Effects of TiC, TiH2, Al, and Carbon on Production of Ti3AlC2 by Self-Sustaining Combustion Synthesis
Highlights
- An elemental Ti-Al-C reaction system is sufficiently exothermic to synthesize Ti3AlC2 through SHS.
- With 20% excess Al as 3Ti-1.2Al-2C, the formation of Ti3AlC2 improved from 56.3 to 80.3 wt%.
- In the TiC-added sample of 2.5Ti + 1.2Al + 1.5C + 0.5TiC, the yield of Ti3AlC2 was further improved to 90 wt%.
- A carbon-deficient composition and the addition of TiH2 resulted in negative effects on the formation of Ti3AlC2.
Abstract
1. Introduction
2. Materials and Methods
3. Results
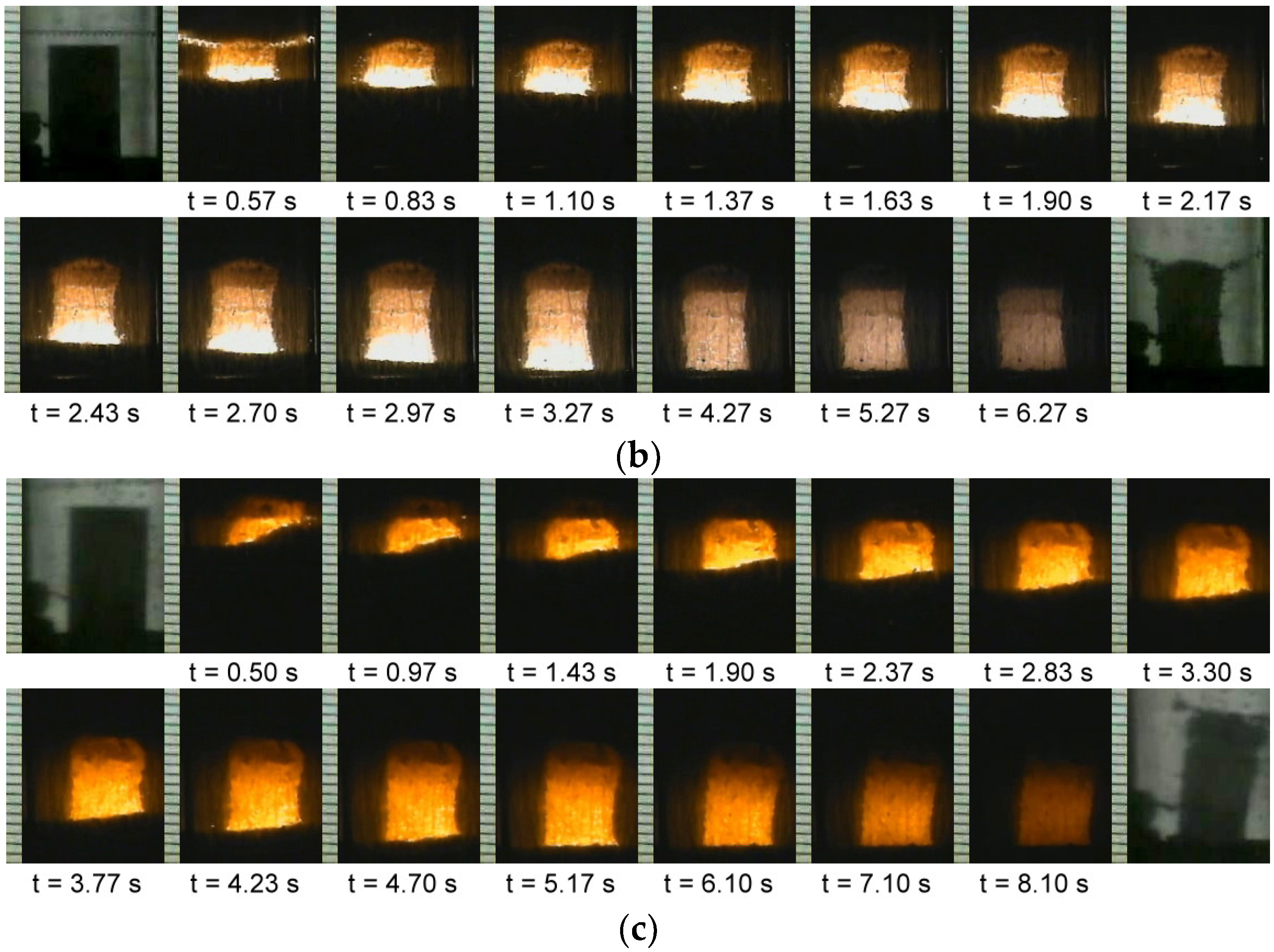
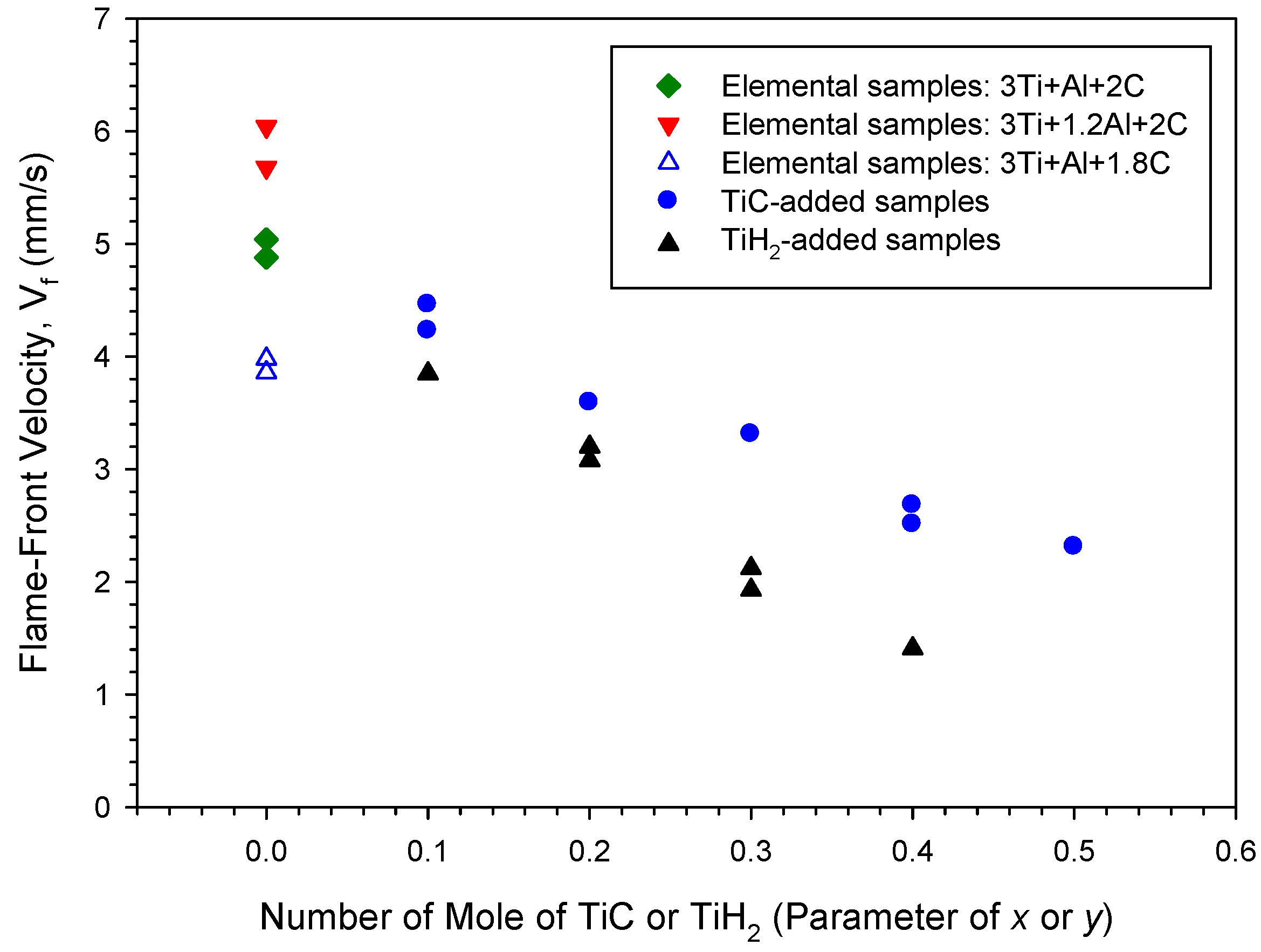
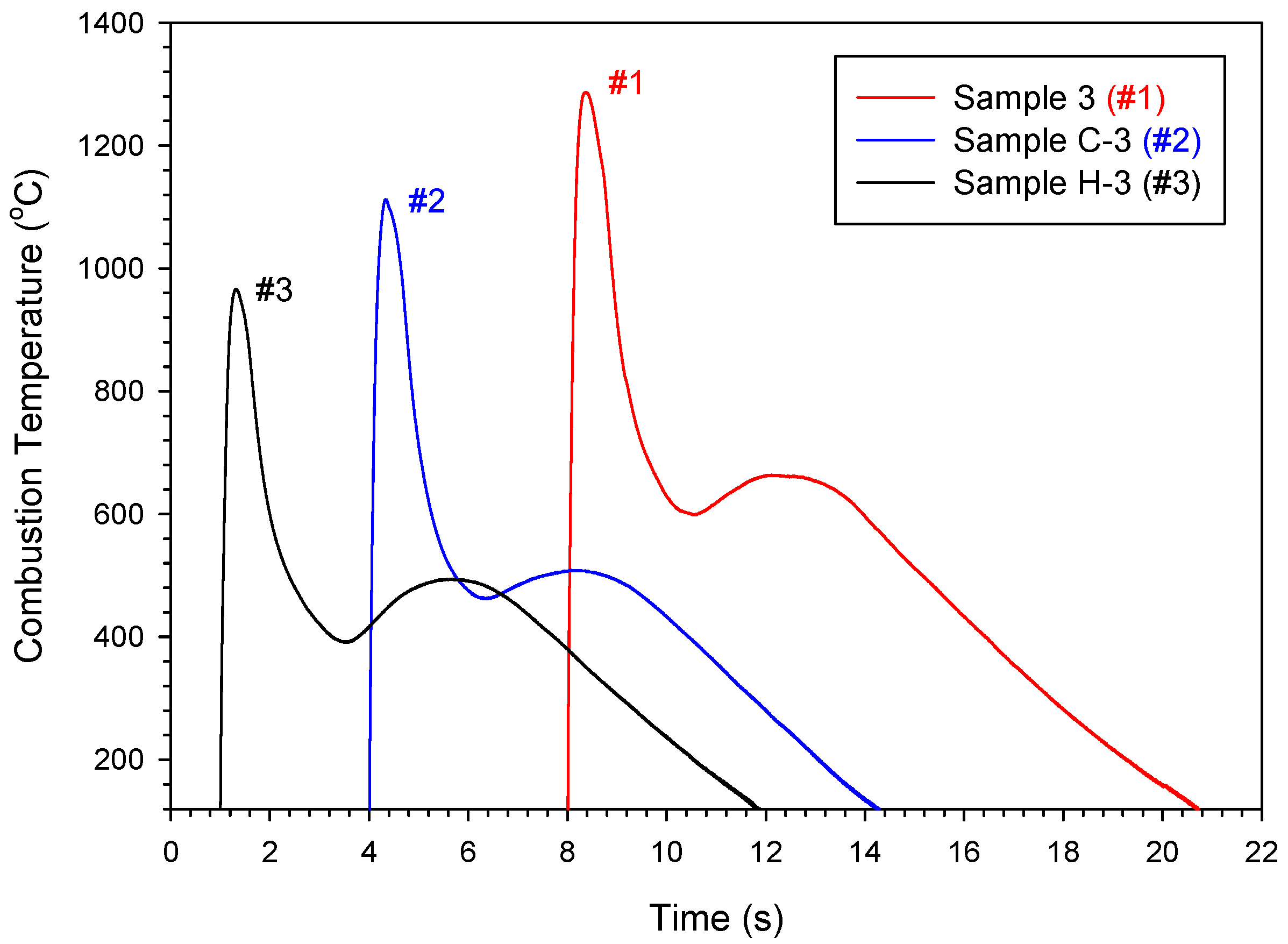
3.1. Combustion Wave Velocity and Combustion Temperature
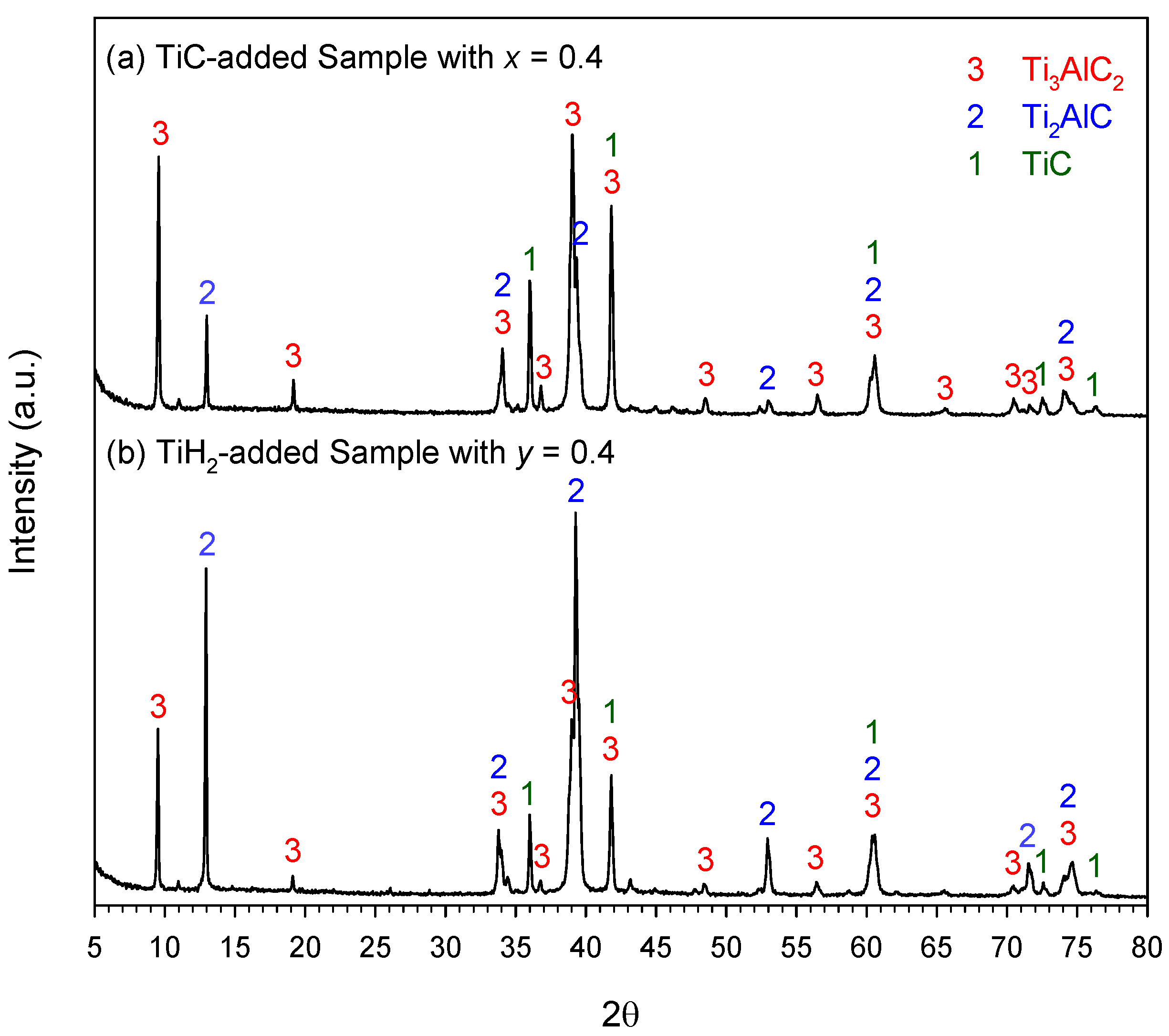
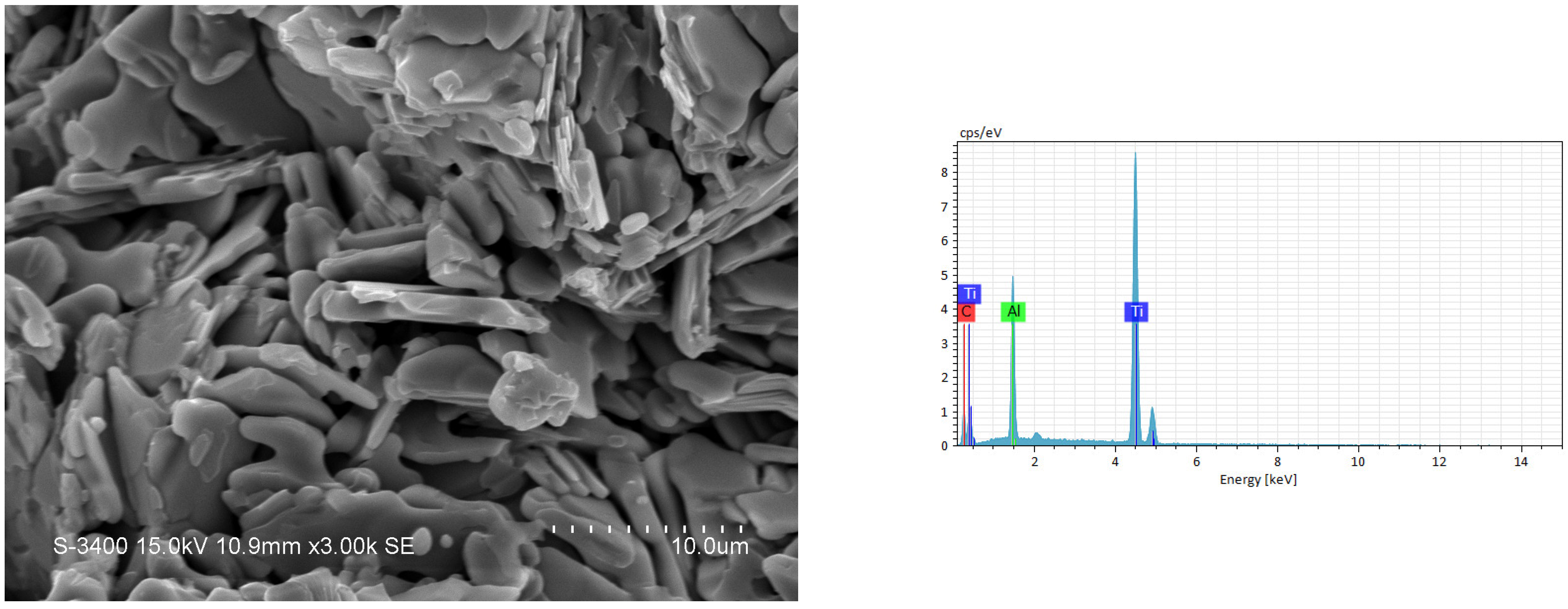
3.2. Phase Composition and Microstructure Analyses of Synthesized Products
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barsoum, M.W. MN+1AXN phases: A new class of solids; thermodynamically stable nanolaminates. Prog. Solid State Chem. 2000, 28, 201–281. [Google Scholar] [CrossRef]
- Pietzka, M.A.; Schuster, J.C. Summary of constitutional data on the Al-C-Ti system. J. Phase Equilib. 1994, 15, 392–400. [Google Scholar] [CrossRef]
- Zhang, Z.; Duan, X.; Jia, D.; Zhou, Y.; van der Zwaag, S. On the formation mechanisms and properties of MAX phases: A review. J. Eur. Ceram. Soc. 2021, 41, 3851–3878. [Google Scholar] [CrossRef]
- Tzenov, N.V.; Barsoum, M.W. Synthesis and characterization of Ti3AlC2. J. Am. Ceram. Soc. 2000, 83, 825–832. [Google Scholar] [CrossRef]
- Barsoum, M.W. MAX Phases: Properties of Machinable Ternary Carbides and Nitrides; Wiley-VCH: Weinheim, Germany, 2013. [Google Scholar]
- Wang, X.H.; Zhou, Y.C. Layered machinable and electrically conductive Ti2AlC and Ti3AlC2 ceramics: A review. J. Mater. Sci. Technol. 2010, 26, 385–416. [Google Scholar] [CrossRef]
- Wang, X.H.; Zhou, Y.C. Microstructure and properties of Ti3AlC2 prepared by the solid-liquid reaction synthesis and simultaneous in-situ hot pressing process. Acta Mater. 2002, 50, 3143–3151. [Google Scholar] [CrossRef]
- Guo, K.Y.; Meng, G.H.; Yang, G.J. Enhanced oxidation resistance in Ti3AlC2 via selective self-healing. J. Eur. Ceram. Soc. 2024, 44, 116676. [Google Scholar] [CrossRef]
- Cui, J.; Hu, X.; Zhang, L.; Yang, Y.; Li, Y.; Chen, G.; Tang, C.; Ke, P. Highly efficient self-healing of fractured Ti3AlC2 MAX phase nanowires. Adv. Funct. Mater. 2024, 2422697. [Google Scholar] [CrossRef]
- Kewate, O.J.; Punniyakoti, S. Ti3AlC2 MAX phase and Ti3C2Tx MXene-based composites towards supercapacitor applications: A comprehensive review of synthesis, recent progress, and challenges. J. Energy Storage 2023, 72, 108501. [Google Scholar] [CrossRef]
- Anayee, M.; Shuck, C.E.; Shekhirev, M.; Goad, A.; Wang, R.; Gogotsi, Y. Kinetics of Ti3AlC2 etching for Ti3C2Tx MXene synthesis. Chem. Mater. 2022, 34, 9589–9600. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, G.; Fang, L.; Wang, Z.; Wu, F.; Liu, G.; Wang, Q.; Nian, H. Surface-modification strategy to produce highly anticorrosive Ti3C2Tx MXene-based polymer composite coatings: A mini-review. Materials 2025, 18, 653. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Julian, J. Processing of MAX phases: From synthesis to applications. J. Am. Ceram. Soc. 2021, 104, 659–690. [Google Scholar] [CrossRef]
- Chen, H.; Du, Y.; Wang, D.; Zhang, C.; Yang, G.; Liu, B.; Gao, Y.; Shi, S. TiC/Ti3AlC2–Co plasma-sprayed coatings with excellent high-temperature tribological properties. Ceram. Int. 2018, 44, 22520–22528. [Google Scholar] [CrossRef]
- Gao, H.; Benitez, R.; Son, W.; Arroyave, R.; Radovic, M. Structural, physical and mechanical properties of Ti3(Al1−xSix)C2 solid solution with x = 0–1. Mater. Sci. Eng. A 2016, 676, 197–208. [Google Scholar] [CrossRef]
- Yu, H.; Suo, X.; Gong, Y.; Zhu, Y.; Zhou, J.; Li, H.; Eklund, P.; Huang, Q. Ti3AlC2 coatings deposited by liquid plasma spraying. Surf. Coat. Technol. 2016, 299, 123–128. [Google Scholar] [CrossRef]
- Gonzalez-Julian, J.; Mauer, G.; Sebold, D.; Mack, D.E.; Vassen, R. Cr2AlC MAX phase as bond coat for thermal barrier coatings: Processing, testing under thermal gradient loading, and future challenges. J. Am. Ceram. Soc. 2020, 103, 2362–2375. [Google Scholar] [CrossRef]
- Galvin, T.; Hyatt, N.C.; Rainforth, W.M.; Reaney, I.M.; Shepherd, D. Slipcasting of MAX phase tubes for nuclear fuel cladding applications. Nucl. Mater. Energy 2020, 22, 100725. [Google Scholar] [CrossRef]
- Sarwar, J.; Shrouf, T.; Srinivasa, A.; Gao, H.; Radovic, M.; Kakosimos, K. Characterization of thermal performance, flux transmission performance and optical properties of MAX phase materials under concentrated solar irradiation. Sol. Energy Mater. Sol. Cells 2018, 182, 76–91. [Google Scholar] [CrossRef]
- Fashandi, H.; Andersson, M.; Eriksson, J.; Lu, J.; Smedfors, K.; Zetterling, C.M.; Spetz, A.L.; Eklund, P. Single-step synthesis process of Ti3SiC2 ohmic contacts on 4H-SiC by sputter-deposition of Ti. Scr. Mater. 2015, 99, 53–56. [Google Scholar] [CrossRef]
- Goc, K.; Prendota, W.; Chlubny, L.; Strączek, T.; Tokarz, W.; Borowiak, P.; Witulska, K.; Bućko, M.M.; Przewoźnik, J.; Lis, J. Structure, morphology and electrical transport properties of the Ti3AlC2 materials. Ceram. Int. 2018, 44, 18322–18328. [Google Scholar] [CrossRef]
- Ng, W.H.; Gnanakumar, E.S.; Batyrev, E.; Sharma, S.K.; Pujari, P.K.; Greer, H.F.; Zhou, W.; Sakidja, R.; Rothenberg, G.; Barsoum, M.W.; et al. The Ti3AlC2 MAX phase as an efficient catalyst for oxidative dehydrogenation of n-butane. Angew. Chem. 2018, 130, 1501–1506. [Google Scholar] [CrossRef]
- Wang, K.; Du, H.; Wang, Z.; Gao, M.; Pan, H.; Liu, Y. Novel MAX-phase Ti3AlC2 catalyst for improving the reversible hydrogen storage properties of MgH2. Int. J. Hydrogen Energy 2017, 42, 4244–4251. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. In MXenes; Jenny Stanford Publishing: Singapore, 2023; pp. 15–29. [Google Scholar]
- Gao, L.; Han, T.; Guo, Z.; Zhang, X.; Pan, D.; Zhou, S.; Chen, W.; Li, S. Preparation and performance of MAX phase Ti3AlC2 by in-situ reaction of Ti-Al-C system. Adv. Powder Technol. 2020, 31, 3533–3539. [Google Scholar] [CrossRef]
- Yang, C.; Jin, S.; Liang, B.; Liu, G.; Duan, L.; Jia, S. Synthesis of Ti3AlC2 by spark plasma sintering of mechanically milled 3Ti/xAl/2C powder mixtures. J. Alloys Compd. 2009, 472, 79–83. [Google Scholar] [CrossRef]
- Yunus, M.; Kumar, R.; Maji, B.C.; Krishnan, M. An optimized method for synthesizing phase-pure Ti3AlC2 MAX-phase through spark plasma sintering. J. Eur. Ceram. Soc. 2022, 42, 354–363. [Google Scholar] [CrossRef]
- Kandrotaitė Janutienė, R.; Syzonenko, O.; Mažeika, D.; Gegeckienė, L.; Venytė, I.; Torpakov, A. Microstructure and phase composition of Ti-Al-C materials obtained by high voltage electrical discharge/spark plasma sintering. Materials 2024, 17, 115. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, J.; Xi, X.; Luo, W.; Zhou, J. Molten salt dynamic sealing synthesis of MAX phases (Ti3AlC2, Ti3SiC2 et al.) powder in air. Ceram. Int. 2023, 49, 168–178. [Google Scholar] [CrossRef]
- Zhong, Y.; Liu, Y.; Jin, N.; Lin, Z.; Ye, J. Molten salt synthesis and formation mechanism of Ti3AlC2: A new path from Ti2AlC to Ti3AlC2. J. Am. Ceram. Soc. 2023, 106, 5567–5579. [Google Scholar] [CrossRef]
- Peng, C.; Wang, C.A.; Song, Y.; Huang, Y. A novel simple method to stably synthesize Ti3AlC2 powder with high purity. Mater. Sci. Eng. A 2006, 428, 54–58. [Google Scholar] [CrossRef]
- Yang, J.; Liao, C.; Wang, J.; Jiang, Y.; He, Y. Reactive synthesis for porous Ti3AlC2 ceramics through TiH2, Al and graphite powders. Ceram. Int. 2014, 40, 6739–6745. [Google Scholar] [CrossRef]
- Shahin, N.; Kazemi, S.; Heidarpour, A. Mechanochemical synthesis mechanism of Ti3AlC2 MAX phase from elemental powders of Ti, Al and C. Adv. Powder Technol. 2016, 27, 1775–1780. [Google Scholar] [CrossRef]
- Zhou, A.; Wang, C.A.; Ge, Z.; Wu, L. Preparation of Ti3AlC2 and Ti2AlC by self-propagating high-temperature synthesis. J. Mater. Sci. Lett. 2001, 20, 1971–1973. [Google Scholar] [CrossRef]
- Ge, Z.; Chen, K.; Guo, J.; Zhou, H.; Ferreira, J.M. Combustion synthesis of ternary carbide Ti3AlC2 in Ti–Al–C system. J. Eur. Ceram. Soc. 2003, 23, 567–574. [Google Scholar] [CrossRef]
- Yeh, C.L.; Shen, Y.G. Combustion synthesis of Ti3AlC2 from Ti/Al/C/TiC powder compacts. J. Alloys Compd. 2008, 466, 308–313. [Google Scholar] [CrossRef]
- Yeh, C.L.; Shen, Y.G. Effects of using Al4C3 as a reactant on formation of Ti3AlC2 by combustion synthesis in SHS mode. J. Alloys Compd. 2009, 473, 408–413. [Google Scholar] [CrossRef]
- Akhlaghi, M.; Tayebifard, S.A.; Salahi, E.; Asl, M.S.; Schmidt, G. Self-propagating high-temperature synthesis of Ti3AlC2 MAX phase from mechanically-activated Ti/Al/graphite powder mixture. Ceram. Int. 2018, 44, 9671–9678. [Google Scholar] [CrossRef]
- Aydinyan, S. Combustion synthesis of MAX phases: Microstructure and properties inherited from the processing pathway. Crystals 2023, 13, 1143. [Google Scholar] [CrossRef]
- Yeh, C.L.; Lai, K.L. Effects of TiC, Si, and Al on combustion synthesis of Ti3SiC2/TiC/Ti5Si3 composites. Materials 2023, 16, 6142. [Google Scholar] [CrossRef]
- Yeh, C.L.; Lai, K.L. Effects of excess Si and Al on synthesis of Ti3SiC2 by self-sustaining combustion in the Ti-Si–C-Al system. J. Aust. Ceram. Soc. 2024, 60, 959–969. [Google Scholar] [CrossRef]
- Wang, C.A.; Zhou, A.; Qi, L.; Huang, Y. Quantitative phase analysis in the Ti–Al–C ternary system by X-ray diffraction. Powder Diffr. 2003, 20, 218–223. [Google Scholar] [CrossRef]
- Binnewies, M.; Milke, E. Thermochemical Data of Elements and Compounds; Wiley-VCH: Weinheim, Germany, 2002. [Google Scholar]
- Ivleva, T.P.; Merzhanov, A.G. Three-dimensional spinning waves in the case of gas-free combustion. Dokl. Phys. 2000, 45, 136–141. [Google Scholar] [CrossRef]
- Munir, Z.A.; Anselmi-Tamburini, U. Self-propagating exothermic reactions: The synthesis of high-temperature materials by combustion. Mater. Sci. Rep. 1989, 3, 277–365. [Google Scholar] [CrossRef]
- Yunus, M.; Maji, B.C. An experimental approach to delineate the reaction mechanism of Ti3AlC2 MAX-phase formation. J. Eur. Ceram. Soc. 2023, 43, 3131–3145. [Google Scholar] [CrossRef]
- Cobbinah, P.V.; Matizamhuka, W.R. Solid-state processing route, mechanical behaviour, and oxidation resistance of TiAl alloys. Adv. Mater. Sci. Eng. 2019, 2019, 4251953. [Google Scholar] [CrossRef]
- Lei, C.; Xu, Q.; Sun, Y.Q. Phase orientation relationships in the TiAl–TiAl2 region. Mater. Sci. Eng. A 2001, 313, 227–236. [Google Scholar] [CrossRef]
- Thomas, T.; Bowen, C.R. Thermodynamic predictions for the manufacture of Ti2AlC MAX-phase ceramic by combustion synthesis. J. Alloys Compd. 2014, 602, 72–77. [Google Scholar] [CrossRef]
- Yeh, C.L.; Zheng, F.Y. Formation of TiB2-MgAl2O4 composites by SHS metallurgy. Materials 2023, 16, 1615. [Google Scholar] [CrossRef]



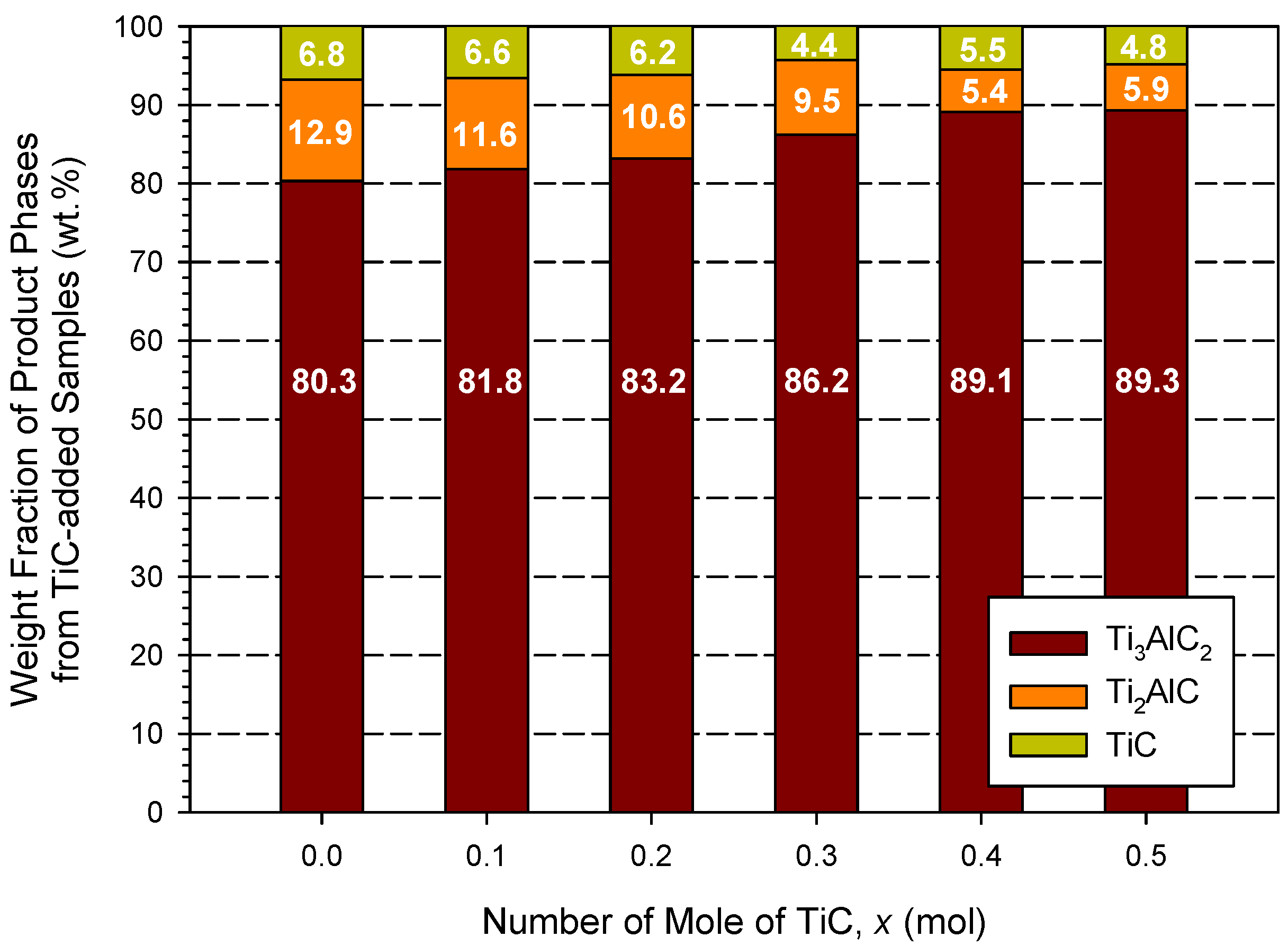
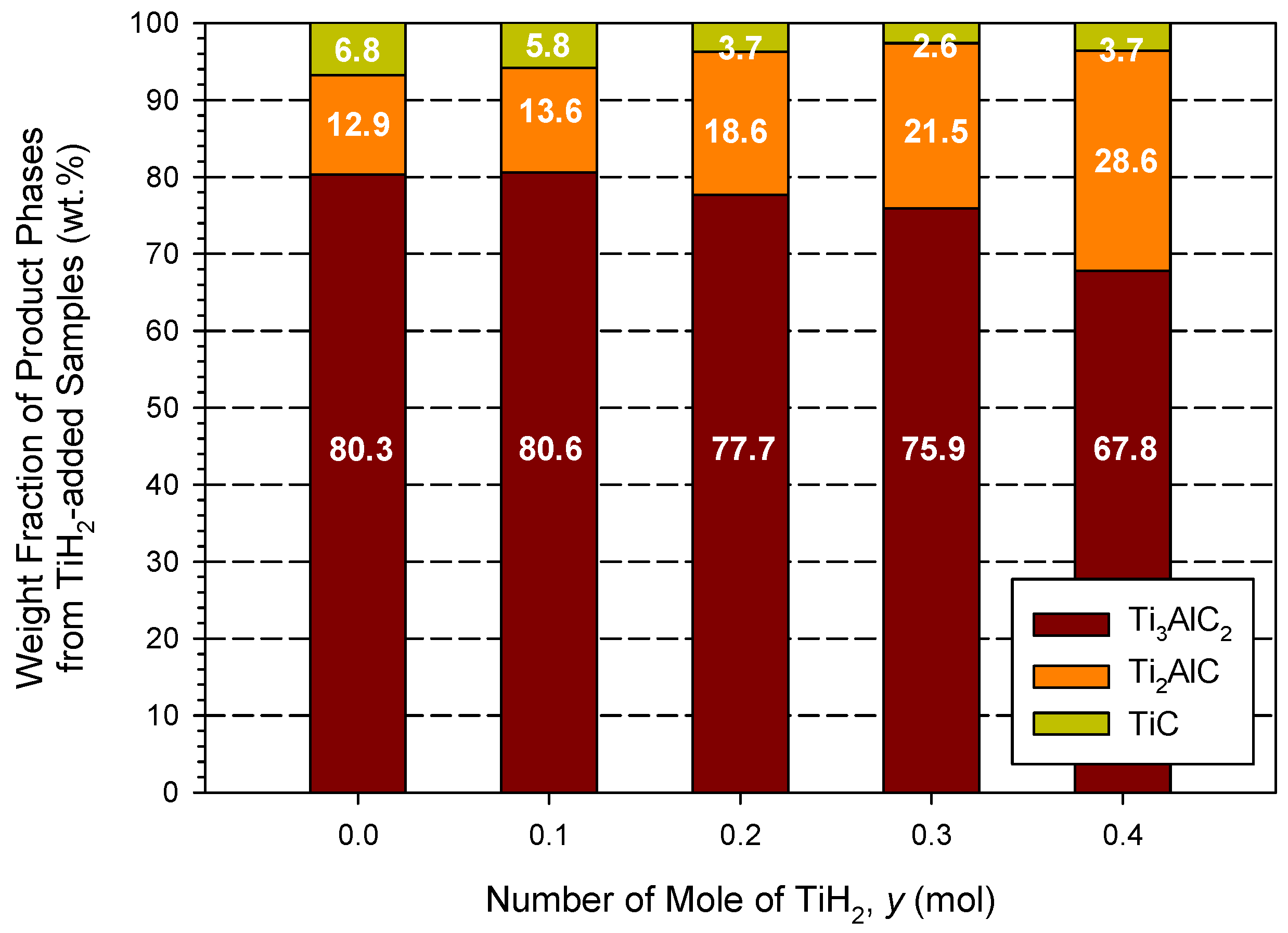
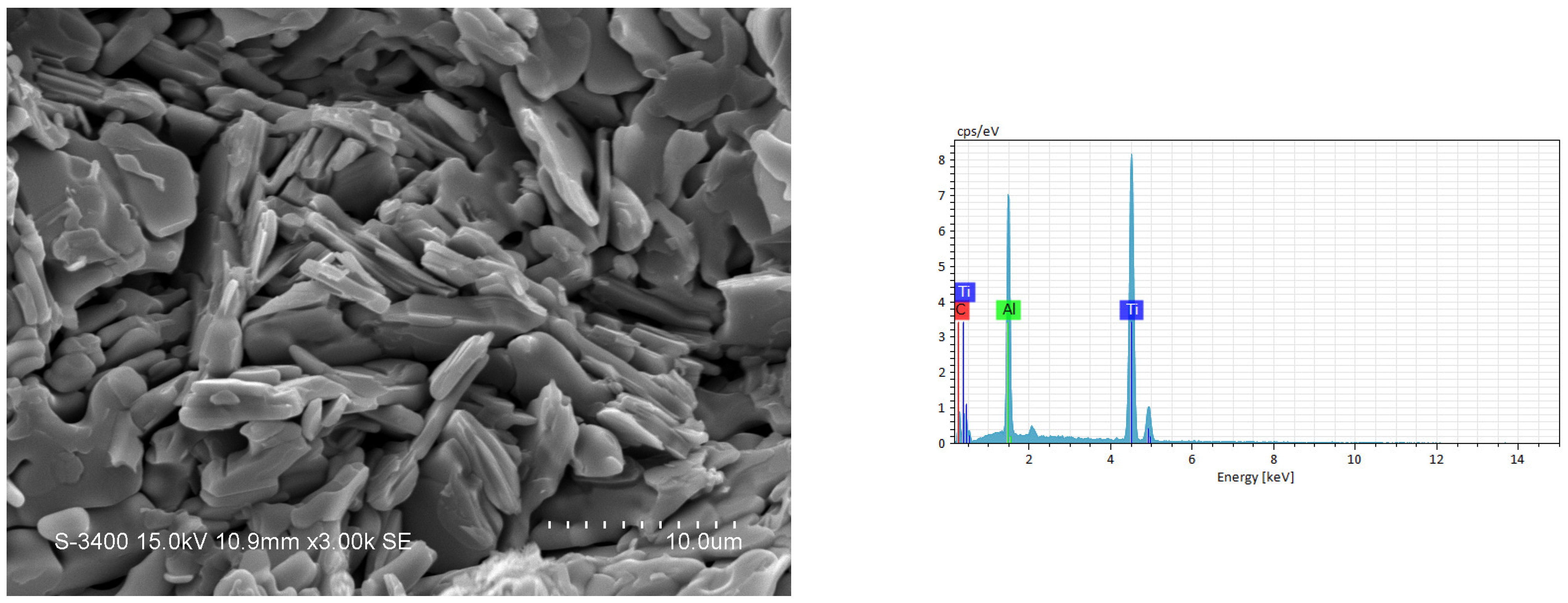
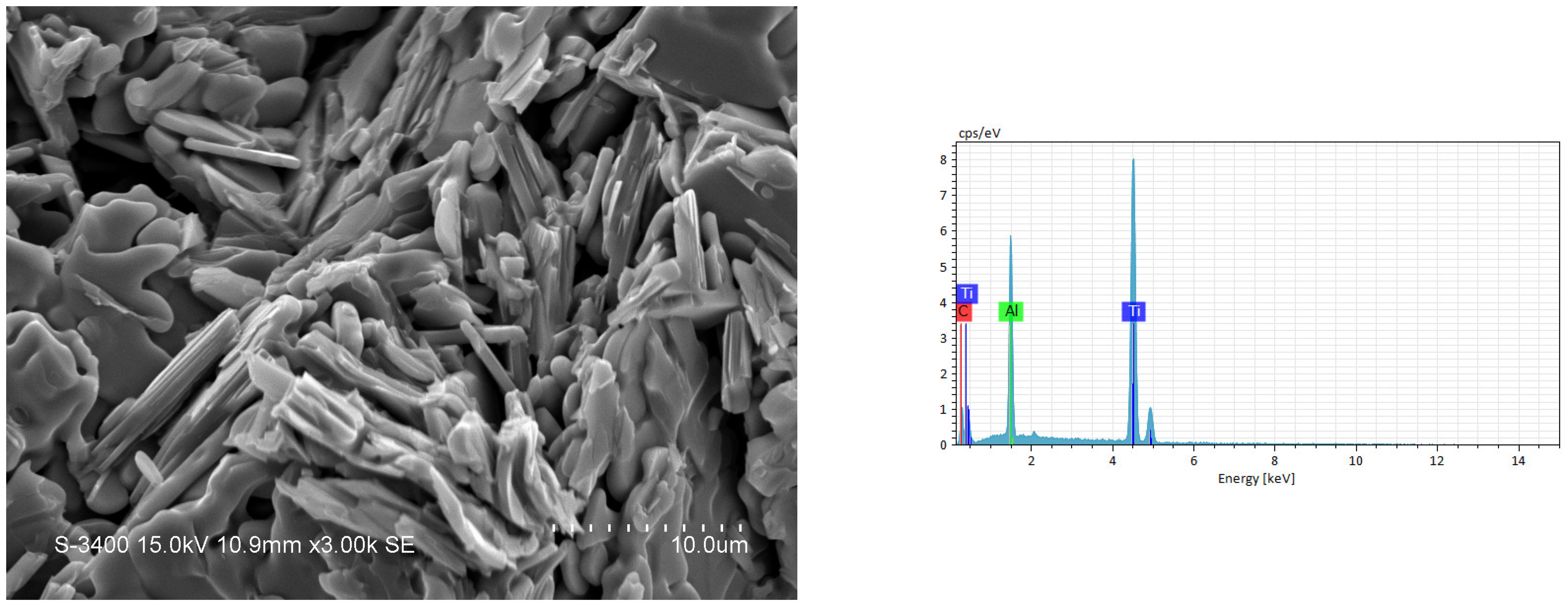
| Combustion Systems | Sample No. | Parameters |
|---|---|---|
| Equation (1): Elemental samples | 1 | m = 1.0, n = 2.0 |
| 2 | m = 1.1, n = 2.0 | |
| 3 | m = 1.2, n = 2.0 | |
| 4 | m = 1.3, n = 2.0 | |
| 5 | m = 1.0, n = 1.9 | |
| 6 | m = 1.1, n = 1.9 | |
| 7 | m = 1.2, n = 1.9 | |
| 8 | m = 1.0, n = 1.8 | |
| 9 | m = 1.1, n = 1.8 | |
| 10 | m = 1.2, n = 1.8 | |
| Equation (2): TiC-added samples | C-1 | x = 0.1 |
| C-2 | x = 0.2 | |
| C-3 | x = 0.3 | |
| C-4 | x = 0.4 | |
| C-5 | x = 0.5 | |
| Equation (3): TiH2-added samples | H-1 | y = 0.1 |
| H-2 | y = 0.2 | |
| H-3 | y = 0.3 | |
| H-4 | y = 0.4 |
| Sample No. of Equation (1) | Ti:Al:C | Weight Percentage (wt.%) | ||
|---|---|---|---|---|
| Ti3AlC2 | Ti2AlC | TiC | ||
| 1 | 3:1:2 | 56.3 | 31.5 | 12.2 |
| 2 | 3:1.1:2 | 78.0 | 15.8 | 6.2 |
| 3 | 3:1.2:2 | 80.3 | 12.9 | 6.8 |
| 4 | 3:1.3:2 | 71.7 | 11.3 | 17.9 |
| 5 | 3:1:1.9 | 30.2 | 43.0 | 26.8 |
| 6 | 3:1.1:1.9 | 33.5 | 52.4 | 14.1 |
| 7 | 3:1.2:1.9 | 48.9 | 31.3 | 19.8 |
| 8 | 3:1:1.8 | 12.8 | 38.9 | 48.3 |
| 9 | 3:1.1:1.8 | 10.9 | 75.7 | 13.4 |
| 10 | 3:1.2:1.8 | 9.4 | 80.9 | 9.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeh, C.-L.; Chen, Y.-T. Effects of TiC, TiH2, Al, and Carbon on Production of Ti3AlC2 by Self-Sustaining Combustion Synthesis. Materials 2025, 18, 1293. https://doi.org/10.3390/ma18061293
Yeh C-L, Chen Y-T. Effects of TiC, TiH2, Al, and Carbon on Production of Ti3AlC2 by Self-Sustaining Combustion Synthesis. Materials. 2025; 18(6):1293. https://doi.org/10.3390/ma18061293
Chicago/Turabian StyleYeh, Chun-Liang, and Yu-Ting Chen. 2025. "Effects of TiC, TiH2, Al, and Carbon on Production of Ti3AlC2 by Self-Sustaining Combustion Synthesis" Materials 18, no. 6: 1293. https://doi.org/10.3390/ma18061293
APA StyleYeh, C.-L., & Chen, Y.-T. (2025). Effects of TiC, TiH2, Al, and Carbon on Production of Ti3AlC2 by Self-Sustaining Combustion Synthesis. Materials, 18(6), 1293. https://doi.org/10.3390/ma18061293







