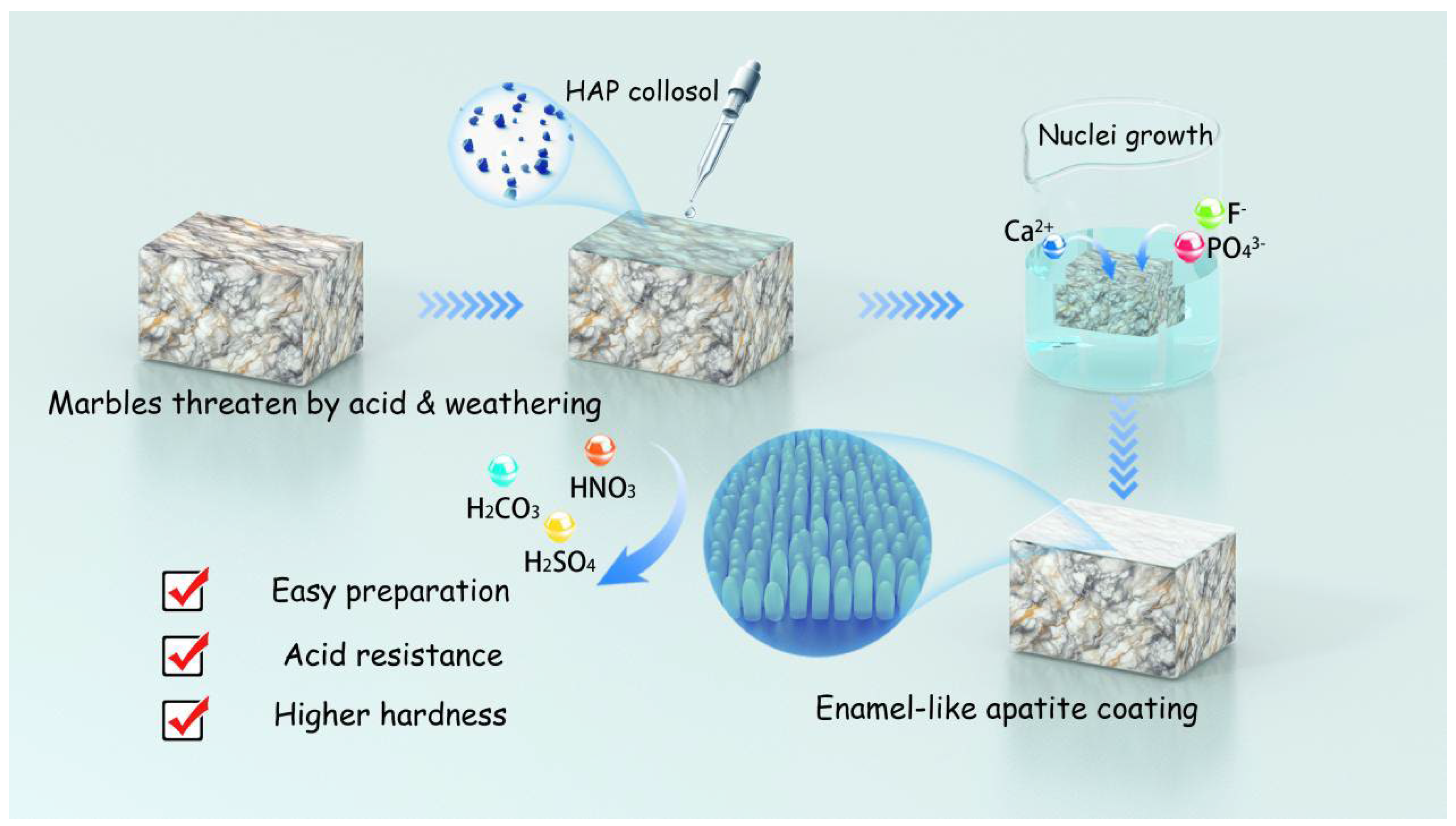
In Situ Growth of Enamel-like Apatite Coating for Marble Protection
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of HAP Collosol
2.3. In Situ Growth of Enamel-like Apatite Coating
2.4. Phosphate Coatings Prepared by DAP Methods
2.5. Scanning Electron Microscopy with Energy Dispersive Spectroscopy (SEM-EDS)
2.6. Grazing Incidence X-Ray Diffraction (GIXRD)
2.7. Polarized Microscopy
2.8. Acid Resistance Measurement
2.9. Nano Indentation
3. Results and Discussion
3.1. Optimization Against Cracking
3.2. Morphology and Phase Characterization
3.3. Acid Resistance
3.4. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, J.K.; Paswan, S.; Saha, D.; Pandya, A.; Singh, D.D.N. Role of air pollutant for deterioration of Taj Mahal by identifying corrosion products on surface of metals. Int. J. Environ. Sci. Technol. 2022, 19, 829–838. [Google Scholar] [CrossRef]
- Straulino, L.; Sedov, S.; Michelet, D.; Balanzario, S. Weathering of carbonate materials in ancient Maya constructions (Río Bec and Dzibanché): Limestone and stucco deterioration patterns. Quat. Int. 2013, 315, 87–100. [Google Scholar] [CrossRef]
- Brimblecombe, P.; Grossi, C.M.; Harris, I. Long term trends in dampness in England. Weather 2006, 61, 278–281. [Google Scholar] [CrossRef]
- Viles, H.A. Implications of future climate change for stone consolidation. In Natural Stone, Weathering Phenomena, Conservation Strategies and Case Studies; Siegesmund, S., Weiss, T., Vollbrecht, A., Eds.; Special Publications; Geological Society: London, UK, 2002; Volume 205, pp. 407–418. [Google Scholar]
- Grossi, C.M.; Brimblecombe, P. Effect of long-term changes in air pollution and climate on the decay and blackening of European stone buildings. In Building Stone Decay: From Diagnosis to Conservation; Přikryl, R., Smith, B.J., Eds.; Special Publication; Geological Society: London, UK, 2007; Volume 271, pp. 117–130. [Google Scholar]
- Peta, K.; Stemp, W.J.; Chen, R.; Love, G.; Brown, C.A. Multiscale characterizations of topographic measurements on lithic materials and microwear using a GelSight Max: Investigating potential archaeological applications. J. Archaeol. Sci. Rep. 2024, 57, 104637. [Google Scholar] [CrossRef]
- Maravelaki-Kalaitzaki, P. Black crusts and patinas on Pentelic marble from the Parthenon and Erechtheum (Acropolis, Athens): Characterization and origin. Anal. Chim. Acta 2005, 532, 187–198. [Google Scholar] [CrossRef]
- Chapoulie, R.; Cazenave, S.; Duttine, M. Laser cleaning of historical limestone buildings in Bordeaux appraisal using Cathodoluminescence and Electron Paramagnetic Resonance. Environ. Sci. Pollut. Res. 2008, 15, 237–243. [Google Scholar] [CrossRef]
- Salvini, S.; Coletti, C.; Maritan, L.; Massironi, M.; Pieropan, A.; Spiess, R.; Mazzoli, C. Petrographic characterization and durability of carbonate stones used in UNESCO World Heritage sites in northeastern Italy. Environ. Earth Sci. 2023, 82, 49. [Google Scholar] [CrossRef]
- Apostolopoulou, M.; Drakopoulou, E.; Karoglou, M.; Bakolas, A. Consolidation treatments for marble sugaring: Reinforced lime versus nanolime. Mater. Struct. 2021, 54, 195. [Google Scholar] [CrossRef]
- Carretti, E.; Dei, L. Physicochemical characterization of acrylic polymeric resins coating porous materials of artistic interest. Prog. Org. Coat. 2004, 49, 282–289. [Google Scholar] [CrossRef]
- Maravelaki-Kalaitzaki, P.; Kallithrakas-Kontos, N.; Agioutantis, Z.; Maurigiannakis, S.; Korakaki, D. A comparative study of porous limestones treated with silicon-based strengthening agents. Prog. Org. Coat. 2008, 62, 49–60. [Google Scholar] [CrossRef]
- Gemelli, G.M.C.; Zarzuela, R.; Fernandez, F.; Mosquera, M.J. Compatibility, effectiveness and susceptibility to degradation of alkoxysilane-based consolidation treatments on a carbonate stone. J. Build. Eng. 2021, 42, 102840. [Google Scholar] [CrossRef]
- Del Monte, M.; Sabbioni, C. A study of the patina called ‘scialbatura’ on imperial Roman marbles. Stud. Conserv. 1987, 32, 114–121. [Google Scholar] [CrossRef]
- Mifsud, T. The Treatment of Weathered Globigerina Limestone: The Surface Conversion of Calcium Carbonate to Calcium Oxalate. Master’s Thesis, University of Malta, Msida, Malta, 2006. [Google Scholar]
- Burgos-Cara, A.; Ruiz-Agudo, E.; Rodriguez-Navarro, C. Effectiveness of oxalic acid treatments for the protection of marble surfaces. Mater. Des. 2017, 115, 82–92. [Google Scholar] [CrossRef]
- Cezar, T.M. Calcium Oxalate: A Surface Treatment for Limestone. J. Conserv. Mus. Stud. 1998, 4, 6. [Google Scholar] [CrossRef]
- Dreyfuss, T. Interactions on site between powdering porous limestone, natural salt mixtures and applied ammonium oxalate. Herit. Sci. 2019, 7, 5. [Google Scholar] [CrossRef]
- Sassoni, E. Hydroxyapatite and Other Calcium Phosphates for the Conservation of Cultural Heritage: A Review. Materials 2018, 11, 557. [Google Scholar] [CrossRef]
- Sassoni, E.; Naidu, S.; Scherer, G.W. The use of hydroxyapatite as a new inorganic consolidant for damaged carbonate stones. J. Cult. Herit. 2011, 12, 346–355. [Google Scholar] [CrossRef]
- Graziani, G.; Sassoni, E.; Franzoni, E.; Scherer, G.W. Hydroxyapatite coatings for marble protection: Optimization of calcite covering and acid resistance. Appl. Surf. Sci. 2016, 368, 241–257. [Google Scholar] [CrossRef]
- Graziani, G.; Sassoni, E.; Scherer, G.W.; Franzoni, E. Resistance to simulated rain of hydroxyapatite and calcium oxalate-based coatings for protection of marble against corrosion. Corros. Sci. 2017, 127, 168–174. [Google Scholar] [CrossRef]
- Naidu, S.; Scherer, G.W. Nucleation, growth and evolution of calcium phosphate films on calcite. J. Colloid Interface Sci. 2014, 435, 128–137. [Google Scholar] [CrossRef]
- Sassoni, E.; Graziani, G.; Franzoni, E.; Scherer, G.W. Calcium phosphate coatings for marble conservation: Influence of ethanol and isopropanol addition to the precipitation medium on the coating microstructure and performance. Corros. Sci. 2018, 136, 255–267. [Google Scholar] [CrossRef]
- Xu, F.; Li, D. The use of CTAB as an addition of DAP for improvement resisting acid rain on limestone. Appl. Surf. Sci. 2017, 422, 1059–1066. [Google Scholar] [CrossRef]
- Naidu, S.; Blair, J.; Scherer, G.W. Acid-Resistant Coatings on Marble. J. Am. Ceram. Soc. 2016, 99, 3421–3428. [Google Scholar] [CrossRef]
- Gu, Y.; Zha, J.; Wang, F.; Han, H.; Lu, J.; Wei, S. Preparation of a nano aluminum phosphate enhanced hydroxyapatite coating for marble conservation. J. Cult. Herit. 2024, 67, 512–521. [Google Scholar] [CrossRef]
- Yamagishi, K.; Onuma, K.; Suzuki, T.; Okada, F.; Tagami, J.; Otsuki, M.; Senawangse, P. A synthetic enamel for rapid tooth repair. Nature 2005, 433, 819. [Google Scholar] [CrossRef]
- Mohabatpour, F.; Chen, X.; Papagerakis, S.; Papagerakis, P. Novel trends, challenges and new perspectives for enamel repair and regeneration to treat dental defects. Biomater. Sci. 2022, 10, 3062. [Google Scholar] [CrossRef]
- Shao, C.; Jin, B.; Mu, Z.; Lu, H.; Zhao, Y.; Wu, Z.; Yan, L.; Zhang, Z.; Zhou, Y.; Pan, H.; et al. Repairof tooth enamel by a biomimetic mineralization frontier ensuring epitaxial growth. Sci. Adv. 2019, 5, eaaw9569. [Google Scholar] [CrossRef]
- Chen, H.; Tang, Z.; Liu, J.; Sun, K.; Chang, S.; Peters, M.C.; Mansfield, J.F.; Czajka-Jakubowska, A.; Clarkson, B.H. Acellular Synthesis of a Human Enamel-like Microstructure. Adv. Mater. 2006, 18, 1846–1851. [Google Scholar] [CrossRef]
- Huang, W.; Restrepo, D.; Jung, J.; Su, F.Y.; Liu, Z.; Ritchie, R.O.; McKittrick, J.; Zavattieri, P.; Kisailus, D. Multiscale toughening mechanisms in biological materials and bioinspired designs. Adv. Mater. 2019, 31, 1901561. [Google Scholar] [CrossRef]
- Onuma, K.; Iijima, M. Artificial enamel induced by phase transformation of amorphous nanoparticles. Sci. Rep. 2017, 7, 2711. [Google Scholar] [CrossRef]
- Olson, T.Y.; Chernov, A.A.; Drabek, B.A.; Satcher, J.H.; Han, T.Y.-J. Experimental validation of the geometrical selection model for hydrothermally grown zinc oxide nanowire arrays. Chem. Mater. 2013, 25, 1363–1371. [Google Scholar] [CrossRef]
- Yokoi, T.; Hara, M.; Seki, T.; Terasaka, S.; Kamitakahara, M.; Matsubara, H. Synthesis of layered double hydroxide coatings with an oriented structure and controllable thickness on aluminum substrate. Cryst. Eng. Comm. 2016, 18, 1207–1214. [Google Scholar] [CrossRef]
- Wang, K.; Hu, D. Hydroxyapatite: Collagen biomimetic composite material in conservation of tortoise shell relics. J. Nat. Mus. China 2013, 116, 141–152. (In Chinese) [Google Scholar]
- Noori, A.; Hoseinpour, M.; Kolivand, S.; Lotfibakhshaiesh, N.; Ebrahimi-Barough, S.; Ai, J.; Azami, M. Exploring the various effects of Cu doping in hydroxyapatite nanoparticle. Sci. Rep. 2024, 14, 3421. [Google Scholar] [CrossRef]
- He, L.; Fujisawa, N.; Swain, M.V. Elastic modulus and stress-strain response of human enamel by nano-indentation. Biomaterials 2006, 27, 4388–4398. [Google Scholar] [CrossRef]
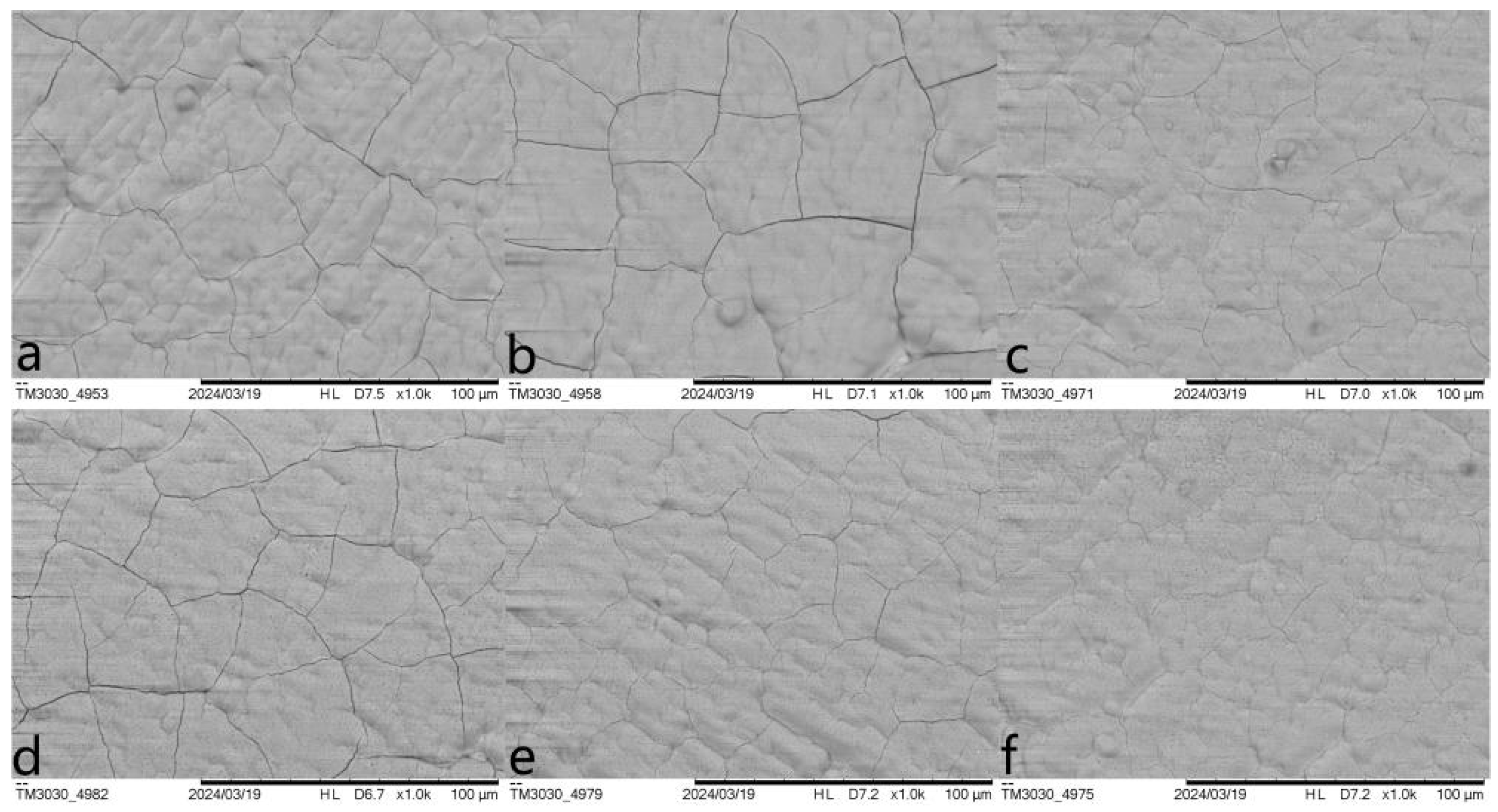
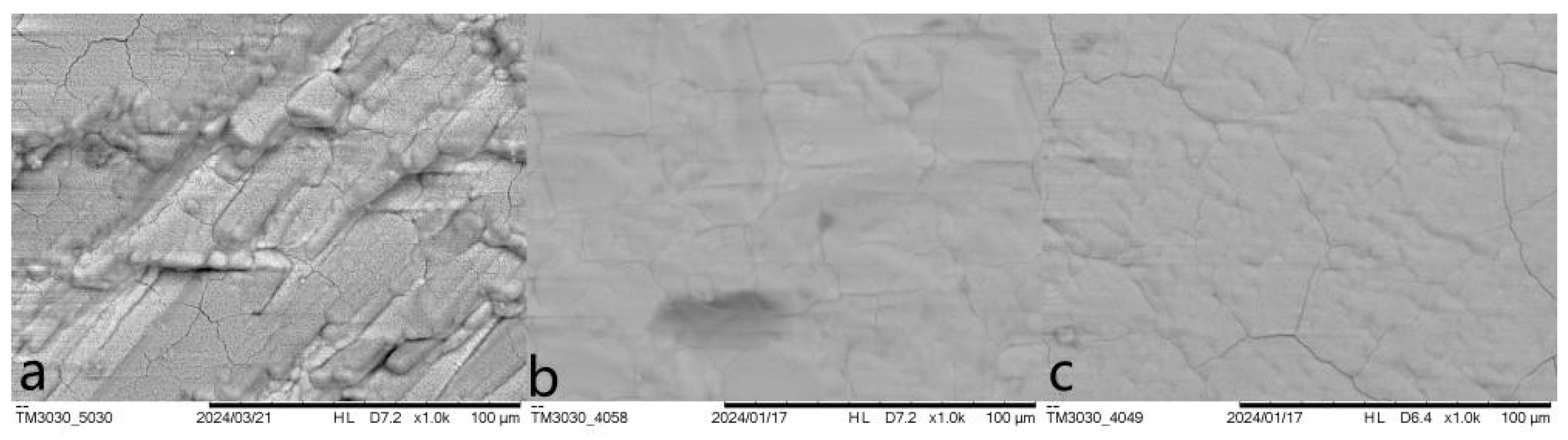
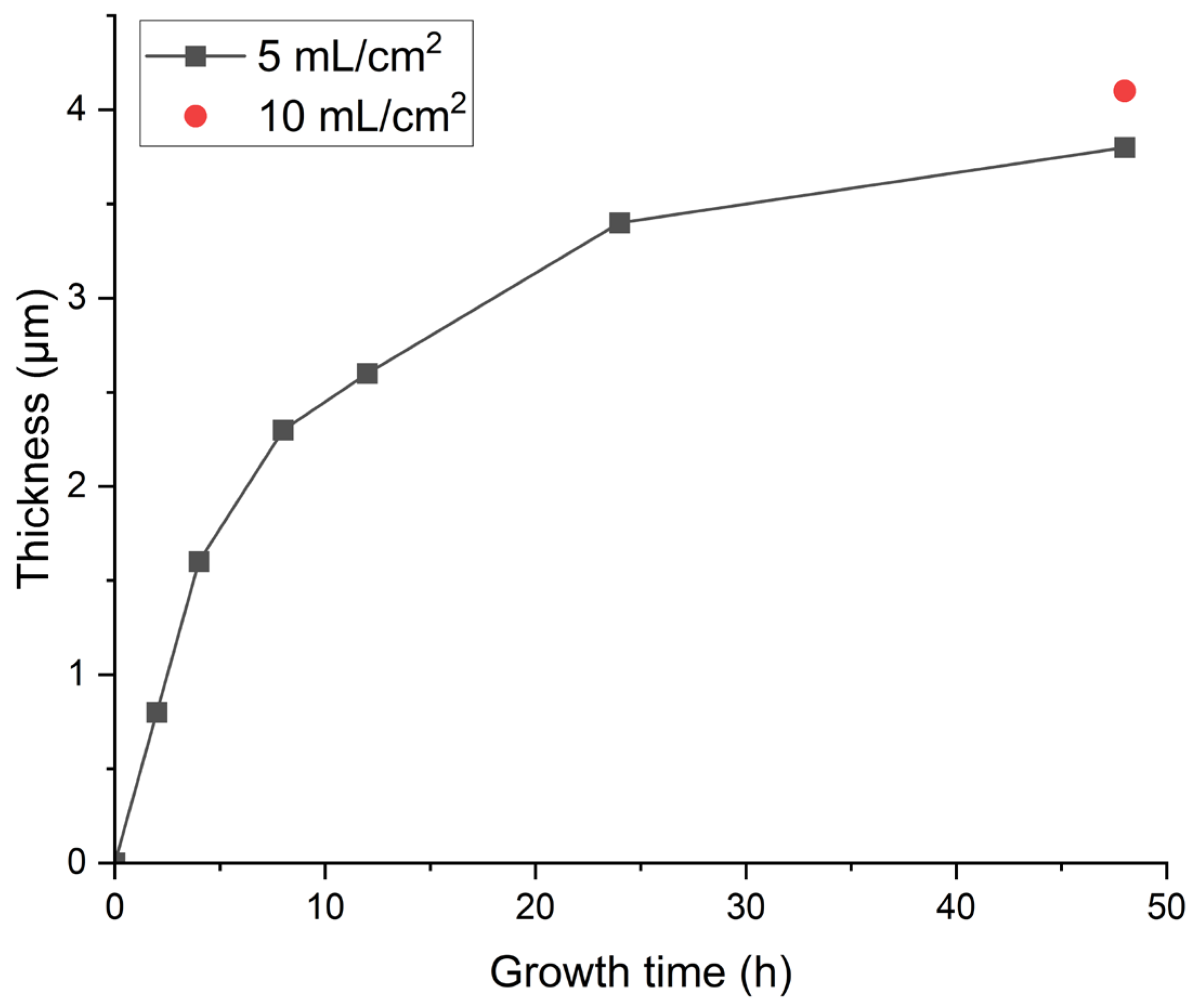
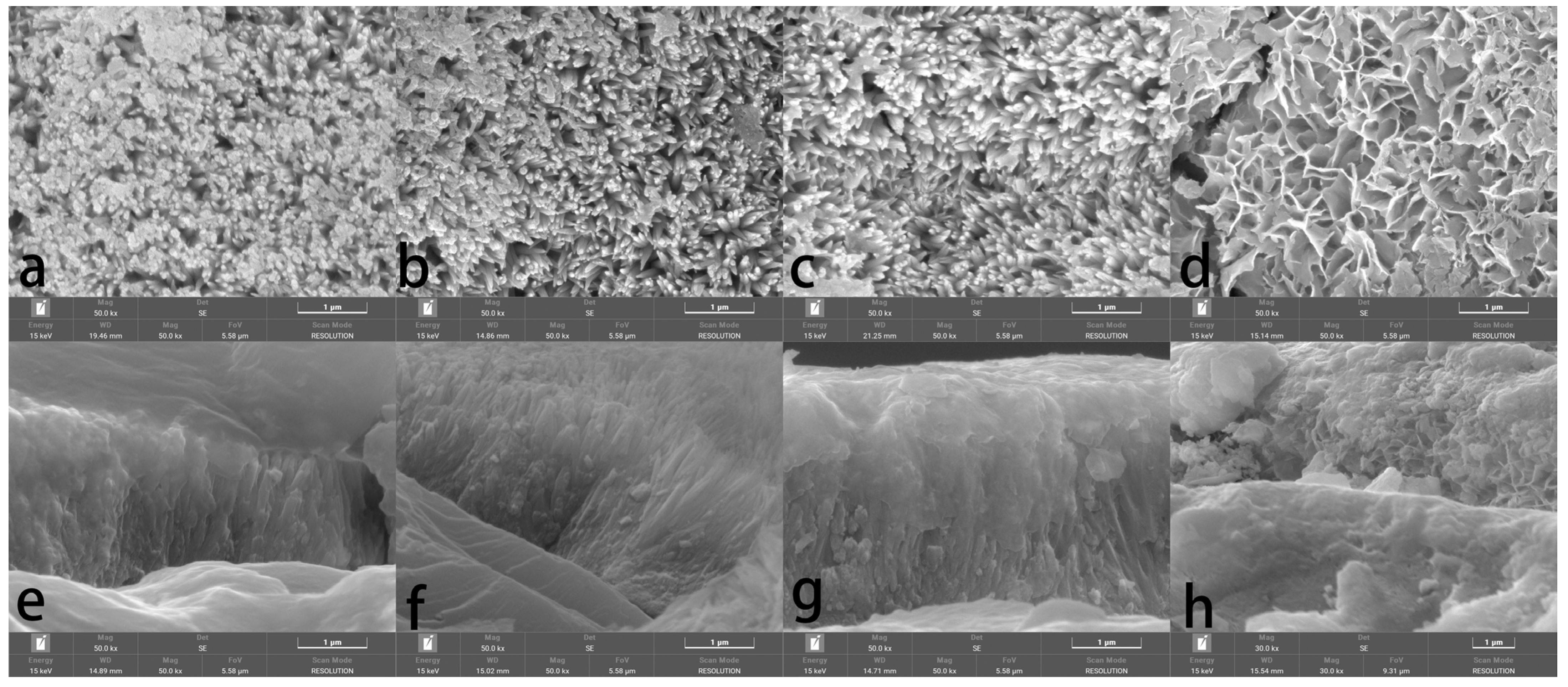
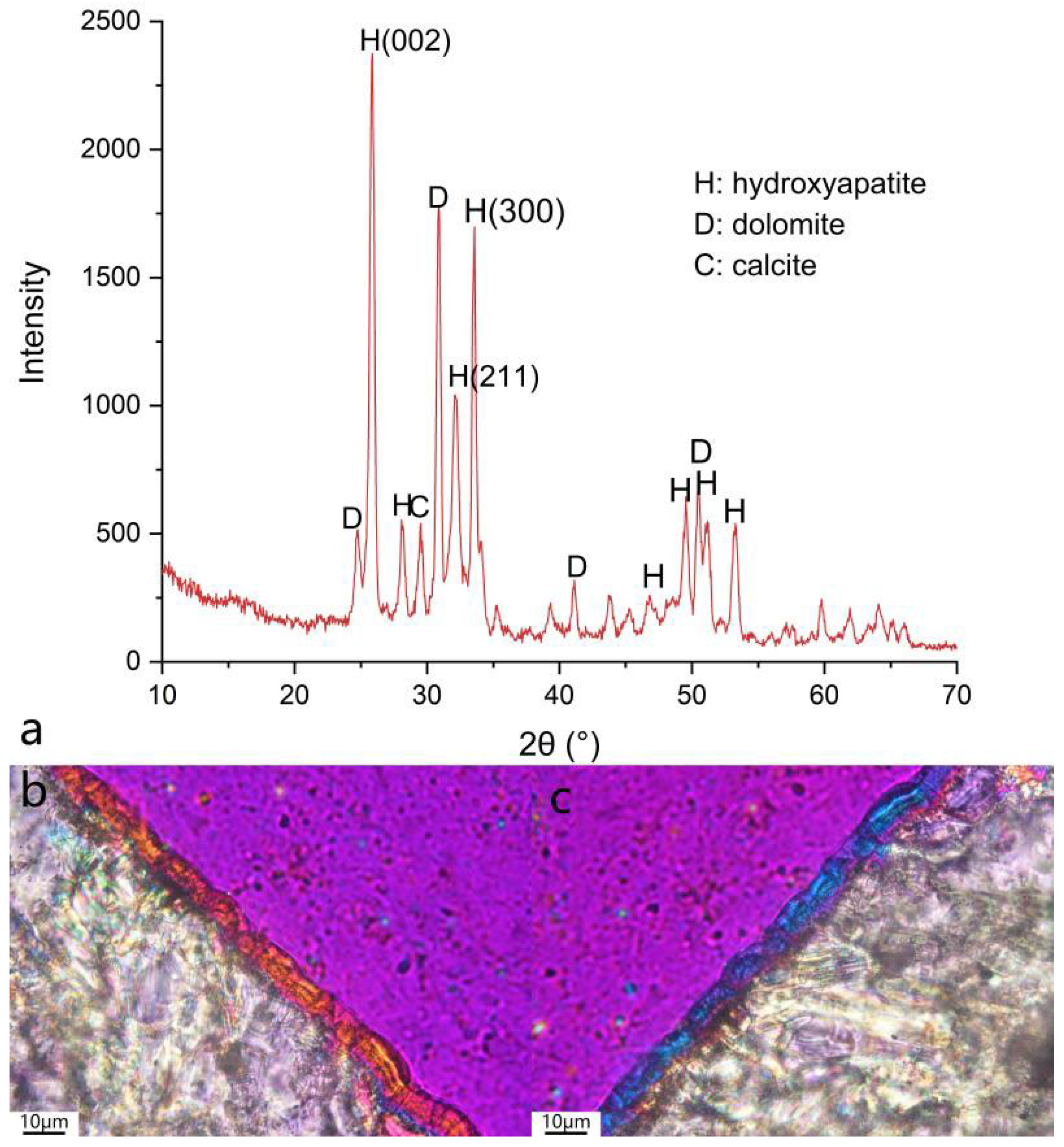

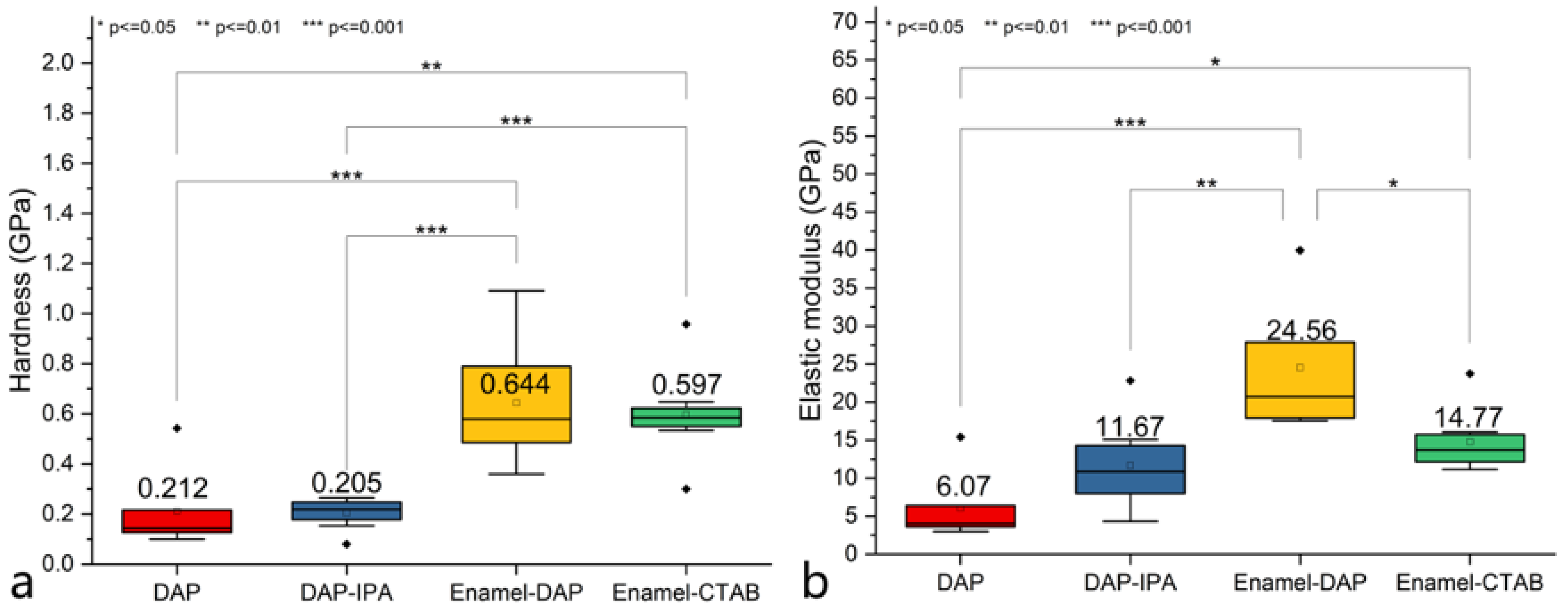
| Entry | Pretreatment | HAP Collosol Applied (Solid Content, μg/cm2) | Additives in Mother Solution | Growth Time (h) | Coating Status |
|---|---|---|---|---|---|
| 1 | none | 105.8 | none | 24 | partially opaque and flaking |
| 2 | none | 72.0 | none | 24 | transparent but flaking |
| 3 | none | 50.4 | none | 24 | transparent, well attached, and with micro cracks |
| 4 | none | 34.2 | none | 24 | transparent, well attached, and micro cracks |
| 5 | none | 10.2 | none | 24 | transparent, well attached, and with micro cracks |
| 6 | none | 0 | none | 24 | No coating |
| 7 | none | 35 * | ethanol | 24 | transparent, well attached, and with micro cracks |
| 8 | none | 10.3 | CTAB | 24 | transparent, well attached, and with minor micro cracks |
| 9 | 1 mol/L DAP for 1 h | 10.6 | none | 24 | transparent, well attached, and with minimal micro cracks |
| 10 | 1 mol/L DAP for 1 h | 0 | none | 24 | No coating |
| 11 | 1 mol/L DAP for 1 h | 35 * | none | 2 | transparent, well attached, and with minor micro cracks |
| 12 | 1 mol/L DAP for 1 h | 35 * | none | 4 | transparent, well attached, and with minor micro cracks |
| 13 | 1 mol/L DAP for 1 h | 35 * | none | 8 | transparent, well attached, and with minor micro cracks |
| 14 | 1 mol/L DAP for 1 h | 35* | none | 12 | transparent, well attached, and with minor micro cracks |
| 15 | 1 mol/L DAP for 1 h | 35 * | none | 24 | transparent, well attached, and with minor micro cracks |
| 16 | 1 mol/L DAP for 1 h | 35 * | none | 48 | transparent, well attached, and with minor micro cracks |
| Preparation Method | Ca (at. %) | O (at. %) | P (at. %) | C (at. %) | F (at. %) | Na (at. %) | Mg (at. %) | Ca/P | P/F |
|---|---|---|---|---|---|---|---|---|---|
| Enamel | 23.25 ± 0.33 | 52.99 ± 1.51 | 13.25 ± 0.58 | 6.38 ± 1.69 | 3.38 ± 0.33 | 0.69 ± 0.60 | 0.05 ± 0.05 | 1.75 | 3.92 |
| Enamel-CTAB | 23.90 ± 0.38 | 53.11 ± 1.58 | 13.43 ± 0.83 | 5.52 ± 2.28 | 3.53 ± 0.17 | 0.46 ± 0.40 | 0.04 ± 0.04 | 1.78 | 3.80 |
| Enamel-DAP | 23.19 ± 0.27 | 54.24 ± 0.69 | 12.99 ± 0.74 | 5.56 ± 0.80 | 3.39 ± 0.15 | 0.56 ± 0.49 | 0.08 ± 0.08 | 1.79 | 3.83 |
| Sample Label | pH | ||
|---|---|---|---|
| 30 min | 60 min | 120 min | |
| Marble (calcite) | 6.80 | 8.10 | 9.24 |
| Marble (dolomite + calcite) | 6.05 | 6.67 | 7.22 |
| DAP | 6.40 | 7.08 | 7.62 |
| DAP-IPA | 6.06 | 6.47 | 6.93 |
| Enamel | 4.82 | 5.39 | 5.52 |
| Enamel-CTAB | 4.97 | 5.31 | 5.69 |
| Enamel-DAP | 5.64 | 6.16 | 6.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Li, W.; Wang, Y.; Wang, K. In Situ Growth of Enamel-like Apatite Coating for Marble Protection. Materials 2025, 18, 880. https://doi.org/10.3390/ma18040880
Zhou Y, Li W, Wang Y, Wang K. In Situ Growth of Enamel-like Apatite Coating for Marble Protection. Materials. 2025; 18(4):880. https://doi.org/10.3390/ma18040880
Chicago/Turabian StyleZhou, Yihang, Wenfei Li, Yue Wang, and Kai Wang. 2025. "In Situ Growth of Enamel-like Apatite Coating for Marble Protection" Materials 18, no. 4: 880. https://doi.org/10.3390/ma18040880
APA StyleZhou, Y., Li, W., Wang, Y., & Wang, K. (2025). In Situ Growth of Enamel-like Apatite Coating for Marble Protection. Materials, 18(4), 880. https://doi.org/10.3390/ma18040880






