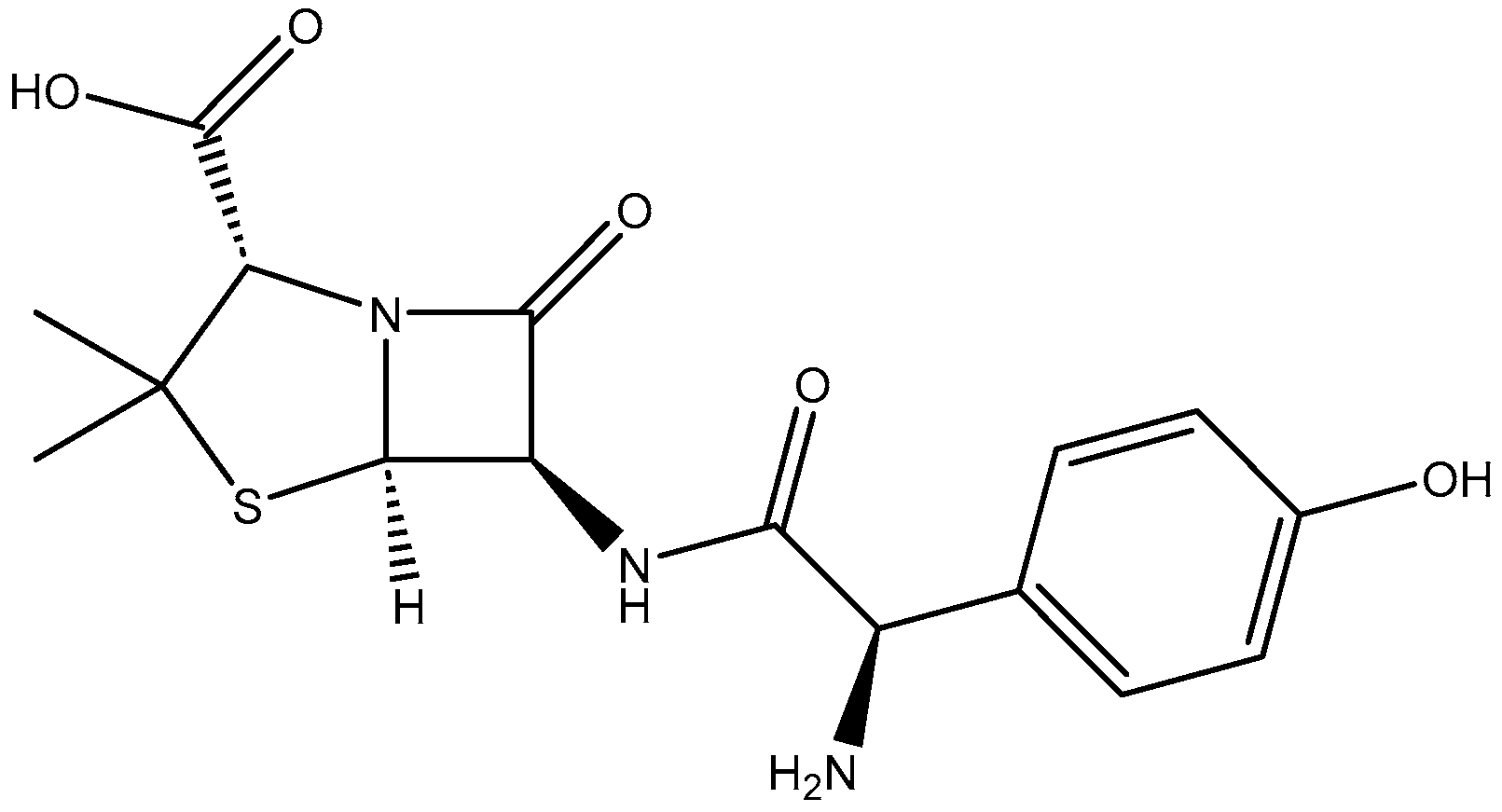
Treatment of Aqueous Amoxicillin Solutions with Sunlight Using a Pelletized Macrocomposite Photocatalyst
Abstract
1. Introduction
2. Experimental Procedure
2.1. Materials
2.2. Catalyst Synthesis
2.3. Photocatalyts Characterization
2.4. Photocatalytic Degradation Procedure
3. Results and Discussion
3.1. Catalyst Characterization
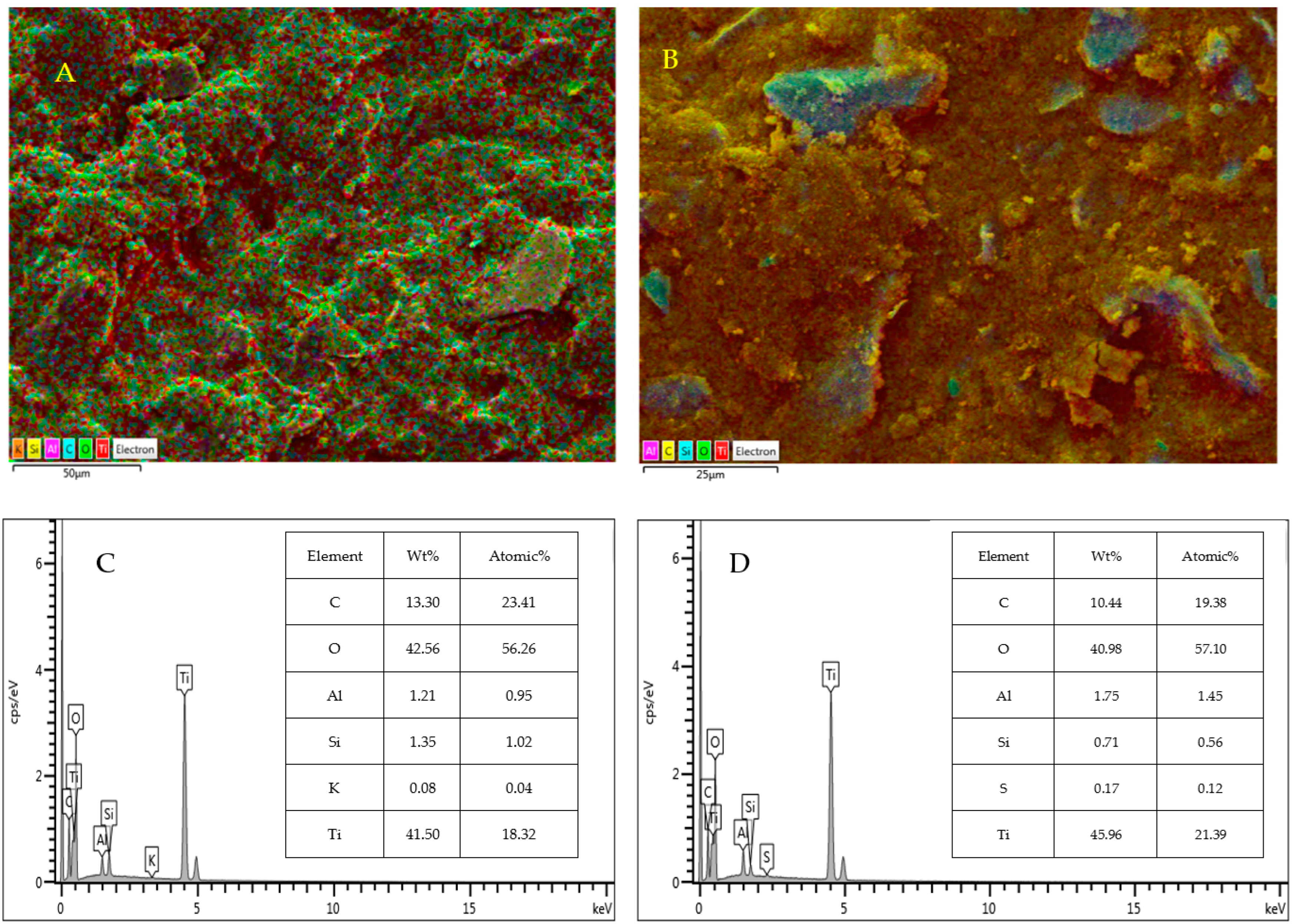
3.1.1. Scanning Electron Microscopy (SEM) and Energy-Dispersive Spectroscopy (EDS)
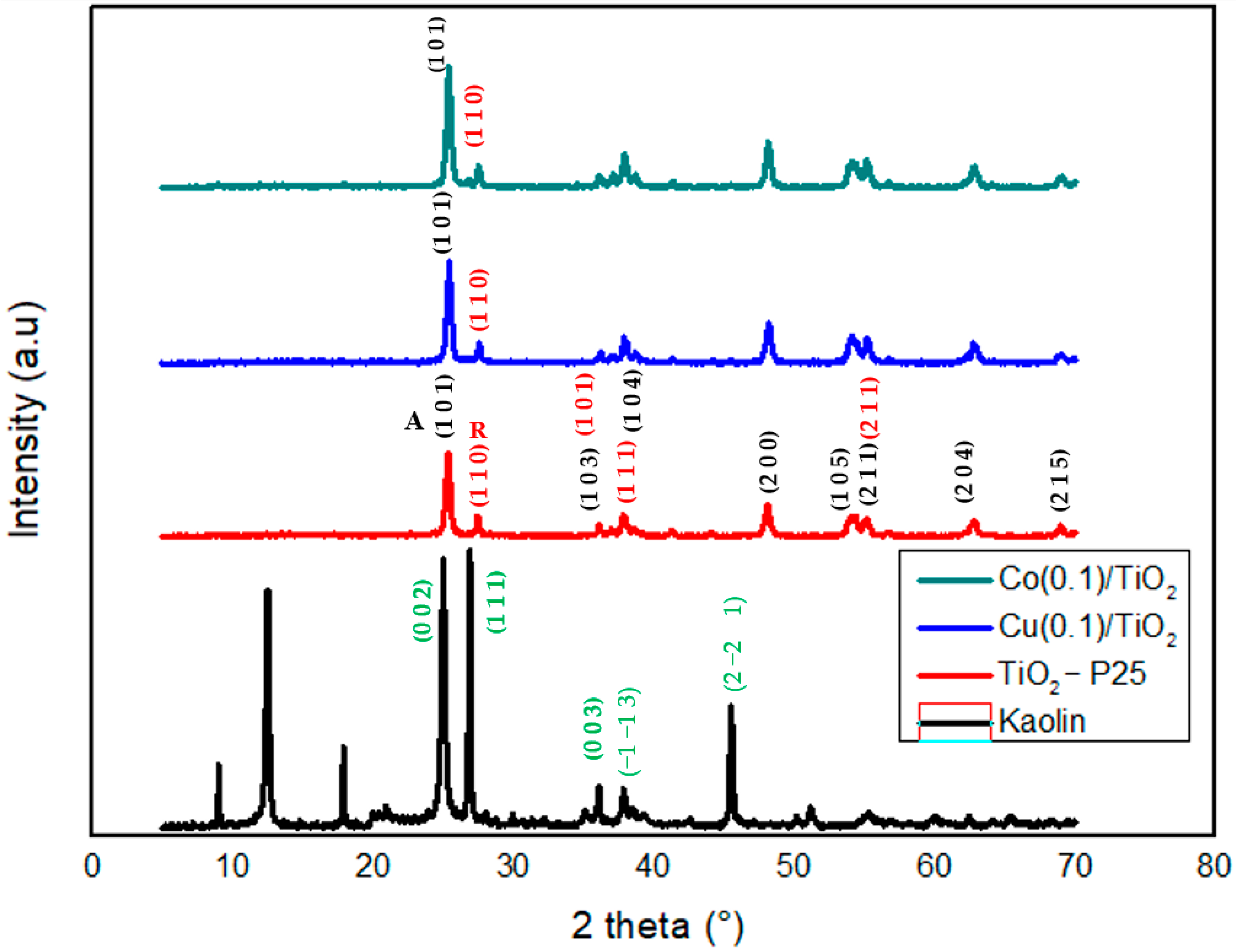
3.1.2. X-Ray Diffraction (XRD) and Fluorescence (XRF)
3.1.3. Textural Properties
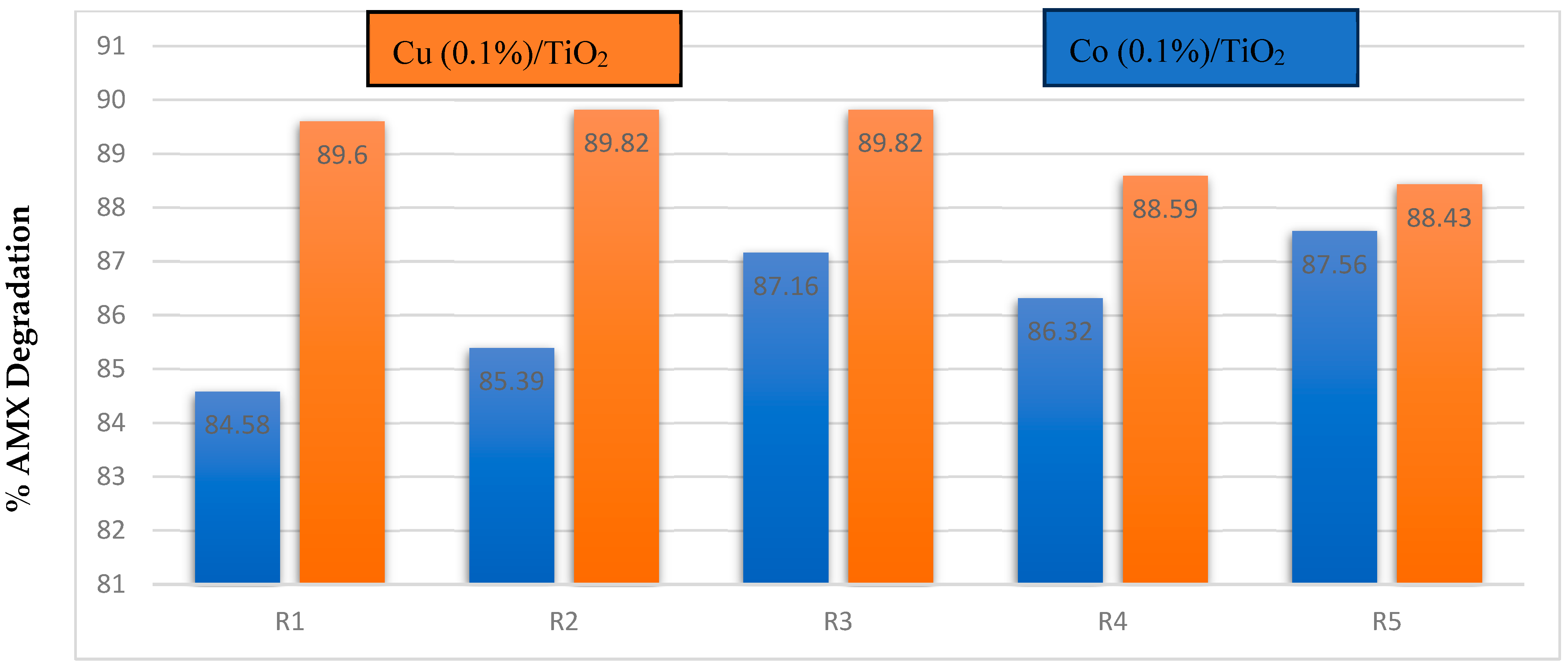
3.2. Photodegradation of AMX upon Sunlight Irradiation
3.3. Parameters Affecting Photocatalytic Degradation of AMX
3.3.1. Direct Photolysis vs. Photocatalysis
3.3.2. pH Effect
3.3.3. Effect of AMX Concentration
4. Degradation Pathways
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Christensen, A.; Gurol, M.D.; Garoma, T. Treatment of persistent organic compounds by integrated advanced oxidation processes and sequential batch reactor. Water Res. 2009, 43, 3910–3921. [Google Scholar] [CrossRef] [PubMed]
- Martín de Vidales, M.J.; Millán, M.; Saez, C.; Canizares, P.; Rodrigo, M.A. Irradiated-assisted electrochemical processes for the removal of persistent pollutants from real wastewater. Sep. Purif. Technol. 2017, 175, 428–434. [Google Scholar] [CrossRef]
- Miwa, T.; Kaneco, S.; Katsumata, H.; Suzuki, T. Photocatalytic hydrogen production from aqueous methanol solution with CuO/Al2O3/TiO2 nanocomposite. Int. J. Hydrogen Energy 2010, 35, 6554–6560. [Google Scholar] [CrossRef]
- Jiang, X.; Manawan, M.; Feng, T.; Qian, R.; Zhao, T.; Zhou, G.; Kong, F.; Wang, Q.; Dai, S.; Pan, J.H. Anatase and rutile in Evonik Aeroxide P25: Heterojunctioned or individual nanoparticles? Catal. Today 2018, 300, 12–17. [Google Scholar] [CrossRef]
- Belekbir, S.; El Azzouzi, M.; El Hamidi, A.; Rodríguez-Lorenzo, L.; Santaballa, J.A.; Canle, M. Improved photocatalyzed degradation of phenol, as a model pollutant, over metal-impregnated nanosized TiO2. Nanomaterials 2020, 10, 996. [Google Scholar] [CrossRef]
- Belekbir, S.; El Azzouzi, M.; Rodríguez-Lorenzo, L.; El Hamidi, A.; Santaballa, J.A.; Canle, M. Cobalt impregnation on titania photocatalysts enhances Vis phenol photodegradation. Materials 2023, 16, 4134. [Google Scholar] [CrossRef]
- Gaim, Y.T.; Yimanuh, S.M.; Kidanu, Z.G. Enhanced photocatalytic degradation of amoxicillin with Mn-doped Cu2O under sunlight irradiation. J. Compos. Sci. 2022, 6, 317. [Google Scholar] [CrossRef]
- Verma, M.; Haritash, A.K. Photocatalytic degradation of Amoxicillin in pharmaceutical wastewater: A potential tool to manage residual antibiotics. Environ. Technol. Innov. 2020, 20, 101072. [Google Scholar] [CrossRef]
- Sddiqa, A.; Masih, D.; Anjum, D.; Siddiq, M. Cobalt and sulfur co-doped nano-size TiO2 for photodegradation of various dyes and phenol. J. Environ. Sci. (China) 2015, 37, 100–109. [Google Scholar] [CrossRef]
- Andreozzi, R.; Canterino, M.; Marotta, R.; Paxeus, N. Antibiotic removal from wastewaters: The ozonation of amoxicillin. J. Hazard. Mater. 2005, 122, 243–250. [Google Scholar] [CrossRef]
- Yu, J.; Lv, L.; Lan, P.; Zhang, S.; Pan, B.; Zhang, W. Effect of effluent organic matter on the adsorption of perfluorinated compounds onto activated carbon. J. Hazard. Mater. 2012, 225–226, 99–106. [Google Scholar] [CrossRef]
- Konstantinou, I.K.; Albanis, T.A. TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: Kinetic and mechanistic investigations: A review. Appl. Catal. B Environ. 2004, 49, 1–14. [Google Scholar] [CrossRef]
- Romeiro, A.; Freitas, D.; Azenha, M.E.; Canle, M.; Burrows, H.D. Effect of the calcination temperature on the photocatalytic efficiency of acidic sol-gel synthesized TiO2 nanoparticles in the degradation of alprazolam. Photochem. Photobiol. Sci. 2017, 16, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Directive (EU) 2020/2184 of the European Parliament and of the Council of 16 December 2020 on the Quality of Water Intended for Human Consumption. Available online: http://data.europa.eu/eli/dir/2020/2184/oj (accessed on 2 January 2025).
- Lace, A.; Cleary, J. A review of microfluidic detection strategies for heavy metals in water. Chemosensors 2021, 9, 60. [Google Scholar] [CrossRef]
- Olama, N.; Dehghani, M.; Malakootian, M. The removal of amoxicillin from aquatic solutions using the TiO2/UV-C nanophotocatalytic method doped with trivalent iron. Appl. Water Sci. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Balarak, D.; Mostafapour, F.K. Photocatalytic degradation of amoxicillin using UV/Synthesized NiO from pharmaceutical wastewater. Indones. J. Chem. 2019, 19, 211–218. [Google Scholar] [CrossRef]
- Carp, O.; Huisman, C.L.; Reller, A. Photoinduced reactivity of titanium dioxide. Prog. Solid State Chem. 2004, 32, 33–177. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Y.L.; Liu, R.; Tsai, D.P. Plasmonic photocatalysis. Rep. Prog. Phys. 2013, 76, 046401. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Chen, Z.; Hu, J.; Li, S.; Wang, Z.; Liu, J.; Wang, X. Semiconductor heterojunction photocatalysts: Design, construction, and photocatalytic performances. Chem. Soc. Rev. 2014, 43, 5234–5244. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Jaroniec, M. Hierarchical photocatalysts. Chem. Soc. Rev. 2016, 45, 2603–2636. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 photocatalysis: Mechanisms and materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Etacheri, V.; Di Valentin, C.; Schneider, J.; Bahnemann, D.; Pillai, S.C. Visible-light activation of TiO2 photocatalysts: Advances in theory and experiments. J. Photochem. Photobiol. C Photochem. Rev. 2015, 25, 1–29. [Google Scholar] [CrossRef]
- Liu, G.; Wang, L.; Yang, H.G.; Cheng, H.-M.; Lu, G.Q. Titania-based photocatalysts—Crystal growth, doping and heterostructuring. J. Mater. Chem. 2010, 20, 831–843. [Google Scholar] [CrossRef]
- Zhao, X.W.; Jin, W.Z.; Cai, J.G.; Ye, J.F.; Li, Z.H.; Ma, Y.R.; Xie, J.L.; Qi, L.M. Shape- and Size-Controlled Synthesis of Uniform Anatase TiO2 Nanocuboids Enclosed by Activity {100} and {001}. Adv. Funct. Mater. 2011, 21, 3554–3563. [Google Scholar]
- Shieh, D.L.; Lin, Y.S.; Yeh, J.H.; Chen, S.C.; Lin, B.C.; Lin, J.L. N-doped, porous TiO2 with rutile phase and visible light sensitive photocatalytic activity. Chem. Commun. 2012, 48, 2528–2530. [Google Scholar] [CrossRef]
- Pongwan, P.; Wetchakun, K.; Phanichphant, S.; Wetchakun, N. Enhancement of visible-light photocatalytic activity of Cu-doped TiO2 nanoparticles. Res. Chem. Intermed. 2016, 42, 2815–2830. [Google Scholar] [CrossRef]
- Ganesh, I.; Kumar, P.P.; Annapoorna, I.; Sumliner, J.M.; Ramakrishna, M.; Hebalkar, N.Y.; Padmanabham, G.; Sundararajan, G. Preparation and characterization of Cu-doped TiO2 materials for electrochemical, photoelectrochemical, and photocatalytic applications. Appl. Surf. Sci. 2014, 293, 229–247. [Google Scholar] [CrossRef]
- Ohtani, B.; Prieto-Mahaney, O.O.; Li, D.; Abe, R. What is Degussa (Evonik) P25? Crystalline composition analysis, reconstruction from isolated pure particles and photocatalytic activity test. J. Photochem. Photobiol. A Chem. 2010, 216, 179–182. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodríguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC technical report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Emmett, P.H. Gases in multimolecular layers. J. Am. Chem. Soc. 1936, 58, 407. [Google Scholar]
- Chiang, K.; Amal, R.; Tran, T. Photocatalytic degradation of cyanide using titanium dioxide modified with copper oxide. Adv. Environ. Res. 2002, 6, 471–485. [Google Scholar]
- Tianping, L.V.; Zhao, J.; Chen, M.; Shen, K.; Zhang, D.; Zhang, J.; Zhang, G.; Liu, Q. Boosted visible-light photodegradation of methylene blue by V and Co co-doped TiO2. Materials 2018, 11, 1946. [Google Scholar] [CrossRef] [PubMed]
- Bougarrani, S.; Skadell, K.; Arndt, R.; El Azzouzi, M.; Glaser, R. Novel CaxMnOy/TiO2 composites for efficient photocatalytic degradation of methylene blue and the herbicide imazapyr in aqueous solution under visible light irradiation. J. Environ. Chem. Eng. 2018, 6, 1934–1942. [Google Scholar] [CrossRef]
- Kristin, H.; Bettina, S.; Gerrit, S.; Thorsten, R. New hydrolysis products of the beta-lactam antibiotic amoxicillin, their pH-dependent formation and search in municipal wastewater. Water Res. 2016, 88, 880–888. [Google Scholar] [CrossRef]
- Trovó, A.G.; Nogueira, R.F.P.; Agüera, A.; Fernandez-Alba, A.R.; Malato, S. Degradation of the antibiotic amoxicillin by photo-Fenton process—Chemical and toxicological assessment. Water Res. 2011, 45, 1394–1402. [Google Scholar] [CrossRef]
- Ali, M.A.; Maafa, I.M. Photodegradation of amoxicillin in aqueous systems: A review. Int. J. Mol. Sci. 2024, 25, 9575. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Q.; Guo, Y.; Shi, H.; Wang, J.; Cheng, X. Synthesis and characterization of Cu and Co doped TiO2/kaolin composites for photocatalytic degradation of organic pollutants. J. Environ. Chem. Eng. 2017, 5, 4876–4884. [Google Scholar] [CrossRef]
- Elmolla, E.S.; Chaudhuri, M. Photocatalytic degradation of amoxicillin, ampicillin and cloxacillin antibiotics in aqueous solution using UV/TiO2 and UV/H2O2/TiO2 photocatalysis. Desalination 2010, 252, 46–52. [Google Scholar] [CrossRef]
- Kümmerer, K. Antibiotics in the aquatic environment—A review—Part I. Chemosphere 2009, 75, 417–434. [Google Scholar] [CrossRef]
- Wang, X.; Li, S.; Yu, H.; Yu, J.; Liu, S. Ag2O as a new visible-light photocatalyst: Self-stability and high photocatalytic activity. Chem. Eur. J. 2011, 17, 7777–7780. [Google Scholar]
- Linic, S.; Christopher, P.; Ingram, D.B. Plasmonic-metal nanostructures for efficient conversion of solar to chemical energy. Nat. Mater. 2011, 10, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xu, Q.; Feng, Z.; Li, M.; Li, C. Importance of the relationship between surface phases and photocatalytic activity of TiO2. Angew. Chem. Int. Ed. 2008, 47, 1766–1769. [Google Scholar] [CrossRef]
- Norte, T.H.; Marcelino, R.B.P.; Medeiros, F.H.A.; Moreira, R.P.L.; Amorim, C.C.; Lago, R.M. Ozone oxidation of β-lactam antibiotic molecules and toxicity decrease in aqueous solution and industrial wastewaters heavily contaminated. Ozone Sci. Eng. 2018, 40, 385–391. [Google Scholar] [CrossRef]



| % | TiO2 | SiO2 | Al2O3 | Fe2O3 | K2O | SO3 | CO2 | CaO | CuO | CoO | NiO | Cr2O3 | V2O5 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cu | 92.7 | 2.7 | 2.1 | 0.23 | 0.18 | 0.11 | 1.7 | 0.039 | 0.14 | --- | 0.013 | 0.067 | --- |
| Co | 93.5 | 2.9 | 2.2 | 0.22 | 0.19 | 0.12 | 0.51 | 0.022 | --- | 0.070 | --- | --- | 0.25 |
| Photocatalyst | Cu (0.1%)/TiO2 | Co (0.1%)/TiO2 | ||
|---|---|---|---|---|
| Property | Ref. [32] | This Work | This Work | Ref. [33] |
| SBET (m2·g−1) | 51 a | 47.0 ± 0.1 | 45.5 ± 0.1 | 22.8 a |
| Constant C | 139.5 | 119.7 | ||
| Pore volume (cm3·g−1) | 0.101 | 0.095 | 0.033 | |
| Average pore width (4 V/A) (nm) | 96 b | 94 b | 65 c | |
| Average particle size (A) | 127.5 | 131.7 | ||
| Median pore width (nm) | 0.7758 | 0.7788 | ||
| Time/min | 15 | 30 | 60 | 120 | 180 |
|---|---|---|---|---|---|
| % AMX adsorption on Cu (0.1)/TiO2 | 0.54 | 2.56 | 5.42 | 8.40 | 13.82 |
| % AMX adsorption on Co (0.1)/TiO2 | 3.05 | 5.51 | 2.50 | 14.51 | 18.01 |
| Photocatalyst | k·103/min−1 | % Removal at 120 min | % Removal at 180 min |
|---|---|---|---|
| Photolysis | 39.6 | 24.2 | 31.1 |
| Cu (0.1%)/TiO2 | 152.5 | 86.7 | 86.4 |
| Cu (0.5%)/TiO2 | 102.5 | 65.3 | 79.2 |
| Cu (1.0%)/TiO2 | 128.4 | 88.0 | 87.6 |
| Co (0.1%)/TiO2 | 107.2 | 83.4 | 84.4 |
| Co (0.5%)/TiO2 | 76.9 | 81.7 | 82.3 |
| Co (1.0%)/TiO2 | 28.6 | 56.7 | 69.1 |
| Photocatalyst | Property | pH | ||
|---|---|---|---|---|
| 3.3 | 5.9 | 8.6 | ||
| Cu (0.1%)/TiO2 | k·103/min−1 | 64.1 | 152.5 | 9.2 |
| % removal at 180 min | 90.9 | 86.4 | 86.1 | |
| Co (0.1%)/TiO2 | k·103/min−1 | 133.7 | 107.2 | 37.0 |
| % removal at 180 min | 89.5 | 84.4 | 76.8 | |
| [AMX]0/mg·L−1 | 1 | 3 | 5 | 7 | 9 |
|---|---|---|---|---|---|
| Cu (0.1%)/TiO2 | 95.4 | 73.9 | 86.4 | 87.8 | 82.0 |
| Co (0.1%)/TiO2 | 96.4 | 75.3 | 84.4 | 66.9 | 82.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slimani Tlemcani, S.; Marín, Z.; Santaballa, J.A.; Canle, M. Treatment of Aqueous Amoxicillin Solutions with Sunlight Using a Pelletized Macrocomposite Photocatalyst. Materials 2025, 18, 1394. https://doi.org/10.3390/ma18071394
Slimani Tlemcani S, Marín Z, Santaballa JA, Canle M. Treatment of Aqueous Amoxicillin Solutions with Sunlight Using a Pelletized Macrocomposite Photocatalyst. Materials. 2025; 18(7):1394. https://doi.org/10.3390/ma18071394
Chicago/Turabian StyleSlimani Tlemcani, Saad, Zenydia Marín, J. Arturo Santaballa, and Moisés Canle. 2025. "Treatment of Aqueous Amoxicillin Solutions with Sunlight Using a Pelletized Macrocomposite Photocatalyst" Materials 18, no. 7: 1394. https://doi.org/10.3390/ma18071394
APA StyleSlimani Tlemcani, S., Marín, Z., Santaballa, J. A., & Canle, M. (2025). Treatment of Aqueous Amoxicillin Solutions with Sunlight Using a Pelletized Macrocomposite Photocatalyst. Materials, 18(7), 1394. https://doi.org/10.3390/ma18071394









