1999, 45, 921–929. [Google Scholar] [CrossRef]
- Ghicov, A.; Schmuki, P. Self-Ordering Electrochemistry: A Review on Growth and Functionality of TiO2 nanotubes and Other Self-Aligned MOx Structures. Chem. Commun. 2009, 2791–2808. [Google Scholar] [CrossRef]
- Rani, S.; Roy, S.C.; Paulose, M.; Varghese, O.K.; Mor, G.K.; Kim, S.; Yoriya, S.; Latempa, T.J.; Grimes, C.A. Synthesis and Applications of Electrochemically Self-Assembled Titania Nanotube Arrays. Phys. Chem. Chem. Phys. 2010, 12, 2780–2800. [Google Scholar] [CrossRef] [PubMed]
- Von Wilmowsky, C.; Bauer, S.; Lutz, R.; Meisel, M.; Neukam, F.W.; Toyoshima, T.; Schmuki, P.; Nkenke, E.; Schlegel, K.A. In Vivo Evaluation of Anodic TiO2 Nanotubes: An Experimental Study in the Pig. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 89, 165–171. [Google Scholar] [CrossRef]
- Hu, Y.; Cai, K.; Luo, Z.; Xu, D.; Xie, D.; Huang, Y.; Yang, W.; Liu, P. TiO2 Nanotubes as Drug Nanoreservoirs for the Regulation of Mobility and Differentiation of Mesenchymal Stem Cells. Acta Biomater. 2012, 8, 439–448. [Google Scholar] [CrossRef]
- Wang, L.N.; Jin, M.; Zheng, Y.; Guan, Y.; Lu, X.; Luo, J.L. Nanotubular Surface Modification of Metallic Implants via Electrochemical Anodization Technique. Int. J. Nanomed. 2014, 9, 4421–4435. [Google Scholar] [CrossRef] [PubMed]
- Minagar, S.; Berndt, C.C.; Wang, J.; Ivanova, E.; Wen, C. A Review of the Application of Anodization for the Fabrication of Nanotubes on Metal Implant Surfaces. Acta Biomater. 2012, 8, 2875–2888. [Google Scholar] [CrossRef] [PubMed]
- Soumetz, F.C.; Pastorino, L.; Ruggiero, C. Human Osteoblast-like Cells Response to Nanofunctionalized Surfaces for Tissue Engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 84B, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Sundelacruz, S.; Kaplan, D.L. Stem Cell- and Scaffold-Based Tissue Engineering Approaches to Osteochondral Regenerative Medicine. Semin. Cell Dev. Biol. 2009, 20, 646–655. [Google Scholar] [CrossRef]
- El-wassefy, N.A.; Hammouda, I.M.; Habib, A.N.E.D.A.; El-awady, G.Y.; Marzook, H.A. Assessment of Anodized Titanium Implants Bioactivity. Clin. Oral Implants Res. 2014, 25, e1–e9. [Google Scholar] [CrossRef]
- Josset, Y.; Oum’hamed, Z.; Zarrinpour, A.; Lorenzato, M.; Adnet, J.J.; Laurent-Maquin, D. In Vitro Reactions of Human Osteoblasts in Culture with Zirconia and Alumina Ceramics. J. Biomed. Mater. Res. 1999, 47, 481–493. [Google Scholar] [CrossRef]
- Kaluderović, M.R.; Krajnović, T.; Maksimović-Ivanić, D.; Graf, H.L.; Mijatović, S. Ti-SLActive and TiZr-SLActive Dental Implant Surfaces Promote Fast Osteoblast Differentiation. Coatings 2017, 7, 102. [Google Scholar] [CrossRef]
- Kalucrossed, D.; Signerović, M.R.; Schreckenbach, J.P.; Graf, H.L. Zirconia Coated Titanium for Implants and Their Interactions with Osteoblast Cells. Mater. Sci. Eng. C 2014, 44, 254–261. [Google Scholar] [CrossRef]
- Ahmadi, S.M.; Campoli, G.; Amin Yavari, S.; Sajadi, B.; Wauthle, R.; Schrooten, J.; Weinans, H.; Zadpoor, A.A. Mechanical Behavior of Regular Open-Cell Porous Biomaterials Made of Diamond Lattice Unit Cells. J. Mech. Behav. Biomed. Mater. 2014, 34, 106–115. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D Biomaterial Scaffolds and Osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Moroni, L.; De Wijn, J.R.; Van Blitterswijk, C.A. 3D Fiber-Deposited Scaffolds for Tissue Engineering: Influence of Pores Geometry and Architecture on Dynamic Mechanical Properties. Biomaterials 2006, 27, 974–985. [Google Scholar] [CrossRef]
- Apachitei, I.; Leoni, A.; Riemslag, A.C.; Fratila-Apachitei, L.E.; Duszczyk, J. Enhanced Fatigue Performance of Porous Coated Ti6Al4V Biomedical Alloy. Appl. Surf. Sci. 2011, 257, 6941–6944. [Google Scholar] [CrossRef]
- Hedayati, R.; Sadighi, M.; Mohammadi-Aghdam, M.; Zadpoor, A.A. Effect of Mass Multiple Counting on the Elastic Properties of Open-Cell Regular Porous Biomaterials. Mater. Des. 2016, 89, 9–20. [Google Scholar] [CrossRef]
- Leoni, A.; Apachitei, I.; Riemslag, A.C.; Fratila-Apachitei, L.E.; Duszczyk, J. In Vitro Fatigue Behavior of Surface Oxidized Ti35Zr10Nb Biomedical Alloy. Mater. Sci. Eng. C 2011, 31, 1779–1783. [Google Scholar] [CrossRef]
- Amirkhani, S.; Bagheri, R.; Zehtab Yazdi, A. Effect of Pore Geometry and Loading Direction on Deformation Mechanism of Rapid Prototyped Scaffolds. Acta Mater. 2012, 60, 2778–2789. [Google Scholar] [CrossRef]
- Kadkhodapour, J.; Montazerian, H.; Darabi, A.C.; Anaraki, A.P.; Ahmadi, S.M.; Zadpoor, A.A.; Schmauder, S. Failure Mechanisms of Additively Manufactured Porous Biomaterials: Effects of Porosity and Type of Unit Cell. J. Mech. Behav. Biomed. Mater. 2015, 50, 180–191. [Google Scholar] [CrossRef]
- Apachitei, I.; Lonyuk, B.; Fratila-Apachitei, L.E.; Zhou, J.; Duszczyk, J. Fatigue Response of Porous Coated Titanium Biomedical Alloys. Scr. Mater. 2009, 61, 113–116. [Google Scholar] [CrossRef]
- Lonyuk, B.; Apachitei, I.; Duszczyk, J. The Effect of Oxide Coatings on Fatigue Properties of 7475-T6 Aluminium Alloy. Surf. Coat. Technol. 2007, 201, 8688–8694. [Google Scholar] [CrossRef]
- Hussein, R.O.; Nie, X.; Northwood, D.O. An Investigation of Ceramic Coating Growth Mechanisms in Plasma Electrolytic Oxidation (PEO) Processing. Electrochim. Acta 2013, 112, 111–119. [Google Scholar] [CrossRef]
- Song, W.H.; Jun, Y.K.; Han, Y.; Hong, S.H. Biomimetic Apatite Coatings on Micro-Arc Oxidized Titania. Biomaterials 2004, 25, 3341–3349. [Google Scholar] [CrossRef]
- Sun, J.; Han, Y.; Huang, X. Hydroxyapatite Coatings Prepared by Micro-Arc Oxidation in Ca- and P-Containing Electrolyte. Surf. Coat. Technol. 2007, 201, 5655–5658. [Google Scholar] [CrossRef]
- Montazeri, M.; Dehghanian, C.; Shokouhfar, M.; Baradaran, A. Investigation of the Voltage and Time Effects on the Formation of Hydroxyapatite-Containing Titania Prepared by Plasma Electrolytic Oxidation on Ti–6Al–4V Alloy and Its Corrosion Behavior. Appl. Surf. Sci. 2011, 257, 7268–7275. [Google Scholar] [CrossRef]
- Shokouhfar, M.; Dehghanian, C.; Montazeri, M.; Baradaran, A. Preparation of Ceramic Coating on Ti Substrate by Plasma Electrolytic Oxidation in Different Electrolytes and Evaluation of Its Corrosion Resistance: Part II. Appl. Surf. Sci. 2012, 258, 2416–2423. [Google Scholar] [CrossRef]
- Abbasi, S.; Golestani-Fard, F.; Mirhosseini, S.M.M.; Ziaee, A.; Mehrjoo, M. Effect of Electrolyte Concentration on Microstructure and Properties of Micro Arc Oxidized Hydroxyapatite/Titania Nanostructured Composite. Mater. Sci. Eng. C 2013, 33, 2555–2561. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.C.; Liu, P.; Chen, Y.; Li, W.; Liu, X.K.; Chen, X.H.; He, D.H. Various Morphologies Hydroxyapatite Crystals on Ti MAO Film Prepared by Hydrothermal Treatment. Phys. Procedia 2013, 50, 442–448. [Google Scholar] [CrossRef]
- Fattah-alhosseini, A.; Molaei, M. A review of functionalizing plasma electrolytic oxidation (PEO) coatings on titanium substrates with laser surface treatments. Appl. Surf. Sci. Adv. 2023, 18, 100506. [Google Scholar] [CrossRef]
- Cheng, Y.; Matykina, E.; Skeldon, P.; Thompson, G. Characterization of Plasma Electrolytic Oxidation Coatings on Zircaloy-4 Formed in Different Electrolytes with AC Current Regime. Electrochim. Acta 2011, 56, 8467–8476. [Google Scholar] [CrossRef]
- Cengiz, S.; Gencer, Y. The characterization of the oxide based coating synthesized on pure zirconium by plasma electrolytic oxidation. Surf. Coat. Technol. 2014, 242, 132–140. [Google Scholar] [CrossRef]
- Matykina, E.; Arrabal, R.; Skeldon, P.; Thompson, G.E.; Wang, P.; Wood, P. Plasma Electrolytic Oxidation of a Zirconium Alloy under AC Conditions. Surf. Coat. Technol. 2010, 204, 2142–2151. [Google Scholar] [CrossRef]
- Tian, Z.; Zhang, Y.; Wang, L.; Nan, K. Apatite-Forming Ability of Pure Titanium Implant after Micro-Arc Oxidation Treatment. Nan Fang Yi Ke Xue Xue Bao 2013, 33, 1554–1556. [Google Scholar]
- Faghihi-Sani, M.A.; Arbabi, A.; Mehdinezhad-Roshan, A. Crystallization of Hydroxyapatite during Hydrothermal Treatment on Amorphous Calcium Phosphate Layer Coated by PEO Technique. Ceram. Int. 2013, 39, 1793–1798. [Google Scholar] [CrossRef]
- Okido, M.; Kuroda, K.; Ishikawa, M.; Ichino, R.; Takai, O. Hydroxyapatite Coating on Titanium by Means of Thermal Substrate Method in Aqueous Solutions. Solid State Ionics 2002, 151, 47–52. [Google Scholar] [CrossRef]
- Abbasi, S.; Golestani-Fard, F.; Rezaie, H.R.; Mirhosseini, S.M.M.; Ziaee, A. MAO-Derived Hydroxyapatite–TiO2 Nanostructured Bio-Ceramic Films on Titanium. Mater. Res. Bull. 2012, 47, 3407–3412. [Google Scholar] [CrossRef]
- Nisar, S.S.; Choe, H.-C. Formation of MoS2/HA ceramic film on plasma electrolytic oxidized Ti-6Al-4V using mechanically synthesized method: Enhancing surface characteristics and biocompatibility for bio-implant. Ceram. Int. 2024, 51, 4806–4827. [Google Scholar] [CrossRef]
- Meirav, U.; Foxman, E.B. Single-Electron Phenomena in Semiconductors. Semicond. Sci. Technol. 1996, 11, 255–284. [Google Scholar] [CrossRef]
- Kim, S.H.; Jeong, Y.H.; Choe, H.C.; Brantley, W.A. Morphology Change of HA Films on Highly Ordered Nanotubular Ti–Nb–Hf Alloys as a Function of Electrochemical Deposition Cycle. Surf. Coat. Technol. 2014, 259, 281–289. [Google Scholar] [CrossRef]
- Suzuki, O. Octacalcium Phosphate: Osteoconductivity and Crystal Chemistry. Acta Biomater. 2010, 6, 3379–3387. [Google Scholar] [CrossRef]
- Vlasa, A.; Varvara, S.; Pop, A.; Bulea, C.; Muresan, L.M. Electrodeposited Zn-TiO2 Nanocomposite Coatings and Their Corrosion Behavior. J. Appl. Electrochem. 2010, 40, 1519–1527. [Google Scholar] [CrossRef]
- Park, T.E.; Choe, H.C.; Brantley, W.A. Bioactivity Evaluation of Porous TiO2 Surface Formed on Titanium in Mixed Electrolyte by Spark Anodization. Surf. Coat. Technol. 2013, 235, 706–713. [Google Scholar] [CrossRef]
- Llobet, J.M.; Domingo, J.L.; Colomina, M.T.; Mayayo, E.; Corbella, J. Subchronic Oral Toxicity of Zinc in Rats. In Bulletin of Environmental Contamination and Toxicology; Springer: Berlin/Heidelberg, Geramany, 1988; Volume 41, pp. 36–43. [Google Scholar] [CrossRef]
- Lusvardi, G.; Zaffe, D.; Menabue, L.; Bertoldi, C.; Malavasi, G.; Consolo, U. In Vitro and In Vivo Behaviour of Zinc-Doped Phosphosilicate Glasses. Acta Biomater. 2009, 5, 419–428. [Google Scholar] [CrossRef]
- Rudnev, V.S.; Morozova, V.P.; Lukiyanchuk, I.V.; Adigamova, M.V. Calcium-Containing Biocompatible Oxide-Phosphate Coatings on Titanium. Russ. J. Appl. Chem. 2010, 83, 671–679. [Google Scholar] [CrossRef]
- Dzhurinskiy, D.; Gao, Y.; Yeung, W.-K.; Strumban, E.; Leshchinsky, V.; Chu, P.-J.; Maev, R.G. Characterization and corrosion evaluation of TiO2:n-HA coatings on titanium alloy formed by plasma electrolytic oxidation. Surf. Coat. Technol. 2015, 269, 258–265. [Google Scholar] [CrossRef]
- Zyman, Z.; Weng, J.; Liu, X.; Li, X.; Zhang, X. Phase and Structural Changes in Hydroxyapatite Coatings under Heat Treatment. Biomaterials 1994, 15, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Quintero, D.; Galvis, O.; Calderón, J.A.; Gómez, M.A.; Castaño, J.G.; Echeverría, F.; Habazaki, H. Control of the Physical Properties of Anodic Coatings Obtained by Plasma Electrolytic Oxidation on Ti6Al4V Alloy. Surf. Coat. Technol. 2015, 283, 210–222. [Google Scholar] [CrossRef]
- Khorasanian, M.; Dehghan, A.; Shariat, M.H.; Bahrololoom, M.E.; Javadpour, S. Microstructure and Wear Resistance of Oxide Coatings on Ti–6Al–4V Produced by Plasma Electrolytic Oxidation in an Inexpensive Electrolyte. Surf. Coat. Technol. 2011, 206, 1495–1502. [Google Scholar] [CrossRef]
- Xing, J.-H.; Xia, Z.-B.; Hu, J.-F.; Zhang, Y.-H.; Zhong, L. Growth and Crystallization of Titanium Oxide Films at Different Anodization Modes. J. Electrochem. Soc. 2013, 160, C239–C246. [Google Scholar] [CrossRef]
- Capek, D.; Gigandet, M.P.; Masmoudi, M.; Wery, M.; Banakh, O. Long-Time Anodisation of Titanium in Sulphuric Acid. Surf. Coat. Technol. 2008, 202, 1379–1384. [Google Scholar] [CrossRef]
- Nisar, S.S.; Choe, H.-C. Mechanical hydroxyapatite coatings on PEO-treated Ti–6Al–4V alloy for enhancing implant’s surface bioactivity. Ceram. Int. 2024, 50, 17703–17719. [Google Scholar] [CrossRef]
- Kazek-Kȩsik, A.; Krok-Borkowicz, M.; Pamuła, E.; Simka, W. Electrochemical and Biological Characterization of Coatings Formed on Ti-15Mo Alloy by Plasma Electrolytic Oxidation. Mater. Sci. Eng. C. Mater. Biol. Appl. 2014, 43, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Henstock, J.R.; Canham, L.T.; Anderson, S.I. Silicon: The Evolution of Its Use in Biomaterials. Acta Biomater. 2015, 11, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, M.; Kermanpur, A.; Kharaziha, M.; Bagherifard, S. Engineering of multilayered coating on additively manufactured Ti-6Al-4V porous implants to promote tribological and fatigue performances. Surf. Coat. Technol. 2024, 494, 131400. [Google Scholar] [CrossRef]
- Bloyce, A.; Qi, P.Y.; Dong, H.; Bell, T. Surface Modification of Titanium Alloys for Combined Improvements in Corrosion and Wear Resistance. Surf. Coat. Technol. 1998, 107, 125–132. [Google Scholar] [CrossRef]
- Bakin, B.; Koc Delice, T.; Tiric, U.; Birlik, I.; Ak Azem, F. Bioactivity and Corrosion Properties of Magnesium-Substituted CaP Coatings Produced via Electrochemical Deposition. Surf. Coat. Technol. 2016, 301, 29–35. [Google Scholar] [CrossRef]
- Belluci, M.M.; Giro, G.; del Barrio, R.A.L.; Pereira, R.M.R.; Marcantonio, E.; Orrico, S.R.P. Effects of Magnesium Intake Deficiency on Bone Metabolism and Bone Tissue around Osseointegrated Implants. Clin. Oral Implants Res. 2011, 22, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.Q.; Ivanisenko, Y.; Diemant, T.; Caron, A.; Chuvilin, A.; Jiang, J.Z.; Valiev, R.Z.; Qi, M.; Fecht, H.J. Synthesis and Properties of Hydroxyapatite-Containing Porous Titania Coating on Ultrafine-Grained Titanium by Micro-Arc Oxidation. Acta Biomater. 2010, 6, 2816–2825. [Google Scholar] [CrossRef]
- Teker, D.; Muhaffel, F.; Menekse, M.; Karaguler, N.G.; Baydogan, M.; Cimenoglu, H. Characteristics of Multi-Layer Coating Formed on Commercially Pure Titanium for Biomedical Applications. Mater. Sci. Eng. C 2015, 48, 579–585. [Google Scholar] [CrossRef]
- Necula, B.S.; Fratila-Apachitei, L.E.; Zaat, S.A.J.; Apachitei, I.; Duszczyk, J. In Vitro Antibacterial Activity of Porous TiO2–Ag Composite Layers against Methicillin-Resistant Staphylococcus aureus. Acta Biomater. 2009, 5, 3573–3580. [Google Scholar] [CrossRef]
- Kim, J.; Fiore, A.M.; Lee, H.H. Influences of Online Store Perception, Shopping Enjoyment, and Shopping Involvement on Consumer Patronage Behavior towards an Online Retailer. J. Retail. Consum. Serv. 2007, 14, 95–107. [Google Scholar] [CrossRef]
- Sahni, G.; Gopinath, P.; Jeevanandam, P. A Novel Thermal Decomposition Approach to Synthesize Hydroxyapatite–Silver Nanocomposites and Their Antibacterial Action against GFP-Expressing Antibiotic Resistant E. coli. Colloids Surf. B Biointerfaces 2013, 103, 441–447. [Google Scholar] [CrossRef]
- Shishir, R.; Nasiruddin, U.; Manojkumar, P.; Ponnilavan, V.; Lokeshkumar, E.; Rama Krishna, L.; Rameshbabu, N. Development of bioactive ceramic composite coating with bactericidal property on Zn–1Mg alloy by plasma electrolytic oxidation for temporary orthopaedic implant applications. Ceram. Int. 2024, 50, 15538–15550. [Google Scholar] [CrossRef]
- Molaei, M.; Fattah-alhosseini, A.; Nouri, M.; Nourian, A. Systematic Optimization of Corrosion, Bioactivity, and Biocompatibility Behaviors of Calcium-Phosphate Plasma Electrolytic Oxidation (PEO) Coatings on Titanium Substrates. Ceram. Int. 2022, 48, 6322–6337. [Google Scholar] [CrossRef]
- Zhai, D.; Qiu, T.; Shen, J.; Feng, K. Mechanism of Tetraborate and Silicate Ions on the Growth Kinetics of Microarc Oxidation Coating on a Ti6Al4V Alloy. RSC Adv. 2023, 13, 5382. [Google Scholar] [CrossRef]
- Carlisle, E.M. Silicon: A Possible Factor in Bone Calcification. Science 1970, 167, 279–280. [Google Scholar] [CrossRef]
- Schwartz, A.; Kossenko, A.; Zinigrad, M.; Danchuk, V.; Sobolev, A. Cleaning Strategies of Synthesized Bioactive Coatings by PEO on Ti-6Al-4V Alloys of Organic Contaminations. Materials 2023, 16, 4624. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yerokhin, A.; Matthews, A. DC Plasma Electrolytic Oxidation of Biodegradable Cp-Mg: In-Vitro Corrosion Studies. Surf. Coat. Technol. 2013, 234, 132–142. [Google Scholar] [CrossRef]
- Williams, D.F. Tissue-Biomaterial Interactions. J. Mater. Sci. 1987, 22, 3421–3445. [Google Scholar] [CrossRef]
- Wang, K. The Use of Titanium for Medical Applications in the USA. Mater. Sci. Eng. A 1996, 213, 134–137. [Google Scholar] [CrossRef]
- Hong, M.H.; Lee, D.H.; Kim, K.M.; Lee, Y.K. Improved Bonding Strength between TiO2 Film and Ti Substrate by Microarc Oxidation. Surf. Interface Anal. 2010, 42, 492–496. [Google Scholar] [CrossRef]
- Karthika, A. Aliovalent Ions Substituted Hydroxyapatite Coating on Titanium for Improved Medical Applications. Mater. Today Proc. 2018, 5, 8768–8774. [Google Scholar] [CrossRef]
- Sajadifar, S.V.; Yapici, G.G. Elevated Temperature Mechanical Behavior of Severely Deformed Titanium. J. Mater. Eng. Perform. 2014, 23, 1834–1844. [Google Scholar] [CrossRef]
- Rafieerad, A.R.; Ashra, M.R.; Mahmoodian, R.; Bushroa, A.R. Surface characterization and corrosion behavior of calcium phosphate-base composite layer on titanium and its alloys via plasma electrolytic oxidation: A review paper. Mater. Sci. Eng. C 2015, 57, 397–413. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Ohsaki, K.; Li, K.; Li, D.J.; Zhu, C.S.; Ogawa, T.; Tenshin, S.; Takano-Yamamoto, T. Histological Reaction to Hydroxyapatite in the Middle Ear of Rats. Auris Nasus Larynx 2001, 28, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Habibovic, P.; Kruyt, M.C.; Juhl, M.V.; Clyens, S.; Martinetti, R.; Dolcini, L.; Theilgaard, N.; Van Blitterswijk, C.A. Comparative In Vivo Study of Six Hydroxyapatite-Based Bone Graft Substitutes. J. Orthop. Res. 2008, 26, 1363–1370. [Google Scholar] [CrossRef]
- Gu, Y.W.; Khor, K.A.; Cheang, P. Bone-like Apatite Layer Formation on Hydroxyapatite Prepared by Spark Plasma Sintering (SPS). Biomaterials 2004, 25, 4127–4134. [Google Scholar] [CrossRef] [PubMed]
- Ooi, C.Y.; Hamdi, M.; Ramesh, S. Properties of Hydroxyapatite Produced by Annealing of Bovine Bone. Ceram. Int. 2007, 33, 1171–1177. [Google Scholar] [CrossRef]
- Shin, K.R.; Ko, Y.G.; Shin, D.H. Effect of Electrolyte on Surface Properties of Pure Titanium Coated by Plasma Electrolytic Oxidation. J. Alloys Compd. 2011, 509, S478–S481. [Google Scholar] [CrossRef]
- Søballe, K.; Hansen, E.S.; B.-Rasmussen, H.; Jørgensen, P.H.; Bünger, C. Tissue Ingrowth into Titanium and Hydroxyapatite-Coated Implants during Stable and Unstable Mechanical Conditions. J. Orthop. Res. 1992, 10, 285–299. [Google Scholar] [CrossRef]
- Afshar-Mohajer, M.; Yaghoubi, A.; Ramesh, S.; Bushroa, A.R.; Chin, K.M.C.; Tin, C.C.; Chiu, W.S. Electrophoretic Deposition of Magnesium Silicates on Titanium Implants: Ion Migration and Silicide Interfaces. Appl. Surf. Sci. 2014, 307, 1–6. [Google Scholar] [CrossRef]
- Chen, J.Z.; Shi, Y.L.; Wang, L.; Yan, F.Y.; Zhang, F.Q. Preparation and Properties of Hydroxyapatite-Containing Titania Coating by Micro-Arc Oxidation. Mater. Lett. 2006, 60, 2538–2543. [Google Scholar] [CrossRef]
- Nikoomanzari, E.; Karbasi, M.; Melo, W.C.; Moris, H.; Babaei, K.; Giannakis, S.; Fattah-alhosseini, A. Impressive strides in antibacterial performance amelioration of Ti-based implants via plasma electrolytic oxidation (PEO): A review of the recent advancements. Chem. Eng. J. 2022, 441, 136003. [Google Scholar] [CrossRef]
- Hu, H.; Liu, X.; Ding, C. Preparation and Formation Mechanism of Porous and Nanostructured TiO2/BCP Coatings on Titanium. In Proceedings of the 2010 3rd International Nanoelectronics Conference (INEC), Hong Kong, China, 3–8 January 2010. [Google Scholar] [CrossRef]
- Yang, X.; Yu, S.; Li, W. Preparation of Bioceramic Films Containing Hydroxyapatites on Ti–6Al–4V Alloy Surfaces by the Micro-Arc Oxidation Technique. Mater. Res. Bull. 2009, 44, 947–949. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, Y.; Haapasalo, M. Dental Materials with Antibiofilm Properties. Dent. Mater. 2014, 30, e1–e16. [Google Scholar] [CrossRef]
- Goudouri, O.M.; Kontonasaki, E.; Lohbauer, U.; Boccaccini, A.R. Antibacterial Properties of Metal and Metalloid Ions in Chronic Periodontitis and Peri-Implantitis Therapy. Acta Biomater. 2014, 10, 3795–3810. [Google Scholar] [CrossRef] [PubMed]
- Murakami, A.; Arimoto, T.; Suzuki, D.; Iwai-Yoshida, M.; Otsuka, F.; Shibata, Y.; Igarashi, T.; Kamijo, R.; Miyazaki, T. Antimicrobial and Osteogenic Properties of a Hydrophilic-Modified Nanoscale Hydroxyapatite Coating on Titanium. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.C.; Llama-Palacios, A.; Fernández, E.; Figuero, E.; Marín, M.J.; León, R.; Blanc, V.; Herrera, D.; Sanz, M. An in Vitro Biofilm Model Associated to Dental Implants: Structural and Quantitative Analysis of in Vitro Biofilm Formation on Different Dental Implant Surfaces. Dent. Mater. 2014, 30, 1161–1171. [Google Scholar] [CrossRef]
- Xia, Y.; Rogers, J.A.; Paul, K.E.; Whitesides, G.M. Unconventional Methods for Fabricating and Patterning Nanostructures. Chem. Rev. 1999, 99, 1823–1848. [Google Scholar] [CrossRef]
- Whitesides, G.M. Nanoscience, Nanotechnology, and Chemistry. Small 2005, 1, 172–179. [Google Scholar] [CrossRef]
- Durán-Lobato, M.; Muñoz-Rubio, I.; Holgado, M.Á.; Álvarez-Fuentes, J.; Fernández-Arévalo, M.; Martín-Banderas, L. Enhanced Cellular Uptake and Biodistribution of a Synthetic Cannabinoid Loaded in Surface-Modified Poly(Lactic-Co-Glycolic Acid) Nanoparticles. J. Biomed. Nanotechnol. 2014, 10, 1068–1079. [Google Scholar] [CrossRef] [PubMed]
- Love, J.C.; Estroff, L.A.; Kriebel, J.K.; Nuzzo, R.G.; Whitesides, G.M. Self-Assembled Monolayers of Thiolates on Metals as a Form of Nanotechnology. Chem. Rev. 2005, 105, 1103–1169. [Google Scholar] [CrossRef]
- MacDonald, R.A.; Laurenzi, B.F.; Viswanathan, G.; Ajayan, P.M.; Stegemann, J.P. Collagen-Carbon Nanotube Composite Materials as Scaffolds in Tissue Engineering. J. Biomed. Mater. Res. A 2005, 74, 489–496. [Google Scholar] [CrossRef]
- Sundfeldt, M.; Carlsson, L.V.; Johansson, C.B.; Thomsen, P.; Gretzer, C. Aseptic Loosening, Not Only a Question of Wear: A Review of Different Theories. Acta Orthop. 2006, 77, 177–197. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.G.; Li, Z.H.; Mao, X.Z.; Wang, W.C. Advances in Bone Repair with Nanobiomaterials: Mini-Review. Cytotechnology 2011, 63, 437–443. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, H.; Webster, T.J. Nanomedicine for Implants: A Review of Studies and Necessary Experimental Tools. Biomaterials 2007, 28, 354–369. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Niu, S.; Yankova, M.; Mecklenburg, M.; King, S.M.; Ravichandran, J.; Kalia, R.K.; Nakano, A.; Vashishta, P.; Setlow, P. Analysis of Killing of Growing Cells and Dormant and Germinated Spores of Bacillus Species by Black Silicon Nanopillars. Sci. Rep. 2017, 7, 17768. [Google Scholar] [CrossRef]
- Izquierdo-Barba, I.; García-Martín, J.M.; Álvarez, R.; Palmero, A.; Esteban, J.; Pérez-Jorge, C.; Arcos, D.; Vallet-Regí, M. Nanocolumnar Coatings with Selective Behavior towards Osteoblast and Staphylococcus aureus Proliferation. Acta Biomater. 2015, 15, 20–28. [Google Scholar] [CrossRef]
- Kelleher, S.M.; Habimana, O.; Lawler, J.; O’reilly, B.; Daniels, S.; Casey, E.; Cowley, A. Cicada Wing Surface Topography: An Investigation into the Bactericidal Properties of Nanostructural Features. ACS Appl. Mater. Interfaces 2016, 8, 14966–14974. [Google Scholar] [CrossRef]
- Ivanova, E.P.; Hasan, J.; Webb, H.K.; Gervinskas, G.; Juodkazis, S.; Truong, V.K.; Wu, A.H.F.; Lamb, R.N.; Baulin, V.A.; Watson, G.S.; et al. Bactericidal Activity of Black Silicon. Nat. Commun. 2013, 4, 2838. [Google Scholar] [CrossRef] [PubMed]
- Nowlin, K.; Boseman, A.; Covell, A.; LaJeunesse, D. Adhesion-Dependent Rupturing of Saccharomyces Cerevisiae on Biological Antimicrobial Nanostructured Surfaces. J. R. Soc. Interface 2015, 12, 20140999. [Google Scholar] [CrossRef] [PubMed]
- Truong, V.K.; Geeganagamage, N.M.; Baulin, V.A.; Vongsvivut, J.; Tobin, M.J.; Luque, P.; Crawford, R.J.; Ivanova, E.P. The Susceptibility of Staphylococcus aureus CIP 65.8 and Pseudomonas Aeruginosa ATCC 9721 Cells to the Bactericidal Action of Nanostructured Calopteryx Haemorrhoidalis Damselfly Wing Surfaces. Appl. Microbiol. Biotechnol. 2017, 101, 4683–4690. [Google Scholar] [CrossRef] [PubMed]
- Hizal, F.; Choi, C.H.; Busscher, H.J.; Van Der Mei, H.C. Staphylococcal Adhesion, Detachment and Transmission on Nanopillared Si Surfaces. ACS Appl. Mater. Interfaces 2016, 8, 30430–30439. [Google Scholar] [CrossRef] [PubMed]
- Nisar, S.S.; Arun, S.; Choe, H.-C. Plasma electrolytic oxidation coatings on femtosecond laser-treated Ti-6Al-4V alloy for bio-implant use. Surf. Coat. Technol. 2023, 464, 129553. [Google Scholar] [CrossRef]
- He, F.; Liao, Y.; Lin, J.; Song, J.; Qiao, L.; Cheng, Y.; He, F.; Sugioka, K. Femtosecond Laser Fabrication of Monolithically Integrated Microfluidic Sensors in Glass. Sensors 2014, 14, 19402. [Google Scholar] [CrossRef]
- Schlie, S.; Fadeeva, E.; Koroleva, A.; Ovsianikov, A.; Koch, J.; Ngezahayo, A.; Chichkov, B.N. Laser-Based Nanoengineering of Surface Topographies for Biomedical Applications. Photonics Nanostructures-Fundam. Appl. 2011, 9, 159–162. [Google Scholar] [CrossRef]
- Vorobyev, A.Y.; Guo, C. Colorizing Metals with Femtosecond Laser Pulses. Appl. Phys. Lett. 2008, 92, 041914. [Google Scholar] [CrossRef]
- Nathala, C.S.R.; Ajami, A.; Ionin, A.A.; Kudryashov, S.I.; Makarov, S.V.; Ganz, T.; Assion, A.; Husinsky, W. Experimental Study of Fs-Laser Induced Sub-100-Nm Periodic Surface Structures on Titanium. Opt. Express 2015, 23, 5915. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.-W.; Zhang, C.-Y.; Liu, H.-Y.; Dai, Q.-F.; Wu, L.-J.; Lan, S.; Gopal, A.V.; Trofimov, V.A.; Lysak, T.M. High Spatial Frequency Periodic Structures Induced on Metal Surface by Femtosecond Laser Pulses. Opt. Express 2012, 20, 905. [Google Scholar] [CrossRef] [PubMed]

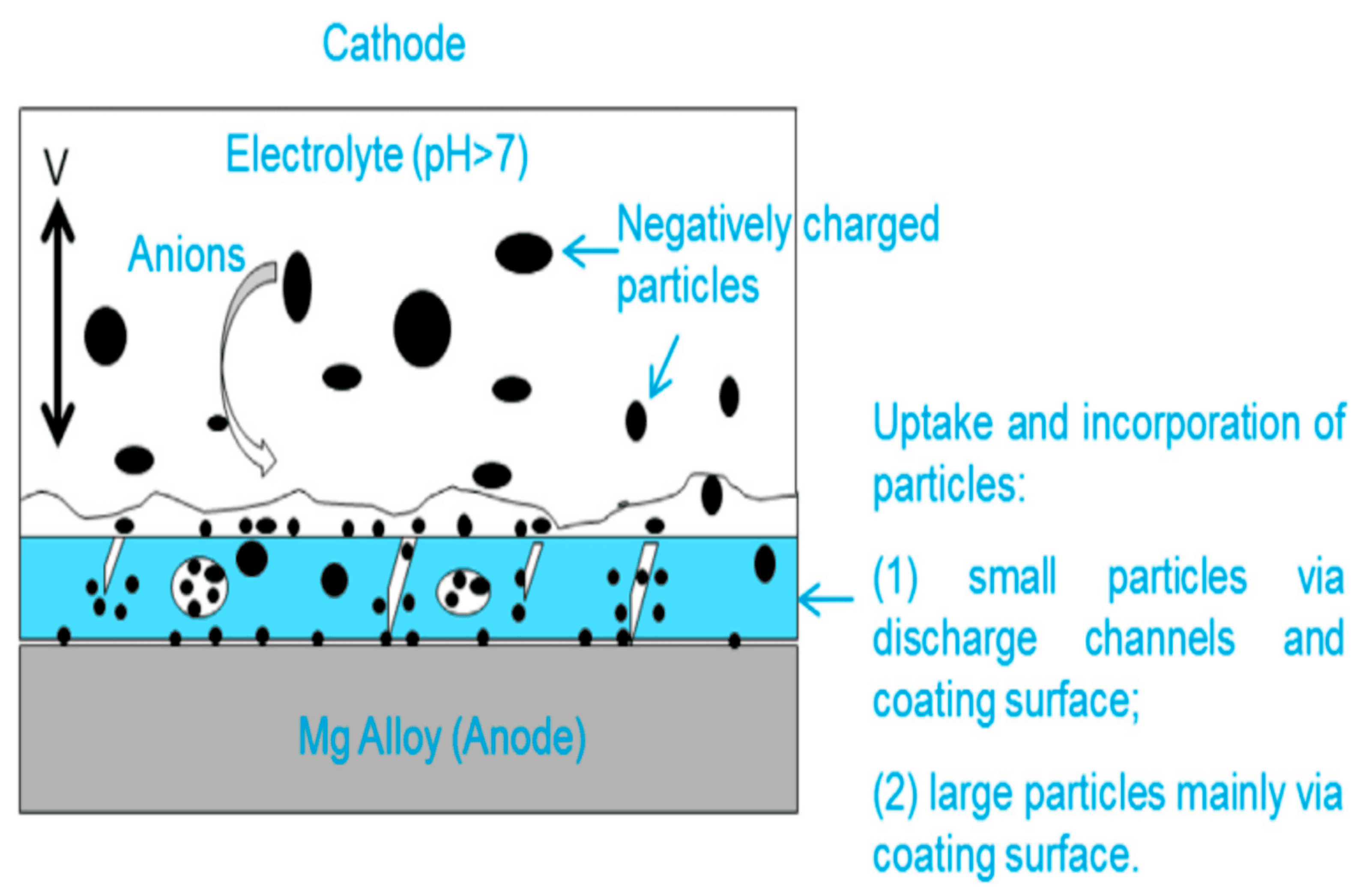
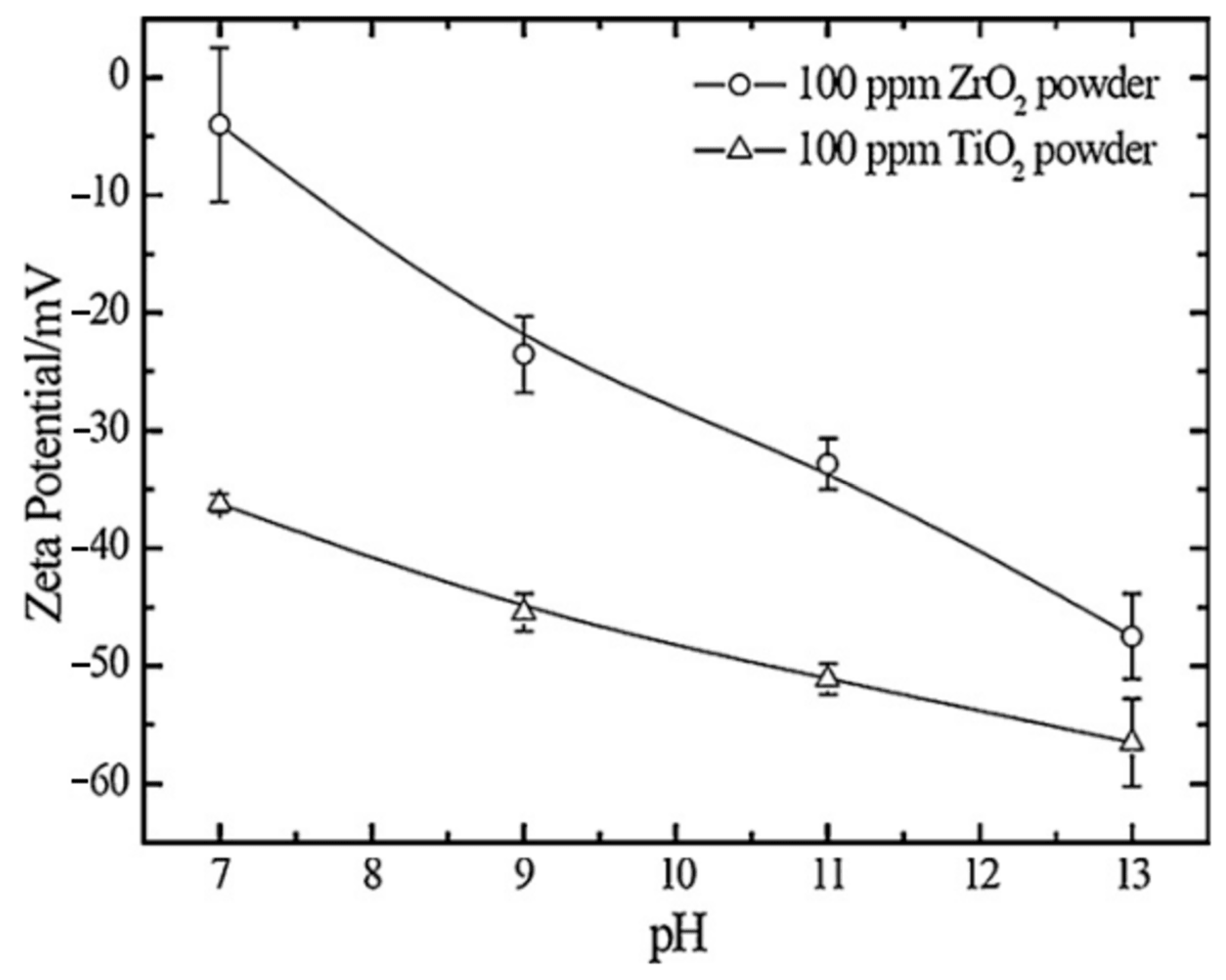

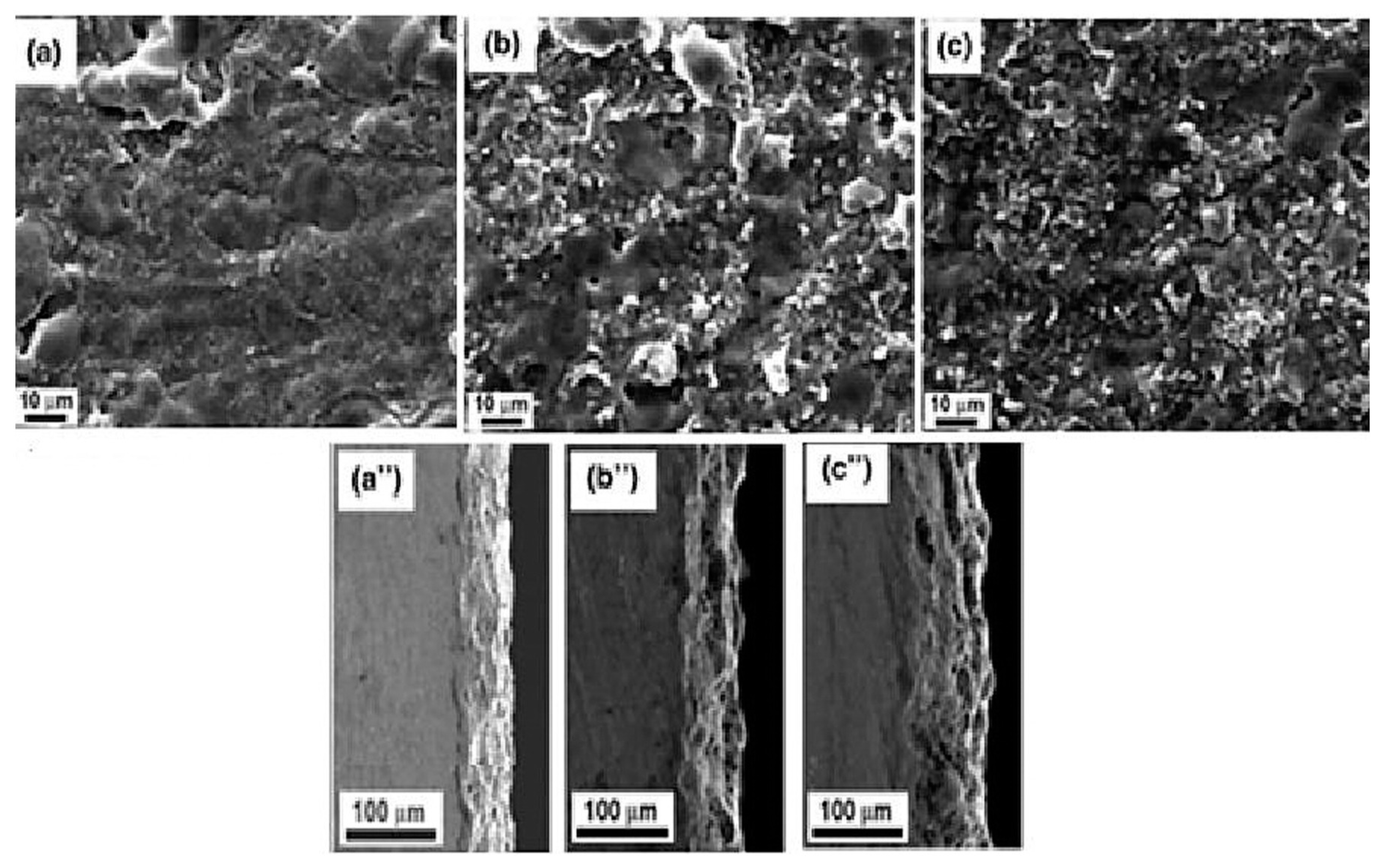


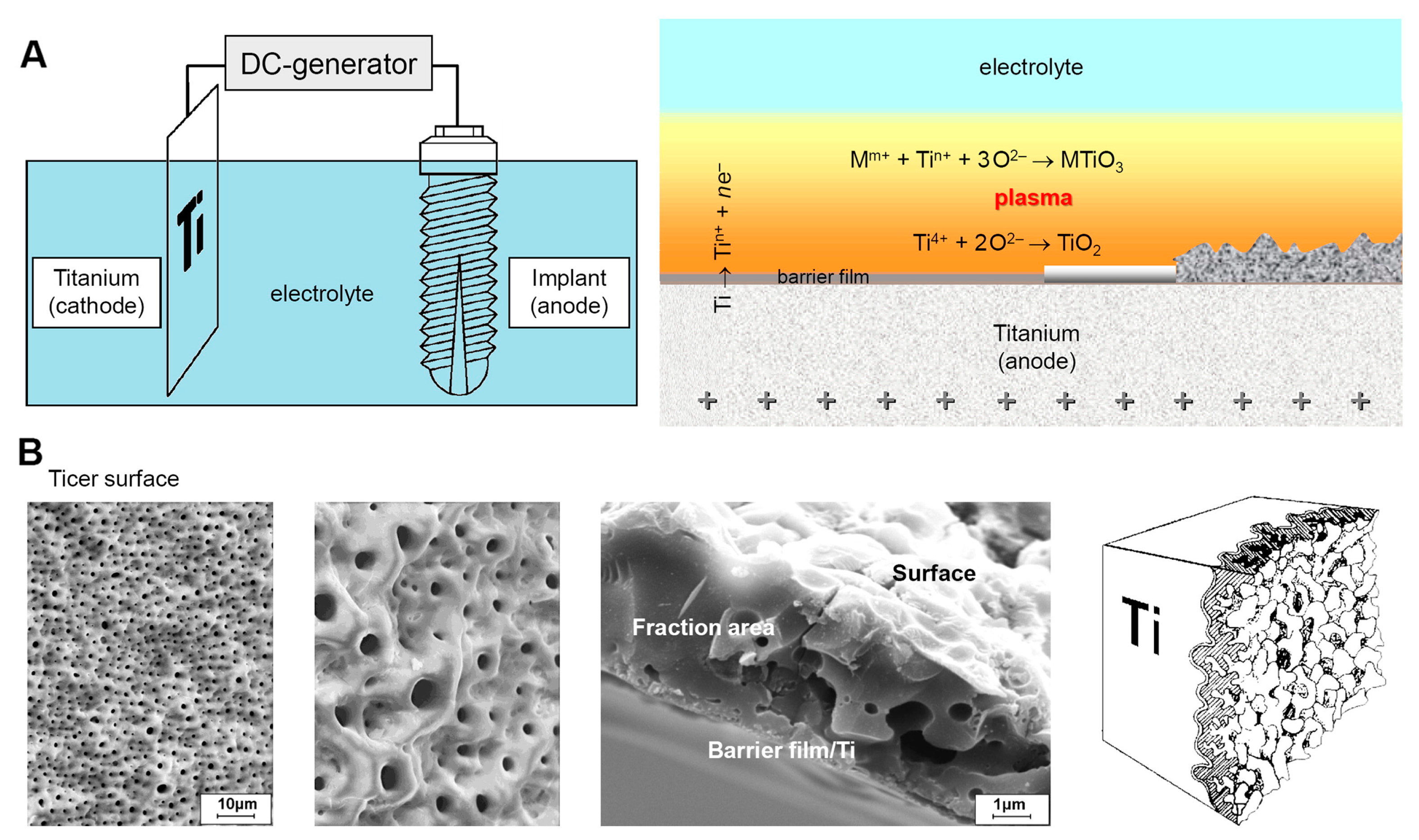
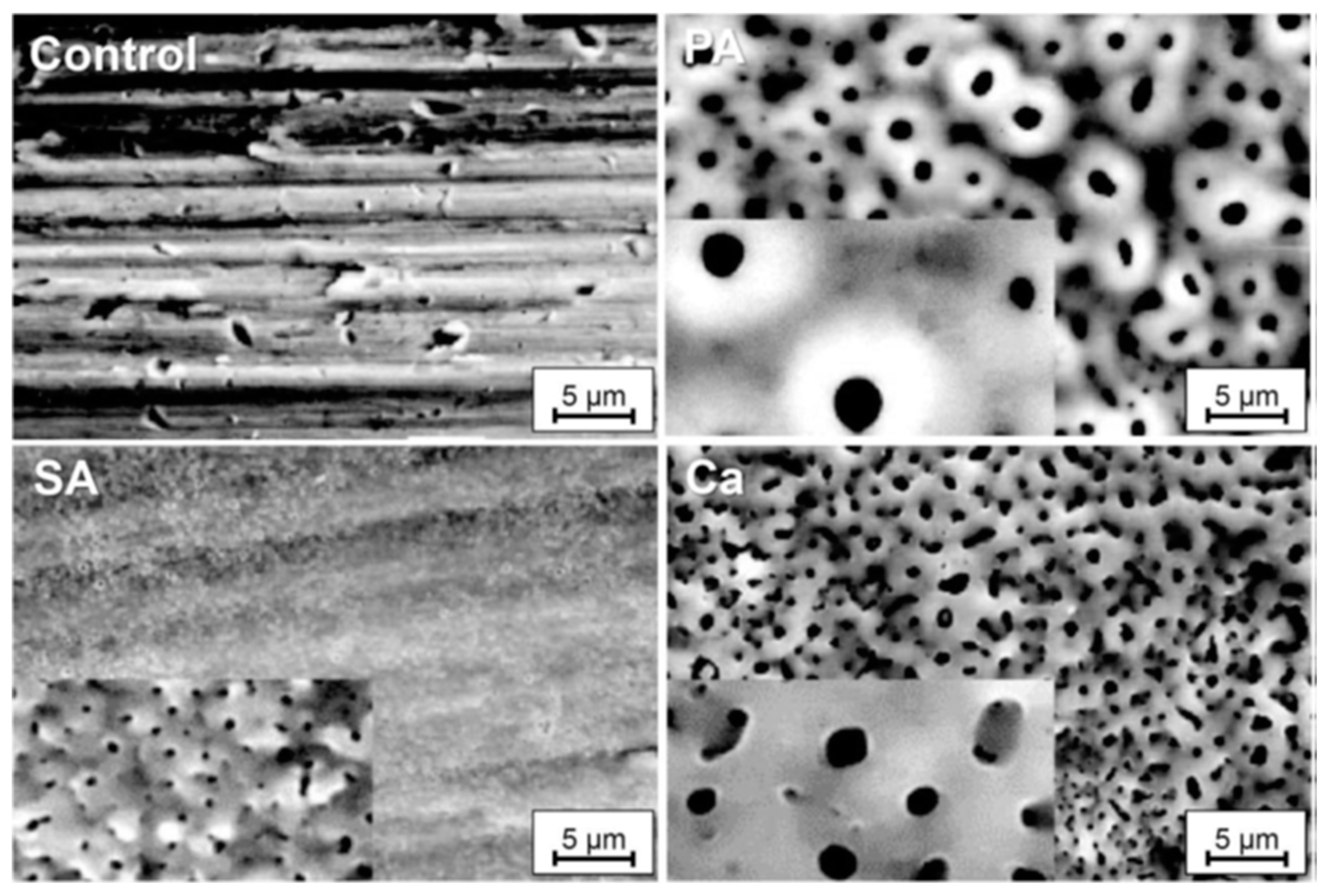

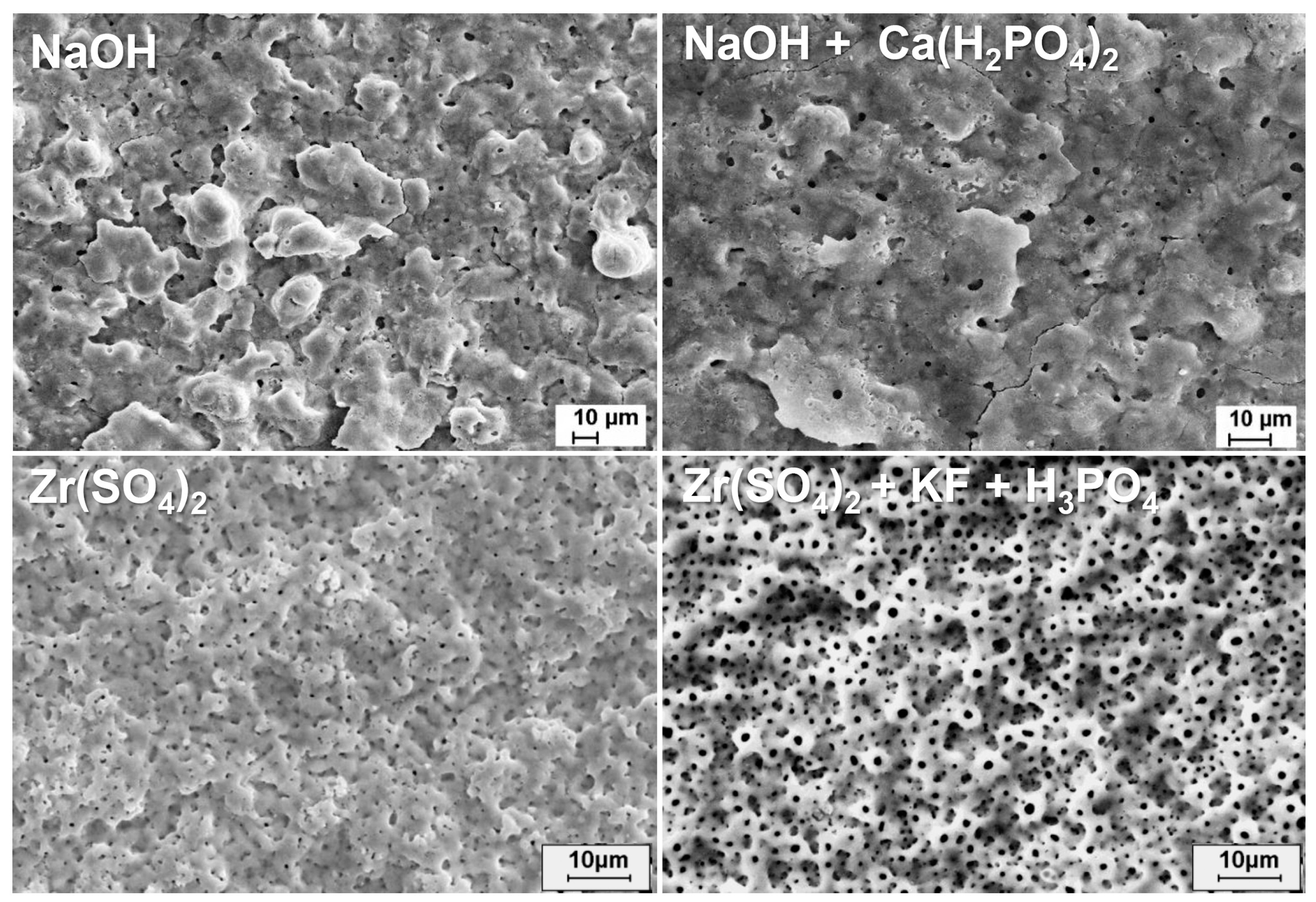
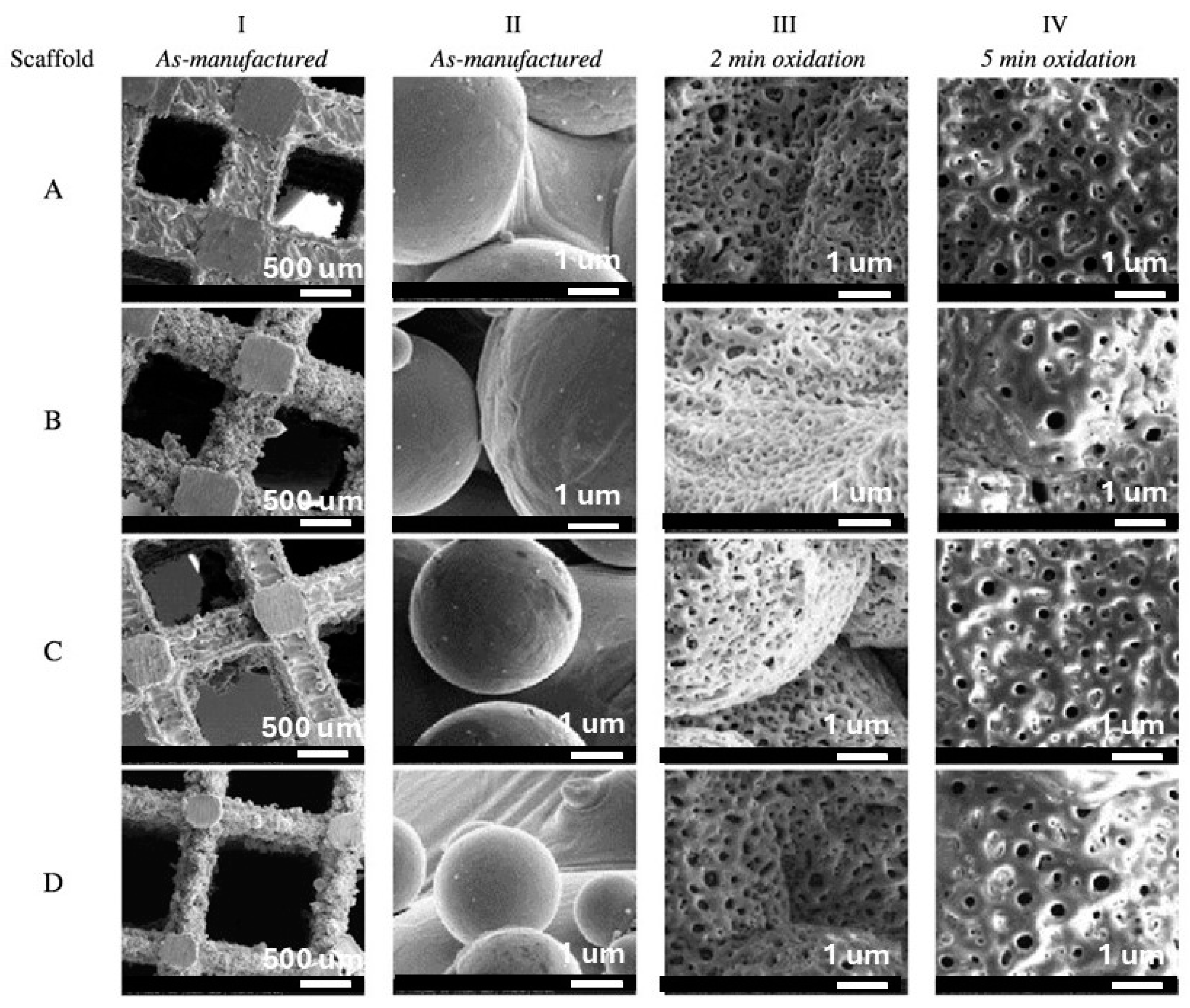

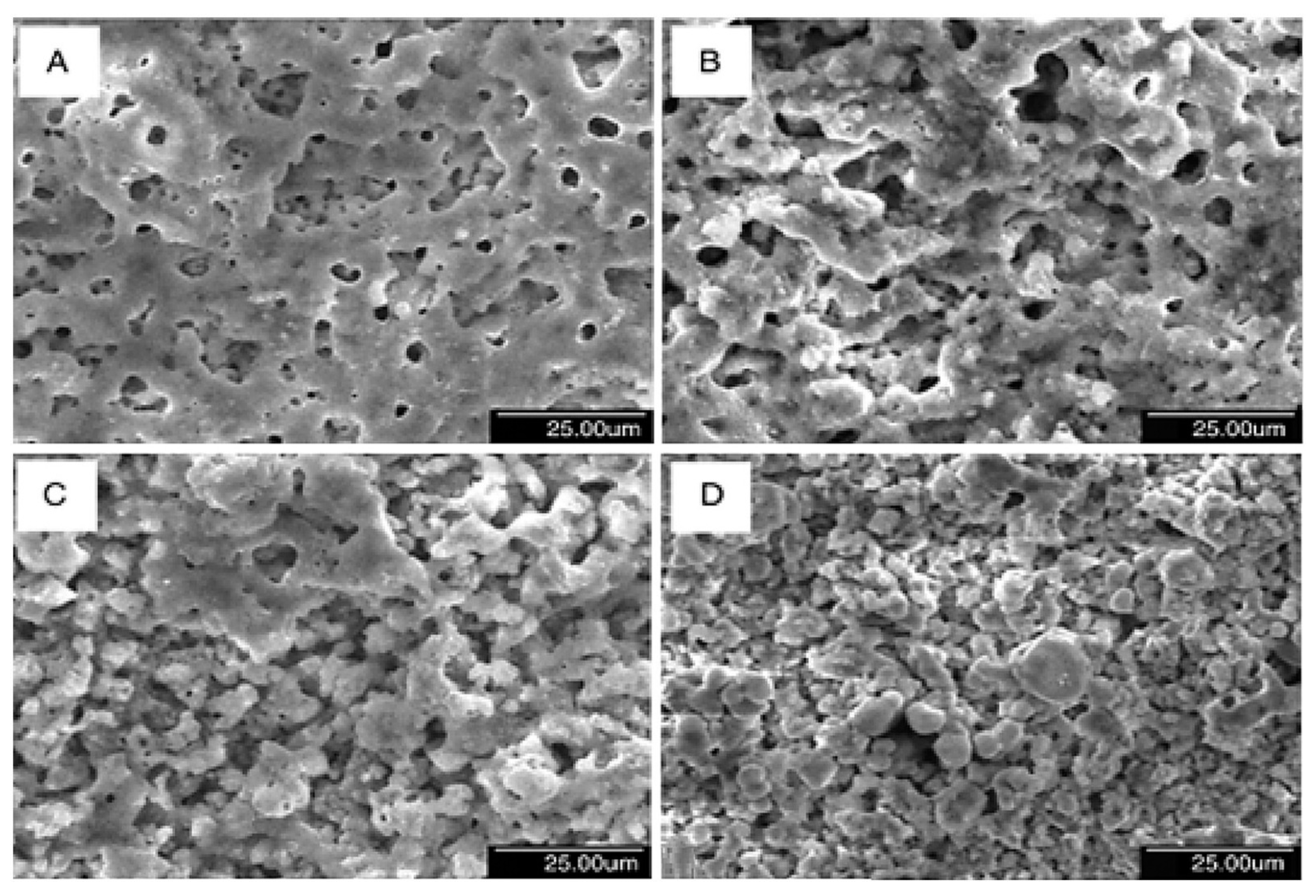

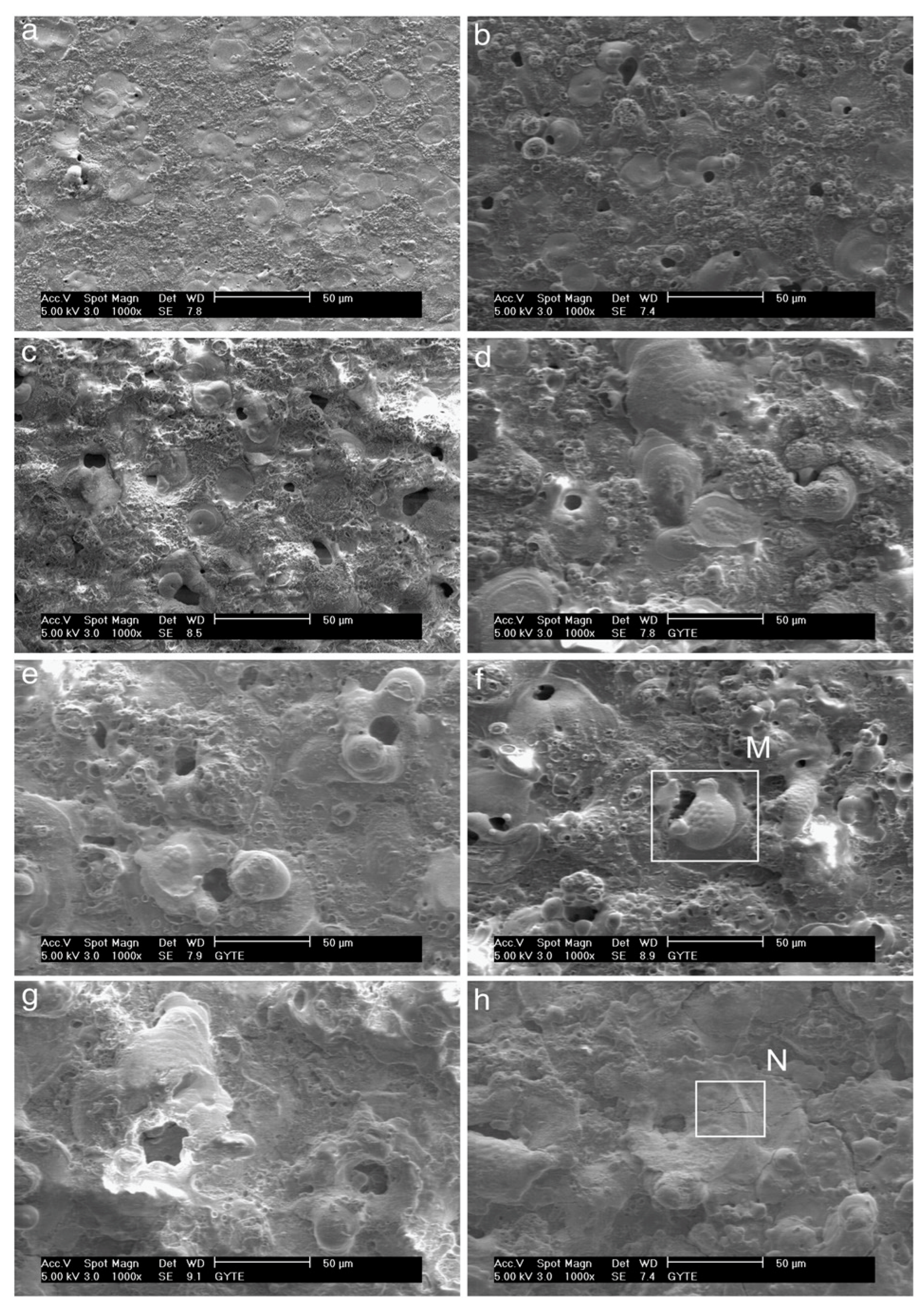

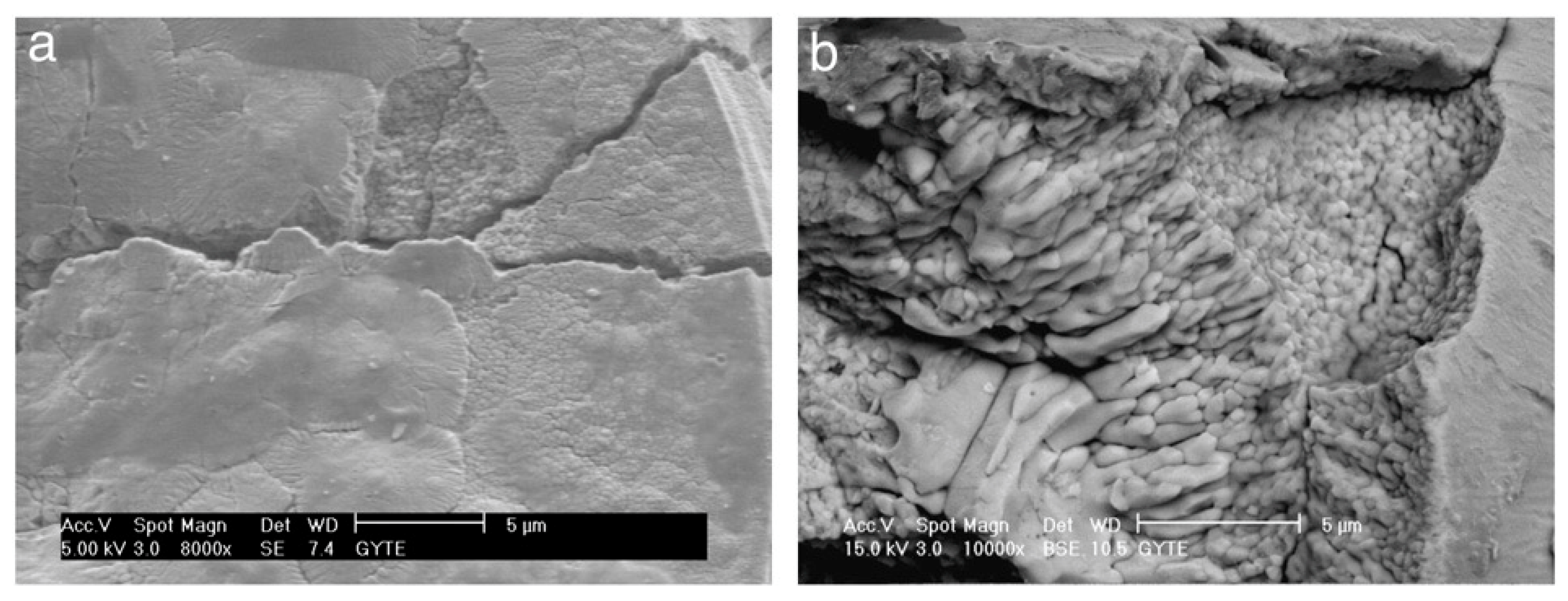
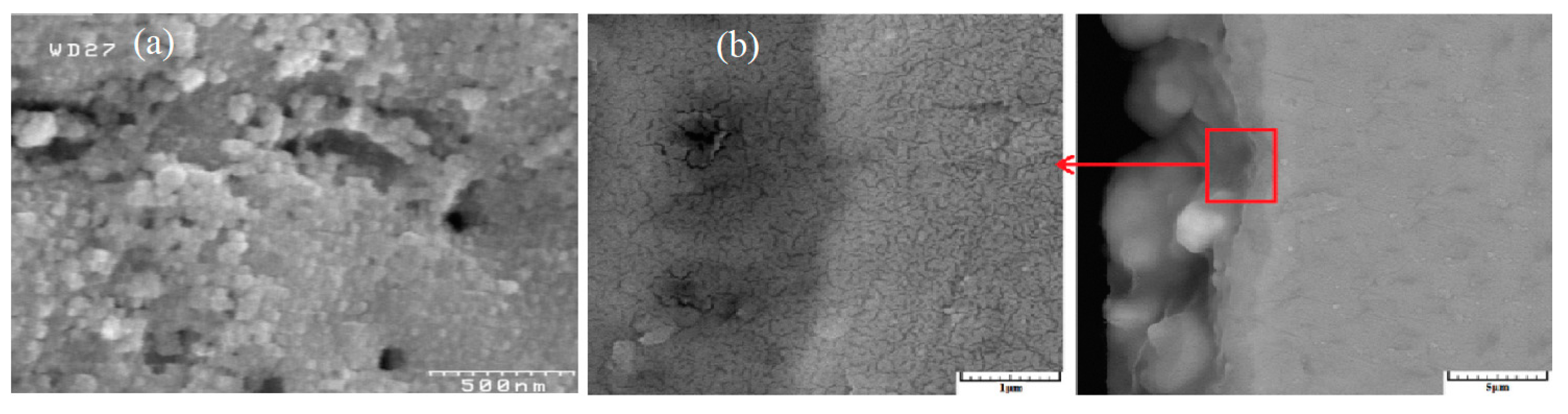

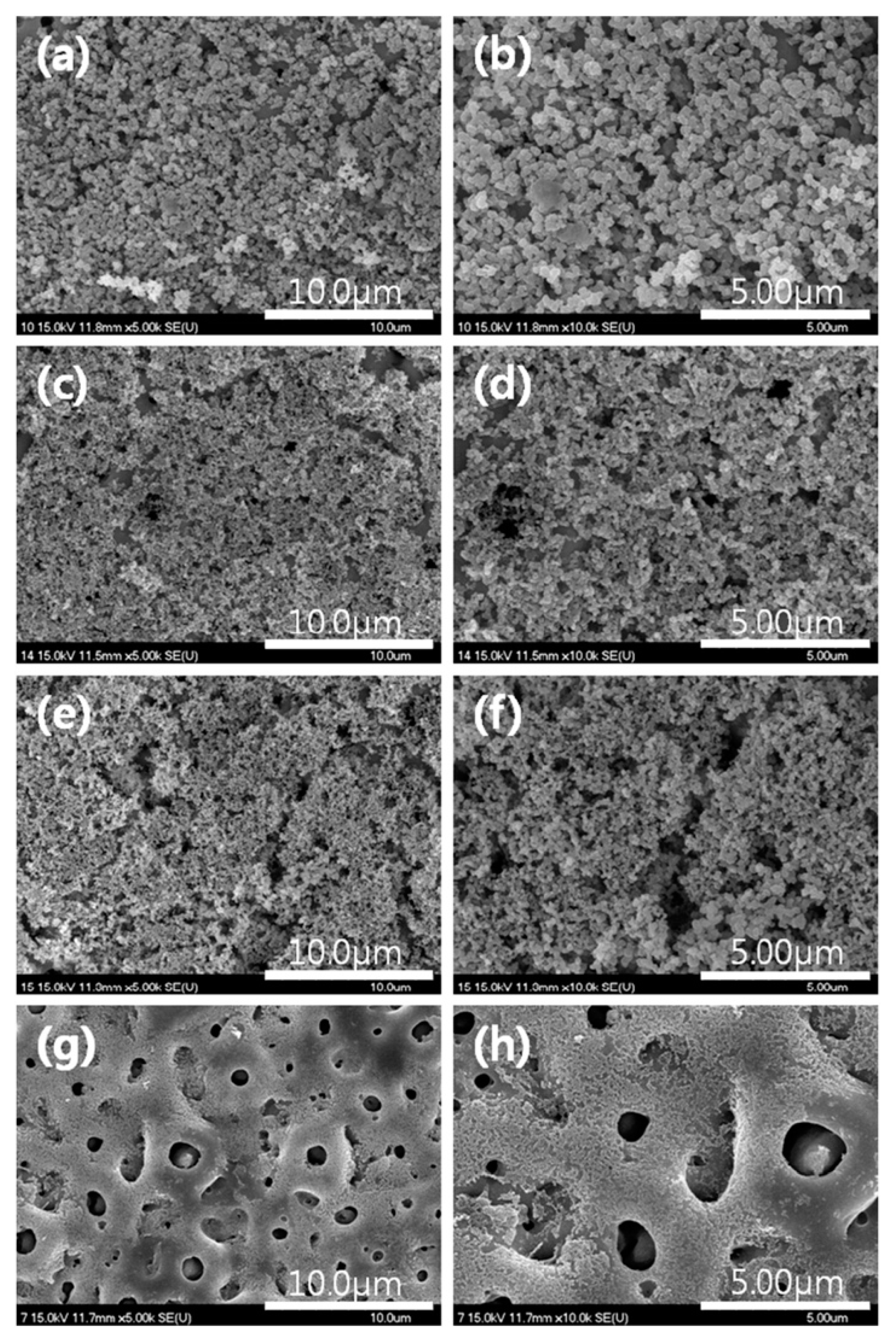

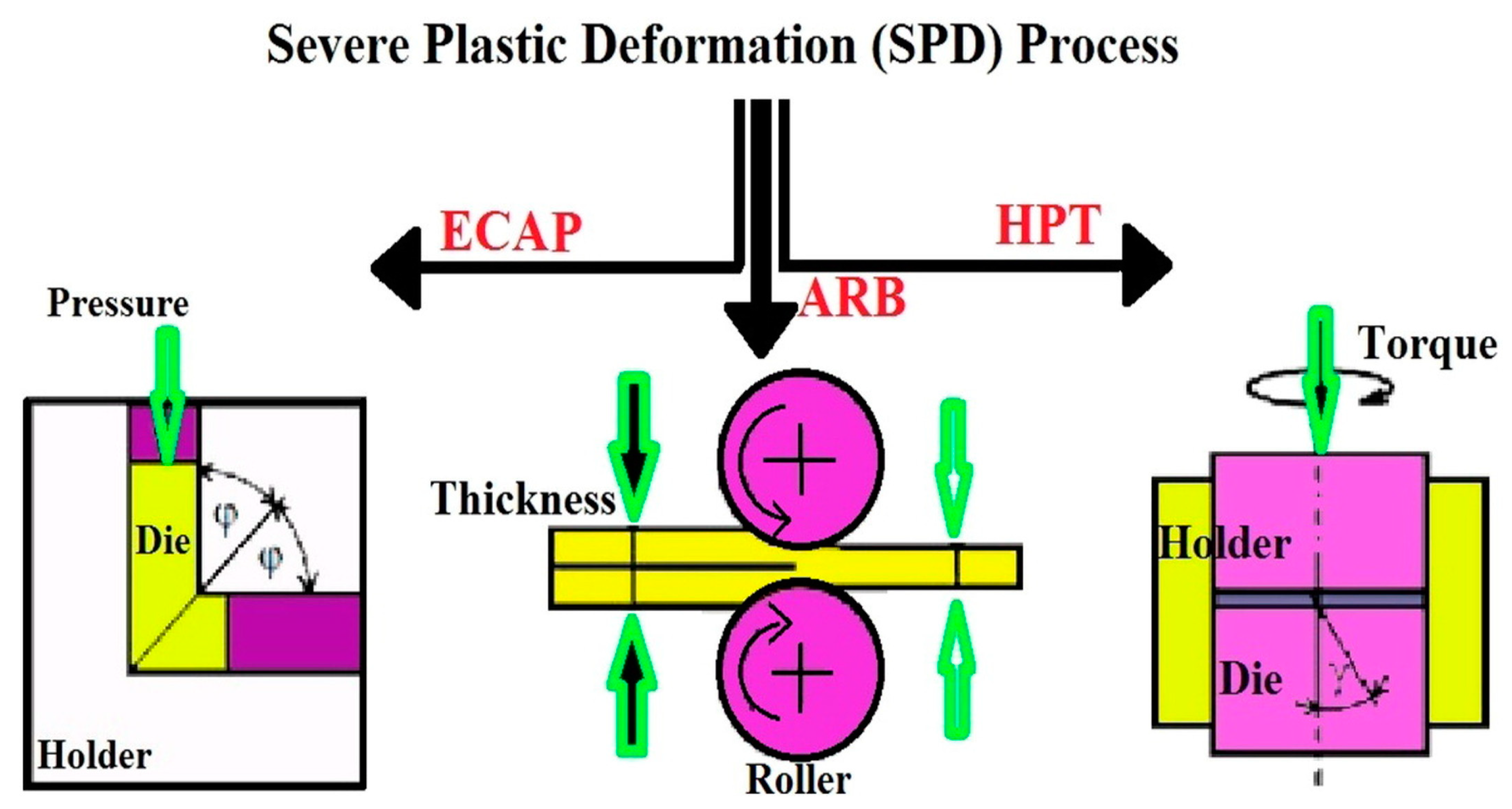
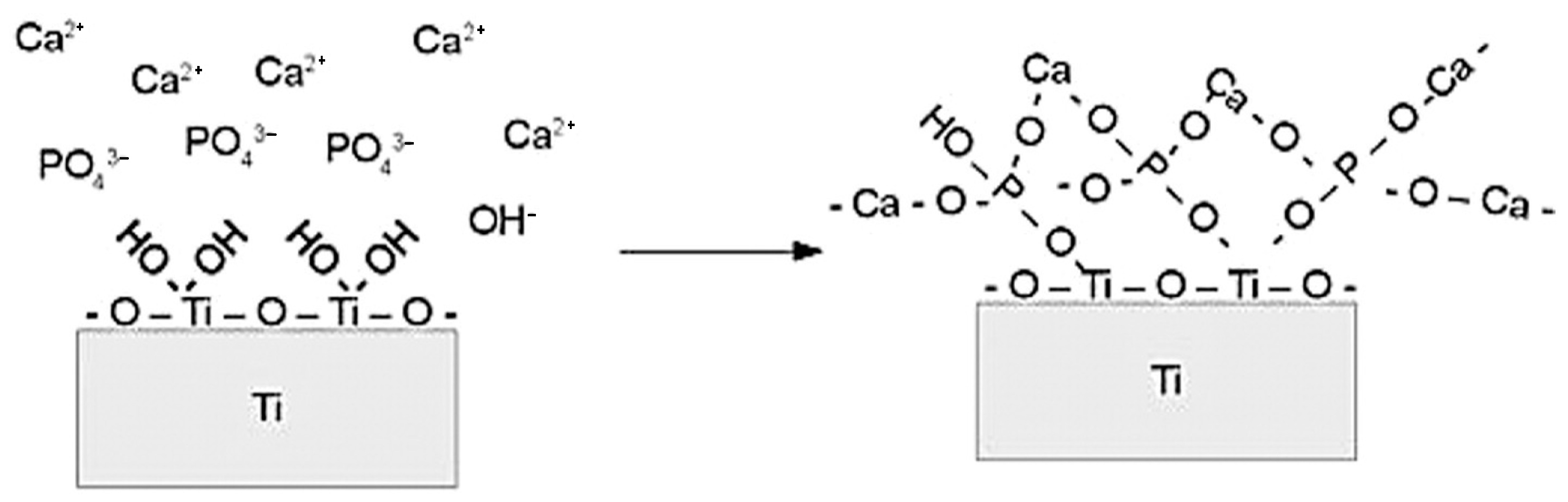
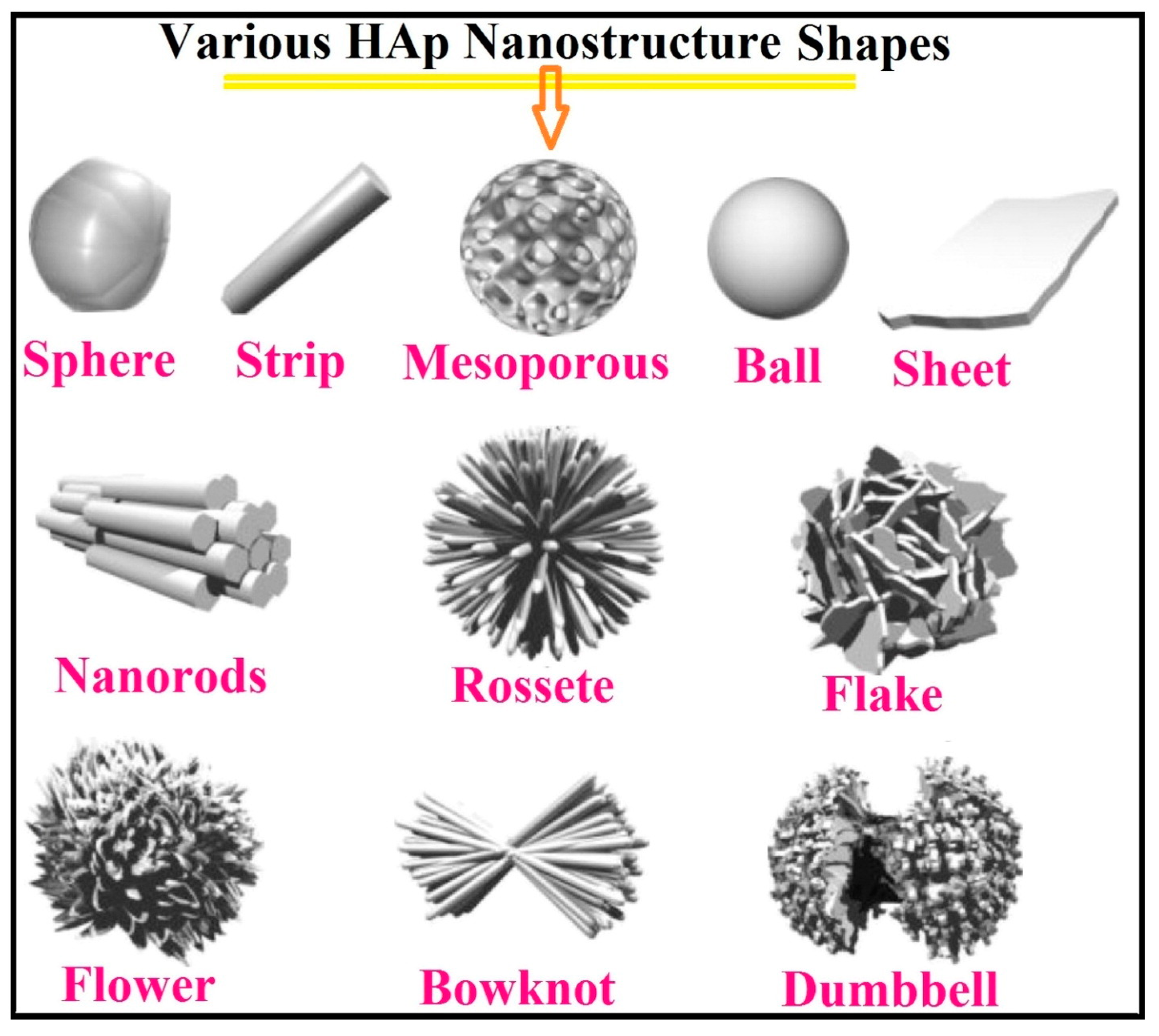

| Particles | Properties and Field of Applications | Reference |
|---|---|---|
| Polytetrafluoroethylene | Lower friction coefficient, chemical inertness and hydrophobicity | [224] |
| Ag | Antibacterial activity | [225] |
| Hydroxyapatite (HA) | Osteogenesis and biomaterial | [226] |
| MoS2 | Solid lubricant | [227] |
| Clay minerals | Absorption capacities and filler material | [217] |
| ZrO2 (monoclinic, tetragonal, and cubic) | High chemical stability | [228,229] |
| SiO2 | High heat and chemical resistance | [230,231] |
| TiO2 | High chemical stability and heat resistance | [232] |
| Si3N4 | High hardness and wear resistance | [233] |
| Al2O3 | High hardness and insulator | [232] |
| CeO2/Ce2O3 | High chemical stability, superconductors and sensors | [234,235] |
| SiC | High mechanical strength and chemical inertness | [236,237] |
| Graphite | Solid lubricant | [238] |
| Calcium phosphates | Natural bone component | [239] |
| Fe/Fe2O3 | Ferromagnetic material | [240] |
| Co | Ferromagnetic material | [241] |
| Cu | Antibacterial activity | [242] |
| Ni/NiO, MnO2/Mn2O3 | Catalytic activity | [243] |
| Surface | Protein | Study | Investigations | Results | Literature |
|---|---|---|---|---|---|
| TiUnite | rhBMP-2 | In vivo | TiUnite-coated screw implants in 12 Labrador dogs | TiUnite surfaces coated with rhBMP-2 possess significant potential to stimulate bone growth | [333] |
| TiO2 | BMP-2 | In vitro | Human osteoblasts growth on surfaces: (non)anodized (un)coated Ti plates | Anodized surfaces coated with BMP-2 induced better osteoblast adhesion | [334] |
| Ti cp and Ticer | BSP, Collagen type I Fibronectin | In vitro | Materials’ influence on adult human maxillary bone cells’ behavior | Coating Ti cp induces better biological properties than a rough ceramic surface material; the best improvement for materials coated with BSP | [335] |
| Ti cp and Ticer | BSP, Collagen type I | In vitro | Effect of protein coated surfaces on bone-derived cells | Collagen surfaces—unsuitable for the cell attachment; BSP surfaces—advance osteoinduction process | [336] |
| Ti Alloy | Electrolyte | Voltage (V) | Time (min) | XRD Detected Phase | Preheat, Oxidation Annealing Temp (°C) | Literature |
|---|---|---|---|---|---|---|
| Cp2Ti | Ca(CH3COO)2, 0.028–0.085 M Na β-glycerophosphate, 0.005–0.02 M | 350 | 3 | Ti TiO2 Anatase HAα-TCP CaTiO3 | No Preheating Oxidation at 70 ± 3 No heat treatment | [366] |
| Ti6Al4V | Ca(CH3COO)2·H2O, 0.26 M Na2HPO4·2H2O, 0.12 M | 400 | 15 | TiO2 Anatase TiO2 Rutile TiV Al0.3Ti1.7 HA | No preheating Oxidation at room temperature No heat treatment | [422] |
| Ti6Al4V | Ca(CH3COO)2·H2O, 0.26 M Na2HPO4·2H2O, 0.12 M | 400 | 60 | TiO2 Anatase TiO2 Rutile TiV Al0.3Ti1.7 HA CaTiO3 Al2O3 Ca10(PO4)6(OH)2 | No preheating Oxidation at room temperature No Heat treatment | [422] |
| Cp2Ti | Ca(CH3COO)2, 0.015 mol/L Ca β-glycerophosphate, 0.02 mol/L | 450 | 7.5 | Ti TiO2 Anatase TiO2 Rutile HA | Preheated at 300 Oxidation at room temperature Heat treatment for 10 h at 190 with autoclave | [423] |
| Cp2Ti | Ca(CH3COO)2, 0.03 M Ca β-glycerophosphate, 0.02 M | 400 | 60 | TiO2 Anatase TiO2 Rutile Ca2Ti2O6 | No preheating Oxidation at 15 Heat treatment for 4 h at 220 with autoclave | [423] |
| Cp2Ti | Ca(CH3COO)2, 0.2 mol/L Ca β-glycerophosphate, 0.02 mol/L | 350 | 3 | TiO2 Anatase TiO2 Rutile HA | No preheating Oxidation at 70 ± 3 No heat treatment | [272] |
| Cp2Ti | Ca(CH3COO)2, 0.2 mol/L Ca β-glycerophosphate, 0.02 mol/L | 350 | 6 | TiO2 Anatase TiO2 Rutile HA CaTiO3 α-TCP | No preheating. Oxidation at 70 ± 3. No heat treatment. | [272] |
| Cp2Ti | Ca(CH3COO)2, 0.2 mol/L Ca β-glycerophosphate, 0.02 mol/L | 350 | 10 | TiO2 Anatase TiO2 Rutile HA CaTiO3 | No preheating Oxidation at 70 ± 3 No heat treatment | [272] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mishchenko, O.; Volchykhina, K.; Maksymov, D.; Manukhina, O.; Pogorielov, M.; Pavlenko, M.; Iatsunskyi, I. Advanced Strategies for Enhancing the Biocompatibility and Antibacterial Properties of Implantable Structures. Materials 2025, 18, 822. https://doi.org/10.3390/ma18040822
Mishchenko O, Volchykhina K, Maksymov D, Manukhina O, Pogorielov M, Pavlenko M, Iatsunskyi I. Advanced Strategies for Enhancing the Biocompatibility and Antibacterial Properties of Implantable Structures. Materials. 2025; 18(4):822. https://doi.org/10.3390/ma18040822
Chicago/Turabian StyleMishchenko, Oleg, Kristina Volchykhina, Denis Maksymov, Olesia Manukhina, Maksym Pogorielov, Mykola Pavlenko, and Igor Iatsunskyi. 2025. "Advanced Strategies for Enhancing the Biocompatibility and Antibacterial Properties of Implantable Structures" Materials 18, no. 4: 822. https://doi.org/10.3390/ma18040822
APA StyleMishchenko, O., Volchykhina, K., Maksymov, D., Manukhina, O., Pogorielov, M., Pavlenko, M., & Iatsunskyi, I. (2025). Advanced Strategies for Enhancing the Biocompatibility and Antibacterial Properties of Implantable Structures. Materials, 18(4), 822. https://doi.org/10.3390/ma18040822







