Transforming Waste into Valuable Resources: Mo2C Nanoparticles Modified Waste Pinecone-Derived Carbon as an Effective Sulfur Host for Lithium–Sulfur Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
2.3. The Pretreatment and Activation of Pinecone
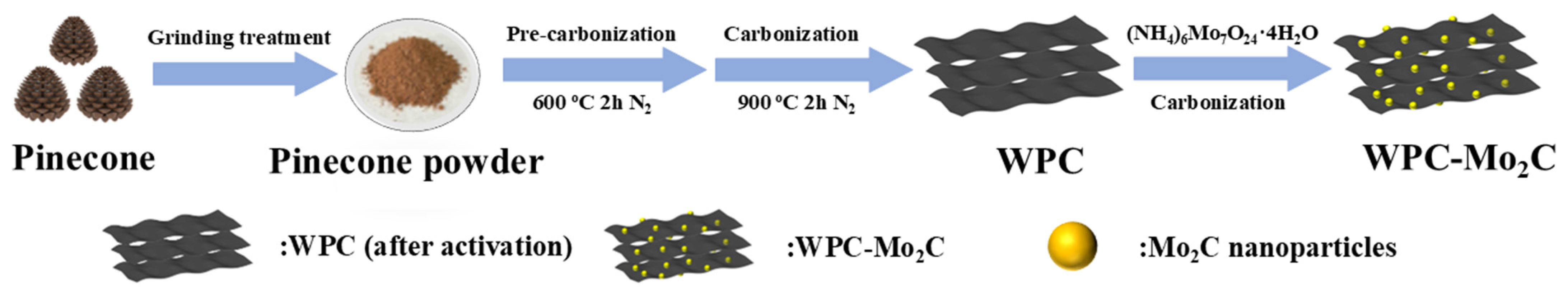
2.4. Synthesis of WPC-Mo2C and WPC
2.5. Synthesis of WPC-Mo2C/S and WPC/S
2.6. Preparation of PC-Mo2C/S Electrode
2.7. Electrochemical Measurements
2.8. Lithium Sulfide Adsorption Test
2.9. Symmetrical Cell Assembly and Measurements
2.10. Li2S Nucleation and Dissolution Experiments
3. Results and Discussions
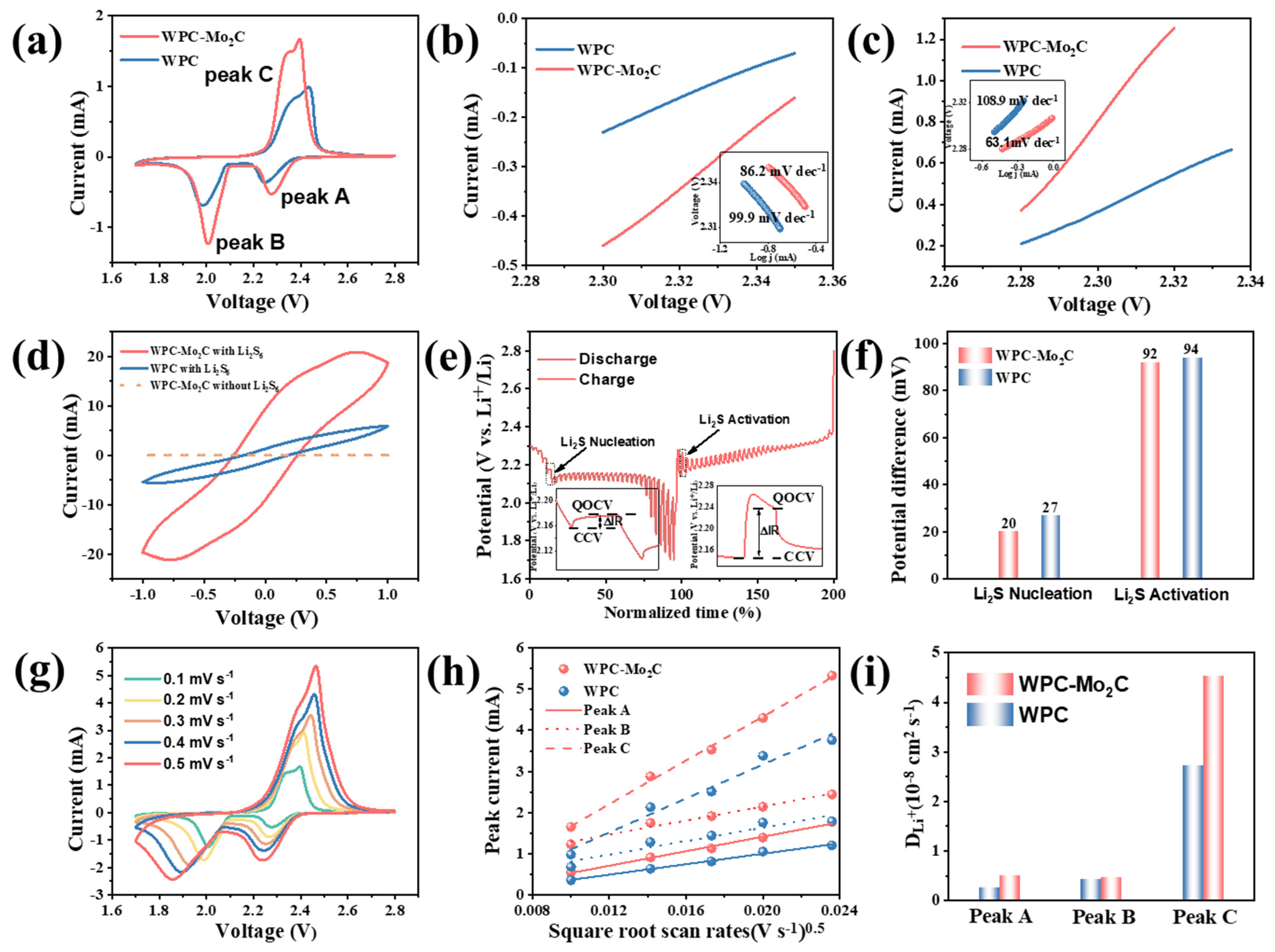
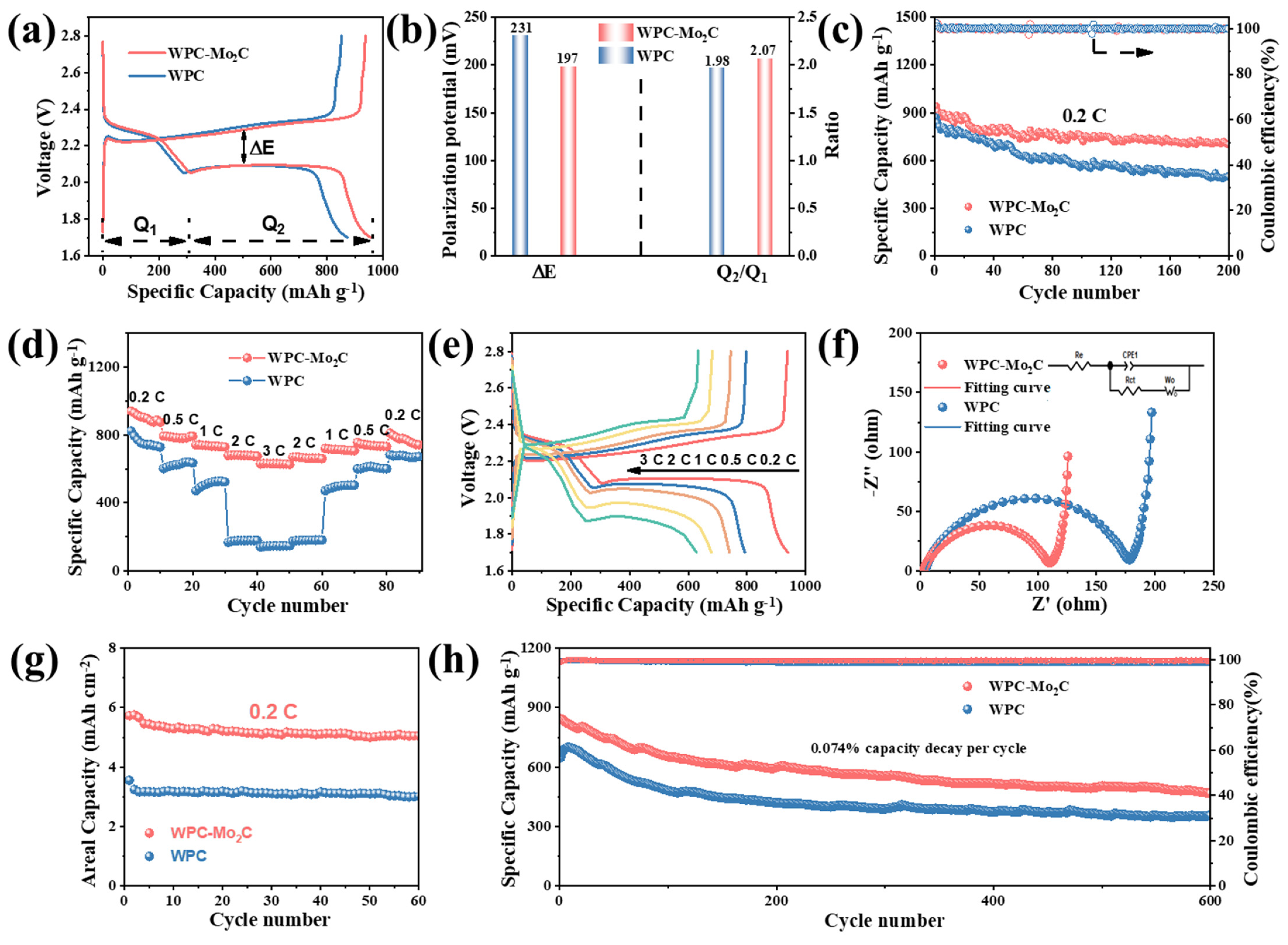
3.1. Results
3.2. Analysis and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, H.; Yang, H.; Ai, X. Routes to electrochemically stable sulfur cathodes for practical Li–S batteries. Adv. Mater. 2023, 2305038. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; He, J.; Zhu, Z.; Cheng, X.-B.; Zhu, J.; Lu, B.; Wu, Y. Heterostructures regulating lithium polysulfides for advanced lithium-sulfur batteries. Adv. Mater. 2023, 35, 2303520. [Google Scholar] [CrossRef] [PubMed]
- Raza, H.; Bai, S.; Cheng, J.; Majumder, S.; Zhu, H.; Liu, Q.; Zheng, G.; Li, X.; Chen, G. Li-S batteries: Challenges, achievements and opportunities. Electrochem. Energy Rev. 2023, 6, 29. [Google Scholar] [CrossRef]
- Zhao, F.; Xue, J.; Shao, W.; Yu, H.; Huang, W.; Xiao, J. Toward high-sulfur-content, high-performance lithium-sulfur batteries: Review of materials and technologies. J. Energy Chem. 2023, 80, 625–657. [Google Scholar] [CrossRef]
- Zuo, X.; Wang, L.; Zhen, M.; You, T.; Liu, D.; Zhang, Y. Multifunctional TiN-MXene-Co@CNTs Networks as sulfur/lithium host for high-areal-capacity lithium-sulfur batteries. Angew. Chem. Int. Ed. 2024, 63, e202408026. [Google Scholar] [CrossRef]
- Chen, R.; Zhou, Y.; Li, X. Nanocarbon-enabled mitigation of sulfur expansion in lithium–sulfur batteries. Energy Storage Mater. 2024, 68, 103353. [Google Scholar] [CrossRef]
- Yang, Z.; Hu, Z.; Yan, G.; Li, M.; Feng, Y.; Qu, X.; Zhang, X. Multi-function hollow nanorod as an efficient sulfur host accelerates sulfur redox reactions for high-performance Li-S batteries. J. Colloid Interface Sci. 2023, 629, 65–75. [Google Scholar] [CrossRef]
- Zhao, M.; Li, B.-Q.; Zhang, X.-Q.; Huang, J.-Q.; Zhang, Q. A perspective toward practical lithium–sulfur batteries. ACS Cent. Sci. 2020, 6, 1095–1104. [Google Scholar] [CrossRef]
- Huang, L.; Li, J.; Liu, B.; Li, Y.; Shen, S.; Deng, S.; Lu, C.; Zhang, W.; Xia, Y.; Pan, G.; et al. Electrode design for lithium–sulfur batteries: Problems and solutions. Adv. Funct. Mater. 2020, 30, 1910375. [Google Scholar] [CrossRef]
- Yang, T.; Xia, J.; Piao, Z.; Yang, L.; Zhang, S.; Xing, Y.; Zhou, G. Graphene-based materials for flexible lithium–sulfur batteries. ACS Nano 2021, 15, 13901–13923. [Google Scholar] [CrossRef]
- Luo, J.; Guan, K.; Lei, W.; Zhang, S.; Jia, Q.; Zhang, H. One dimensional carbon-based composites as cathodes for lithium-sulfur battery. J. Mater. Sci. 2022, 122, 101–120. [Google Scholar] [CrossRef]
- Chen, L.; Yuan, Y.; Orenstein, R.; Yanilmaz, M.; He, J.; Liu, J.; Liu, Y.; Zhang, X. Carbon materials dedicate to bendable supports for flexible lithium-sulfur batteries. Energy Storage Mater. 2023, 60, 102817. [Google Scholar] [CrossRef]
- Xiang, Y.; Lu, L.; Kottapalli, A.G.P.; Pei, Y. Status and perspectives of hierarchical porous carbon materials in terms of high-performance lithium–sulfur batteries. Carbon Energy 2022, 4, 346–398. [Google Scholar] [CrossRef]
- Hou, M.; Chen, K.; Zhang, G.; Liang, X.; Liu, X.; Xing, S. 3D conductive molecular framework derived MnO2/N, P co-doped carbon as sulfur hosts for high-performance lithium-sulfur batteries. J.Energy Storage 2023, 72, 108339. [Google Scholar] [CrossRef]
- Chen, K.; Lin, Z.; Zhang, G.; Zheng, J.; Fan, Z.; Xiao, L.; Xu, Q.; Xu, J. Efficient host materials for lithium-sulfur batteries: Ultrafine CoP nanoparticles in black phosphorus-carbon composite. ChemSusChem 2024, 17, e202400339. [Google Scholar] [CrossRef]
- Li, Y.; Hao, Y.; Ali, U.; Liu, B.; Zhang, Q.; Jin, Z.; Li, L.; Wang, C.; Zhang, L. Ultrafine Ni12P5 nanoparticle-embedded carbon with abundant catalytic activity sites as separator modifiers in high-performance lithium–sulfur batteries. Inorg. Chem. Front. 2023, 10, 5719–5725. [Google Scholar] [CrossRef]
- Yang, Z.; Liang, X.; Chen, K.; Zhang, G.; Xing, S. Incorporation of Mo2C nanoparticles to pollen-derived 3D porous carbon as electrocatalyst for high performance lithium-sulfur batteries. J. Energy Storage 2024, 102, 114218. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, S.; Zeng, X.; Li, X.; Xiao, L.; Chen, K.; Lu, Q.; Xu, Q.; Weng, J.; Xu, J. Holey amorphous FeCoO-coated black phosphorus for robust polysulfide adsorption and catalytic conversion in lithium–sulfur batteries. J. Mater. Chem. A 2022, 10, 11676–11683. [Google Scholar] [CrossRef]
- Wu, Q.; Chen, K.; Shadike, Z.; Li, C. Relay-type catalysis by a dual-metal single-Atom system in a waste biomass derivative host for high-rate and durable Li–S batteries. ACS Nano 2024, 18, 13468–13483. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.; Liu, X.; Zhang, Y.; Zhao, W.; Li, Y.; Qin, C.; Bakenov, Z. High specific surface area bimodal porous carbon derived from biomass reed flowers for high performance lithium-sulfur batteries. J. Colloid Interface Sci. 2020, 569, 22–33. [Google Scholar] [CrossRef]
- Yuan, H.; Liu, T.; Liu, Y.; Nai, J.; Wang, Y.; Zhang, W.; Tao, X. A review of biomass materials for advanced lithium–sulfur batteries. Chem. Sci. 2019, 10, 7484–7495. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, L.; Shen, L.; Zhou, Q.; Chen, Y.; Wu, J.; Wen, Y.; Zheng, J. CoO embedded porous biomass-derived carbon as dual-functional host material for lithium-sulfur batteries. J. Colloid Interface Sci. 2023, 640, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Huang, Z.; Zhang, C.; Wang, T.; Hao, T.; Deng, D.; Sun, Z.; Wang, Y.; Xu, C.; Zeng, J.; et al. Monolithic carbon derived from biomass via zinc-assisted pyrolysis for lithium-sulfur batteries. Green Chem. 2025. [Google Scholar] [CrossRef]
- Dharmesh, A.; Rani, P.; Sharma, A.K. Tamarind peel-derived nitrogen-doped porous carbon: A sustainable sulfur host for enhanced lithium–sulfur battery performance. J. Power Sources 2025, 634, 236429. [Google Scholar] [CrossRef]
- Chen, R.; Shen, J.; Chen, K.; Tang, M.; Zeng, T. Metallic phase MoS2 nanosheet decorated biomass carbon as sulfur hosts for advanced lithium–sulfur batteries. Appl. Surf. Sci. 2021, 566, 150651. [Google Scholar] [CrossRef]
- Han, H.; Park, G.; Kim, S.; Choi, Y.; Park, C.S.; Le, T.-H.; Chae, S.; Kim, Y.A.; Yoon, H. Selective incorporation of aqueous-phase SWNTs into pine cones: A unique route to creating versatile carbon precursors for electrode materials. ACS Sustain.Chem. Eng. 2018, 6, 12426–12435. [Google Scholar] [CrossRef]
- Saikia, D.; Deka, J.R.; Lu, B.-J.; Chen, Y.-C.; Lian, J.-W.; Kao, H.-M.; Yang, Y.-C. Pinecone-derived biomass carbons as anodes for lithium and sodium-ion batteries by template-assisted and chemically activated approaches. J. Power Sources 2023, 580, 233329. [Google Scholar] [CrossRef]
- He, M.; Li, X.; Li, W.; Zheng, M.; Wang, J.; Ma, S.; Ma, Y.; Yin, G.; Zuo, P.; Sun, X. Immobilization and kinetic promotion of polysulfides by molybdenum carbide in lithium-sulfur batteries. Chem. Eng. J. 2021, 411, 128563. [Google Scholar] [CrossRef]
- Li, W.; Chen, K.; Xu, Q.; Li, X.; Zhang, Q.; Weng, J.; Xu, J. Mo2C/C Hierarchical double-shelled hollow spheres as sulfur host for advanced Li-S batteries. Angew. Chem. Int. Ed. 2021, 60, 21512–21520. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y.; Lan, Y.; Lu, G.; Liu, L.; Tang, T.; Li, M.; Cheng, Y.; Xiao, J.; Li, X. Integrated design for discrete sulfur@polymer nanoreactor with tandem connection as lithium–sulfur battery cathodes. Angew. Chem. Int. Ed. 2024, 63, e202406693. [Google Scholar] [CrossRef]
- Chen, M.; Song, C.; Liang, C.; Zhang, B.; Sun, Y.; Li, S.; Lin, L.; Xu, P. Crystalline phase induced Raman enhancement on molybdenum carbides. Inorg. Chem. Front. 2022, 9, 2575–2582. [Google Scholar] [CrossRef]
- Sultanov, F.; Zhumasheva, N.; Dangaliyeva, A.; Zhaisanova, A.; Baikalov, N.; Tatykayev, B.; Yeleuov, M.; Bakenov, Z.; Mentbayeva, A. Enhancing lithium-sulfur battery performance with biomass-derived graphene-like porous carbon and NiO nanoparticles composites. J. Power Sources 2024, 593, 233959. [Google Scholar] [CrossRef]
- Cui, J.; Liu, J.; Chen, X.; Meng, J.; Wei, S.; Wu, T.; Wang, Y.; Xie, Y.; Lu, C.; Zhang, X. Ganoderma Lucidum-derived erythrocyte-like sustainable materials. Carbon 2022, 196, 70–77. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, P.; Dai, L.; Gui, Y.; Yuan, L.; Shen, X.; Zhang, C.; Huo, K. A scalable waste-free biorefinery inspires revenue from holistic lignocellulose valorization. Green Chem. 2021, 23, 6008–6019. [Google Scholar] [CrossRef]
- Choi, J.R.; Kim, E.; Park, B.-I.; Choi, I.; Park, B.-H.; Lee, S.-B.; Lee, J.H.; Yu, S. Meringue-derived hierarchically porous carbon as an efficient polysulfide regulator for lithium-sulfur batteries. J. Ind. Eng. Chem. 2022, 115, 355–364. [Google Scholar] [CrossRef]
- Fan, L.; Li, Z.; Kang, W.; Cheng, B. Biomass-derived tube-like nitrogen and oxygen dual-doped porous carbon in the sulfur cathode for lithium sulfur battery. Renew. Energy 2020, 155, 309–316. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Han, Y.; Chen, K.; Zhang, G.; Xing, S. Transforming Waste into Valuable Resources: Mo2C Nanoparticles Modified Waste Pinecone-Derived Carbon as an Effective Sulfur Host for Lithium–Sulfur Batteries. Materials 2025, 18, 1141. https://doi.org/10.3390/ma18051141
Yang Z, Han Y, Chen K, Zhang G, Xing S. Transforming Waste into Valuable Resources: Mo2C Nanoparticles Modified Waste Pinecone-Derived Carbon as an Effective Sulfur Host for Lithium–Sulfur Batteries. Materials. 2025; 18(5):1141. https://doi.org/10.3390/ma18051141
Chicago/Turabian StyleYang, Zhe, Yicheng Han, Kai Chen, Guodong Zhang, and Shuangxi Xing. 2025. "Transforming Waste into Valuable Resources: Mo2C Nanoparticles Modified Waste Pinecone-Derived Carbon as an Effective Sulfur Host for Lithium–Sulfur Batteries" Materials 18, no. 5: 1141. https://doi.org/10.3390/ma18051141
APA StyleYang, Z., Han, Y., Chen, K., Zhang, G., & Xing, S. (2025). Transforming Waste into Valuable Resources: Mo2C Nanoparticles Modified Waste Pinecone-Derived Carbon as an Effective Sulfur Host for Lithium–Sulfur Batteries. Materials, 18(5), 1141. https://doi.org/10.3390/ma18051141






