The Effect of Ionic Soil Stabilizer on Cement and Cement-Stabilized Iron Tailings Soil: Hydration Difference and Mechanical Properties
Abstract
1. Introduction
2. Materials and Experimental Methods
2.1. Materials
2.1.1. ISS
2.1.2. Cement
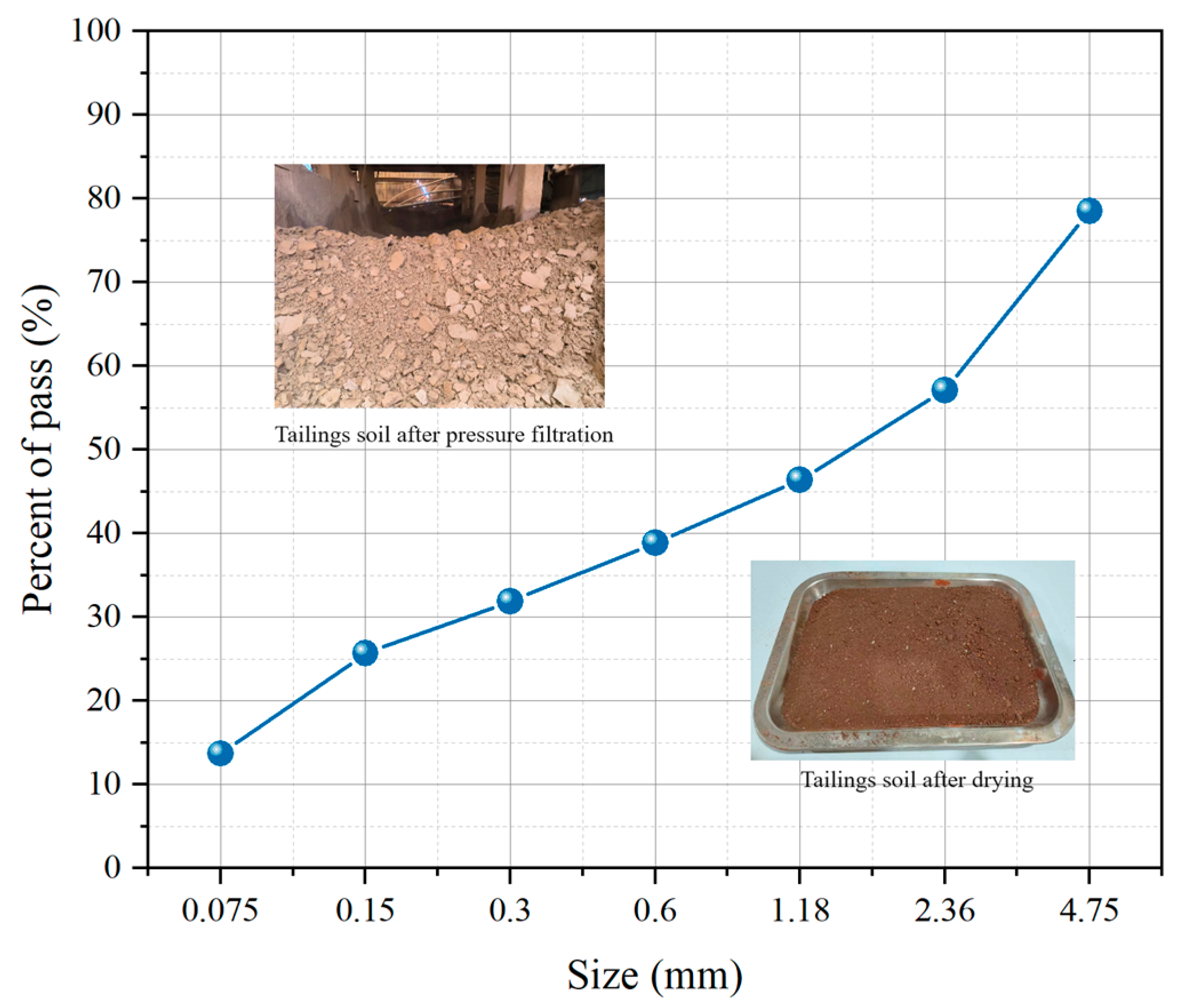
2.1.3. Iron Tailings Soil
2.2. Mix Proportion and Sample Preparation
2.2.1. Experiment of ISS on Cement Hydration
2.2.2. Experiment of ISS on Cement-Stabilized Iron Tailings Soil
2.2.3. The Effect of ISS on Cement Hydration Under Pressure Compaction
2.3. Methods
2.3.1. Test and Characterization Method of ISS in Cement System
Mechanical Properties
Isothermal Calorimetry
X-Ray Diffraction (XRD)
Scanning Electron Microscope (SEM)
Low-Field Nuclear Magnetic Resonance (NMR)
X-Ray Photoelectron Spectroscopy (XPS)
2.3.2. Test and Characterization Method of ISS in Cement-Stabilized Iron Tailings System
Proctor Compaction Test
Unconfined Compression Strength (UCS)
Non-Evaporable Water Content
Zeta Potential
3. Results and Analysis
3.1. Mechanical Properties
3.1.1. Effect of ISS on the Hydration Process and Mechanical Properties of Cement
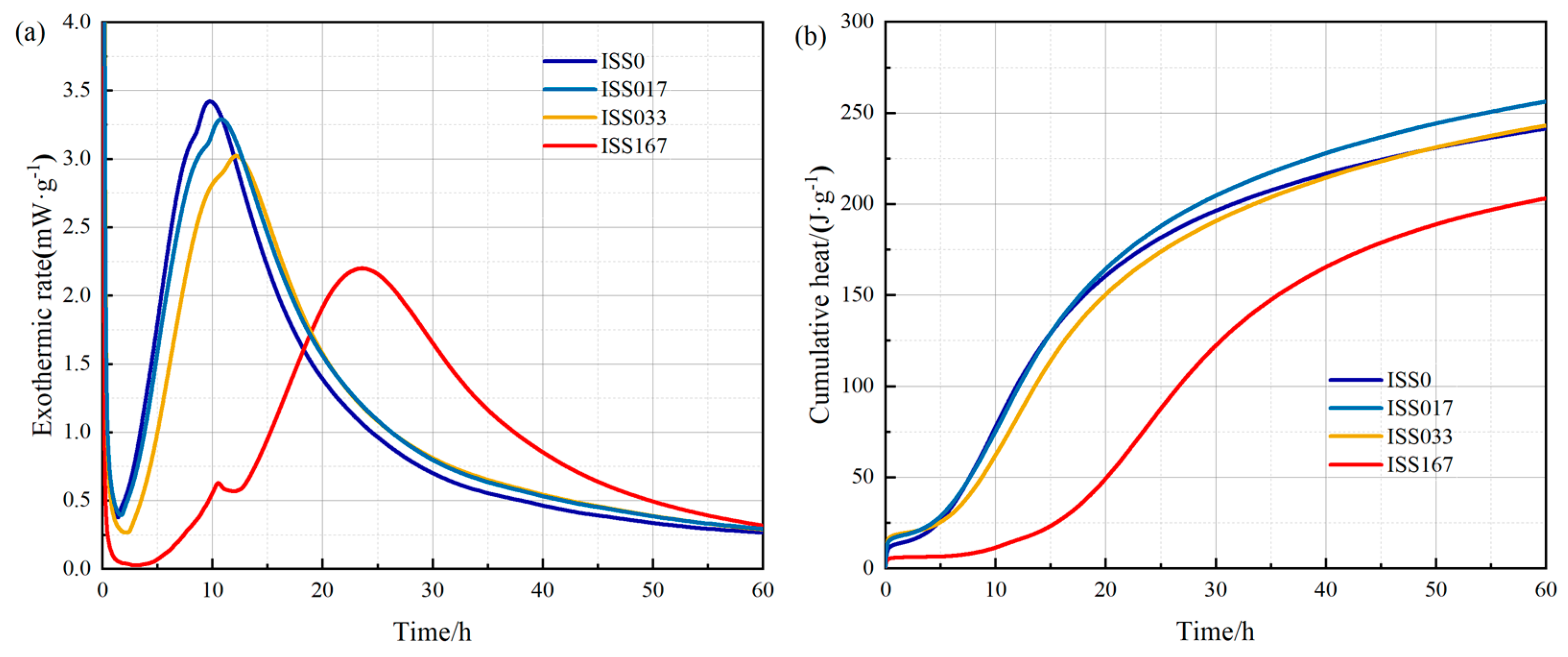
Isothermal Calorimetry and Degree of Cement
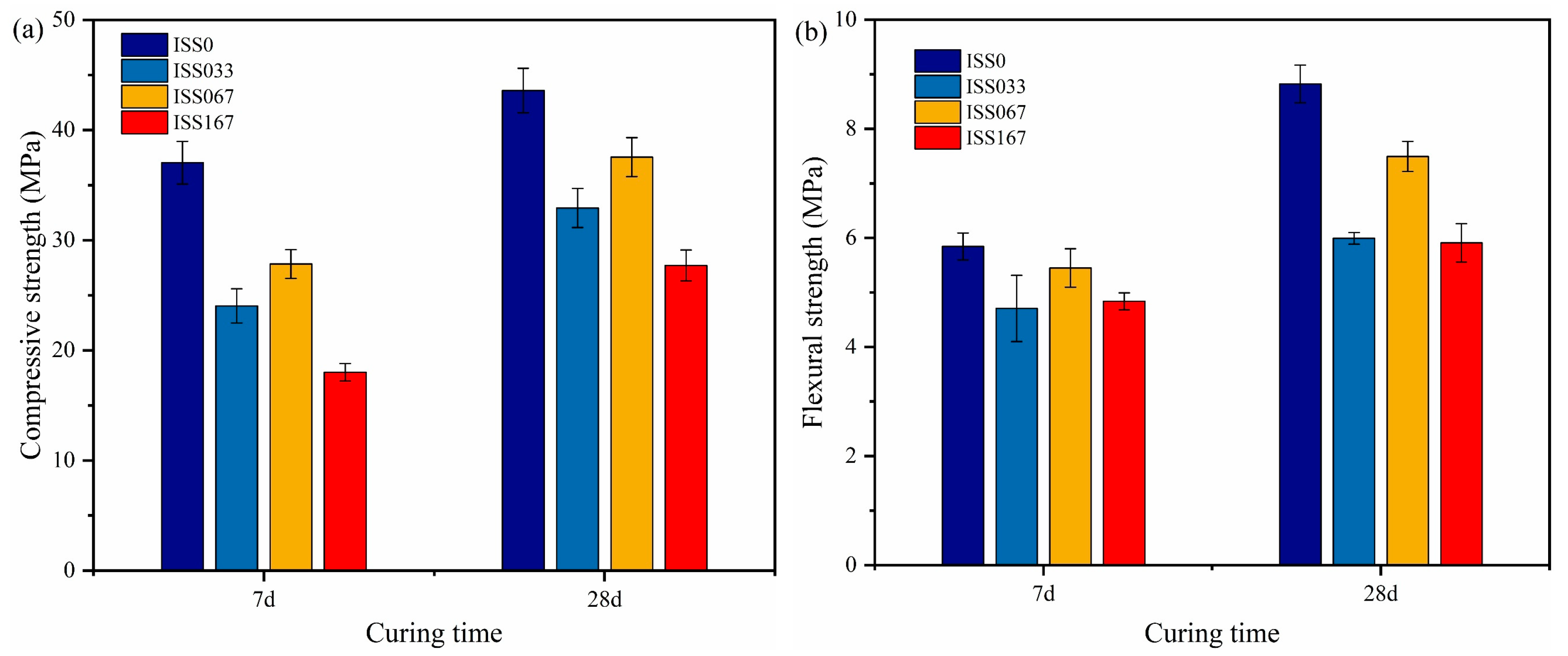
The Effect of ISS on Mechanical Properties
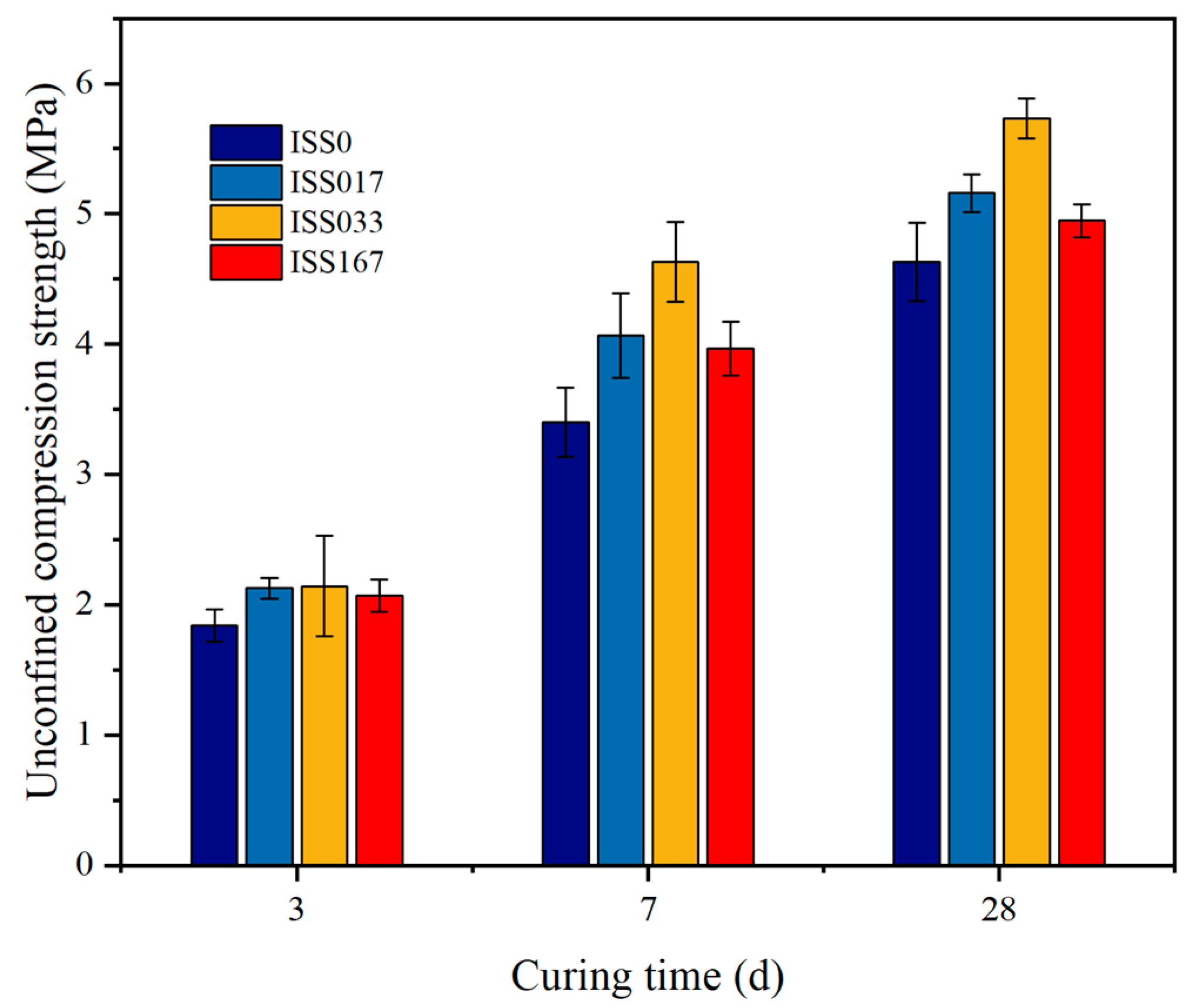
3.1.2. Effect of ISS on Mechanical Properties of Cement-Stabilized Iron Tailings Soil
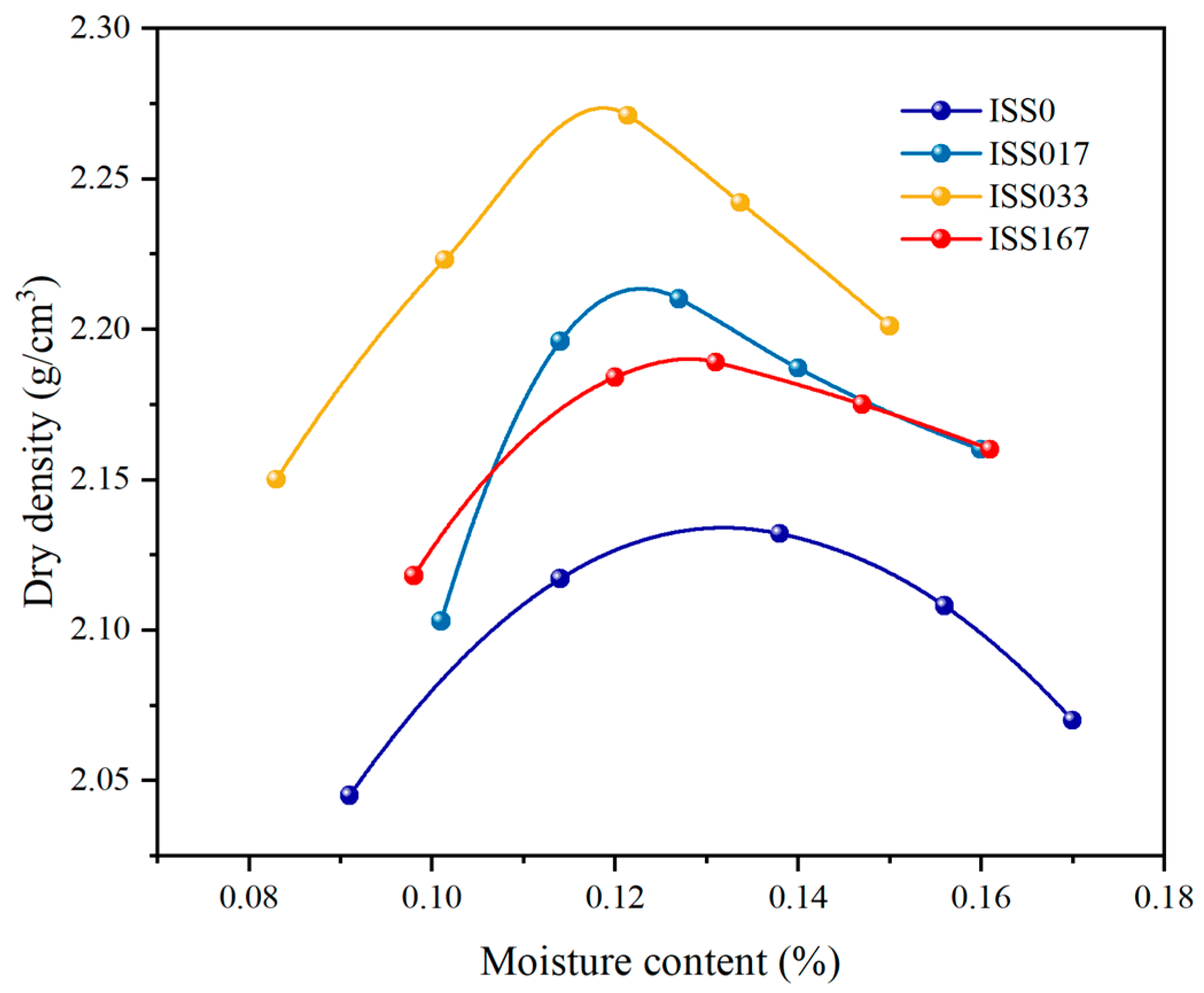
Compaction Characteristic
UCS
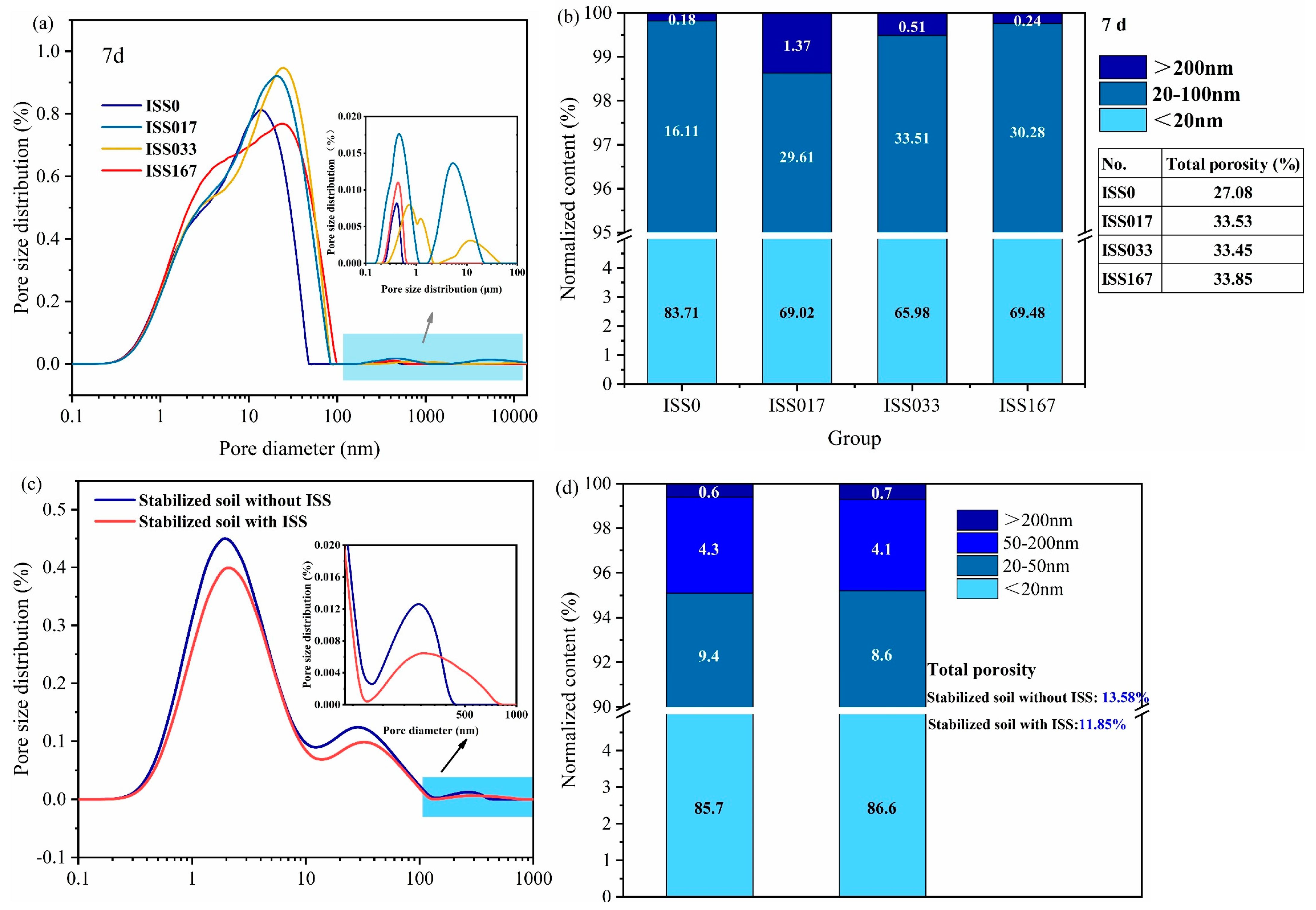
3.2. The Pore Distribution of the Hardened Cement Paste
3.3. Analysis of Hydration Products
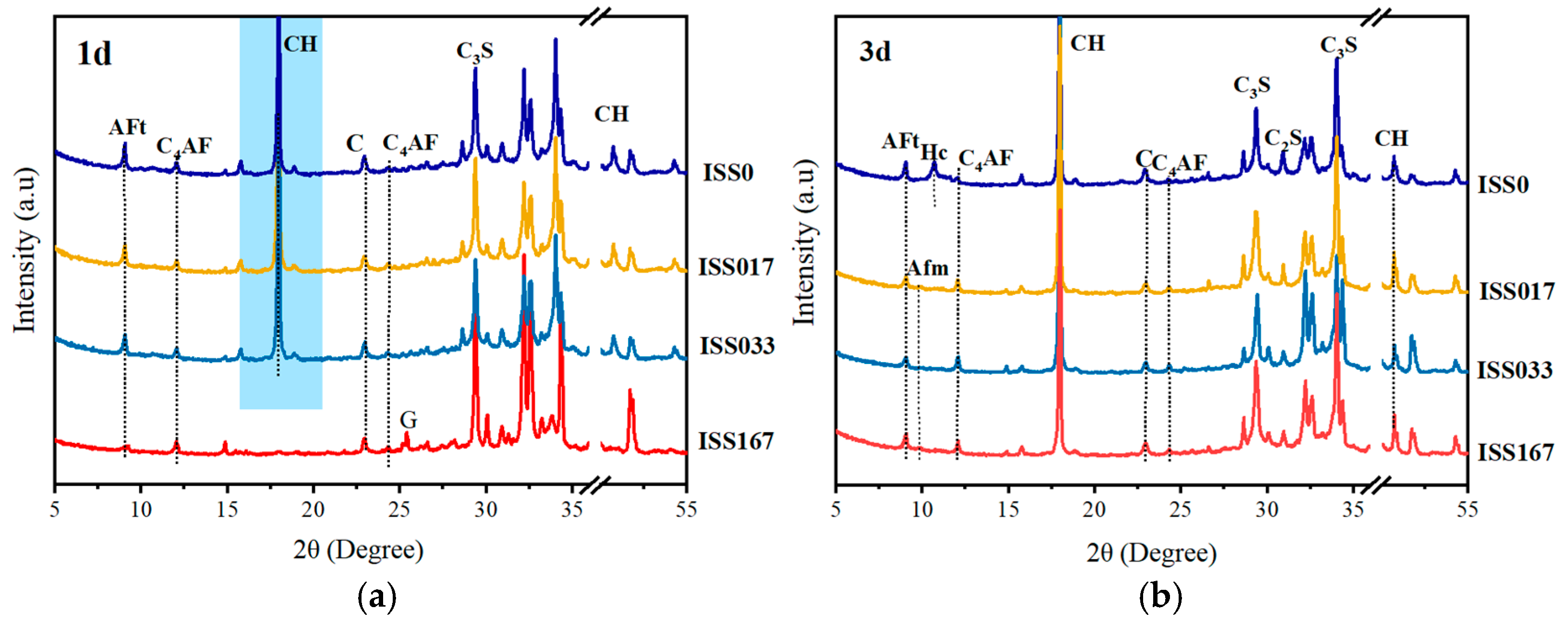
3.3.1. Mineralogical Analysis
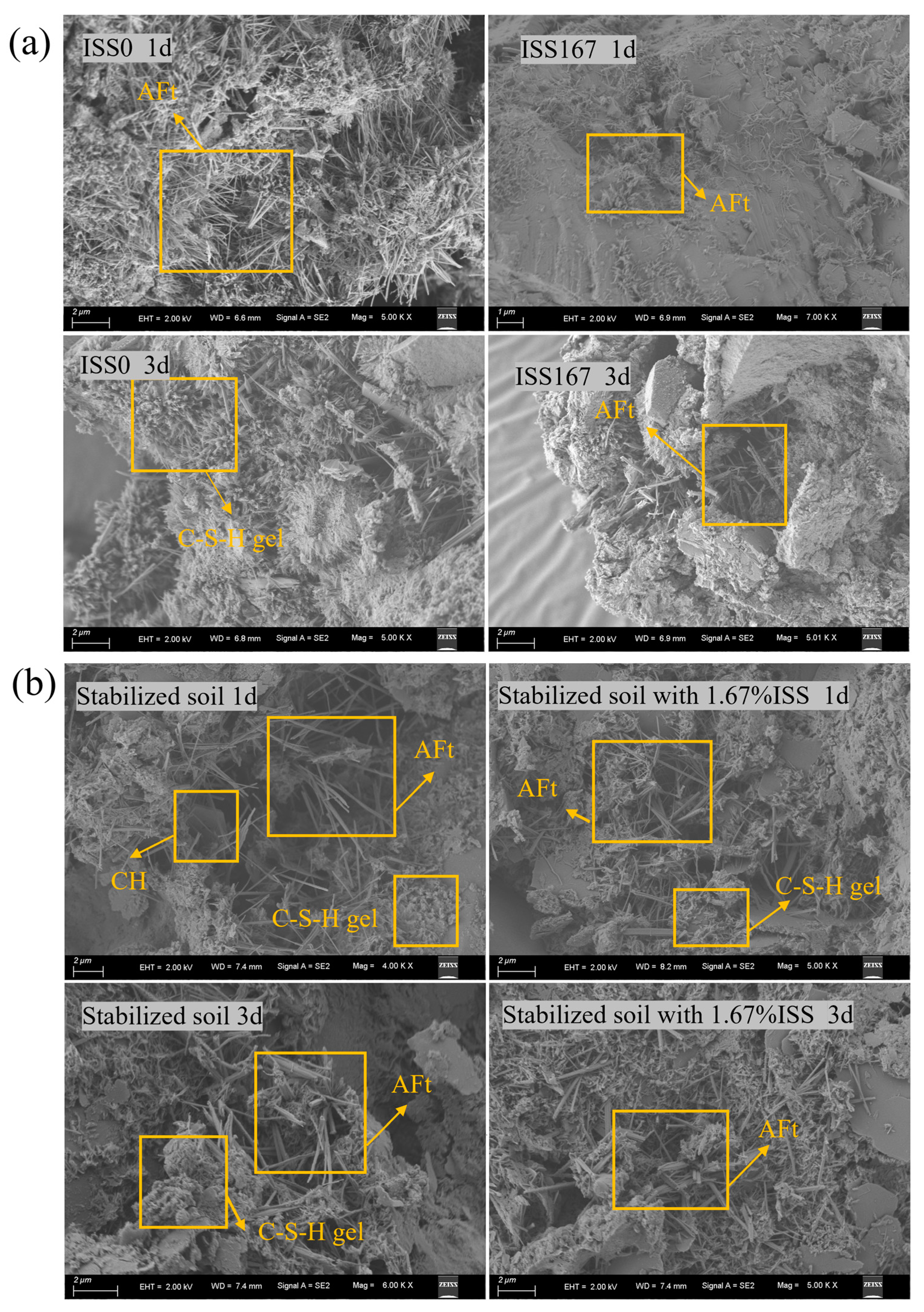
3.3.2. Morphology Analysis
3.4. Quantitative Analysis of Hydration Products
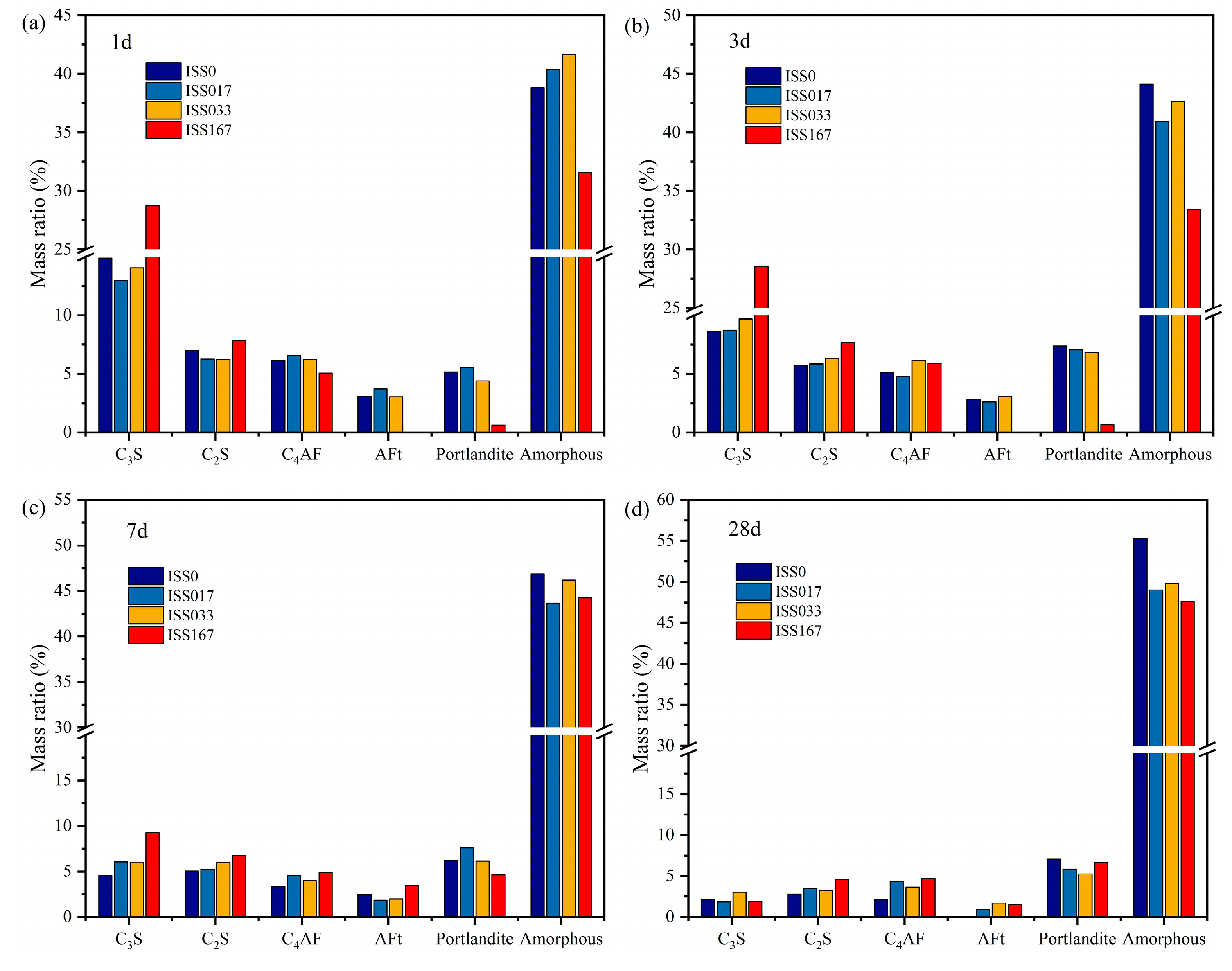
3.4.1. Quantitative Analysis Using the Rietveld Method
3.4.2. Non-Evaporable Water Content in Cement-Stabilized Iron Tailings Soil
3.5. The Role of ISS in Cement-Stabilized Iron Tailings Soil
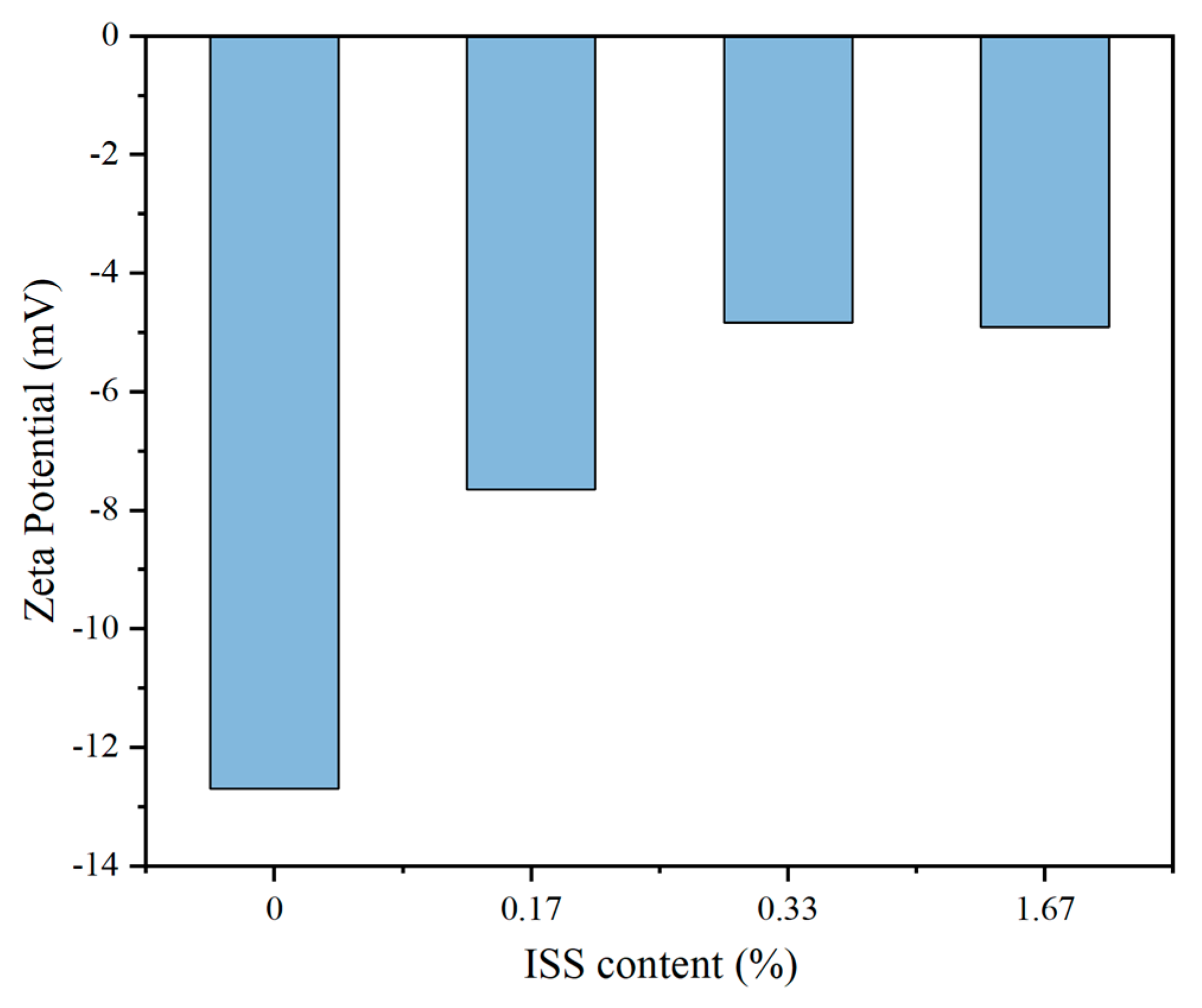
3.5.1. Zeta Potential
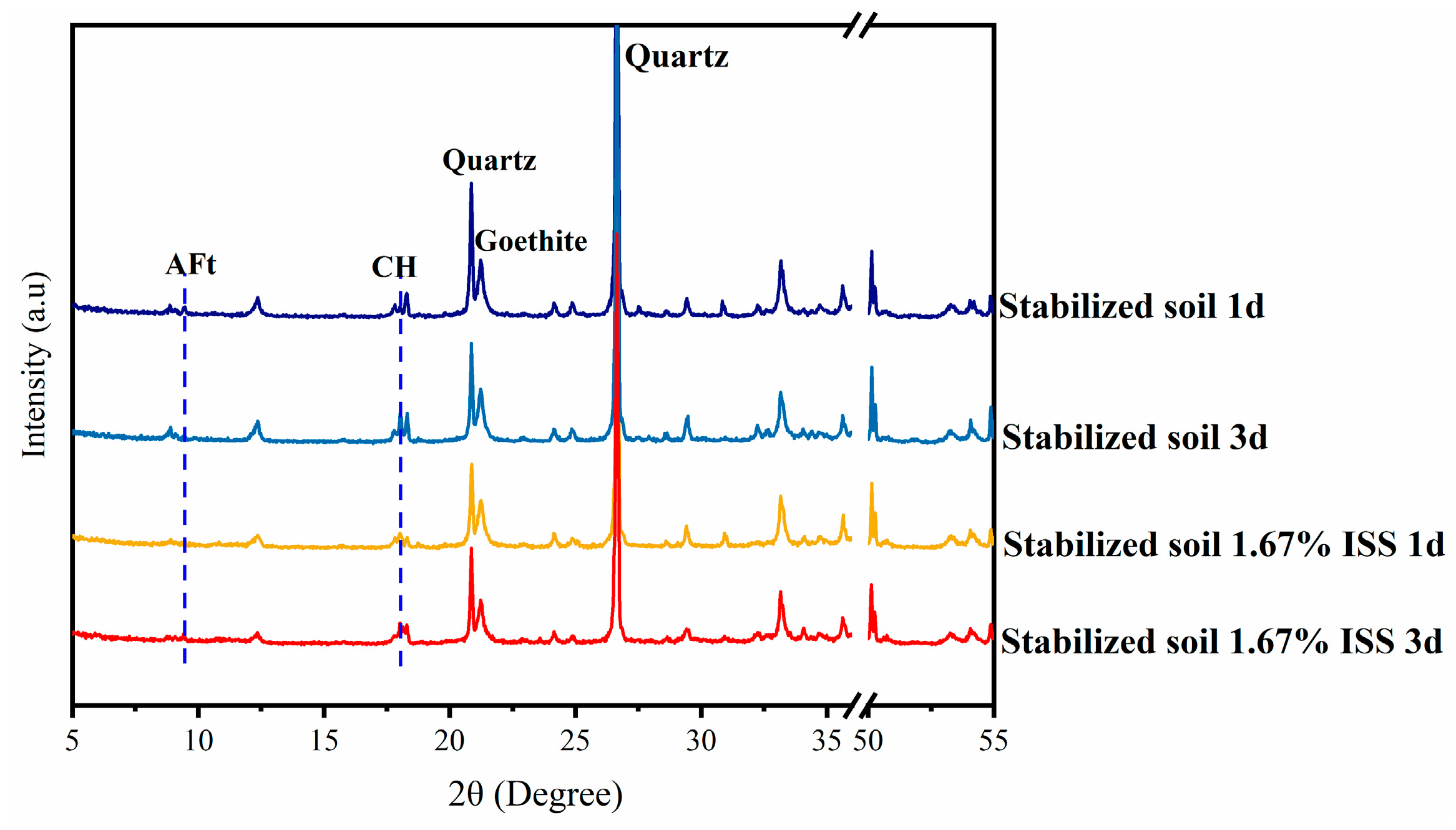
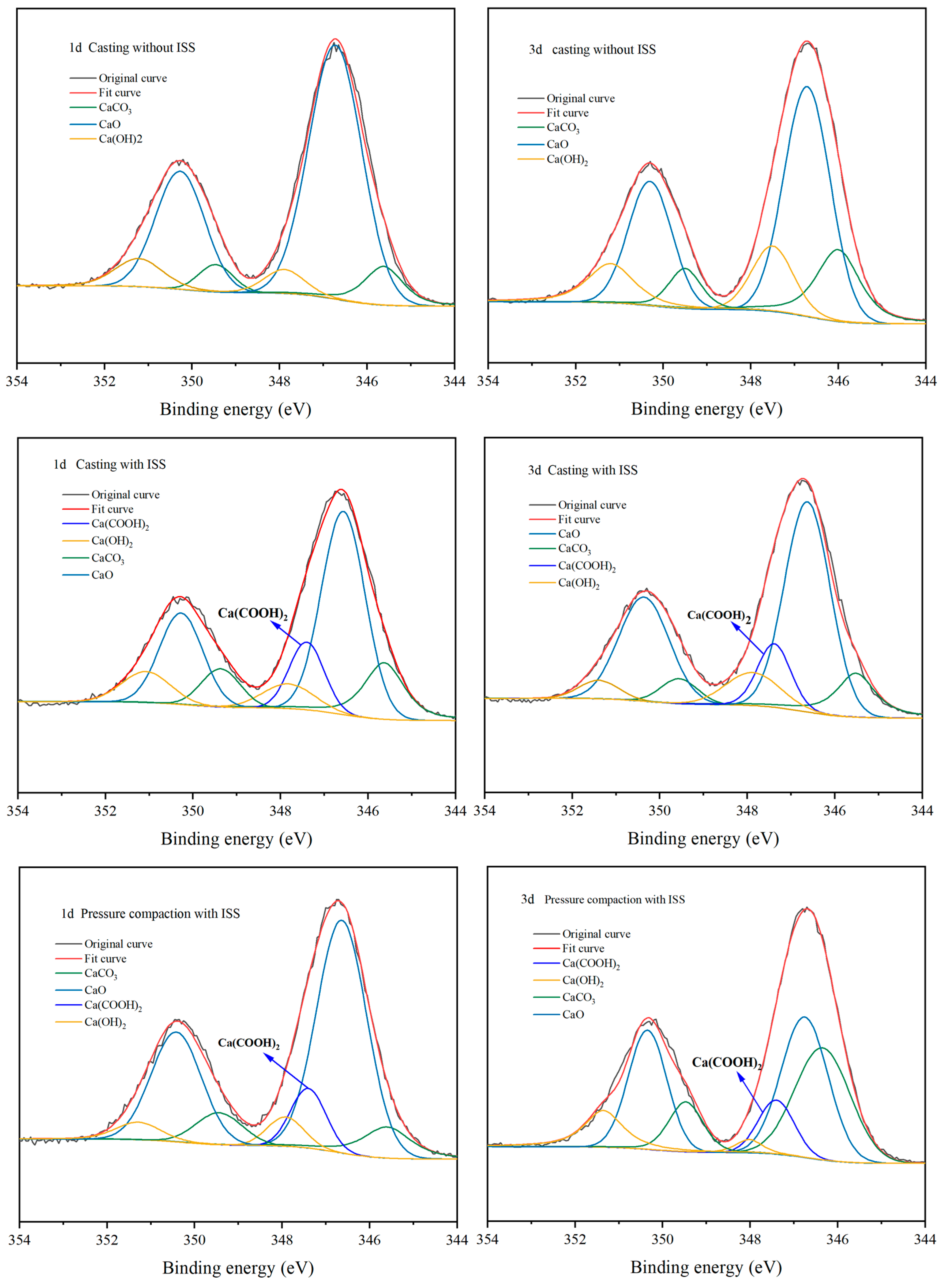
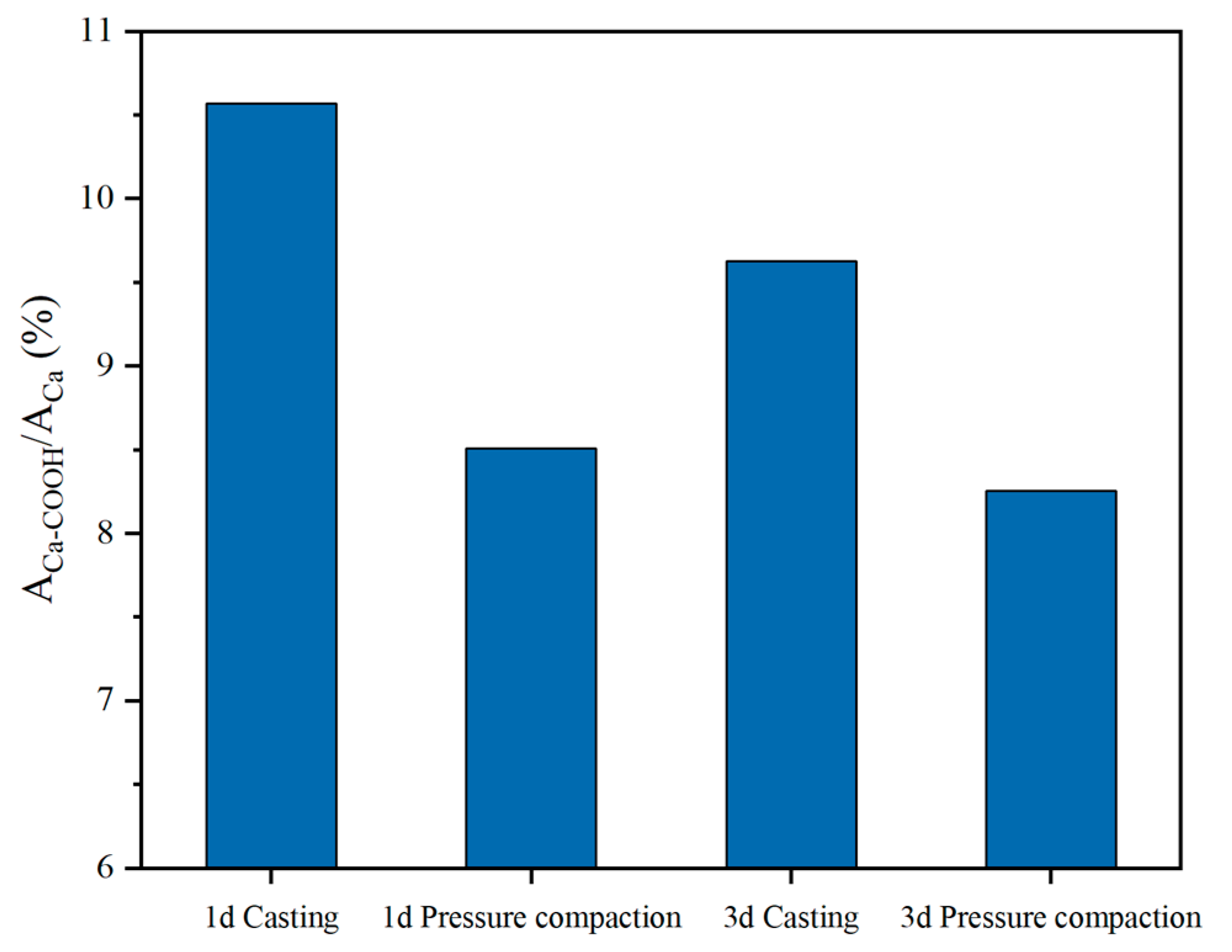
3.5.2. Changes of Hydration Products Under Compaction Conditions
3.6. Discussion
3.6.1. The Effect of ISS on the Hydration Properties of Cement
3.6.2. The Effect of ISS on the Compaction Performance of Cement-Stabilized Soil
3.6.3. The Effect of ISS on the Degree of Cement Hydration in Cement-Stabilized Soil
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, A.; Zhang, D.; Deng, Y. Lateral response of single piles in cement-improved soil: Numerical and theoretical investigation. Comput. Geotech. 2018, 102, 164–178. [Google Scholar] [CrossRef]
- Qiang, Y.; Chen, Y. Experimental Research on the Mechanical Behavior of Lime-Treated Soil under Different Loading Rates. Adv. Mater. Sci. Eng. 2015, 2015, 862106. [Google Scholar] [CrossRef]
- Jallu, M.; Arulrajah, A.; Saride, S.; Evans, R. Flexural fatigue behavior of fly ash geopolymer stabilized-geogrid reinforced RAP bases. Constr. Build. Mater. 2020, 254, 119263. [Google Scholar] [CrossRef]
- Little, D.N.; Nair, S.; Herbert, B. Addressing Sulfate-Induced Heave in Lime Treated Soils. J. Geotech. Geoenviron. Eng. 2010, 136, 110–118. [Google Scholar] [CrossRef]
- Intaj, F.; Liu, Y.; Wu, Z. Application and Evaluation of Micro-Cracking on Cement-Stabilized Bases at Field Projects in Louisiana. Transp. Res. Rec. 2019, 2673, 355–364. [Google Scholar] [CrossRef]
- Al-Dakheeli, H.; Arefin, S.; Bulut, R.; Little, D. Effectiveness of ionic stabilization in the mitigation of soil volume change behavior. Transp. Geotech. 2021, 29, 100573. [Google Scholar] [CrossRef]
- Katz, L.E.; Rauch, A.F.; Liljestrand, H.M.; Harmon, J.S.; Shaw, K.S.; Albers, H. Mechanisms of Soil Stabilization with Liquid Ionic Stabilizer. Transp. Res. Rec. 2001, 1757, 50–57. [Google Scholar] [CrossRef]
- Arefin, S.; Al-Dakheeli, H.; Bulut, R. Stabilization of expansive soils using ionic stabilizer. Bull. Eng. Geol. Environ. 2021, 80, 4025–4033. [Google Scholar] [CrossRef]
- Gautam, S.; Hoyos, L.R.; He, S.; Prabakar, S.; Yu, X. Chemical Treatment of a Highly Expansive Clay Using a Liquid Ionic Soil Stabilizer. Geotech. Geol. Eng. 2020, 38, 4981–4993. [Google Scholar] [CrossRef]
- Huo, J.; Wang, Z.; Zhang, T.; He, R.; Chen, H. Influences of interaction between cement and ionic paraffin emulsion on cement hydration. Constr. Build. Mater. 2021, 299, 123951. [Google Scholar] [CrossRef]
- Khan, R.A.; Murtaza, M.; Mustafa, A.; Abdulraheem, A.; Mahmoud, M.; Kamal, M.S. Ionic liquids as clay stabilizer additive in fracturing fluid. Fuel 2023, 334, 126154. [Google Scholar] [CrossRef]
- Kaur, G.; Kumar, H.; Singla, M. Diverse applications of ionic liquids: A comprehensive review. J. Mol. Liq. 2022, 351, 118556. [Google Scholar] [CrossRef]
- Petry, T.M.; Das, B. Evaluation of Chemical Modifiers and Stabilizers for Chemically Active Soils—Clays. Transp. Res. Rec. 2001, 1757, 43–49. [Google Scholar] [CrossRef]
- Eren, Ş.; Filiz, M. Comparing the conventional soil stabilization methods to the consolid system used as an alternative admixture matter in Isparta Darıdere material. Constr. Build. Mater. 2009, 23, 2473–2480. [Google Scholar] [CrossRef]
- Soltani, A.; Deng, A.; Taheri, A.; Mirzababaei, M. A sulphonated oil for stabilisation of expansive soils. Int. J. Pavement Eng. 2017, 20, 1285–1298. [Google Scholar] [CrossRef]
- Jia, J.; Lu, X.; Zhu, J.; Wang, J.; Zhang, L.; Cheng, X. Effect of ionic soil stabilizer on the properties of lime and fly ash stabilized iron tailings as pavement base. Constr. Build. Mater. 2024, 450, 138558. [Google Scholar] [CrossRef]
- Zhang, B.; Jiang, W.; Xu, Q.; Yuan, D.; Shan, J.; Lu, R. Experimental feasibility study of ethylene-vinyl acetate copolymer (EVA) as cement-stabilized soil curing agent. Road Mater. Pavement Des. 2022, 23, 617–638. [Google Scholar] [CrossRef]
- Xue, C.; Wang, X.; Lian, B.; Luo, L.; Liu, K. Research on the mechanism of composite improvement of loess based on quantitative analysis of microstructure and mechanical strength. Constr. Build. Mater. 2023, 379, 131215. [Google Scholar] [CrossRef]
- Latifi, N.; Eisazadeh, A.; Marto, A. Strength behavior and microstructural characteristics of tropical laterite soil treated with sodium silicate-based liquid stabilizer. Environ. Earth Sci. 2014, 72, 91–98. [Google Scholar] [CrossRef]
- Ma, F.; Wu, B.; Zhang, Q.; Cui, D.; Liu, Q.; Peng, C.; Li, F.; Gu, Q. An innovative method for the solidification/stabilization of PAHs-contaminated soil using sulfonated oil. J. Hazard. Mater. 2018, 344, 742–748. [Google Scholar] [CrossRef]
- Wu, X.-T.; Qi, Y.; Chen, B. Solidification effect and mechanism of ionic soil stabilizer applied on high-water-content clay. B. Eng. Geol. Env. 2021, 80, 8583–8595. [Google Scholar] [CrossRef]
- Ministry of Transport of the People’s Republic of China. Test Methods of Soils for Highway Engineering; Ministry of Transport of the People’s Republic of China: Beijing, China, 2020.
- GB/T 50547-2022; Technical Standard for Geotechnical Engineering of Tailings Embankmen. Ministry of Housing and Urban-Rural Development, People’s Republic of China: Beijing, China, 2022.
- Ministry of Transport of the People’s Republic of China. Test Methods of Materials Stabilized with Inorganic Binders for Highway Engineering; Ministry of Transport of the People’s Republic of China: Beijing, China, 2024.
- Wang, B.; Xing, X.; Lin, F.; Wang, S.; Li, F.; Huang, H.; Wei, J.; Yu, Q. Effect of vacuum–vibration–compaction molding on the properties and performances of polymer-modified cementitious material. Cem. Concr. Compos. 2024, 148, 105450. [Google Scholar] [CrossRef]
- GB/T 17671-2021; Test Method of Cement Mortar Strength (ISO Method). State Administration for Market Regulation. National Standard of the People’s Republic of China: Beijing, China, 2021.
- Zhang, J.; Scherer, G.W. Comparison of methods for arresting hydration of cement. Cem. Concr. Res. 2011, 41, 1024–1036. [Google Scholar] [CrossRef]
- Yan, Y.; Ouzia, A.; Yu, C.; Liu, J.; Scrivener, K.L. Effect of a novel starch-based temperature rise inhibitor on cement hydration and microstructure development. Cem. Concr. Res. 2020, 129, 105961. [Google Scholar] [CrossRef]
- Ma, Y.; Ye, G. The shrinkage of alkali activated fly ash. Cem. Concr. Res. 2015, 68, 75–82. [Google Scholar] [CrossRef]
- Marchuk, A.; Rengasamy, P.; McNeill, A. Influence of organic matter, clay mineralogy, and pH on the effects of CROSS on soil structure is related to the zeta potential of the dispersed clay. Soil Res. 2013, 51, 34–40. [Google Scholar] [CrossRef]
- Farahani, E.; Emami, H.; Fotovat, A.; Khorassani, R. Effect of different K:Na ratios in soil on dispersive charge, cation exchange and zeta potential. Eur. J. Soil Sci. 2019, 70, 311–320. [Google Scholar] [CrossRef]
- Chen, B.; Wu, K.; Yao, W. Conductivity of carbon fiber reinforced cement-based composites. Cem. Concr. Compos. 2004, 26, 291–297. [Google Scholar] [CrossRef]
- Wang, M.; Wang, R.; Yao, H.; Farhan, S.; Zheng, S.; Wang, Z.; Du, C.; Jiang, H. Research on the mechanism of polymer latex modified cement. Constr. Build. Mater. 2016, 111, 710–718. [Google Scholar] [CrossRef]
- Chaudhari, O.; Biernacki, J.J.; Northrup, S. Effect of carboxylic and hydroxycarboxylic acids on cement hydration: Experimental and molecular modeling study. J. Mater. Sci. 2017, 52, 13719–13735. [Google Scholar] [CrossRef]
- Marchon, D.; Flatt, R.J. 12—Impact of chemical admixtures on cement hydration. In Science and Technology of Concrete Admixtures; Aïtcin, P.-C., Flatt, R.J., Eds.; Woodhead Publishing: Sawston, UK, 2016; pp. 279–304. [Google Scholar]
- Danner, T.; Justnes, H.; Geiker, M.; Lauten, R.A. Phase changes during the early hydration of Portland cement with Ca-lignosulfonates. Cem. Concr. Res. 2015, 69, 50–60. [Google Scholar] [CrossRef]
- Jolicoeur, C.; Simard, M.-A. Chemical admixture-cement interactions: Phenomenology and physico-chemical concepts. Cem. Concr. Compos. 1998, 20, 87–101. [Google Scholar] [CrossRef]
- Kiventerä, J.; Piekkari, K.; Isteri, V.; Ohenoja, K.; Tanskanen, P.; Illikainen, M. Solidification/stabilization of gold mine tailings using calcium sulfoaluminate-belite cement. J. Clean. Prod. 2019, 239, 118008. [Google Scholar]
- Onyejekwe, S.; Ghataora, G.S. Soil stabilization using proprietary liquid chemical stabilizers: Sulphonated oil and a polymer. Bull. Eng. Geol. Environ. 2015, 74, 651–665. [Google Scholar] [CrossRef]
- Zhu, G.; Luo, Y.; Chen, C.; Shen, K.; Zhang, Y. Effect of metakaolin on the microstructure of cement-based artificial marble slabs incorporating natural marble offcuts under vacuum vibration compaction method. Constr. Build. Mater. 2023, 369, 130478. [Google Scholar] [CrossRef]

| Setting Time (min) | Compressive Strength (MPa) | Flexural Strength (MPa) | Fineness | |||
|---|---|---|---|---|---|---|
| Initial set | Final set | 3 d | 28 d | 3 d | 28 d | 1.2 |
| 156 | 282 | 22.1 | 44.7 | 5.2 | 9.6 | |
| Chemical Composition | CaO | SiO2 | Al2O3 | Fe2O3 | MgO | SO3 |
|---|---|---|---|---|---|---|
| Content (%) | 58.67 | 24.16 | 6.42 | 4.03 | 0.83 | 2.21 |
| Component | Iron Tailings Soil (%) |
|---|---|
| SiO2 | 51.4 |
| Al2O3 | 13.6 |
| Fe2O3 | 10.2 |
| CaO | 6.58 |
| MgO | 5.07 |
| SO3 | 3.84 |
| K2O | 3.06 |
| Na2O | 2.21 |
| P2O5 | 1.03 |
| TiO2 | 0.83 |
| MnO | 0.17 |
| Loss | 2.64 |
| Cement | Sand | W/C | ISS (%) | |
|---|---|---|---|---|
| ISS0 | 450 | 1350 | 0.5 | 0 |
| ISS017 | 450 | 1350 | 0.5 | 0.17%mcem |
| ISS033 | 450 | 1350 | 0.5 | 0.33%mcem |
| ISS167 | 450 | 1350 | 0.5 | 1.67%mcem |
| Maximum Dry Density (g/cm3) | Optimum Moisture Content (%) | Sample Volume (cm3) | ISS (%) | |
|---|---|---|---|---|
| ISS0 | 2.132 | 13.8 | 98.125 | 0 |
| ISS017 | 2.210 | 12.7 | 98.125 | 0.17%mcem |
| ISS033 | 2.271 | 12.1 | 98.125 | 0.33%mcem |
| ISS167 | 2.189 | 13.1 | 98.125 | 1.67%mcem |
| Samples | Cumulative Heat Cement with Different ISS Content (J/g) | ||||
|---|---|---|---|---|---|
| 1 h | 10 h | 24 h | 45 h | 60 h | |
| ISS0 | 13.3 | 77.4 | 177.7 | 224.3 | 241.3 |
| ISS017 | 17.3 | 75.4 | 183.9 | 236.7 | 256.1 |
| ISS033 | 18.6 | 62.5 | 169.8 | 223.4 | 243.0 |
| ISS167 | 5.9 | 11.6 | 80.2 | 178.8 | 203.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Jia, J.; Lu, X.; Cheng, X.; Zhu, J.; Zhang, L.; Guo, P.; Zhai, G. The Effect of Ionic Soil Stabilizer on Cement and Cement-Stabilized Iron Tailings Soil: Hydration Difference and Mechanical Properties. Materials 2025, 18, 1444. https://doi.org/10.3390/ma18071444
Li H, Jia J, Lu X, Cheng X, Zhu J, Zhang L, Guo P, Zhai G. The Effect of Ionic Soil Stabilizer on Cement and Cement-Stabilized Iron Tailings Soil: Hydration Difference and Mechanical Properties. Materials. 2025; 18(7):1444. https://doi.org/10.3390/ma18071444
Chicago/Turabian StyleLi, Hongtu, Jian Jia, Xiaolei Lu, Xin Cheng, Jiang Zhu, Lina Zhang, Peipei Guo, and Gongning Zhai. 2025. "The Effect of Ionic Soil Stabilizer on Cement and Cement-Stabilized Iron Tailings Soil: Hydration Difference and Mechanical Properties" Materials 18, no. 7: 1444. https://doi.org/10.3390/ma18071444
APA StyleLi, H., Jia, J., Lu, X., Cheng, X., Zhu, J., Zhang, L., Guo, P., & Zhai, G. (2025). The Effect of Ionic Soil Stabilizer on Cement and Cement-Stabilized Iron Tailings Soil: Hydration Difference and Mechanical Properties. Materials, 18(7), 1444. https://doi.org/10.3390/ma18071444






