Preparation of an Fe80P14B6 Bulk Nanocrystalline Alloy via Solidification from a Molten Alloy at Deep Undercooling
Abstract
1. Introduction
2. Experiments
3. Results and Discussion
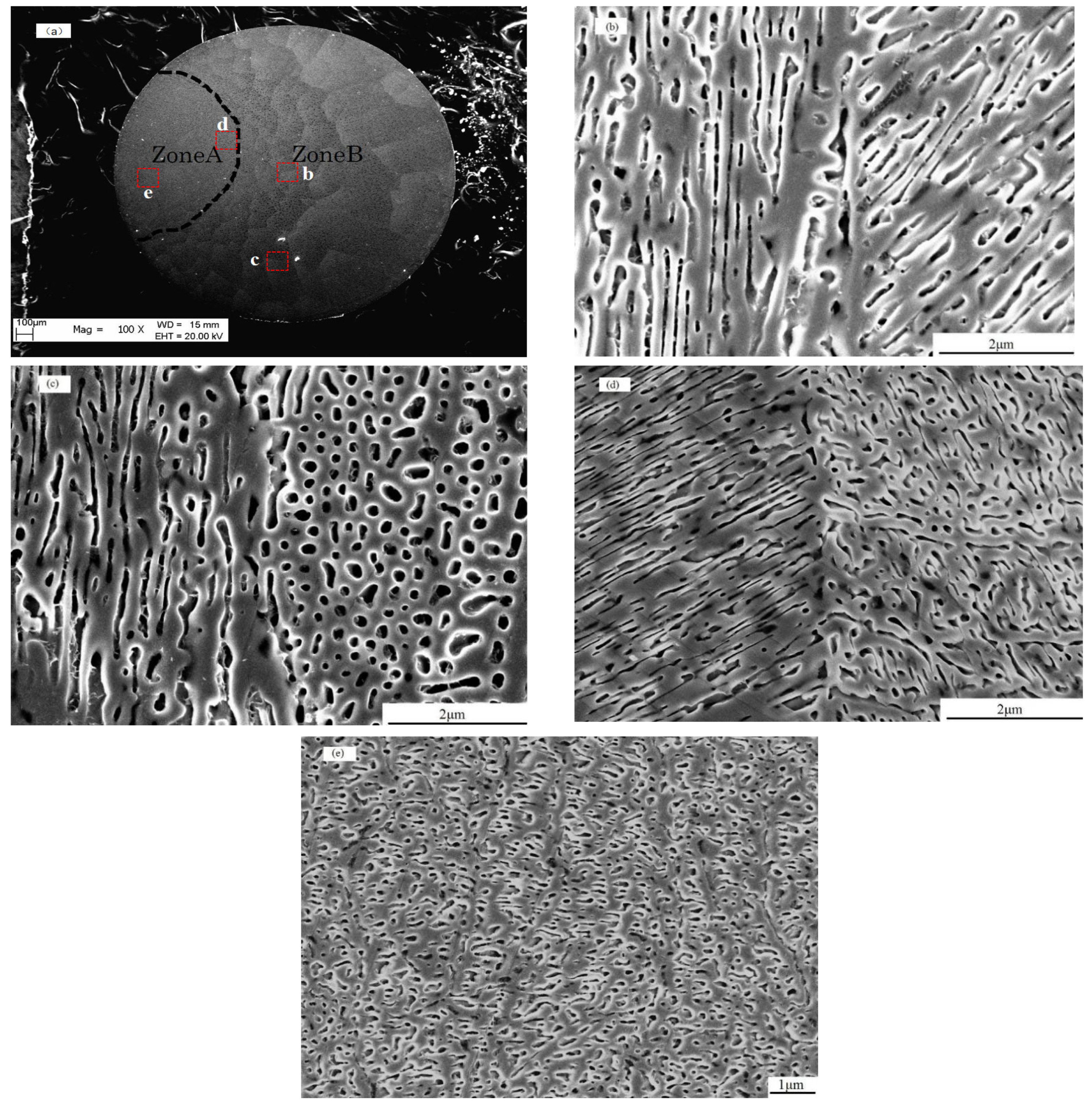
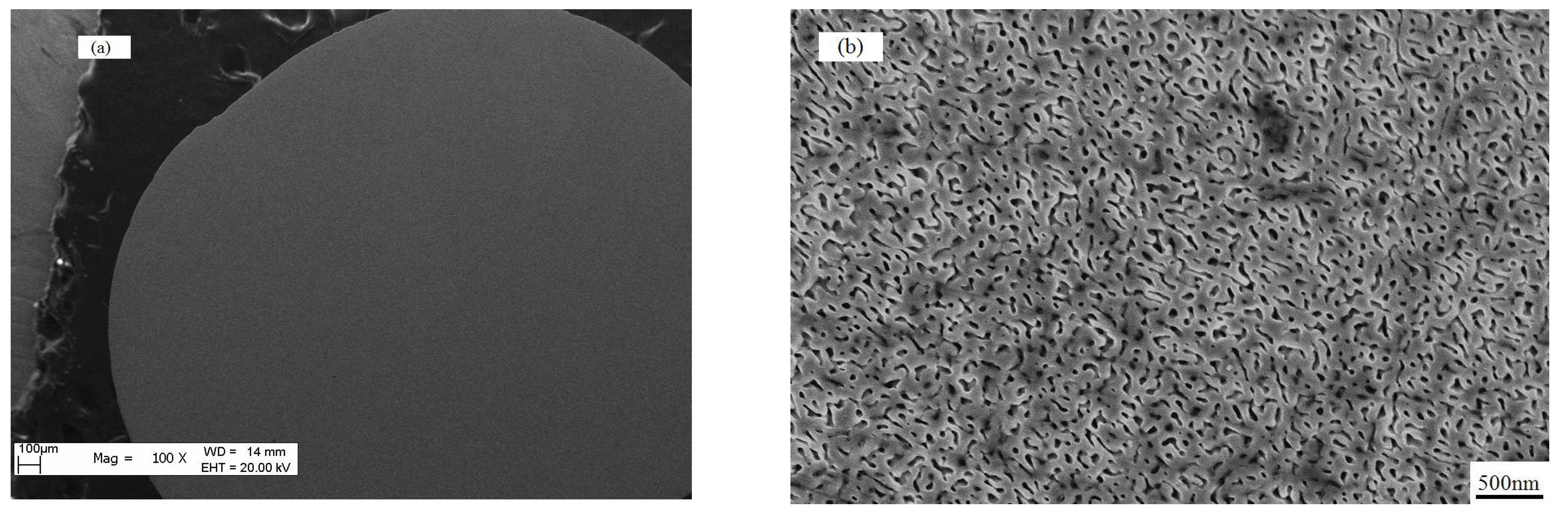
3.1. The Evolution of the Solidified Morphology Under Different Levels of Undercooling
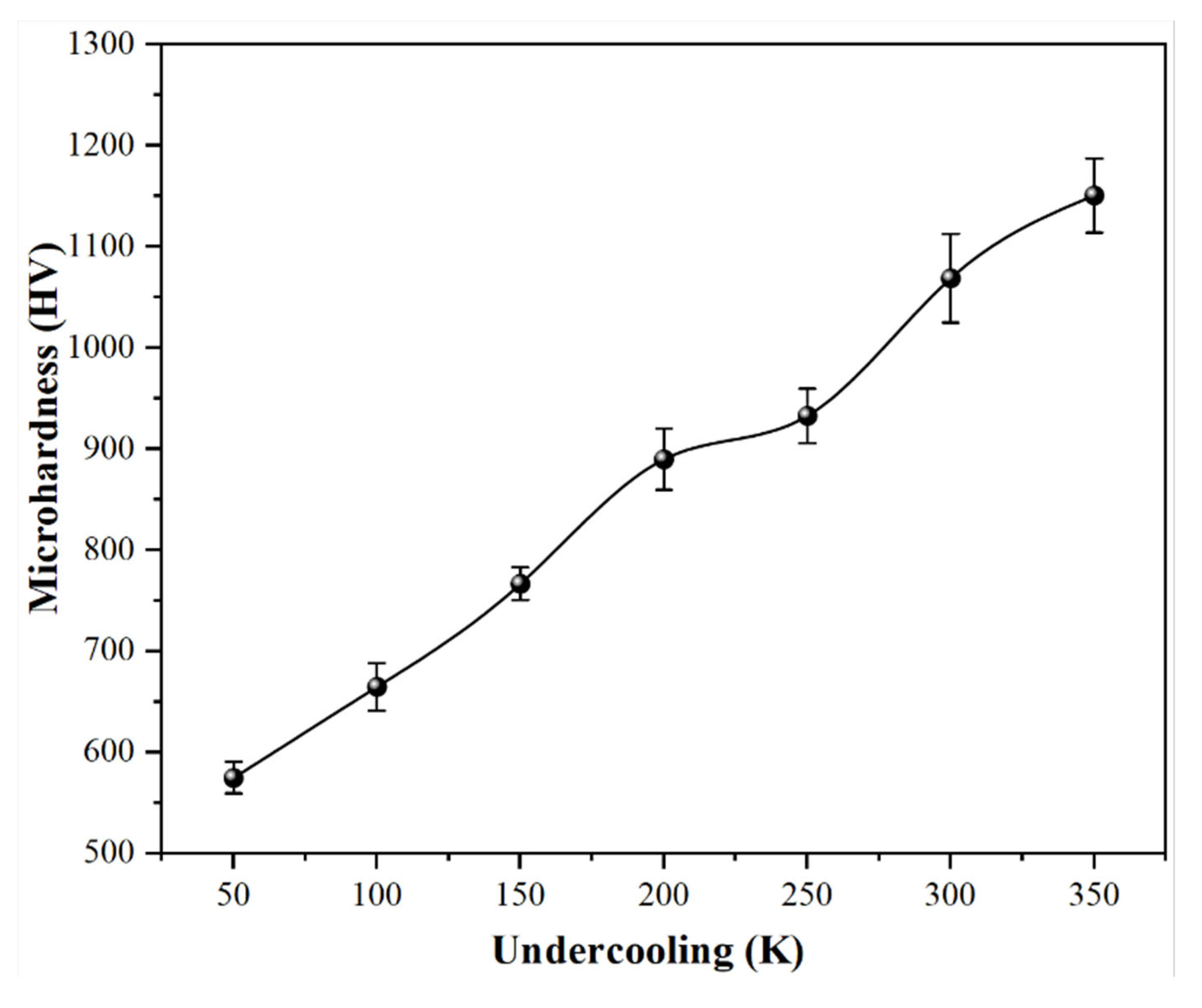
3.2. The Evolution of Microhardness Under Different Levels of Undercooling
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gleiter, H. Nanocrystalline Materials. Prog. Mater. Sci. 1989, 33, 223–315. [Google Scholar] [CrossRef]
- Turnbull, D. Metastable structures in metallurgy. Metall. Trans. 1981, B12, 217–230. [Google Scholar] [CrossRef]
- Shi, L.X.; Yao, K.F. Composition design for Fe-based soft magnetic amorphous and nanocrystalline alloys with high Fe content. Mater. Design 2020, 189, 108511. [Google Scholar] [CrossRef]
- Ding, J.L.; Xu, H.J.; Shi, Z.G.; Li, X.; Zhang, T. Effect of primary α-Fe on soft magnetic properties of FeCuNbSiB amorphous/nanocrystalline alloy. J. Non-Cryst. Solids 2021, 571, 121079. [Google Scholar] [CrossRef]
- Birringer, R.; Gleiter, H.; Klein, H.P.; Marquardt, P. Nanocrystalline materials an approach to a novel solid structure with gas-like disorder? Phys. Lett. A 1984, 102, 365–369. [Google Scholar] [CrossRef]
- Schneider, S.; Thiyagarajan, P.; Johnson, W.L. Formation of nanocrystals based on decomposition in the amorphous Zr41.2Ti13.8Cu12.5 Ni10Be22.5 alloy. Appl. Phys. Lett. 1996, 68, 493–495. [Google Scholar] [CrossRef]
- Guo, W.H.; Kui, H.W. Bulk nanostructured alloy formation with controllable grain size. Acta Mater. 2000, 48, 2117–2121. [Google Scholar] [CrossRef]
- Wu, Z.D.; Lu, X.H.; Wu, Z.H.; Kui, H.W. On the short-range orders in spinodal Pd–Ni–P bulk metallic glasses. J. Non-Cryst. Solids 2014, 385, 40–46. [Google Scholar] [CrossRef]
- Lee, K.L.; Kui, H.W. Phase separation in undercooled molten Pd80 Si20: Part I. J. Mater. Res. 1999, 14, 3653–3662. [Google Scholar] [CrossRef]
- Macchi, J.; Nakonechna, O.; Henry, R.; Castro, C.; Edalati, K.; Geuser, F.D.; Sauvage, X.; Lefebvre, W. Microstructural design by combining nanograins and spinodal decomposition in a Fe-Cr alloy. Scr. Mater. 2024, 252, 116247. [Google Scholar] [CrossRef]
- Bakonyi, I.; Cziráki, Á. Nanocrystalline-forming ability of alloys by melt-quenching. Nanostruct. Mater. 1999, 11, 9–16. [Google Scholar] [CrossRef]
- Lau, M.T.; Lan, S.; Kui, H.W. A metastable liquid state miscibility gap in under- cooled Pd–Ni–P melts. J. Non-Cryst. Solids 2012, 358, 2667–2673. [Google Scholar] [CrossRef]
- Lan, S.; Lau, M.T.; Kui, H.W. The time constant of the spinodal decomposition in Pd41.75Ni41.75P17.5 bulk metallic glasses. J. Non-Cryst. Solids 2013, 361, 1–8. [Google Scholar] [CrossRef]
- Takeuchi, A.; Inoue, A. Classification of Bulk Metallic Glasses by Atomic Size Difference, Heat of Mixing and Period of Constituent Elements and Its Application to Characterization of the Main Alloying Element (Overview). Mater. Trans. 2005, 46, 2817–2829. [Google Scholar] [CrossRef]
- Ho, C.M.; Leung, C.C.; Yip, Y.L.; Mok, S.W.; Kui, H.W. Ductile Fe83C17 Alloys of Ultrafine Networklike Microstructure. Metall. Mater. Trans. A 2010, 41, 3443–3451. [Google Scholar] [CrossRef]
- Ho, C.M.; Kui, H.W. Ductile and High Strength White Cast Iron of Ultrafine Interconnected Network Morphology. Metall. Mater. Trans. A 2011, 42, 3826–3837. [Google Scholar] [CrossRef]
- Chow, W.H.; Leung, C.C.; Yip, Y.L.; Mok, S.W.; Kui, H.W. Scanning Electron Microscopy of Fe79.5B6.5C14 Network Alloys: Part I. Metall. Mater. Trans. A 2013, 44, 3532–3543. [Google Scholar] [CrossRef]
- Guo, W.H.; Chua, L.F.; Leung, C.C.; Kui, H.W. Formation of Bulk Nanostructured Materials by Rapid Solidification. J. Mater. Res. 2000, 15, 1605–1611. [Google Scholar] [CrossRef]
- Yip, Y.L.; Leung, C.C.; Mok, S.W.; Yip, K.H.; Kui, H.W. Transmission Electron Microscopy of Fe79.5B6.5C14 Network Alloys: Part II. Metall. Mater. Trans. A 2014, 45, 1457–1469. [Google Scholar] [CrossRef]
- Cahn, J.W. Spinodal Decomposition. Trans. Metall. Soc. AIME 1968, 242, 166–180. [Google Scholar] [CrossRef]
- Cahn, J.W. Phase Separation by Spinodal Decomposition in Isotropic Systems. J. Chem. Phys. 1965, 42, 93–99. [Google Scholar] [CrossRef]
- Park, H.; Haftlang, F.; Heo, Y.; Seol, J.B.; Wang, Z.; Kim, H.S. Periodic spinodal decomposition in double–strengthened medium–entropy alloy. Nat. Commun. 2024, 15, 5757. [Google Scholar] [CrossRef] [PubMed]
- Cahn, J.W.; Hilliard, J.E. Spinodal decomposition: A repriseDecomposition spinodale: Mise au pointSpinodale entmischung: Eine reprise. Acta Mater. 1971, 19, 151–161. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Wang, T.; Zhang, Z.; Li, Y.; Wang, M.; Lv, K.; Wu, G.; Wang, X.; Li, Z.; Hui, X. Preparation of an Fe80P14B6 Bulk Nanocrystalline Alloy via Solidification from a Molten Alloy at Deep Undercooling. Materials 2025, 18, 1361. https://doi.org/10.3390/ma18061361
Chen X, Wang T, Zhang Z, Li Y, Wang M, Lv K, Wu G, Wang X, Li Z, Hui X. Preparation of an Fe80P14B6 Bulk Nanocrystalline Alloy via Solidification from a Molten Alloy at Deep Undercooling. Materials. 2025; 18(6):1361. https://doi.org/10.3390/ma18061361
Chicago/Turabian StyleChen, Xiaoming, Tuo Wang, Zhe Zhang, Yuluo Li, Mingming Wang, Kuang Lv, Guigen Wu, Xiaoli Wang, Zhangyin Li, and Xidong Hui. 2025. "Preparation of an Fe80P14B6 Bulk Nanocrystalline Alloy via Solidification from a Molten Alloy at Deep Undercooling" Materials 18, no. 6: 1361. https://doi.org/10.3390/ma18061361
APA StyleChen, X., Wang, T., Zhang, Z., Li, Y., Wang, M., Lv, K., Wu, G., Wang, X., Li, Z., & Hui, X. (2025). Preparation of an Fe80P14B6 Bulk Nanocrystalline Alloy via Solidification from a Molten Alloy at Deep Undercooling. Materials, 18(6), 1361. https://doi.org/10.3390/ma18061361




