Abstract
Ammonia zinc refining has the benefits of low energy consumption, high zinc recovery, and good environmental protection compared with traditional acid and alkaline zinc refining. However, in the production process of refining zinc with ammonia, the anode undergoes chlorine precipitation, and then the oxidation of the ammonia precipitation of some nitrogen occurs. Ammonia replenishment is a cumbersome process that results in large amounts of ammonia volatilization and environmental pollution. In ammonia zinc refining, it is important to ensure the concentration of ammonia and chlorine, as the graphite anodes used in conventional ammonia zinc refining do not retain chlorine and ammonia and dissolve slowly due to oxidation. Therefore, this paper proposes a new measure to conserve chlorine and ammonia to reduce anode chlorine generation by adding an anionic barrier layer and selecting manganese anode materials with selective oxygen precipitation. Under the conditions of 50 × 100 mm sized electrodes, a current density of 350 A/m2, and a temperature of 60 °C, a graphite anode and manganese anode were used for electrowinning and for the collection of anode gas under different additive conditions. For the first time, we present a comparative analysis of gas composition, using gas chromatography to demonstrate the feasibility of the different measures used to preserve chlorine, ammonia, and oxygen for industrial applications, as well as the advantages of using these methods in reducing costs. And the experiments show that, by adding the anionic barrier layer, adding urea, and using manganese anode materials with selective oxygen precipitation, the nitrogen precipitation in the anode gas can be reduced to 40–50%, and oxygen precipitation reaches 48.76%.
1. Introduction
As an environmentally friendly metallurgical process, electrolytic, ammonia-leaching, zinc-refining technology has attracted much attention in the field of zinc smelting in recent years due to its advantages, such as the efficient utilisation of low-grade zinc oxide ore and the avoidance of hydrogen sulphide emissions. Its advantages over the traditional sulfuric acid system include the fact that the electrodeposition voltage can be reduced to 2.5–2.8 V (saving energy by 18–26% compared to the sulfuric acid method) and the fact that the electrolyte can be recycled, which reduces the discharge of acidic wastewater [1,2,3]. However, the irreversible depletion of NH3 in the anodic zone and the continuous precipitation of Cl2 during the electrolysis process leads to difficulty in maintaining the ammonia equilibrium, and every 1 ton of zinc produced needs to be supplemented with 0.5–0.8 tons of ammonia. The resulting loss of ammonia volatilization not only increases raw material costs but also poses the risk of fugitive gases and the eutrophication of water bodies. And the root of the problem is the existence of mutual competition between the OER [4] and CER [5] at high Cl− concentrations (5–7 mol/L). Although the standard electrode potential for the oxygen extraction reaction (OER) (1.23 V) is lower than that for the chlorine extraction reaction (CER, 1.36 V) [6,7], the four-electron transfer process involved in the OER has a higher activation energy barrier. The high-Cl−-concentration environment further exacerbates the kinetic dominance of the CER, resulting in the depletion of NH3 due to the dominance of Cl2 in the anodic product and the initiation of the following side reaction: 4NH3 + 3Cl2 ⟶ N2 + 2NH4Cl + 2HCl.
Existing anode material systems generally face a performance–cost–life trade-off dilemma in high-Cl− ammonia media [8,9]. Although graphite anodes have cost advantages, their loose, porous structure makes it difficult to block Cl− penetration. Irreversible corrosion of C + 2Cl2 ⟶ CCl4 occurs under Cl2 oxidation, leading to electrode chalking and electrolyte carbon contamination. A titanium-based IrO2 anode can reduce OER overpotential by noble metal catalysis. However, a reversible Cl− adsorption layer is formed on the IrO2 surface at high Cl− concentrations, which triggers the preferential precipitation of Cl2. The high cost of Ir restricts industrial applications. Although lead-based anodes have better corrosion resistance, the PbO2 passivation layer generated on its surface increases the OER’s potential, while lead ion leaching causes the cathode zinc purity to decrease. Defects in these materials can cause the cost of ammonia replenishment to comprise a disproportionately high percentage of the production costs, and they are susceptible to the risk of heavy metal contamination.
In recent years, studies targeting anode-selective modulation have been conducted in low-Cl− systems (e.g., seawater electrolysis, Cl− ≈ 0.5 mol/L). For example, Ling et al. [10] promoted OER activity by optimising the electronic structure of the OER-active site through transition metal doping (Fe, P-Ni Se2). Guo et al. [11] inhibited the CER by constructing an alkaline microenvironment on the surface of Cr2O3-Cox. However, when the Cl− concentration reaches 5–7 mol/L in an ammonia-refined zinc system, conventional physical barriers (e.g., MnOx coatings) undergo lattice distortions due to high osmotic pressures, resulting in a sudden drop in Cl− shielding efficiency. Therefore, it is difficult to utilize the catalytic materials in the low-Cl− system in the ammonia system for zinc refining, and there is an urgent need to develop anode materials that can be adapted to the high-Cl− environment.
Based on this background, this paper proposes a new anode material, MnOx-IrO2/Ti, and additives for chlorine inhibition. This new anode material is able to synergistically inhibit the precipitation of chlorine gas by the chemisorption of Cl− through the MnOx layer and by a small amount of doped IrO2, as well as the effects of chlorine preservation, ammonia preservation [12,13,14,15], and oxygen precipitation in ammonia zinc refining through the addition of an anion and an anionic barrier layer.
2. Materials and Methods
2.1. Preparation of Experimental Materials and Calculations
All chemical reagents were of analytical grade and procured from Sichuan Xilong Scientific Co., Ltd. (Chengdu, China). The zinc–ammonia–chloride complex electrolyte was synthesized by dissolving analytical-grade zinc oxide (99% purity) in 4 mol/L ammonium chloride solution (99.5% purity), yielding a final Zn(NH3)2Cl2 system with a zinc ion concentration of 50 g/L Zn2+. The introduction of polyethylene glycol 8000 (PEG8000) at 600 mg L−1 significantly enhances the performance of zinc electrowinning through three synergistic mechanisms: (1) facilitating regular zinc deposition at the cathode by modulating interfacial charge transfer kinetics; (2) maintaining electrolyte uniformity through steric stabilization effects; (3) suppressing particle agglomeration via surface adsorption, ultimately achieving a 12.7% improvement in current efficiency compared to additive-free systems. The electrowinning process was conducted at an industry-standard current density of 350 A/m2. Current efficiency (CE) and energy consumption (EC) were determined through continuous potential monitoring between electrodes combined with chronometric quantification of electrolysis duration, as described in Equations (1) and (2) [16,17]:
where n denotes the electron transfer number, F = 96,485 C/mol represents the Faraday constant, I (A) is the applied current, t (h) indicates the duration of electrolysis, m (g) corresponds to the actual cathodic zinc deposition mass, M = 65.38 g/mol stands for zinc’s molar mass, and V (V) refers to the average cell voltage across the electrodes.
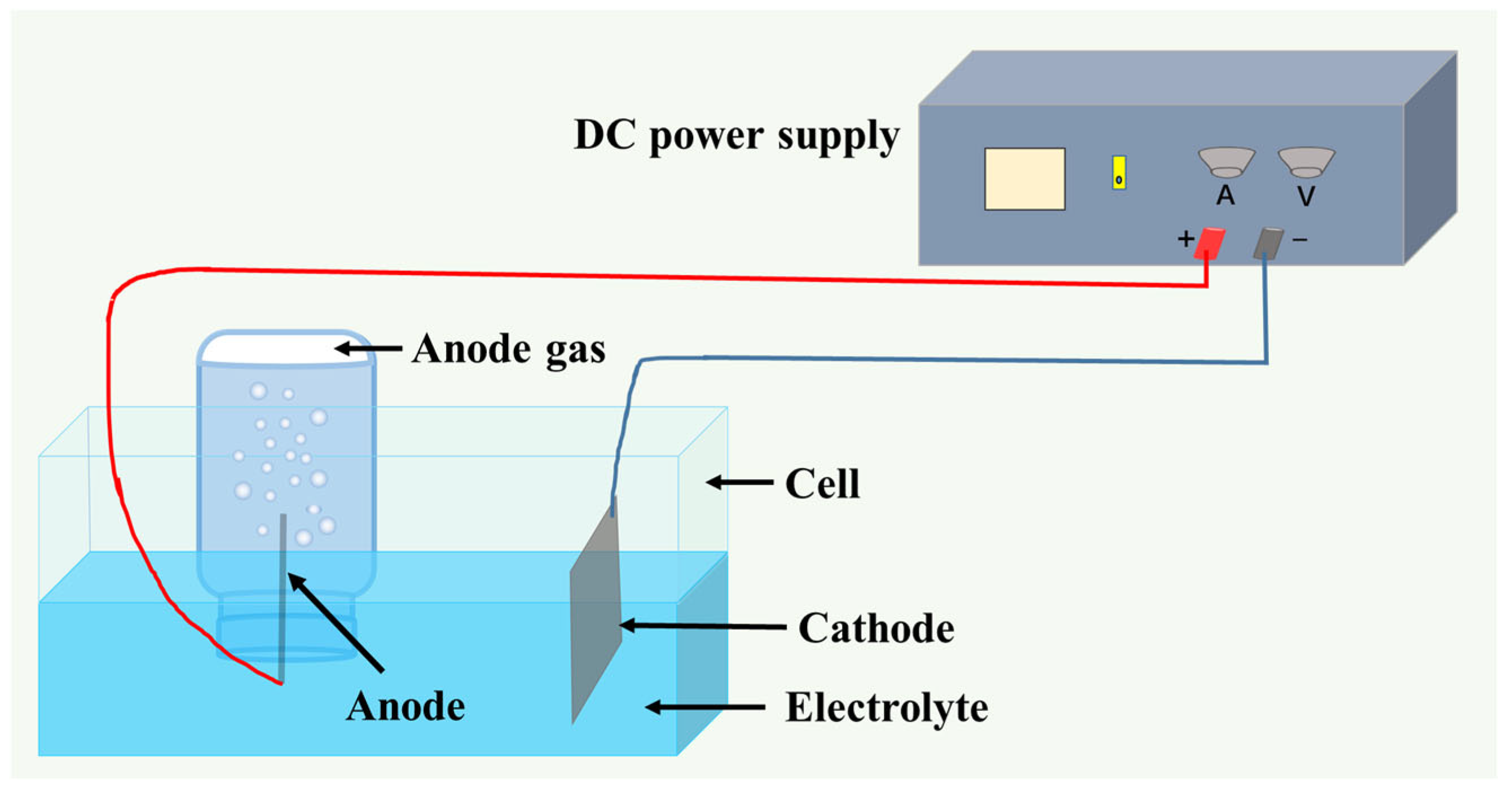
Electrodeposition was conducted in a thermostatically controlled water bath using graphite and MnOx-IrO2/Ti anodes. The process employed 50 × 100 mm electrodes under standardized conditions: 60 °C operating temperature, 350 A/m2 current density, 35 mm interelectrode spacing, and 10 h duration. Triplicate experiments were performed for each additive concentration level, with anodic gases quantitatively collected via the water displacement method. Data were statistically averaged, and the experimental configuration is detailed in Figure 1.
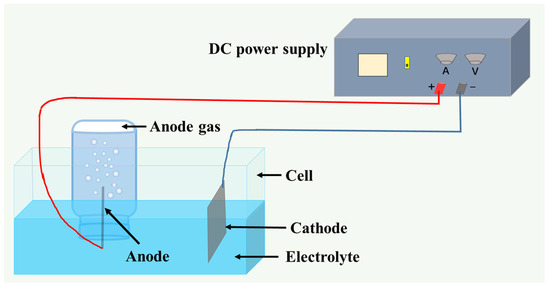
Figure 1.
Schematic diagram of the experimental anode gas collection device.
The MnOx-IrO2/Ti composite anodes were fabricated using 0.5 mm thick titanium sheets (99.9% purity) as substrates. Pretreatment involved sequential surface modifications: (1) ultrasonic degreasing in 5 mol/L NaOH to remove organic contaminants; (2) chemical etching in 1 mol/L oxalic acid at 100 °C for 30 min; (3) plasma-enhanced chemical vapor deposition (PECVD) of a 3 nm Pt interlayer. The active coating was synthesized by dip-coating with a precursor solution containing 50 wt% Mn(NO3)2, 1 wt% TiO2, 2 wt% Na2SiO3, and 0.02 wt% IrO2, followed by multiple coating in a muffle furnace at a decomposition temperature of 200 ℃ and, finally, annealing at 500 ℃.
2.2. Experimental Equipment and Material Characterization
Anode gas composition was quantified using a TRACE 1300 gas chromatograph (Thermo Scientific, Karlsruhe, Germany) with manual peak integration. Phase analysis and crystallographic parameters were determined via X’Pert PRO X-ray diffraction (Panalytical, Almelo, The Netherlands). Material surface morphology was characterized by S-4800 field-emission scanning electron microscopy (Hitachi, Tokyo, Japan), while elemental composition was evaluated using an INCA IE 350 energy-dispersive X-ray spectroscopy system (Oxford Instruments, Oxford, UK).
3. Results and Discussion
3.1. Effect of Graphite Anode and Addition of Sulphate Anion Barrier on Electrolysis
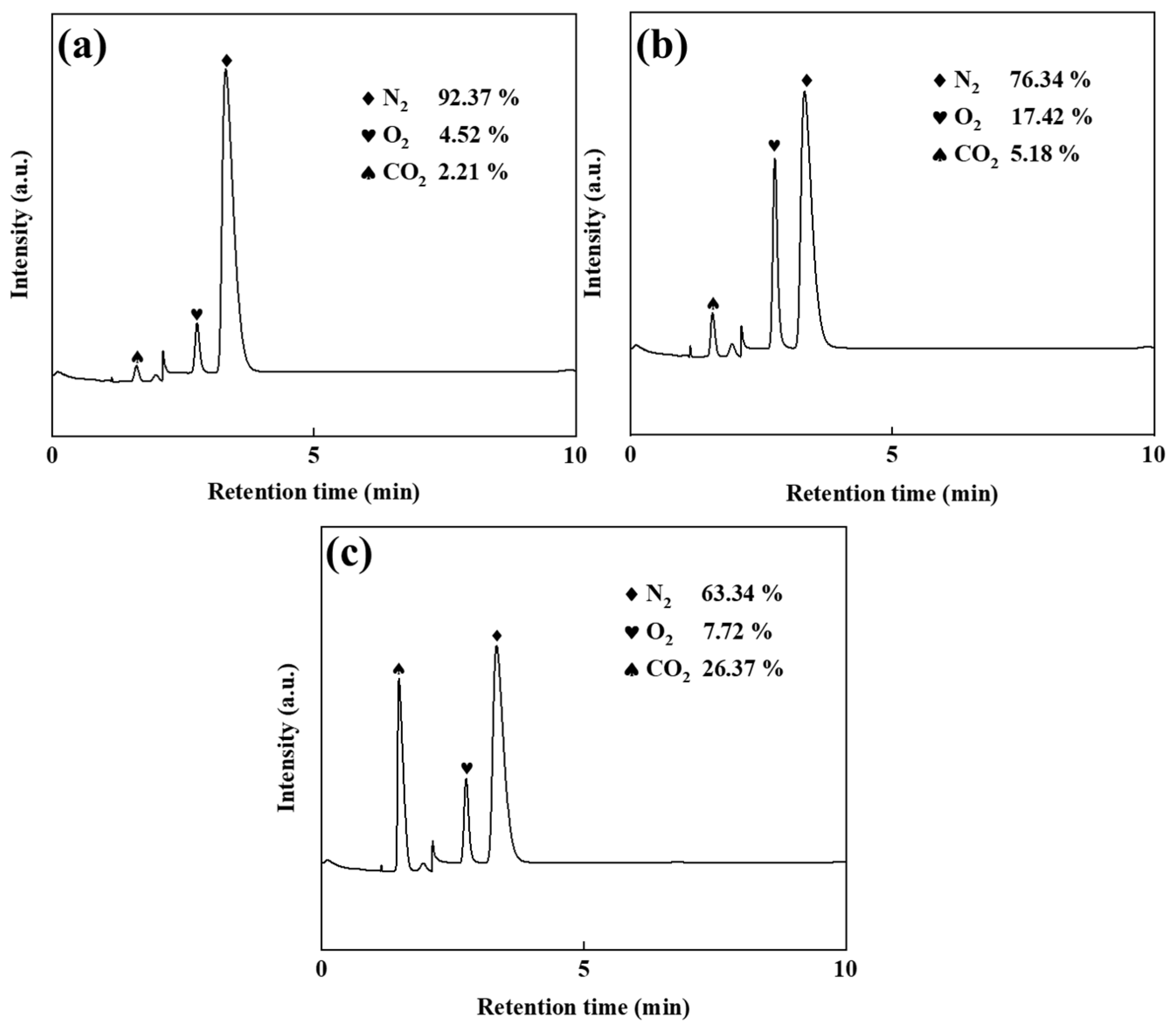
The electrolyte maintained a total Zn2+ concentration of 50 g/L, with Zn(NH3)2Cl2 solutions containing 45 g/L and 40 g/L Zn2+. ZnSO4 additions of 5 and 10 g/L (corresponding to 10% and 20% of the total zinc concentration, respectively) were applied. Electrolysis experiments employed graphite anodes to collect anode gases, and gas chromatography analysis (Figure 2) revealed the influence of ZnSO4 supplementation on gas composition.
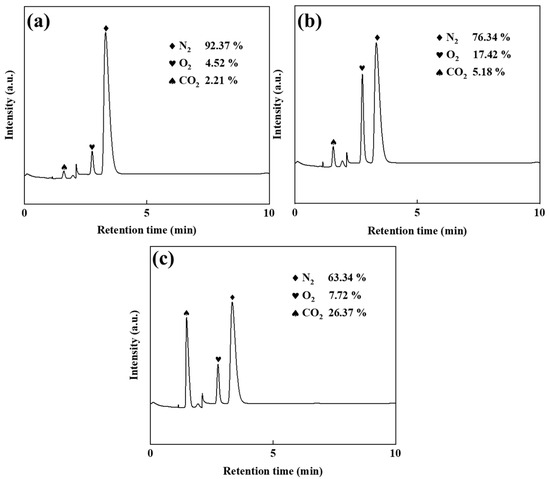
Figure 2.
Gas chromatogram diagrams: ZnSO4 free (a), 10% ZnSO4 (b), and 20% ZnSO4 (c).
Figure 2a reveals, through gas chromatographic analysis, that the anode gas in ZnSO4-free electrolysis primarily consists of N2 (92.37%), with minimal O2 content (4.52%). This composition demonstrates a two-stage anodic mechanism: initial Cl− oxidation through electrochemical discharge, followed by redox interactions with ammonia species (NH3/NH4+). The suppressed oxygen evolution further indicates the limited participation of hydroxide ions (OH−) or water molecules in the oxidation processes. As shown in Figure 2b, the addition of ZnSO4 significantly reduced the anode gas nitrogen content (76.34%) compared to no addition of ZnSO4. The reduction in Cl2 emissions further confirms that ZnSO4 inhibits the chlorine oxidation at the anode, thus limiting the chlorine evolution and subsequent ammonia displacement reaction. Concurrently, the O2 content rose to 17.42%, demonstrating that suppression of Cl− oxidation enhanced OH−/H2O discharge at the anode, thereby elevating gas-phase oxygen generation. Comparative analysis of Figure 2a,b reveals a CO2 content increase from 2.21% to 5.18%, with graphite serving as the exclusive carbon source in the system. This confirms the enhancement of anodic graphite oxidation under ZnSO4 addition, evidenced by the concomitant rise in gas-phase CO2 generation and accelerated carbon corrosion kinetics. As shown in Figure 2c, the anodic N2 content decreased further to 63.34% upon introducing 20% ZnSO4, demonstrating suppressed Cl− oxidation at the anode. Concurrently, the O2 level declined to 7.72%, suggesting sulphate-enhanced carbon oxidation in graphite [18,19,20], which elevated CO2 generation while reducing residual N2 proportion.
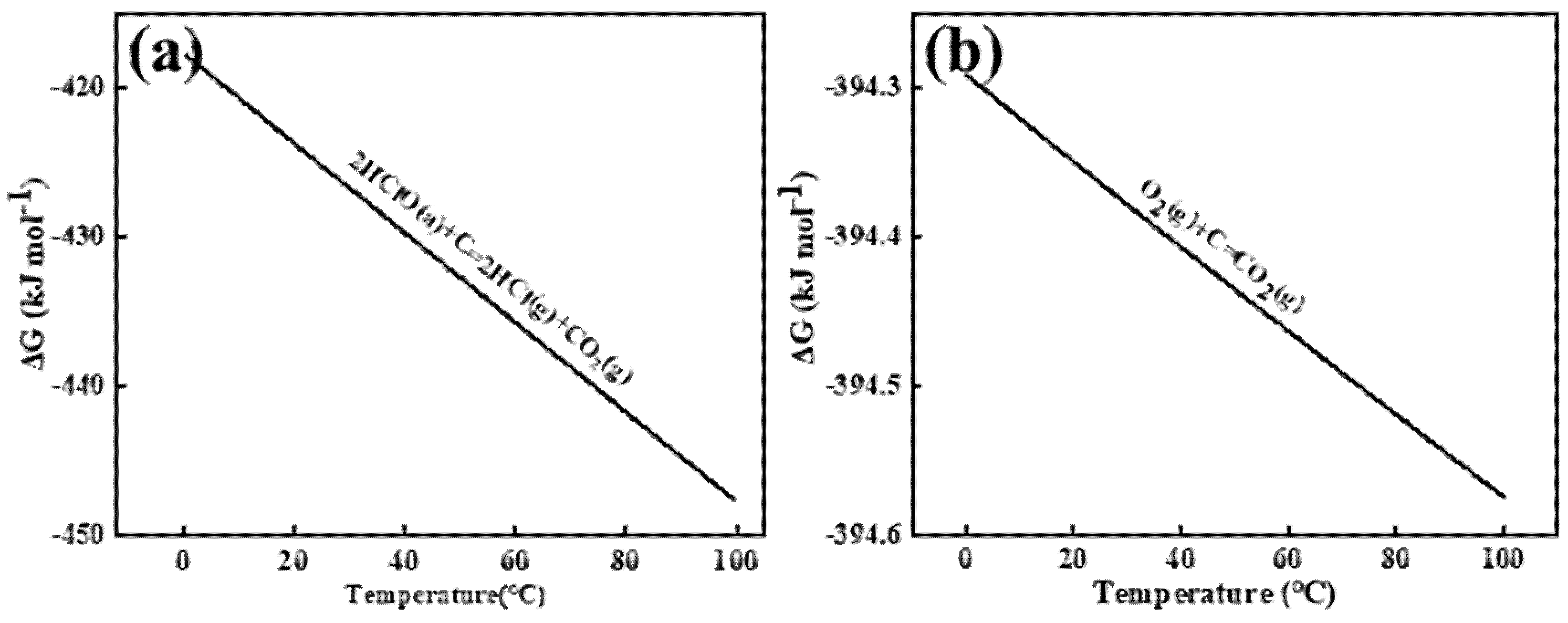
The Gibbs free energy was calculated using the relationship ΔG = −RT ln Kc, where R represents the molar gas constant (8.314 J·mol−¹·K−¹), T denotes absolute temperature, and Kc is the equilibrium constant. Figure 3 illustrates the Gibbs free energy changes for the electrochemical reactions between chlorine (Cl2), oxygen (O2), and carbon (C) in aqueous solution, specifically those occurring at the graphite anode interface. As shown in Figure 3a,b, chlorine gas induces gradual oxidation of the graphite anode carbon to CO2. In aqueous solution, anodically generated Cl2 undergoes rapid hydrolysis near the interface (Cl2 + H2O ⇌ HClO + Cl− + H+ [13]), resulting in hypochlorous acid (HClO) serving as the primary reactive species. HSC 6.0 calculations of Gibbs free energy changes confirm the spontaneity of both reactions. Comparative analysis reveals that oxygen species on the aqueous-phase anode surface exhibit enhanced reactivity, demonstrating faster reaction kinetics with carbon and consequently accelerated graphite consumption.
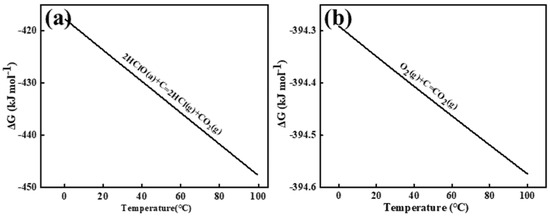
Figure 3.
The Gibbs free energy of HClO (a) and O2 (b) reacts with C in an aqueous solution. a and g in the diagram represent the states of the reactants, with a—representing the ionic form and g—representing the gaseous form.
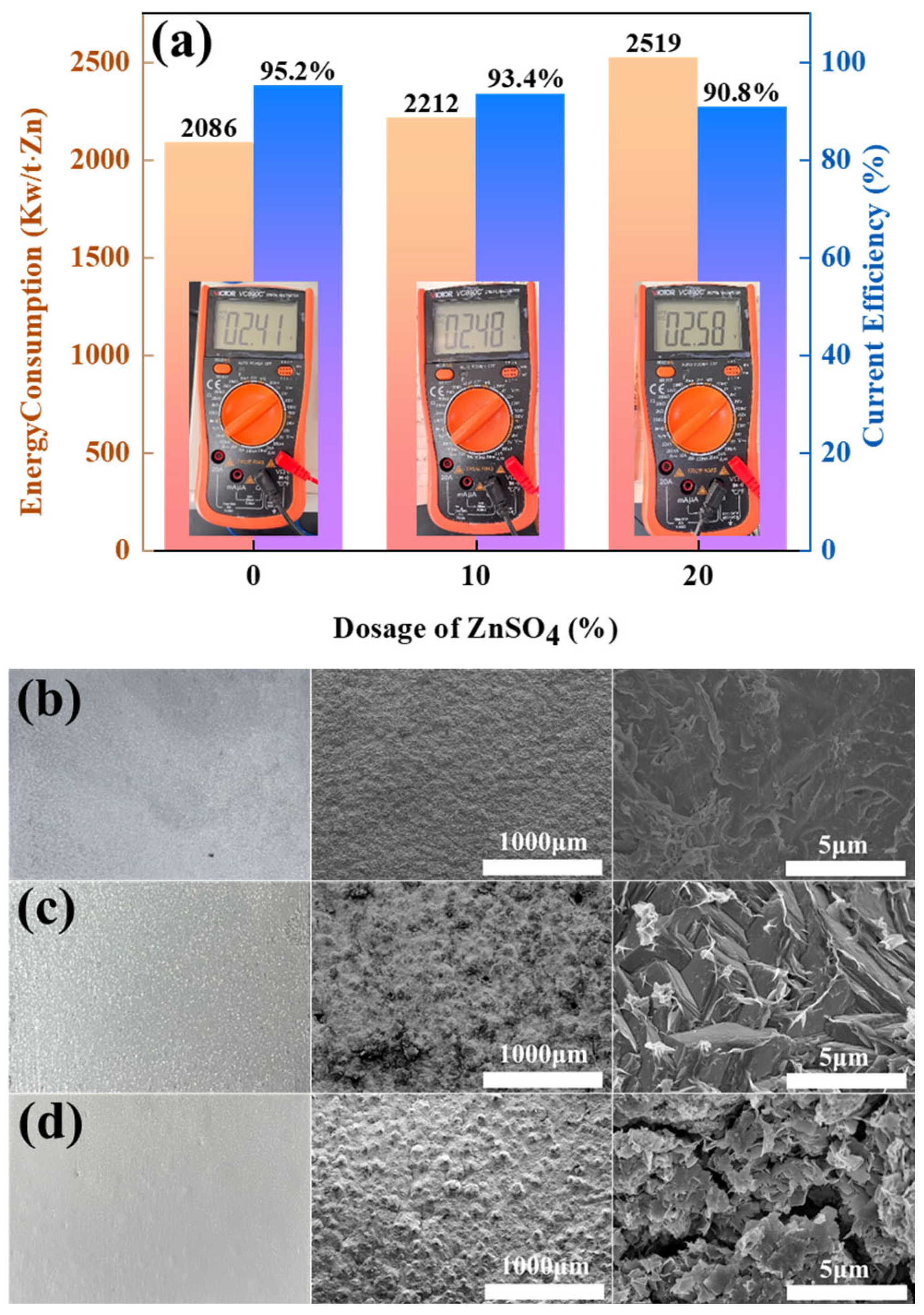
To evaluate the impact of ZnSO4 addition on zinc electrowinning performance in the Zn-NH3-Cl system, current efficiency (CE) and DC energy consumption were systematically analysed under identical operational parameters. Figure 4 quantitatively demonstrates how ZnSO4 supplementation modulates three key metrics: cathodic zinc deposition dynamics, DC electrolysis efficiency, and specific energy expenditure.
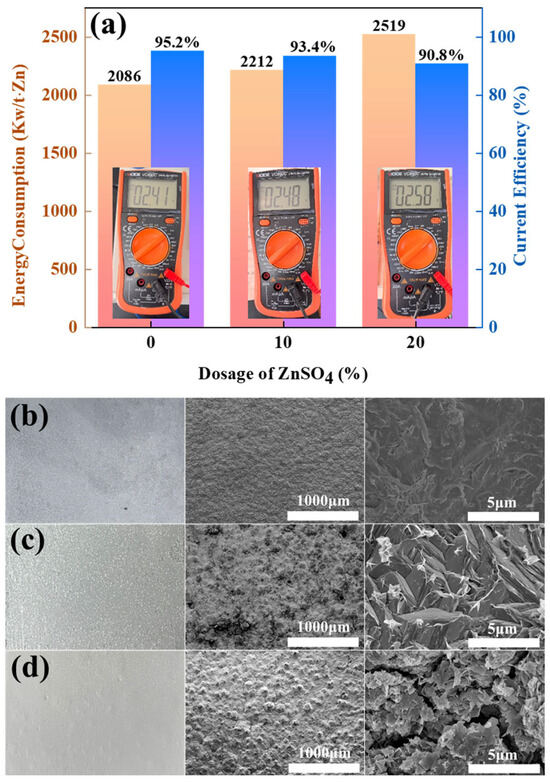
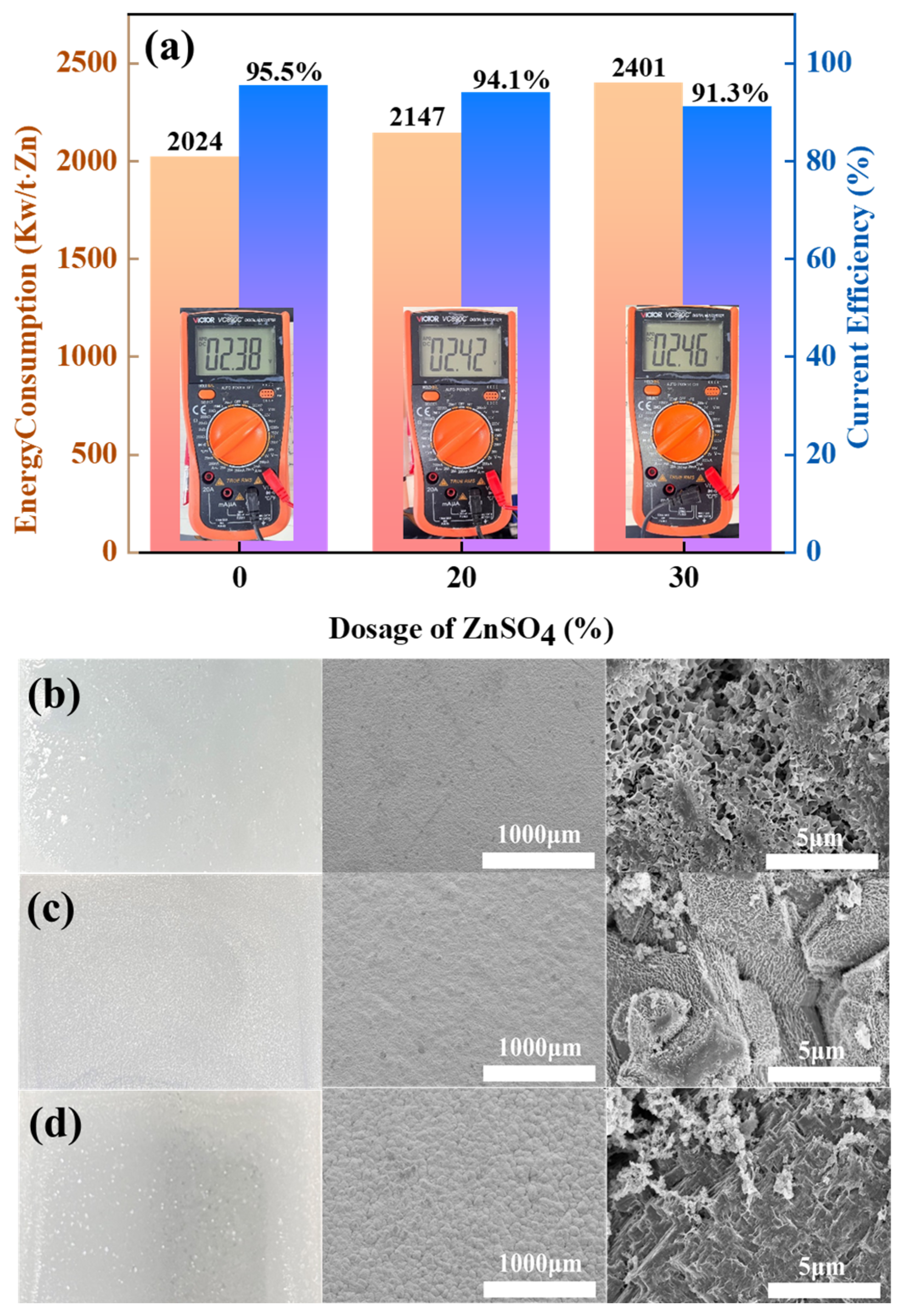
Figure 4.
(a) Energy consumption and CE of ZnSO4-free, 10% ZnSO4 addition, and 20% ZnSO4 addition conditions; (b) macroscopic diagrams of cathode zinc under ZnSO4-free, (c) 10% ZnSO4 addition, and (d) 20% ZnSO4 addition conditions.
Figure 4a shows that the current efficiency (CE) of the amino-hybrid acid system modified with ZnSO4 remains above 90%. Notably, the baseline system without ZnSO4 achieved higher current efficiency (95.2%) and lower energy consumption (2086 kW h/t Zn). However, the introduction of 20% ZnSO4 significantly increased the energy consumption to between 2212 and 2519 kW h/t Zn, which was attributed to the sulphate-induced voltage increase during electrolysis. This arises from sulphate ions (SO42−) exhibiting lower ionic mobility than chloride ions (Cl−), consequently elevating solution resistance. Figure 4b–d reveal that zinc cathodes deposited under all three conditions exhibit macroscopically smooth and dense morphologies. Notably, ZnSO4-supplemented systems yield off-white zinc flakes, distinctively lighter than the greyish-cyan deposits formed without ZnSO4. Electron microscopy further demonstrates that the ZnSO4-free zinc deposits maintain a fully dense microstructure devoid of detectable porosity. At 10% ZnSO4 supplementation, zinc deposits adopt a microstructure of tightly packed cubic blocks with observable intergranular micropores. Elevating ZnSO4 concentration to 20% fundamentally alters crystallization pathways, resulting in loosely stacked nanoflake architectures with extensive cleavage planes.
Therefore, industrial-grade graphite can be used as a functional anode. Gas composition analysis confirmed that the addition of ZnSO4 can effectively inhibit the evolution of Cl2, but excessive addition (≥20%) leads to a decrease in current efficiency along with a significant increase in energy consumption. In addition, the accelerated degradation of graphite anodes under sulphate-enhanced conditions was experimentally verified.
3.2. Manganese Anodes and Their Effect on Electrolysis
3.2.1. Manganese Anode Characterization
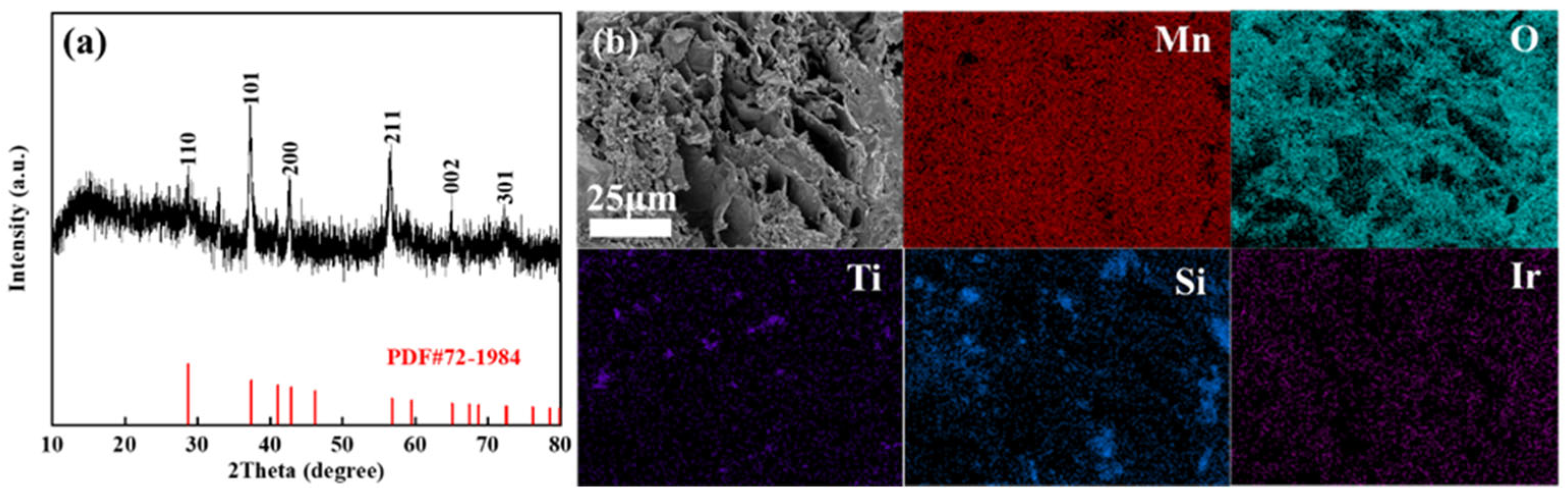
The XRD pattern of the MnOx anode (Figure 5a) demonstrates low crystallinity, with thermally decomposed manganese oxides showing faint diffraction peaks. Alignment with the β-MnO2 reference (PDF#72-1984) confirms weak signals only at the (110), (101), (200), and (211) crystallographic planes. A prominent broad diffraction peak centred at 2θ ≈ 20° further indicates the coexistence of amorphous phases, suggesting that the synthesized MnOx primarily consists of low-crystallinity manganese oxides with significant amorphous content. SEM characterization reveals the MnOx anode exhibits a lamellar porous architecture decorated with polydisperse particulate features. This lamellar porosity yields an expanded surface area configuration, beneficial for boosting the electrode’s electrocatalytic activity. EDS mapping of the MnOx anode confirms the coexistence of Mn, O, Ti, Si, and Ir with distinct distribution patterns. Mn, O, and Ir demonstrate homogeneous dispersion, while Ti and Si exhibit localized clustering—a phenomenon attributed to the particulate nature of titanium and silicon additives undergoing agglomeration during synthesis. Surface-enriched Ti facilitates the formation of an oxyphilic oxide layer, thereby improving interfacial reaction kinetics, while the uniformly distributed Ir enhances bulk electrical conductivity and promotes oxygen evolution reaction (OER) activity.
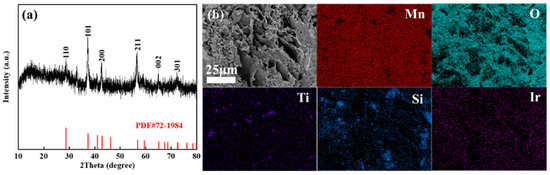
Figure 5.
XRD pattern (a), SEM (b), and corresponding element distribution of the prepared manganese anode.
In order to determine the valence distribution of various major elements in the prepared manganese oxide anode catalytic materials, X-ray photoelectron spectroscopy (XPS) was used to detect and analyse the prepared materials.
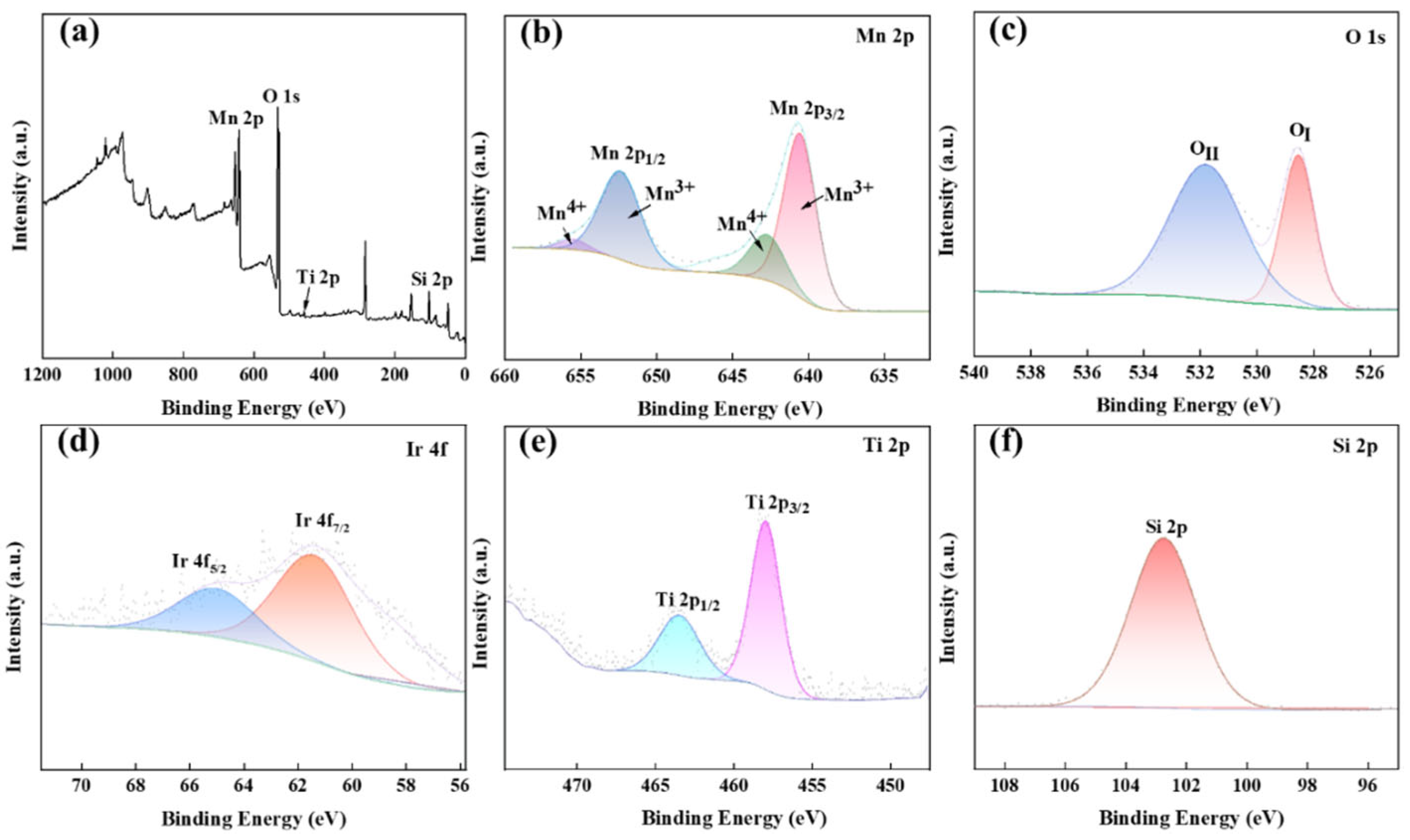
Figure 6 presents the XPS analysis of the MnOx anode catalytic material. The survey spectrum (Figure 6a) confirms the presence of Mn, O, Ti, Si, and Ir, with elemental valence states determined through binding energy analysis. Distinct peaks are observed at 653.3 eV (Mn 2p), 529.6 eV (O 1s), 459.1 eV (Ti 2p), and 102.92 eV (Si 2p), while the Ir 4f signal is absent due to the low IrO2 loading. Figure 6b–f, respectively, display the high-resolution spectra of Mn 2p, O 1s, Ti 2p, Si 2p, and Ir 4f. The Mn 2p spectrum exhibits spin–orbit splitting into 2p3/2 and 2p1/2 components, with the Mn 2p3/2 peaks at 641.05 eV and 642.45 eV corresponding to Mn3+ and Mn4+ oxidation states, respectively [21,22].
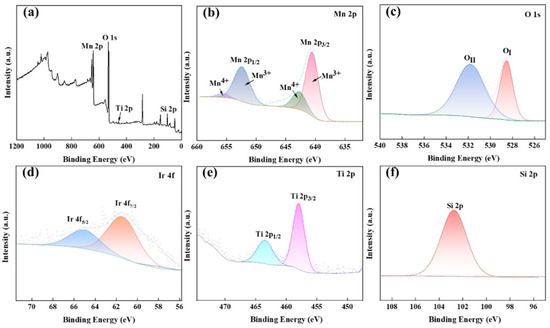
Figure 6.
XPS diagram of the prepared MnOx anode: Full spectrum (a), Mn 2p (b), O 1s (c), Ir 4f (d), Ti 2p (e), Si 2p (f).
The Mn 2p1/2 core level exhibits spin–orbit split components at 652.85 eV and 656.15 eV, assigned to Mn3+ and Mn4+, respectively [23], confirming the coexistence of MnO2 and Mn2O3 phases. The Mn3+/Mn4+ redox pair exhibits strong adsorption capacity for oxygen intermediates, facilitating O-O bond formation and enhancing oxygen evolution reaction (OER) activity. Deconvolution of the O 1s spectrum reveals two dominant peaks at 528.65 eV (Me-O bonds) and 531.8 eV (Si-O bonds) [24]. The 531.8 eV peak’s higher intensity indicates abundant oxygen vacancies in the thermally synthesized MnOx [25,26,27], which promote OER kinetics. Ir 4f analysis shows doublet peaks at 62.55 eV (4f7/2) and 64.8 eV (4f5/2) [28], suggesting IrO2 formation. Titanium-supported Ir sites effectively reduce OER overpotential while suppressing chloride ion oxidation. The Si 2p spectrum exhibits a single peak at 102.75 eV, characteristic of Si-O bonding [29]. The Ti 2p spectrum displays characteristic spin–orbit splitting with 2p3/2 and 2p1/2 peaks at 458.1 eV and 463.75 eV, confirming TiO2 formation [30].
3.2.2. Manganese Anode Gas Characterization
Figure 7 displays gas chromatography profiles of anode off-gases generated during electrolysis with the synthesized MnOx anode under varied operational parameters, where gas sampling was systematically conducted via water displacement methodology.
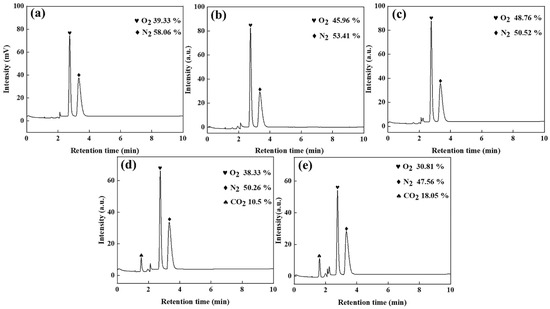
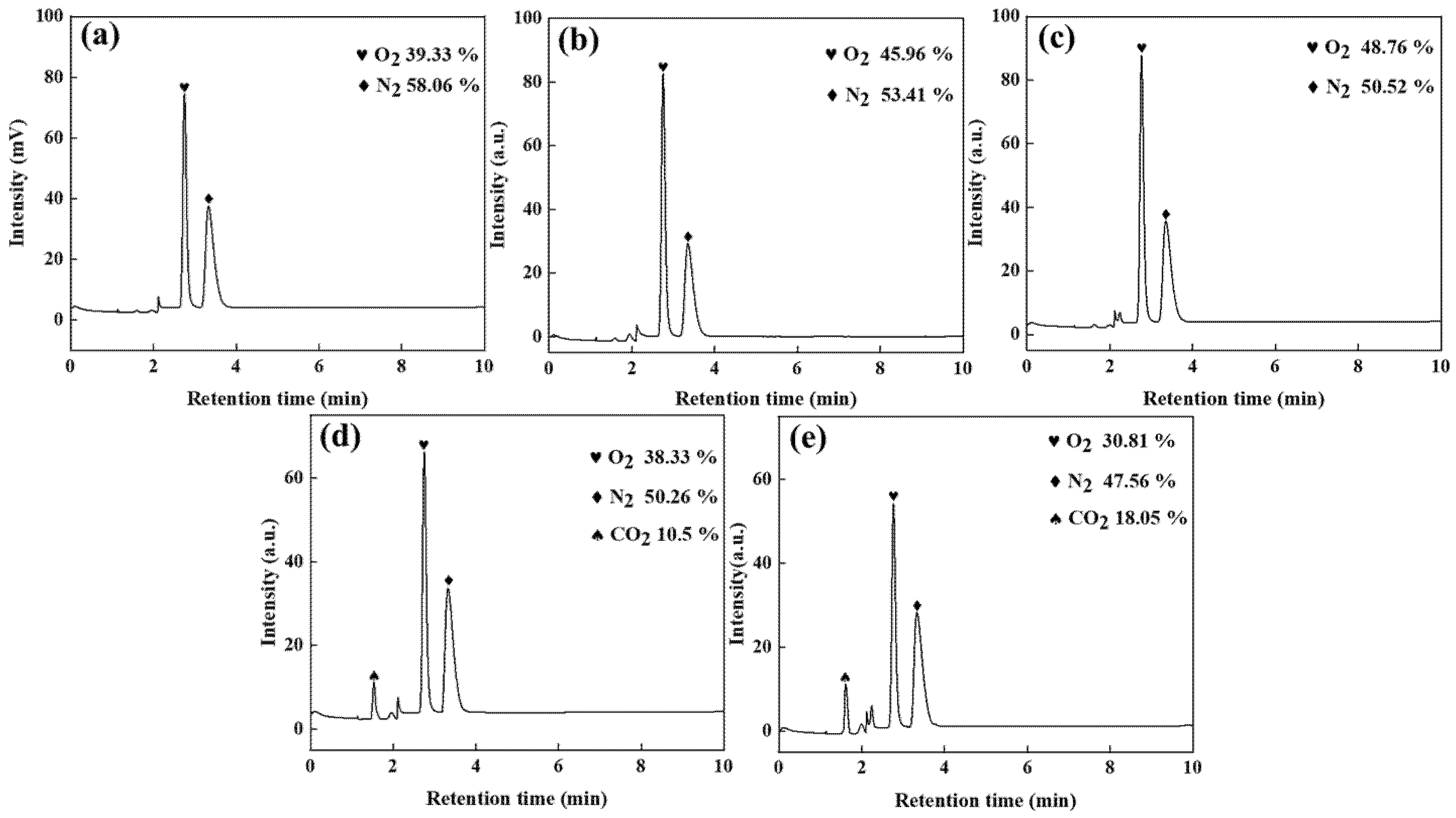
Figure 7.
Gas chromatogram of manganese anode gas: (a) zinc-sulphate-free, (b) 20% zinc sulphate addition, (c) 30% zinc sulphate addition, (d) 20% zinc sulphate and 5 g/L urea addition, and (e) 30% zinc sulphate and 5 g/L urea addition conditions.
As shown in Figure 7a, the oxygen and nitrogen contents in the anode gas produced by electrolysis without ZnSO4 addition are 39.33% and 58.06%, respectively. In contrast, when graphite is used as the anode, the oxygen content drops to 4.52%, with nitrogen rising to 92.37%, demonstrating the manganese anode’s critical role in promoting oxygen-selective evolution in high-chloride solutions. To suppress chlorine evolution and mitigate ammonia oxidation, adding 20% and 30% ZnSO4 elevates oxygen content to 45.96% and 48.76%, respectively, while reducing nitrogen to 53.41% and 50.52%. This occurs because sulphate ions accumulate on the anode surface under electric fields, forming a barrier that inhibits chloride ion oxidation—a mechanism consistent with graphite anode observations. Comparing Figure 7a–c reveals that 20% ZnSO4 addition increases oxygen content to 45.96%, whereas 30% ZnSO4 provides only a marginal 2.8% further increase (45.96% → 48.76%) and reduces nitrogen by merely 2.89% (53.41% → 50.52%). Therefore, 20% ZnSO4 is identified as the optimal concentration.
The introduction of N2H4CO moderately reduces nitrogen concentration while simultaneously decreasing oxygen content. This demonstrates that N2H4CO preferentially undergoes oxidation over ammonia, accelerates CO2 evolution kinetics, and reveals the dual nitrogen origin in anode gas: partial oxidation of N2H4CO and residual ammonia decomposition. Comparative analysis of Figure 7d,e confirms that optimized N2H4CO addition achieves minimal anode nitrogen levels at 47.56%.
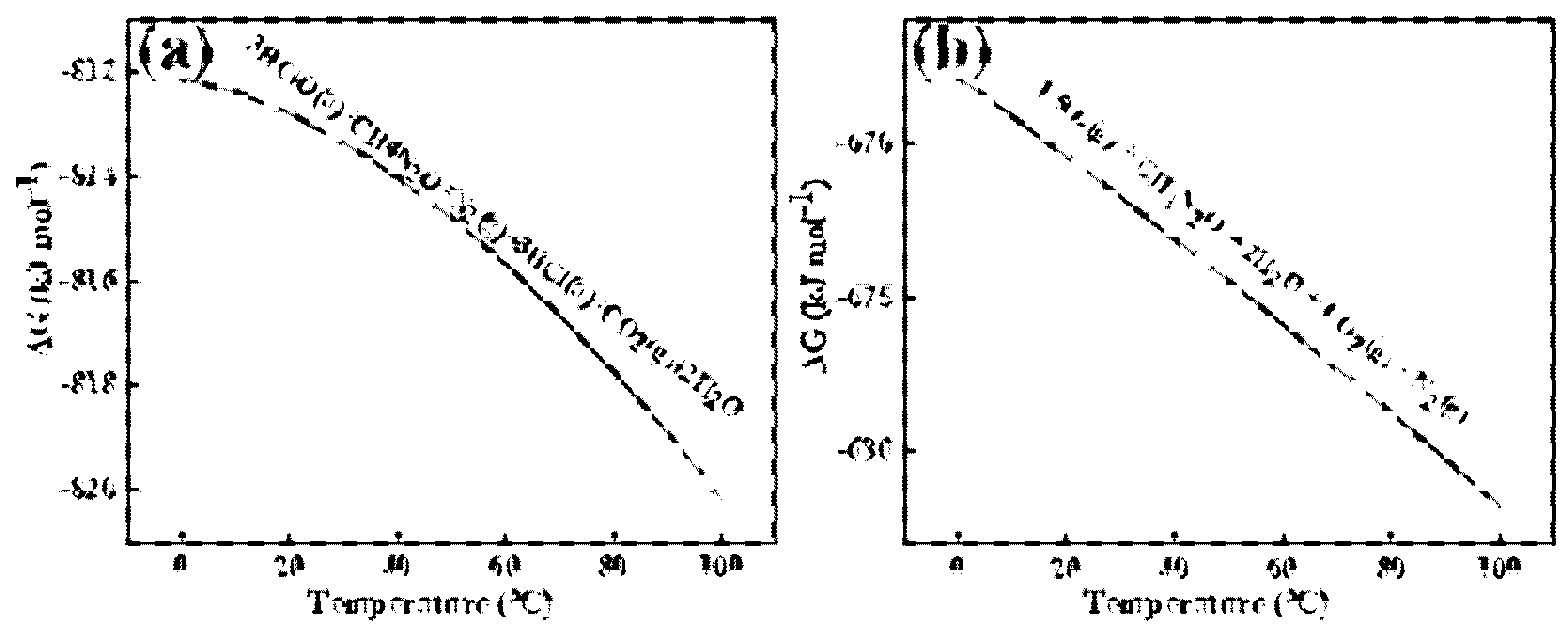
Figure 8 shows the Gibbs free energy changes for the two reactions occurring near the anode interface under this condition, as calculated by HSC 6.0.
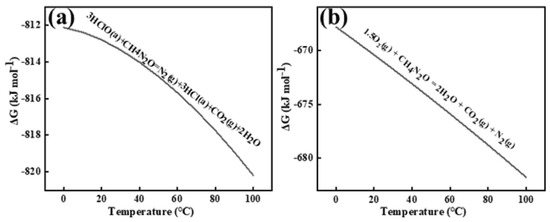
Figure 8.
Reaction of urea with hypochlorous acid (a) and oxygen (b). a and g are used to distinguish between types of states of matter.
Prior studies confirm that electrogenerated chlorine undergoes rapid hydrolysis in aqueous media to form hypochlorous acid (HClO), establishing HClO as the primary oxidative agent for urea decomposition. Electrochemical analysis reveals that N2H4CO competitively reacts with both anode-derived HClO and dissolved oxygen species. The kinetic preference for HClO-mediated oxidation stems from its stronger oxidizing capacity and inherent oxidative instability compared to molecular oxygen.
3.2.3. Effect of Manganese Anodes on Zinc Cathodes
Electrolysis using prepared MnOx anode plates was performed to evaluate the manganese anode’s effects on three critical parameters: zinc deposition at the cathode, DC energy consumption, and current efficiency (CE), with corresponding results detailed in Figure 9.
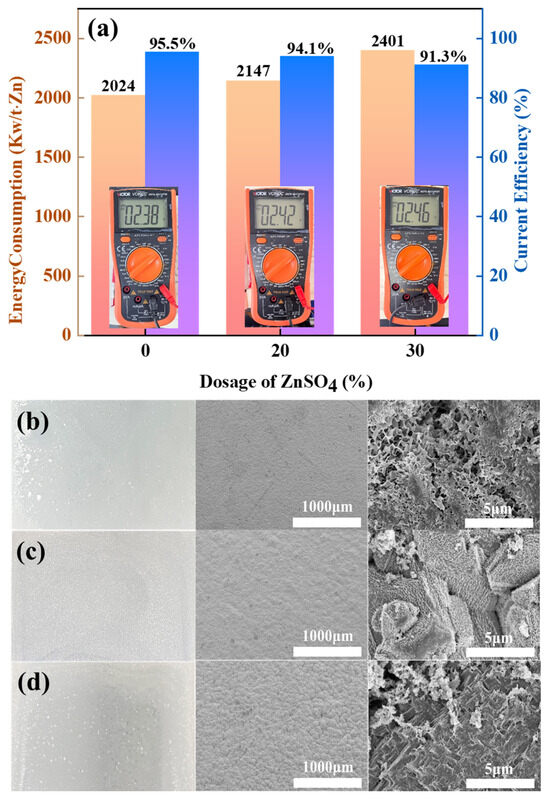
Figure 9.
Difference in energy consumption and current efficiency between ZnSO4-free and 20% ZnSO4 and 30% ZnSO4 added conditions (a); macro- and micrographs of zinc cathode without ZnSO4 added (b); macro- and micrographs of zinc cathode with 20% ZnSO4 added (c); macro- and micrographs of zinc cathode with 30% ZnSO4 added (d).
Direct voltage measurements show that the MnOx anode has a slightly lower electrolysis voltage and a slightly higher current efficiency compared to the graphite anode. Following the same trend as the graphite anode, the CE gradually decreases with increasing ZnSO4 concentration, along with a corresponding increase in DC energy consumption. Importantly, the excellent oxygen evolution activity of the MnOx anode maintains a CE of 91.3% at 30% ZnSO4 load while consuming 4.7% less energy than graphite under the same operating parameters. The zinc cathode yields macroscopically flat and compact metallic deposits. Microscopic analysis reveals that under ZnSO4-free conditions, zinc adopts nanosheet morphology akin to graphite anode growth patterns. However, ZnSO4 supplementation induces crystalline restructuring, producing densely packed bulk zinc agglomerates. Conventional PbO2 anodes in amine-zinc-refining systems have three key limitations: (1) susceptibility to corrosive degradation, which can lead to leaching of lead ions and affect cathode zinc purity; (2) risk of environmental contamination from heavy metal release; and (3) inherently high oxygen evolution overpotential. In contrast, manganese oxide (MnOx) anodes have excellent corrosion resistance and do not undergo toxic metal dissolution, thus effectively maintaining the quality of cathode deposits. In addition, manganese oxide has a lower material cost than PbO2 and can be strategically doped to achieve tuneable oxygen evolution kinetics—both lowering the overpotential and increasing the electrolytic efficiency. These combined advantages make manganese oxide the technically and economically optimal anode choice for ammonia-based zinc refining.
4. Investigation on the Mechanism of Chlorine Inhibition by Graphite and Manganese Anode
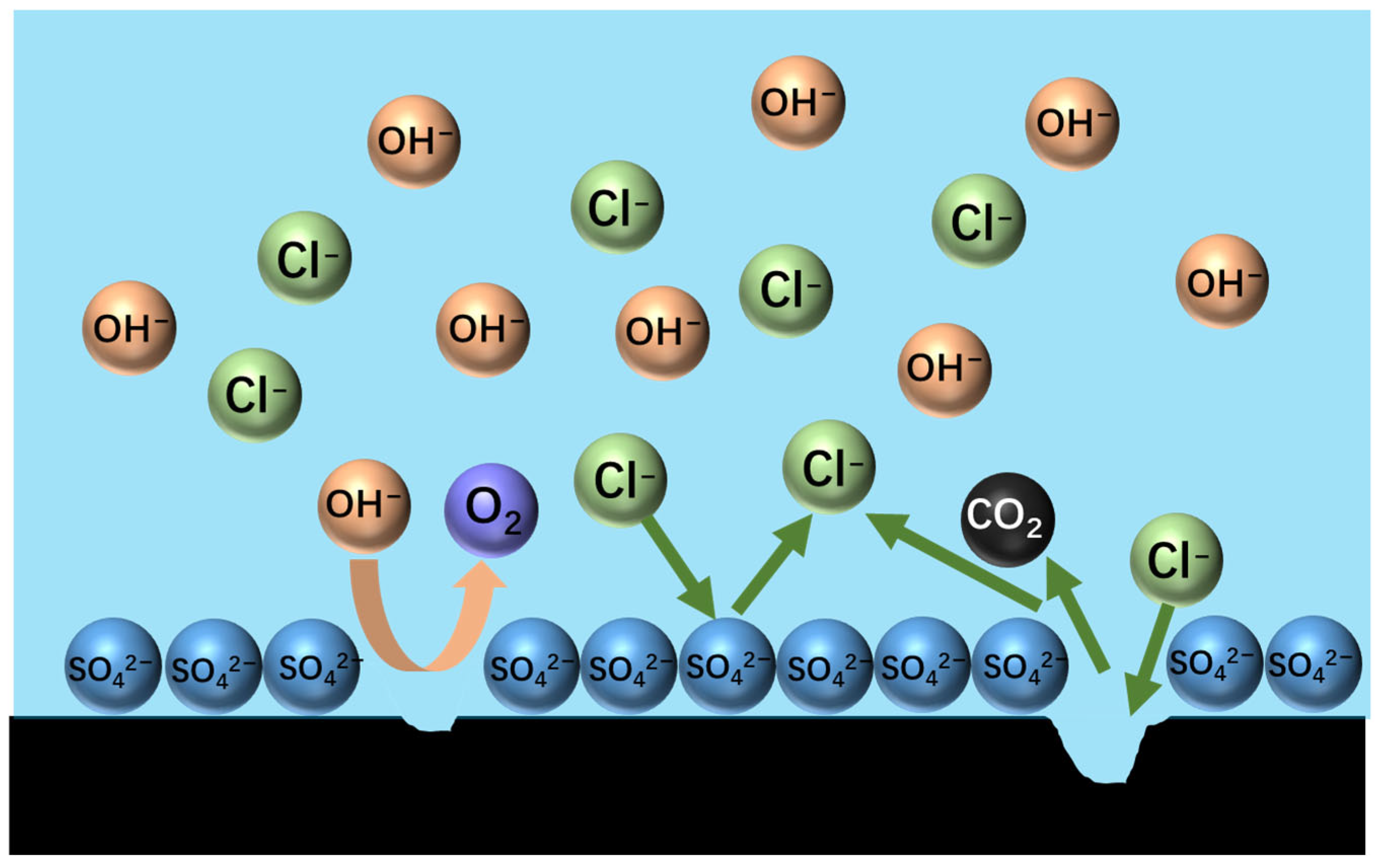
The anode gas consisted mainly of N2, which indicated that the graphite anode had minimal selectivity for chlorine evolution during ammonia electrolysis, and the O2 content was extremely low. When SO42− was introduced, the O2 content rose, suggesting that sulphate-induced competitive adsorption hindered chlorine oxidation. Meanwhile, the increase in CO2 content (Figure 10) provides direct evidence of the accelerated oxidative degradation of graphite via the sulphate-mediated corrosion pathway.
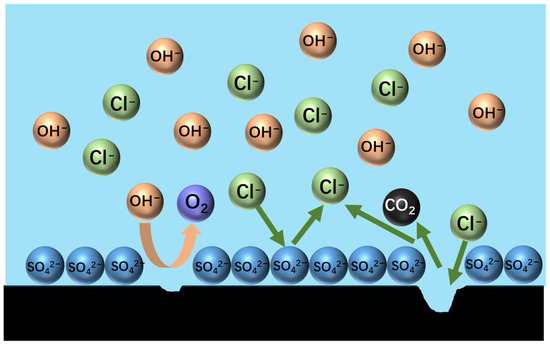
Figure 10.
Diagram of sulphate forming a barrier layer on the surface of a graphite anode.
The elevated CO2 levels primarily result from accelerated carbon oxidation at the anode surface driven by enhanced oxygen evolution. Although chlorine (Cl2) generation predominates on graphite anodes, secondary hypochlorous acid (HClO) formation oxidizes residual carbon to CO2. Experimental observations revealed sulphate-induced graphite degradation, evidenced by increasing anode material loss as fine particulates. Post-electrolysis analysis showed substantial black graphite powder accumulation at the electrolyzer base, particularly pronounced in 20% ZnSO4-supplemented systems.
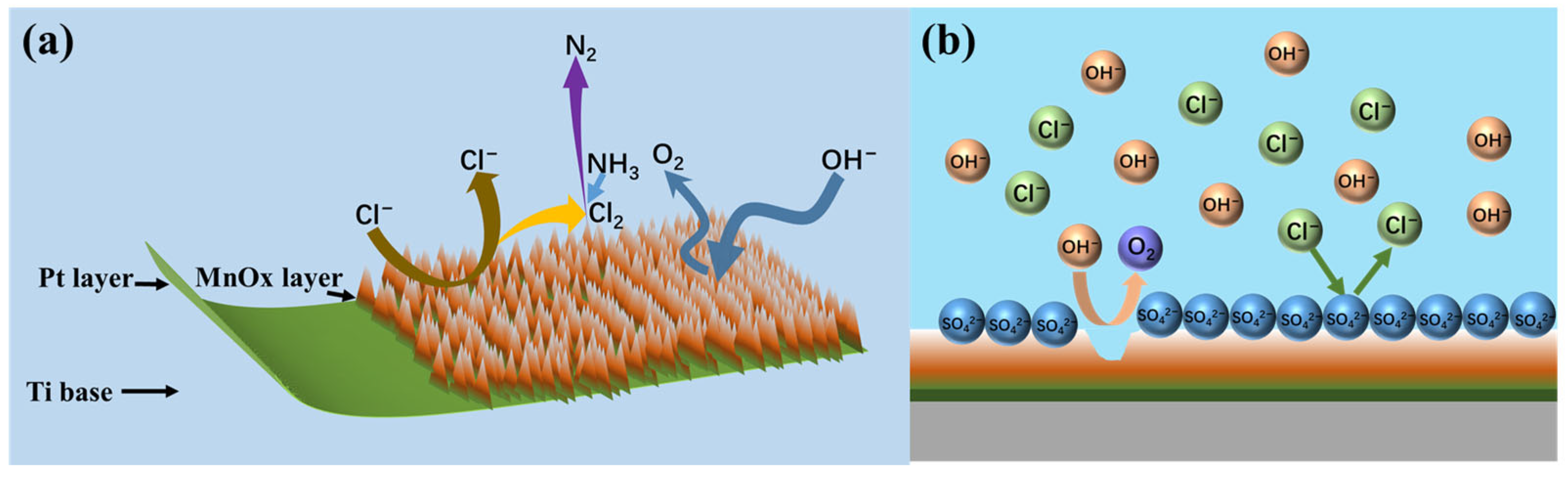
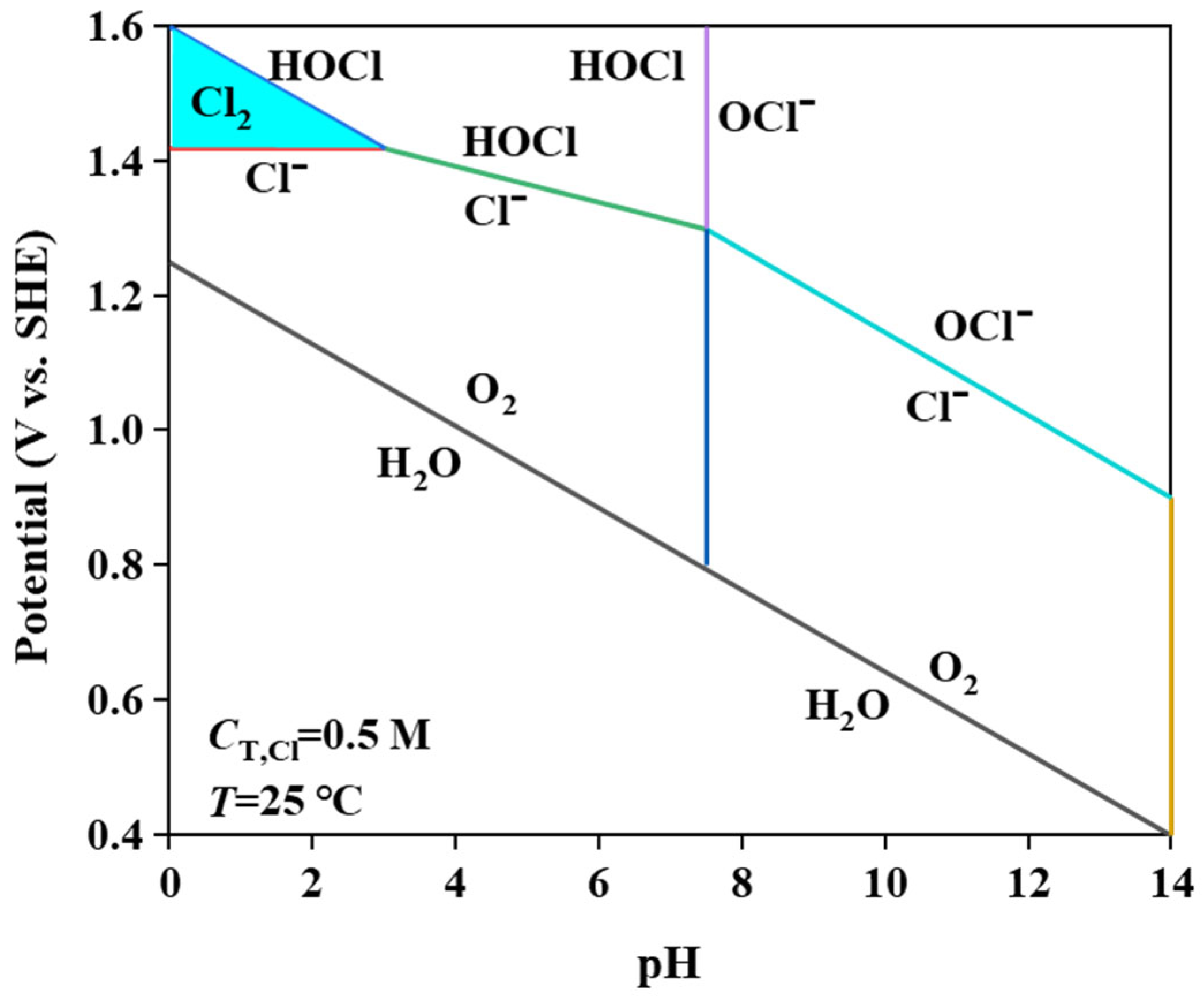
MnO2 and Mn2O3 constitute the primary catalytic phases in MnOx anode materials. The MnO6 octahedral framework serves as the electrochemically active site, where the octahedral coordination geometry dictates both oxygen evolution reaction (OER) activity and structural stability. As evidenced in prior studies [31], the MnO6 octahedron undergoes dynamic surface reconstruction by releasing an oxygen atom to form MnO5 moieties that bind hydroxyl groups (-OH). Concurrently, the layered architecture of MnOx enables selective ion permeation, exclusively permitting H2O and -OH transport through its interlamellar channels. Although IrO2 doping effectively reduces OER overpotential, its extreme catalytic activity creates kinetic disparity: IrO2-driven OER rates exceed proton diffusion rates by >3 orders of magnitude. This kinetic imbalance induces localized alkaline microenvironment formation at the anode interface, thereby autocatalytically enhancing OER performance through hydroxide concentration effects. Previous studies have shown that the catalytic performance of MnOx can be improved by strategic modifications, including surface defect engineering, metal ion doping, carbon-based hybridization, and Mn3+/Mn4+ ratio optimization [32,33,34]. In agreement with these reports, our experimental results confirm that the synthesized MnOx anode has a selective oxygen evolution capability when electrolyzed in a highly chlorinated environment. As shown in Figure 11a, some of the chloride ions are discharged at the anode interface, producing Cl2, which subsequently reacts with NH3 by displacement to produce N2 gas. The other part is released as OH− after discharge. Figure 11b shows that the SO42− anion competitively adsorbs on the anode surface, thereby impeding chloride oxidation and further enhancing oxygen precipitation. Notably, Cl2 produced by electrolysis is rapidly hydrolysed to form HClO, which dissociates protons (H+) that accumulate near the electrode interface. At the same time, the oxidation of hydroxide (OH−) produces additional protons that synergistically form an acidic microenvironment in the anode region. Figure 12 [4,11,35] is a Pourbaix plot for chlorides based on previous studies where solution pH, applied potential, and temperature are considered in complex chloride electrooxidation systems. The plot shows that chloride oxidation dominates at pH values below 3.0. The acidified interfacial layer at the anode (pH less than 3.0) will raise the thermodynamic potential of the oxygen evolution reaction (OER), as indicated by the Equation 2Cl− − 2e− = Cl2; thus, it thermodynamically facilitates the chloride evolution reaction (CER) through the electrochemical series of substitutions.
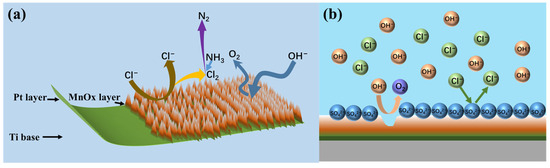
Figure 11.
Selective OER by MnOx anode (a) and schematic diagram of anion barrier layer (b).
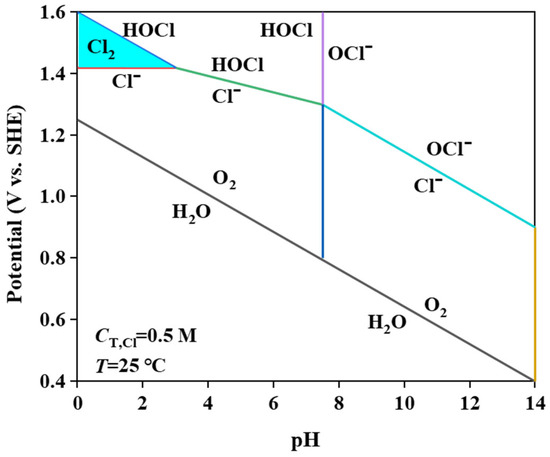
Figure 12.
Pourbaix diagram of brine electrolyte.
While employing MnOx anode materials for electrolysis, the incomplete formation of MnO6 octahedral configurations and Mn3+-active sites limits their catalytic selectivity. This structural imperfection permits partial OH− oxidation for oxygen evolution; yet, it cannot fully suppress the competitive chloride oxidation that generates Cl2. Consequently, the anode gas composition invariably contains chlorine species alongside oxygen, as evidenced by quantitative gas analysis.
5. Conclusions
The amorphous MnOx anode exhibits selective oxygen evolution in a chloride-rich environment, with 48.76% O2 content in the anode gas through its active Mn-O coordination site. The application of an anionic barrier reduces the N2 content to 47.56%, with the residual N2 coming exclusively from the decomposition of NH3. This dual strategy—deployment of a manganese-based anode combined with ion migration inhibition—effectively minimizes ammonia/ammonium oxidative substitution, thereby maintaining the initial ammonia concentration during prolonged electrolysis. The MnOx-IrO2/Ti composite anode developed in this paper has significant potential for industrial applications in high Cl− ammonia electrolytes. Although the initial cost of MnOx-IrO2/Ti will be about 3–5 times higher (mainly originating from Ir doping), it can achieve long-term cost-saving through the lower CER current efficiency, reduced ammonia replenishment, and longer lifetime. In addition, MnOx-IrO2/Ti anode avoids the risk of heavy metal contamination of lead-based materials, improves cathode zinc purity, and has significant premium space against the backdrop of tightening environmental regulations. For future research, the main focus should be on the following three aspects: firstly, the development of non-precious metal alternatives to increase OER activity by doping with non-precious metals; secondly, coating durability is optimized and in situ growth and other methods are used to improve the coating bond strength, thus extending the life of the material; finally, monitoring of coating pore changes by means of electrochemical impedance spectroscopy, etc., reduces the chances of sudden failures leading to production stoppages, etc. The above approaches can be used to promote the upgrading of the zinc-refining process in the ammonia system.
Author Contributions
Conceptualization, K.S., Z.L. and Y.L.; methodology, K.S. and Z.L.; software, K.S. and Z.L.; validation, K.S. and Z.L.; formal analysis, K.S.; investigation, K.S.; resources, Z.L.; data curation, K.S.; writing—original draft preparation, K.S.; writing—review and editing, K.S. and Z.L.; visualization, K.S. and Z.L.; supervision, Z.L., W.Z. and Y.L.; project administration, Y.L.; funding acquisition, W.Z. and Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (U23A20557); the National Natural Science Foundation of China (52464045); the Guangxi Science and Technology Program Projects (Major Special Projects, Guike AA23023033-1); and the Guangxi Science and Technology Program Projects (2023GXNSFBA026140).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Díaz-Arista, P.; Meas, Y.; Ortega, R.; Trejo, G. Electrochemical and AFM study of Zn electrodeposition in the presence of benzylideneacetone in a chloride-based acidic bath. J. Appl. Electrochem. 2005, 35, 217–227. [Google Scholar] [CrossRef]
- Gu, W.; Liu, C.; Tang, J.; Liu, R.; Yang, H.; Hu, J. Improving zinc electrodeposition in ammoniacal electrolytes with the saturated dissolved methyltrioctylammonium chloride. Hydrometallurgy 2018, 175, 43–51. [Google Scholar] [CrossRef]
- Dresp, S.; Dionigi, F.; Klingenhof, M.; Strasser, P. Direct Electrolytic Splitting of Seawater: Opportunities and Challenges. ACS Energy Lett. 2019, 4, 933–942. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, H.; Ang, E.H.; Nie, Z.; Liu, H.; Du, Y.; Han, C.; Zhu, J.; Huang, W. In-situ self-catalyzed growth of bimetallic nanoparticles/carbon nanotubes: A flexible binder-free electrocatalyst for high-performance oxygen evolution reaction. Mater. Today Phys. 2021, 16, 100303. [Google Scholar] [CrossRef]
- Zhang, Q.; Yu, G.; Hong, R.; Qiu, W.; Deng, C.; Yu, C. Electrochemical chlorine evolution reaction to improve the desalination of sea sand. Sci. Total Environ. 2024, 945, 174063. [Google Scholar] [CrossRef]
- Exner, K.S.; Lim, T.; Joo, S.H. Circumventing the OCl versus OOH scaling relation in the chlorine evolution reaction: Beyond dimensionally sTab. anodes. Curr. Opin. Electrochem. 2022, 34, 100979. [Google Scholar] [CrossRef]
- Chang, J.; Wang, G.; Yang, Z.; Li, B.; Wang, Q.; Kuliiev, R.; Orlovskaya, N.; Gu, M.; Du, Y.; Wang, G.; et al. Dual-Doping and Synergism toward High-Performance Seawater Electrolysis. Adv. Mater. 2021, 33, 2101425. [Google Scholar] [CrossRef]
- Rasmus, K.B.; Ann, C. Selectivity between Oxygen and Chlorine Evolution in the Chlor-Alkali and Chlorate Processes. Chem. Rev. 2016, 116, 2982–3028. [Google Scholar]
- Jia, L.; Zhong, Y.; Li, K.; Li, B.; Gao, J.; Liu, T.; Wang, F.; Wu, W.; Feng, J. Recovery of zinc resources from secondary zinc oxide via composite ammonia leaching: Analysis of Zn leaching behavior. Chem. Eng. J. 2023, 472, 144930. [Google Scholar] [CrossRef]
- Ling, T.; Yan, D.Y.; Wang, H.; Jiao, Y.; Hu, Z.; Zheng, Y.; Zheng, L.; Mao, J.; Liu, H.; Du, X.W.; et al. Activating cobalt(II) oxide nanorods for efficient electrocatalysis by strain engineering. Nat. Commun. 2017, 8, 1509. [Google Scholar] [CrossRef]
- Guo, J.; Zheng, Y.; Hu, Z.; Zheng, C.; Mao, J.; Du, K.; Jaroniec, M.; Qiao, S.Z.; Ling, T. Direct seawater electrolysis by adjusting the local reaction environment of a catalyst. Nat. Energy 2023, 8, 264–272. [Google Scholar] [CrossRef]
- Tong, W.; Forster, M.; Dionigi, F.; Dresp, S.; Sadeghi Erami, R.; Strasser, P.; Cowan, A.J.; Farràs, P. Electrolysis of low-grade and saline surface water. Nat. Energy 2020, 5, 367–377. [Google Scholar] [CrossRef]
- Bennett, J.E. Electrodes for generation of hydrogen and oxygen from seawater. Int. J. Hydrogen Energy 1980, 5, 401–408. [Google Scholar] [CrossRef]
- Vos, J.G.; Wezendonk, T.A.; Jeremiasse, A.W.; Koper, M.T. MnOx/IrOx as Selective Oxygen Evolution Electrocatalyst in Acidic Chloride Solution. J. Am. Chem. Soc. 2018, 140, 10270–10281. [Google Scholar] [CrossRef]
- Fujimura, K.; Izumiya, K.; Kawashima, A.; Akiyama, E.; Habazaki, H.; Kumagai, N.; Hashimoto, K. Anodically deposited manganese-molybdenum oxide anodes with high selectivity for evolving oxygen in electrolysis of seawater. J. Appl. Electrochem. 1999, 29, 769–775. [Google Scholar] [CrossRef]
- Freire, N.H.J.; Majuste, D.; Angora, M.A.; Ciminelli, V.S.T. The effect of organic impurities and additive on nickel electrowinning and product quality. Hydrometallurgy 2017, 169, 112–123. [Google Scholar] [CrossRef]
- Padhy, S.K.; Patnaik, P.; Tripathy, B.C.; Ghosh, M.K.; Bhattacharya, I.N. Electrodeposition of manganese metal from sulphate solutions in the presence of sodium octyl sulphate. Hydrometallurgy 2016, 165, 73–80. [Google Scholar] [CrossRef]
- Rabah, M.A.; Abdul Azim, A.A.; Ismail, A. Wear of graphite anodes during electrolysis of add sulphate solutions. J. Appl. Electrochem. 1981, 11, 41–47. [Google Scholar] [CrossRef]
- Hine, F.; Yasuda, M.; Sugiura, I.; Noda, T. Effects of the Active Chlorine and the pH on Consumption of Graphite Anode in Chlor-Alkali Cells. J. Electrochem. Soc. 1974, 121, 220. [Google Scholar] [CrossRef]
- Wranglén, G.; Sjödin, B.; Wallén, B. A new test method for graphite anodes in alkali chloride electrolysis. Electrochim. Acta 1962, 7, 577–587. [Google Scholar] [CrossRef]
- Tian, H.; Zeng, L.; Huang, Y.; Ma, Z.; Meng, G.; Peng, L.; Chen, C.; Cui, X.; Shi, J. In Situ Electrochemical Mn(III)/Mn(IV) Generation of Mn(II)O Electrocatalysts for High-Performance Oxygen Reduction. Nano-Micro Lett. 2020, 12, 161. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Wang, M.; Li, G.; He, Q.; Liu, J.; Li, F. Spherical α-MnO2 Supported on N-KB as Efficient Electrocatalyst for Oxygen Reduction in Al-Air Battery. Materials 2018, 11, 601. [Google Scholar] [CrossRef] [PubMed]
- George, L.; Viji, C.; Maheen, M.; Mohammed, E.M. Enhanced magnetic properties at low temperature of Mn substituted Ni-Zn mixed ferrite doped with Gd ions for magnetoresistive applications. Mater. Res. Bull. 2020, 126, 110833. [Google Scholar] [CrossRef]
- Li, M.; Zeng, L.; Chen, Y.; Zhuang, L.; Wang, X.; Shen, H. Realization of Colored Multicrystalline Silicon Solar Cells with SiO2/SiNx:H Double Layer Antireflection Coatings. Int. J. Photoenergy 2013, 2013, 352473. [Google Scholar] [CrossRef]
- Abe, H.; Murakami, A.; Tsunekawa, S.; Okada, T.; Wakabayashi, T.; Yoshida, M.; Nakayama, M. Selective Catalyst for Oxygen Evolution in Neutral Brine Electrolysis: An Oxygen-Deficient Manganese Oxide Film. ACS Catal. 2021, 11, 6390–6397. [Google Scholar] [CrossRef]
- Kim, J.; Yin, X.; Tsao, K.C.; Fang, S.; Yang, H. Ca2Mn2O5 as Oxygen-Deficient Perovskite Electrocatalyst for Oxygen Evolution Reaction. J. Am. Chem. Soc. 2014, 136, 14646–14649. [Google Scholar] [CrossRef]
- Genuino, H.C.; Meng, Y.; Horvath, D.T.; Kuo, C.H.; Seraji, M.S.; Morey, A.M.; Joesten, R.L.; Suib, S.L. Enhancement of Catalytic Activities of Octahedral Molecular Sieve Manganese Oxide for Total and Preferential CO Oxidation through Vanadium Ion Framework Substitution. ChemCatChem 2013, 5, 2306–2317. [Google Scholar] [CrossRef]
- Martin, R.; Kim, M.; Lee, C.J.; Mehar, V.; Albertin, S.; Hejral, U.; Merte, L.R.; Lundgren, E.; Asthagiri, A.; Weaver, J.F. High-Resolution X-ray Photoelectron Spectroscopy of an IrO2(110) Film on Ir(100). J. Phys. Chem. Lett. 2020, 11, 7184–7189. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Kaur, A.; Chahal, P.; Hogan, T. Selective Fabrication of SiC/Si Diodes by Excimer Laser Under Ambient Conditions. IEEE Electron Device Lett. 2016, 37, 142–145. [Google Scholar] [CrossRef]
- Foo, C.; Li, Y.; Lebedev, K.; Chen, T.; Day, S.; Tang, C.; Tsang, S.C.E. Characterization of oxygen defects and nitrogen impurities in TiO2 photocatalysts using variable-temperature X-ray powder diffraction. Nat. Commun. 2021, 12, 661. [Google Scholar] [CrossRef]
- Ignatans, R.; Mallia, G.; Ahmad, E.A.; Spillane, L.; Stoerzinger, K.A.; Shao-Horn, Y.; Harrison, N.M.; Tileli, V. The Effect of Surface Reconstruction on the Oxygen Reduction Reaction Properties of LaMnO3. J. Phys. Chem. C 2019, 123, 11621–11627. [Google Scholar] [CrossRef]
- Risch, M.; Stoerzinger, K.A.; Han, B.; Regier, T.Z.; Peak, D.; Sayed, S.Y.; Wei, C.; Xu, Z.; Shao-Horn, Y. Redox Processes of Manganese Oxide in Catalyzing Oxygen Evolution and Reduction: An in Situ Soft X-ray Absorption Spectroscopy Study. J. Phys. Chem. C 2017, 121, 17682–17692. [Google Scholar] [CrossRef]
- Lima, F.H.B.; Calegaro, M.L.; Ticianelli, E.A. Electrocatalytic activity of manganese oxides prepared by thermal decomposition for oxygen reduction. Electrochim. Acta 2007, 52, 3732–3738. [Google Scholar] [CrossRef]
- Dionigi, F.; Reier, T.; Pawolek, Z.; Gliech, M.; Strasser, P. Design Criteria, Operating Conditions, and Nickel-Iron Hydroxide Catalyst Materials for Selective Seawater Electrolysis. ChemSusChem 2016, 9, 962–972. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).