The Microstructure, Crystallization Behavior, and Mechanical Performance Evolutions of Li2O-Al2O3-SiO2 Glass and Glass–Ceramics with Different Alkaline Earth Oxide Modifications
Abstract
:1. Introduction
2. Experimental Section
2.1. Glass and Glass–Ceramics’ Preparation
2.2. Characterization
3. Results and Discussion
3.1. The Basic Physical Properties and Microstructures of the Glass
3.2. The Thermal Properties of the Glass
3.3. The Mechanical Performances of the Glass and Glass–Ceramics
| Composition System | Crystallization Process | Main Crystalline Phases | Vickers Hardness (GPa) | Elastic Modulus (GPa) | References |
|---|---|---|---|---|---|
| LAS | 680–750 °C/1–2 h + 800–950 °C/1–4 h | β-quartz, β-Spodumene | 6.5–7.5 | 95–110 | [40] |
| LAS + TiO2 | 720 °C/0.5 h + 850 °C/1 h | β-quartz | 6.8–7.3 | 80–90 | [41] |
| LAS + ZrO2 | 700 °C/1 h + 880 °C/1.5 h | β-quartz | 7.2–7.6 | 105–112 | [42] |
| MLAS | 640 °C/2 h + 780 °C/1 h | MgAl2Si4O12, LixAlxSi3−xO6 | 8.61 | 89.1 | This work |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fu, L.; Engqvist, H.; Xia, W. Glass–ceramics in dentistry: A Review. Materials 2020, 13, 1049. [Google Scholar] [CrossRef]
- Xing, Z.; Ling, J.; Hui, Y. Effects of Li replacement on the nucleation, crystallization and microstructure of Li2O-Al2O3-SiO2 glass. J. Non Cryst. Solids 2008, 354, 4031–4036. [Google Scholar]
- Kleebusch, E.; Patzig, C.; Krause, M.; Hu, Y.; Höche, T.; Rüssel, C. The formation of nanocrystalline ZrO2 nuclei in a Li2O-Al2O3-SiO2 glass— A combined XANES and TEM study. Sci. Rep. 2017, 7, 10869. [Google Scholar]
- Beall, G.H.; Monique, C.; Dejneka, M.J.; Paulo, M.; Philippe, P. Ion-Exchange in glass-ceramics. Front. Mater. 2016, 3, 41. [Google Scholar]
- Venkateswaran, C.; Sreemoolanadhan, H.; Vaish, R. Lithium aluminosilicate (LAS) glass-ceramics: A review of recent progress. Int. Mater. Rev. 2021, 67, 620–657. [Google Scholar]
- Karmakar, B.; Kundu, P.; Jana, S.; Dwivedi, R.N. Crystallization kinetics and mechanism of low-expansion lithium aluminosilicate glass-ceramics by dilatometry. J. Am. Ceram. Soc. 2002, 85, 2572–2574. [Google Scholar]
- Zanotto, E.D. Bright future for glass-ceramics. J. Am. Ceram. Soc. Bull. 2010, 89, 19–27. [Google Scholar]
- Hasselman, D.; Donaldson, K.Y. Thermal conductivity of vapor-liquid-solid and vapor-solid silicon carbide whisker-reinforced lithium aluminosilicate glass-ceramic composites. J. Am. Ceram. Soc. 1996, 79, 742–748. [Google Scholar]
- Soares, V.O.; Serbena, F.C.; Mathias, I.; Crovace, M.C.; Zanotto, E.D. New, tough and strong lithium metasilicate dental glass-ceramic. Ceram. Int. 2021, 47, 2793–2801. [Google Scholar]
- Łączka, K.; Cholewa-Kowalska, K.; Środa, M.; Rysz, J.; Marzec, M.M.; Łączka, M. Glass–ceramics of LAS (Li2O-Al2O3-SiO2) system enhanced by ion-exchange in KNO3 salt bath. J. Non Cryst. Solids 2015, 428, 90–97. [Google Scholar]
- Sasmal, N.; Garai, M.; Molla, A.R.; Tarafder, A.; Singh, S.P.; Karmakar, B. Effects of lanthanum oxide on the properties of barium-free alkaline-earth borosilicate sealant glass. J. Non Cryst. Solids 2014, 387, 62–70. [Google Scholar]
- Nakane, S. Recent research on Li2O-Al2O3-SiO2 glass-ceramics for expansion of applications. J. Non Cryst. Solids X 2022, 16, 100121. [Google Scholar]
- Sun, T.; Zheng, C.; Zhang, F.; Zhang, J.; Han, J.; Xie, J.; He, J.; Jiang, H. Xie. Mixed CaO/MgO effect on microstructure, mechanical properties and crystallization behavior of Li2O-Al2O3-SiO2-ZrO2-P2O5 glass. J. Non Cryst. Solids 2023, 616, 122457. [Google Scholar]
- Zhang, X.; Wang, J.; Han, J. Crystallization kinetics, structural and mechanical properties of transparent lithium alumino-silicate glass-ceramics. J. Non Cryst. Solids 2023, 603, 122113. [Google Scholar]
- Fabris, D.C.N.; Miguel, E.H.; Vargas, R.; Canto, R.B.; Villas-Boas, M.d.O.C.; Peitl, O.; Sglavo, V.M.; Zanotto, E.D. Microstructure, residual stresses, and mechanical performance of surface crystallized translucent glass-ceramics. J. Eur. Ceram. Soc. 2022, 42, 4631–4642. [Google Scholar]
- Yang, J.; Xing, J.; Zheng, Q.; Qiao, A.; Tao, H. Transparent surface crystallized aluminosilicate glasses with an ultrahigh crack resistance. J. Non Cryst. Solids 2024, 635, 122996. [Google Scholar]
- Kim, S.-W.; Lee, H.-S.; Jun, D.-S. Enhancing the Plasma-Resistance Properties of Li2O-Al2O3-SiO2 Glasses for the Semiconductor Etch Process via Alkaline Earth Oxide Incorporation. Materials 2023, 16, 5112. [Google Scholar] [CrossRef]
- Weigel, C.; Le Losq, C.; Vialla, R.; Dupas, C.; Clément, S.; Neuville, D.R.; Rufflé, B. Elastic moduli of XAlSiO4 aluminosilicate glasses: Effects of charge-balancing cations. J. Non Cryst. Solids 2016, 447, 267–272. [Google Scholar] [CrossRef]
- Shelby, J.E. Introduction to Glass Science and Technology; Royal Society of Chemistry: Edinburgh, UK, 2020. [Google Scholar]
- Wada, M.; Furukawa, H.; Fujita, K. Crack resistance of glass on Vickers indentation. Proc. X Int. Congr. Glass. 1974, 11, 39–46. [Google Scholar]
- Fry, A.L.; Krug, C.; Ladinger, L. Fracture toughness of Ca-, Sr-, and Ba-aluminoborosilicate glasses. J. Am. Ceram. Soc. 2023, 107, 830–843. [Google Scholar]
- Furukawa, T.; Fox, K.E.; White, W.B. Raman spectroscopic investigation of the structure of silicate glasses. III. Raman intensities and structural units in sodium silicate glasses. J. Chem. Phys. 1981, 75, 226–3237. [Google Scholar] [CrossRef]
- Ross, S.; Welsch, A.M.; Behrens, H. Lithium conductivity in glasses of the Li2O-Al2O3-SiO2 system. Phys. Chem. Chem. Phys. 2015, 17, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Poe, B.T.; McMillan, P.F.; Coté, B.; Massiot, D.; Coutures, J. Structure and Dynamics in Calcium Aluminate Liquids: High-Temperature 27Al NMR and Raman Spectroscopy. J. Am. Ceram. Soc. 1994, 77, 1832–1838. [Google Scholar] [CrossRef]
- Ke, X.; Wang, X.; Wang, Y.; Ren, X.; Tao, H. Mg and Al mixed effects on thermal properties in aluminosilicate glasses. J. Am. Ceram. Soc. 2023, 106, 7449–7459. [Google Scholar] [CrossRef]
- Qu, W.; Liu, S.; Xiao, Z.; Ma, J.; Wei, M. Rheological and thermodynamic properties of alkaline earth oxide bearing silicate glasses: Implication of structural role of Mg2+ in peralkaline glasses. J. Alloy. Compd. 2016, 661, 1–5. [Google Scholar] [CrossRef]
- Kolay, S.; Bhargava, P. Role of MgO in Lowering Glass Transition Temperature and Increasing Hardness of Lithium Silicate Glass and Glass-Ceramics. Ceram. Int. 2022, 48, 12699–12711. [Google Scholar] [CrossRef]
- Park, S.Y.; Lee, S.K. High-resolution solid-state NMR study of the effect of composition on network connectivity and structural disorder in multi-component glasses in the diopside and jadeite join: Implications for structure of andesitic melts. Geochim. Cosmochim. Acta 2014, 147, 26–42. [Google Scholar] [CrossRef]
- Jakobsen, H.J.; Skibsted, J.; Bildsøe, H.; Nielsen, N.C. Magic-angle spinning NMR spectra of satellite transitions for quadrupolar nuclei in solids. J. Magn. Reson. 1989, 85, 173–180. [Google Scholar] [CrossRef]
- Goswami, M.; Kothiyal, G.P.; Montagne, L.; Delevoye, L. MAS-NMR study of lithium zinc silicate glasses and glass-ceramics with various ZnO content. J. Solid State Chem. 2008, 181, 269–275. [Google Scholar] [CrossRef]
- Stebbins, J.F.; Kroeker, S.; Lee, S.K.; Kiczenski, T. Quantification of five-and six-coordinated aluminum ions in aluminosilicate and fluoride-containing glasses by high-field, high-resolution 27Al NMR. J. Non Cryst. Solids 2000, 275, 1–6. [Google Scholar] [CrossRef]
- Lilensten, L.; Fu, Q.; Wheaton, B.R.; Credle, A.J.; Stewart, R.L.; Kohli, J.T. Kinetic study on lithium-aluminosilicate (LAS) glass-ceramics containing MgO and ZnO. Ceram. Int. 2014, 40, 11657–11661. [Google Scholar]
- Lu, Z.; Lu, J.; Li, X.; Shao, G. Effect of MgO addition on sinter ability, crystallization kinetics, and flexural strength of glass–ceramics from waste materials. Ceram. Int. 2016, 42, 3452–3459. [Google Scholar]
- Shan, Z.; Li, C.; Tao, H. Mixed alkaline-earth effect on the mechanical and rheological properties of Ca–Mg silicate glasses. J. Am. Ceram. Soc. 2017, 100, 4570–4580. [Google Scholar] [CrossRef]
- Hou, Y.; Cheng, J.; Kang, J.; Yuan, J. Structure, glass stability and rheological properties of Na2O-CaO-Al2O3-SiO2 glasses doped with Y2O3. Ceram. Silik. 2018, 62, 173–180. [Google Scholar]
- Atila, A.; Ghardi, E.M.; Ouaskit, S.; Hasnaoui, A. Atomistic insights into the impact of charge balancing cations on the structure and properties of aluminosilicate glasses. Phys. Rev. B 2019, 100, 144109. [Google Scholar]
- Moysan, C.; Riedel, R.; Harshe, R.; Rouxel, T.; Augereau, F. Mechanical characterization of a polysiloxane-derived SiOC glass. J. Eur. Ceram. Soc. 2006, 27, 397–403. [Google Scholar]
- Hermansen, C.; Matsuoka, J.; Yoshida, S.; Yamazaki, H.; Kato, Y.; Yue, Y. Densification and plastic deformation under microindentation in silicate glasses and the relation to hardness and crack resistance. J. Non Cryst. Solids 2013, 364, 40–43. [Google Scholar]
- Rouxel, T.; Yoshida, S. The fracture toughness of inorganic glasses. J. Am. Ceram. Soc. 2017, 100, 4374–4396. [Google Scholar]
- Beall, G.H. Design and Properties of Glass-Ceramics. Annu. Rev. Mater. Sci. 1992, 22, 91–119. [Google Scholar]
- Höland, W.; Rheinberger, V.; Schweiger, M. Studies on Crystal Phase Formation in Lithium Aluminosilicate Glass-Ceramics. J. Eur. Ceram. Soc. 1999, 19, 2277–2283. [Google Scholar]
- Cheng, X.; Li, Y. Effect of ZrO2 on LAS Glass-Ceramics. Ceram. Int. 2018, 44, 16368–16375. [Google Scholar]



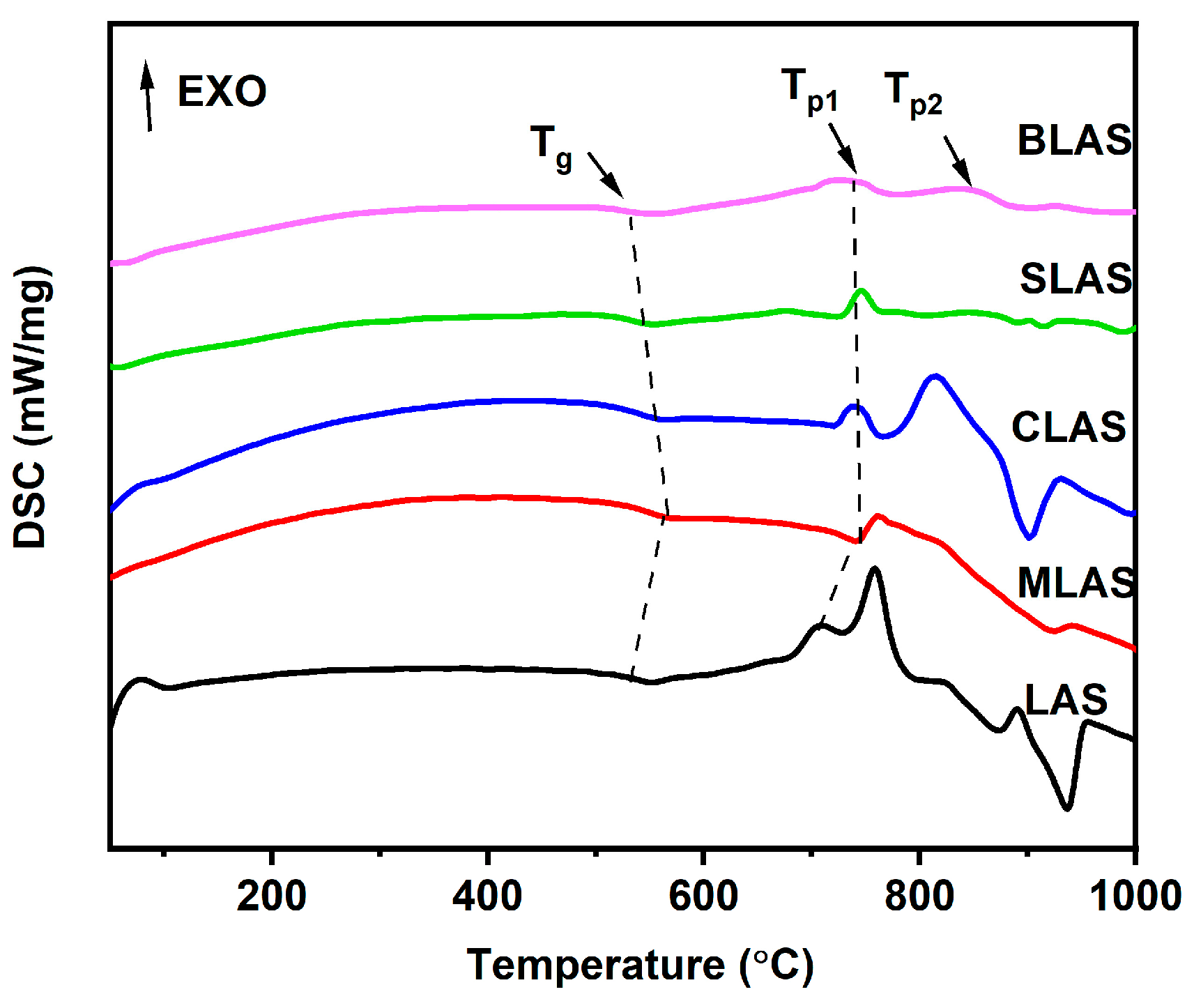
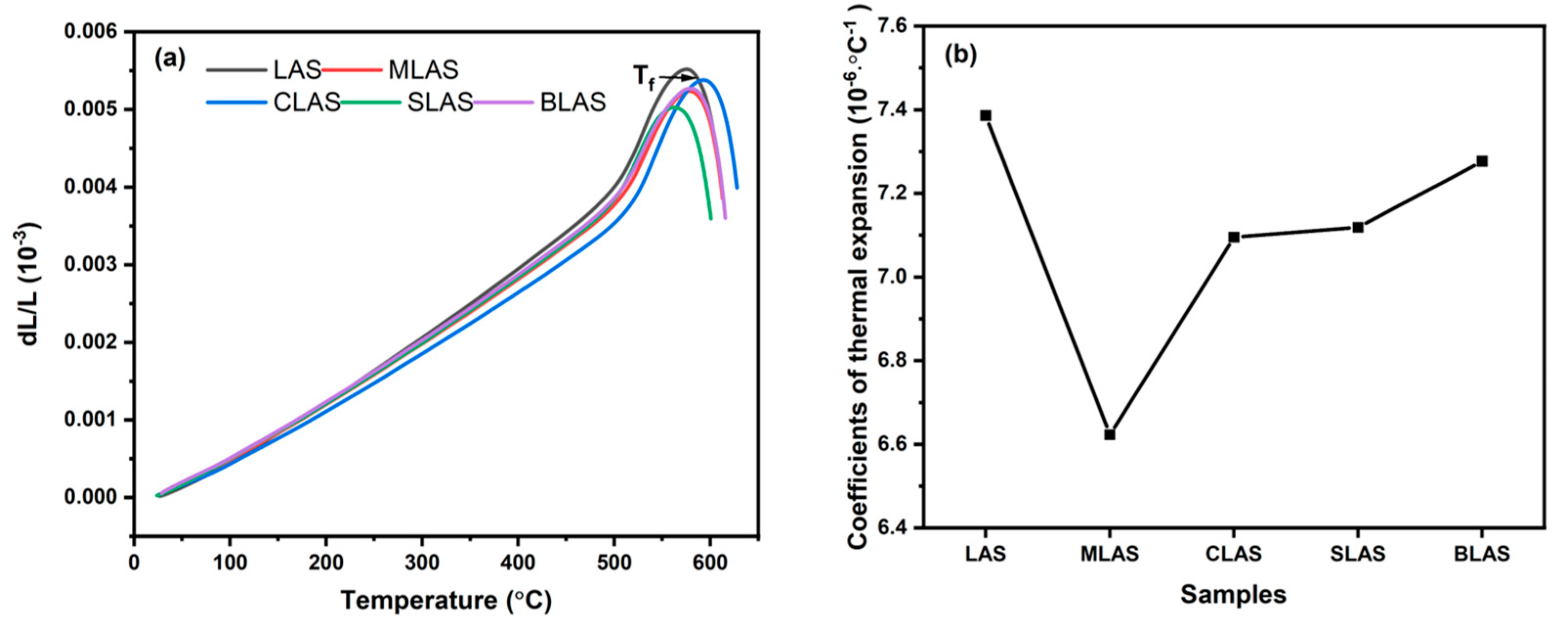
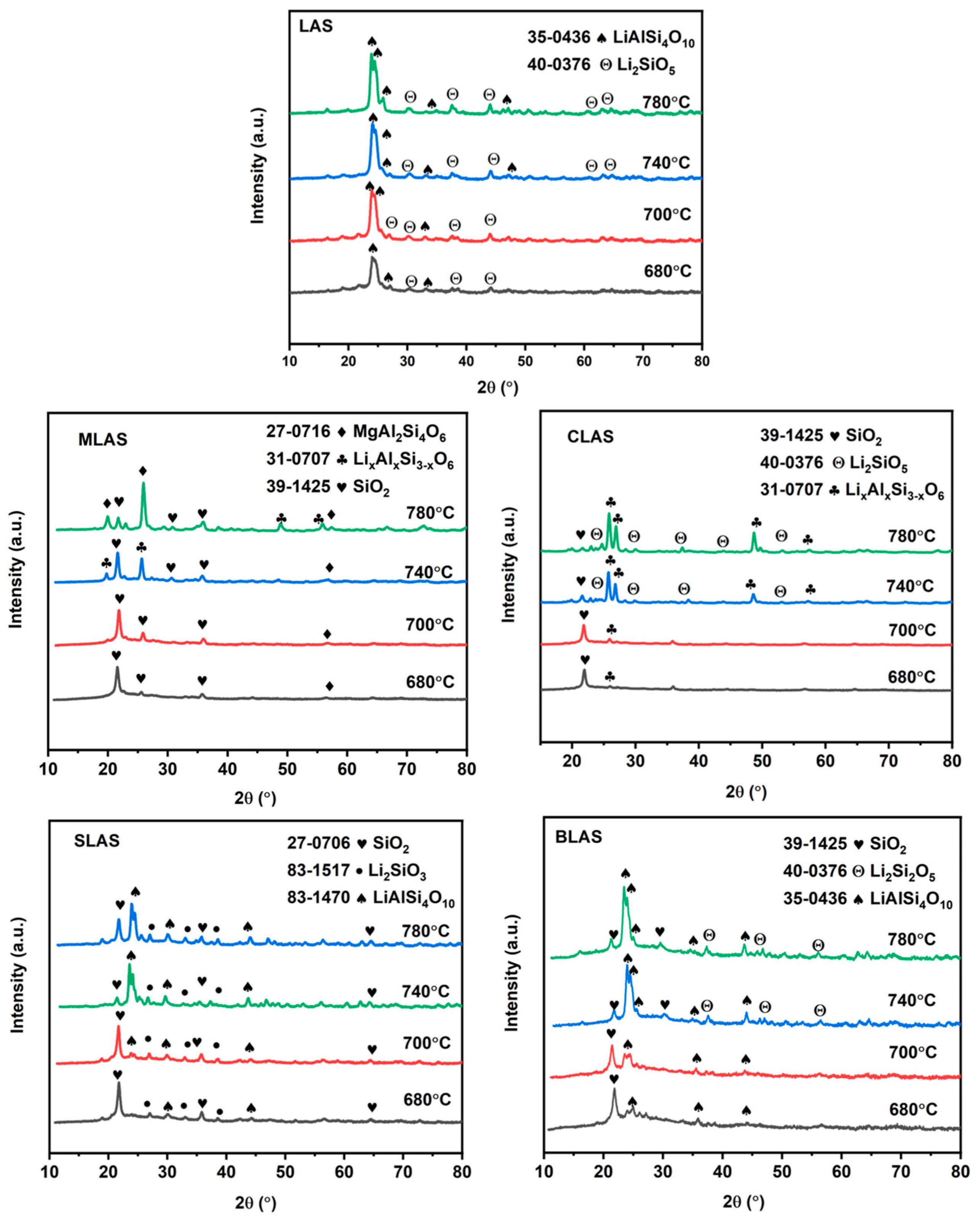
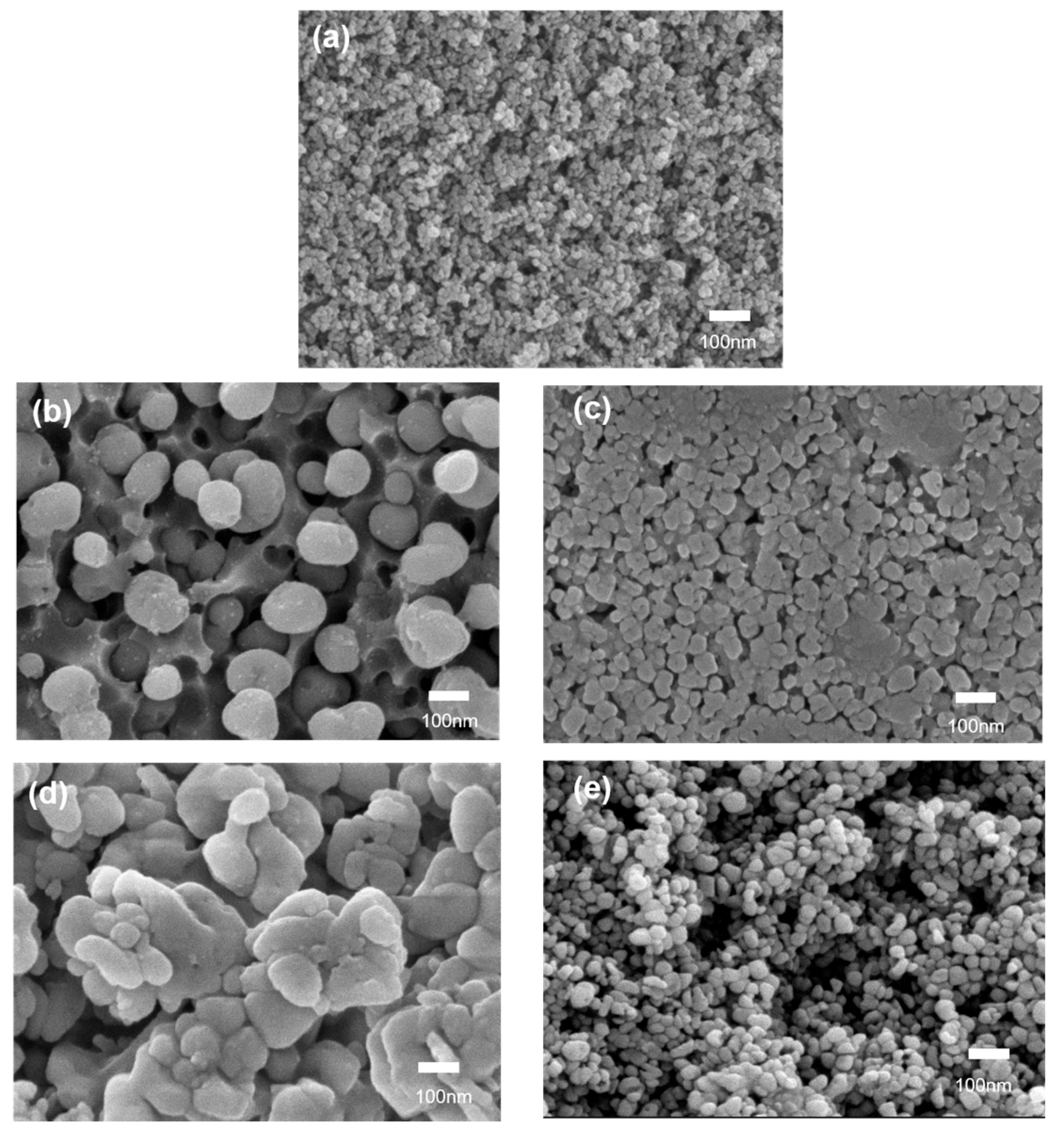
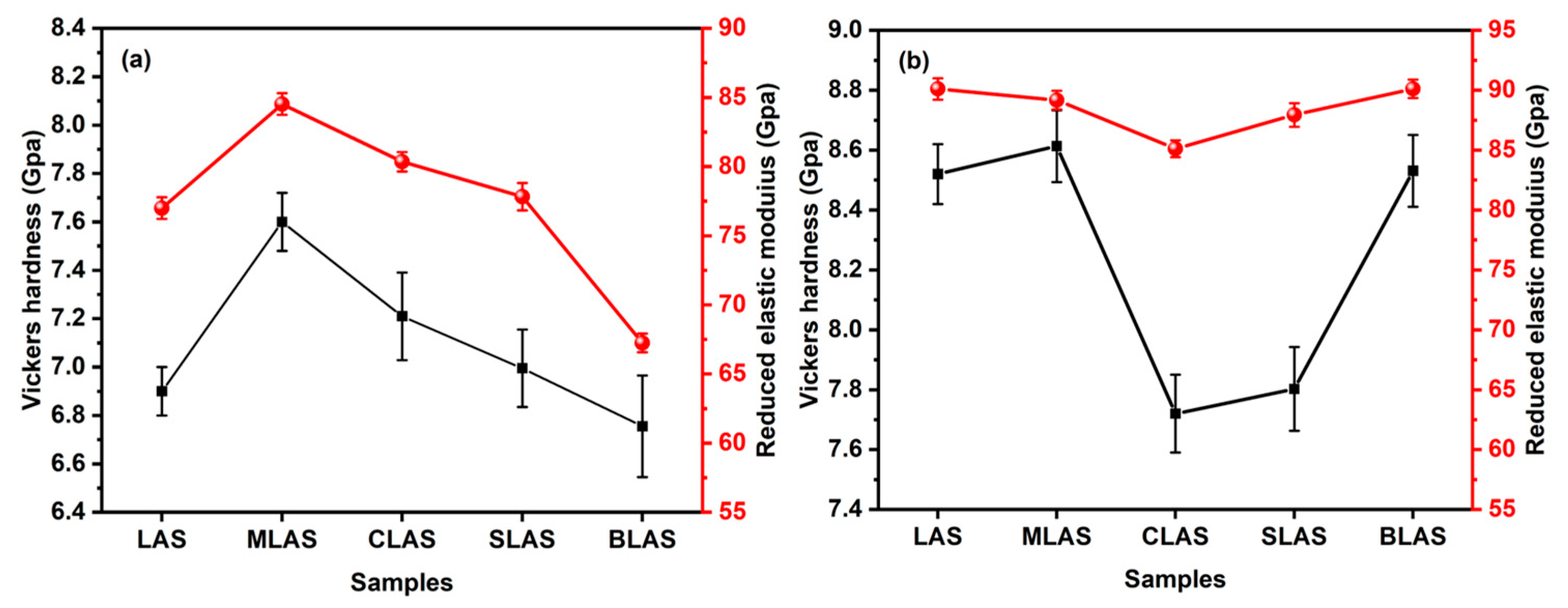
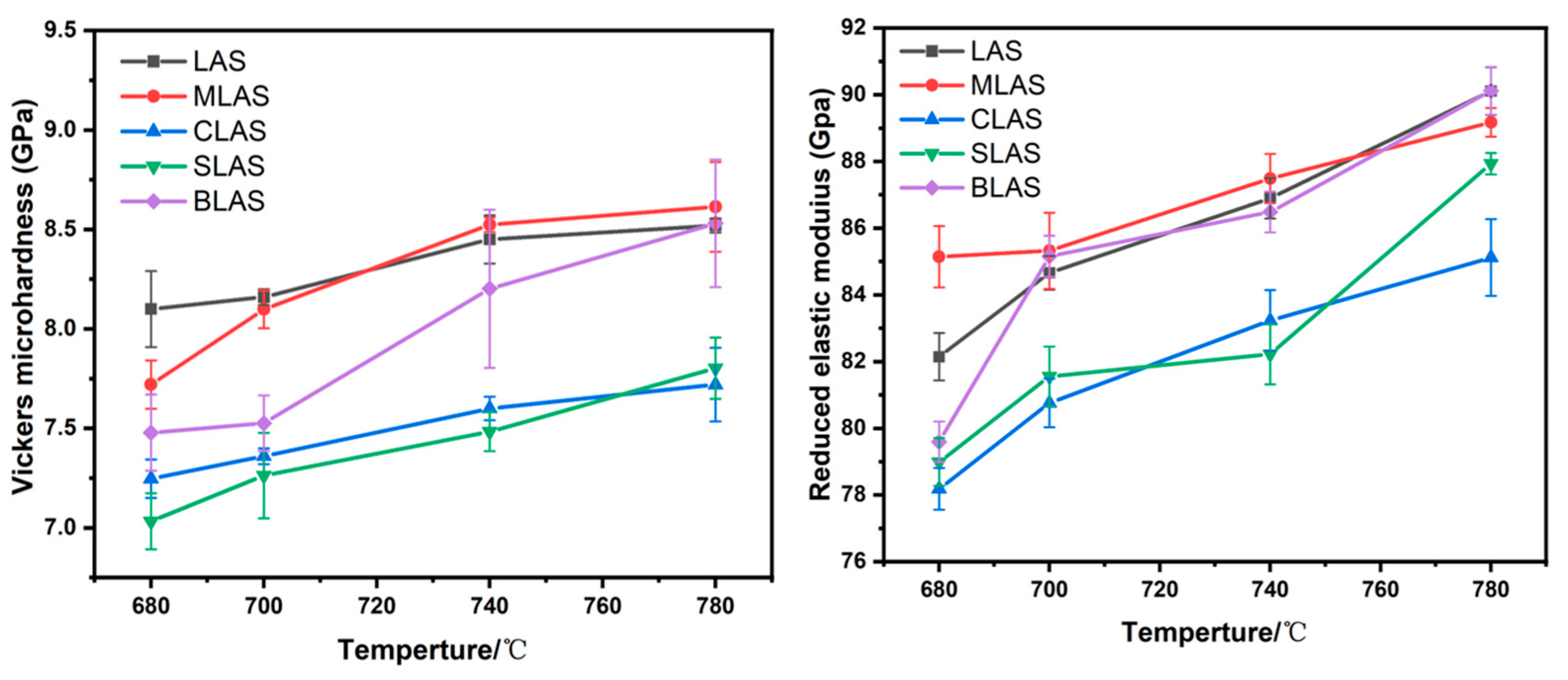
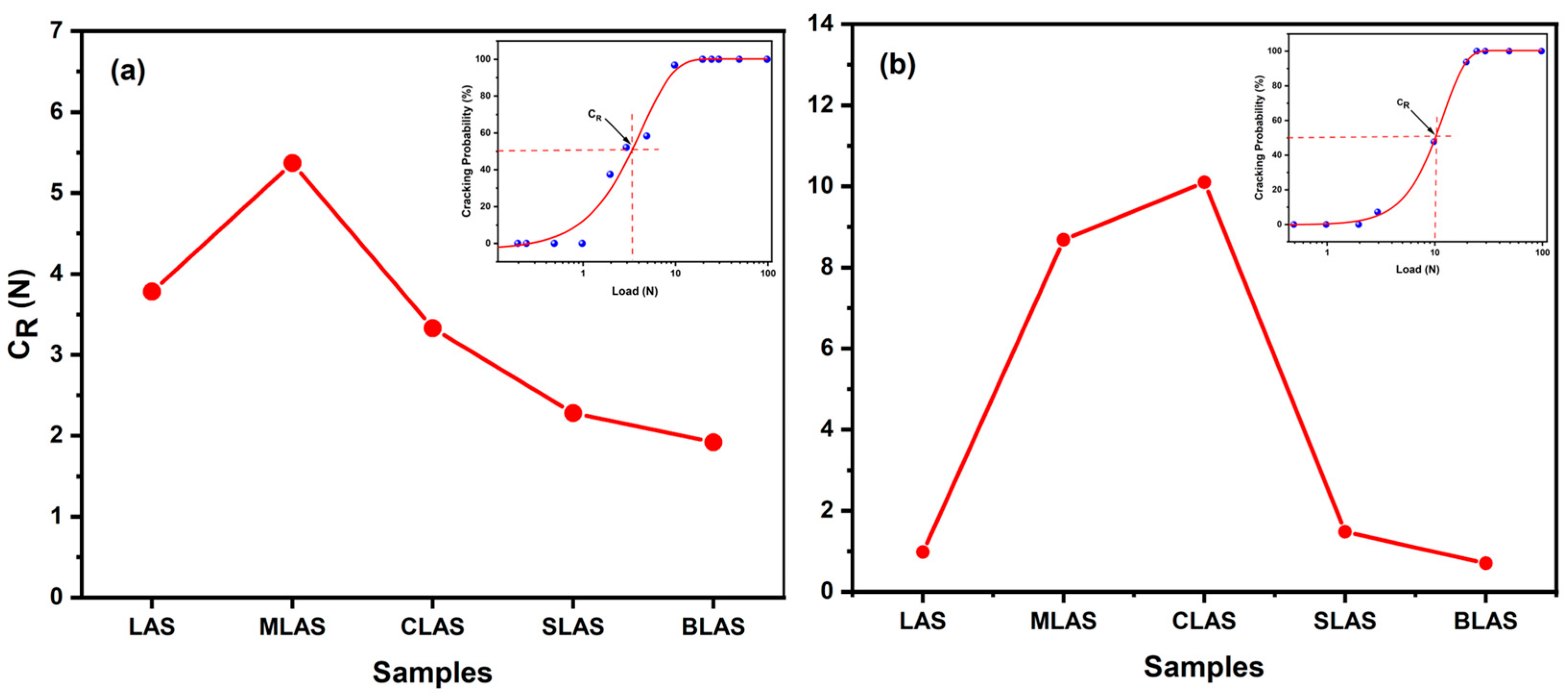
| LAS | MLAS | CLAS | SLAS | BLAS | |
|---|---|---|---|---|---|
| Tg (°C) | 501.4 | 547.3 | 534.3 | 529.7 | 528.4 |
| Tp1 (°C) | 708.5 | 741.4 | 738.7 | 687.8 | 685.9 |
| Tp2 (°C) | 758.3 | 827.7 | 814.2 | 734.0 | 731.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, C.; Li, D.; Guo, M.; Zhang, J.; Xie, J.; Han, J. The Microstructure, Crystallization Behavior, and Mechanical Performance Evolutions of Li2O-Al2O3-SiO2 Glass and Glass–Ceramics with Different Alkaline Earth Oxide Modifications. Materials 2025, 18, 1383. https://doi.org/10.3390/ma18061383
Zheng C, Li D, Guo M, Zhang J, Xie J, Han J. The Microstructure, Crystallization Behavior, and Mechanical Performance Evolutions of Li2O-Al2O3-SiO2 Glass and Glass–Ceramics with Different Alkaline Earth Oxide Modifications. Materials. 2025; 18(6):1383. https://doi.org/10.3390/ma18061383
Chicago/Turabian StyleZheng, Chi, Danni Li, Mengshuo Guo, Jihong Zhang, Jun Xie, and Jianjun Han. 2025. "The Microstructure, Crystallization Behavior, and Mechanical Performance Evolutions of Li2O-Al2O3-SiO2 Glass and Glass–Ceramics with Different Alkaline Earth Oxide Modifications" Materials 18, no. 6: 1383. https://doi.org/10.3390/ma18061383
APA StyleZheng, C., Li, D., Guo, M., Zhang, J., Xie, J., & Han, J. (2025). The Microstructure, Crystallization Behavior, and Mechanical Performance Evolutions of Li2O-Al2O3-SiO2 Glass and Glass–Ceramics with Different Alkaline Earth Oxide Modifications. Materials, 18(6), 1383. https://doi.org/10.3390/ma18061383






