The Investigation of Ni-Doped SrFeO3−δ Perovskite for a Symmetrical Electrode in Proton Ceramic Fuel Cells
Abstract
1. Introduction
2. Experimental and Analysis
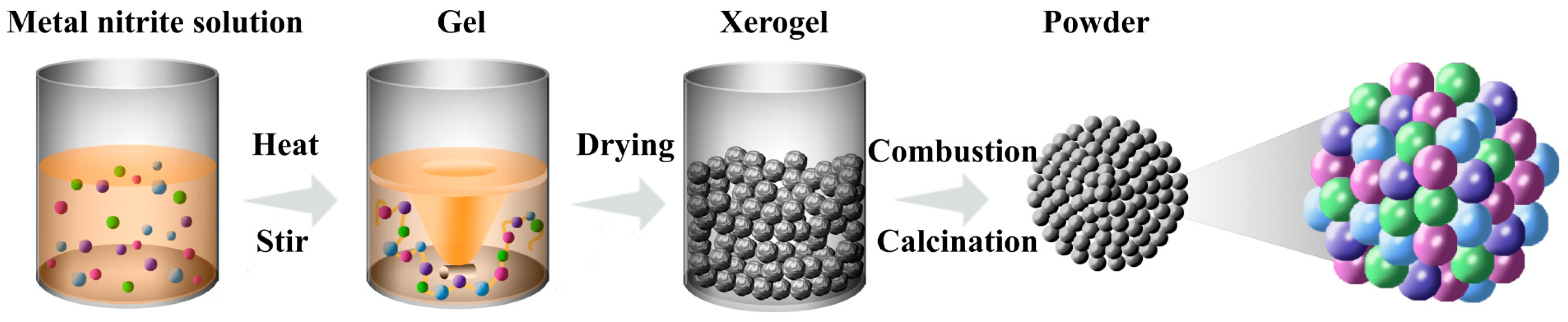
2.1. Materials Synthesis
2.2. Cell Fabrication
2.3. Characterization
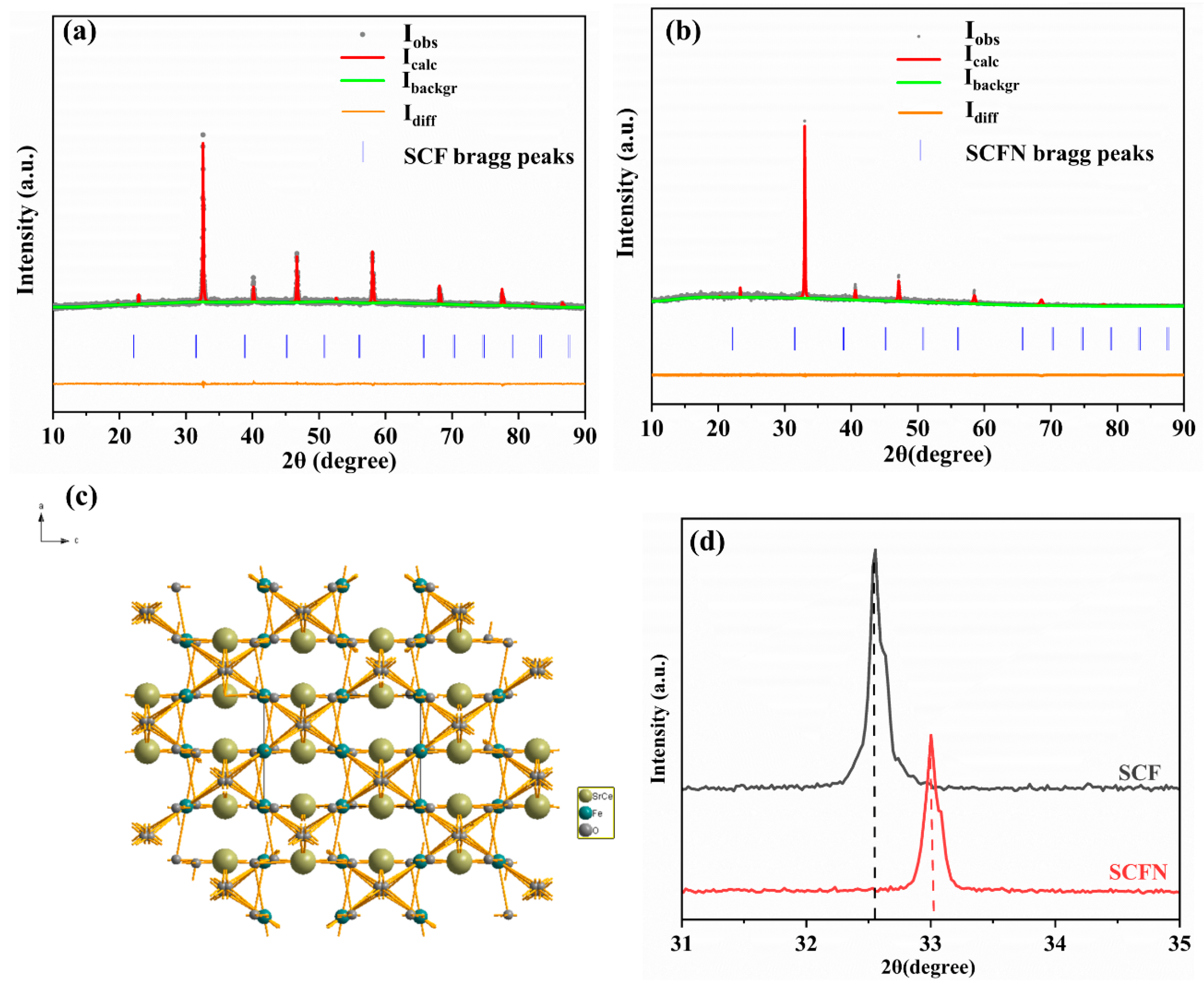
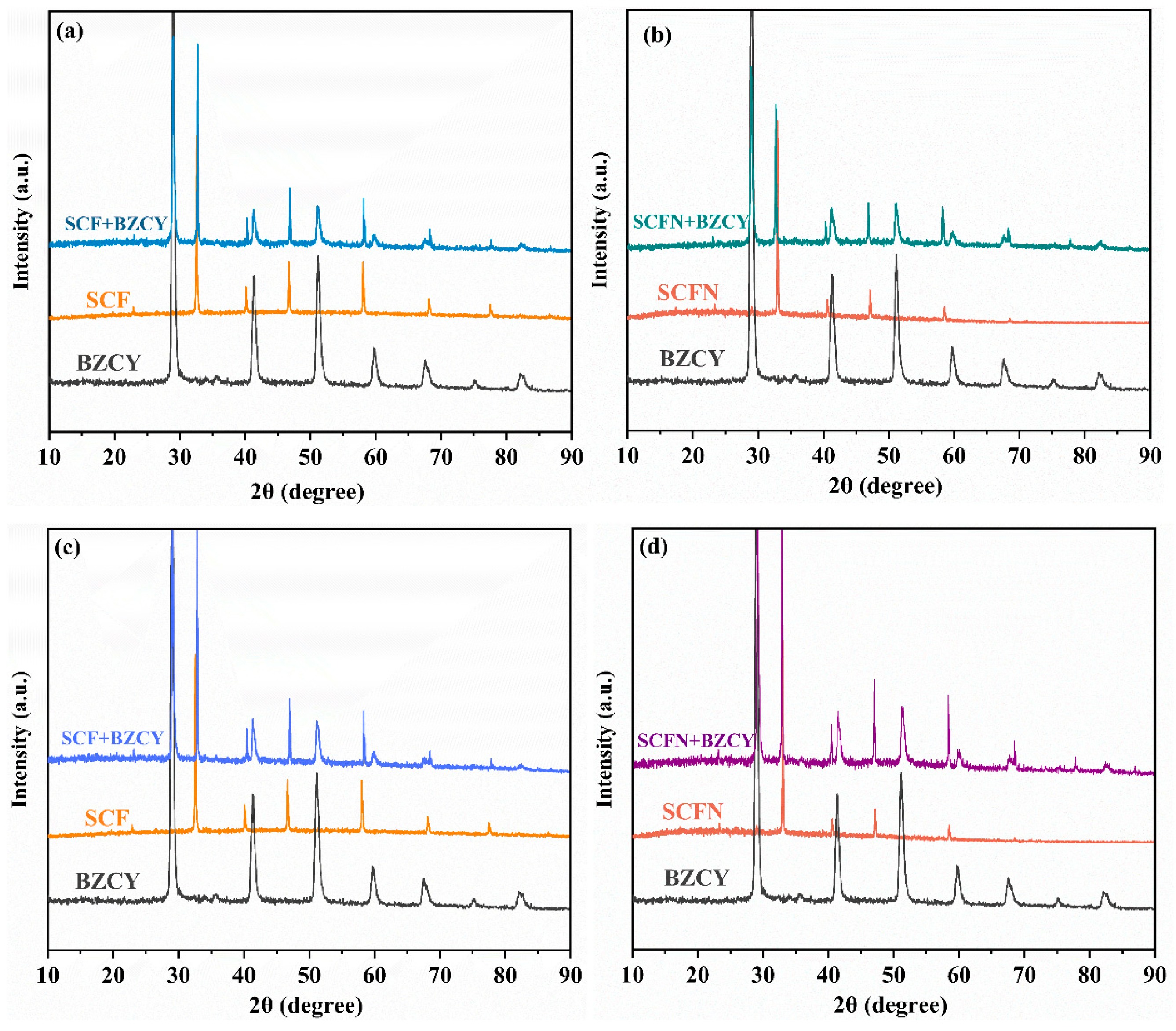
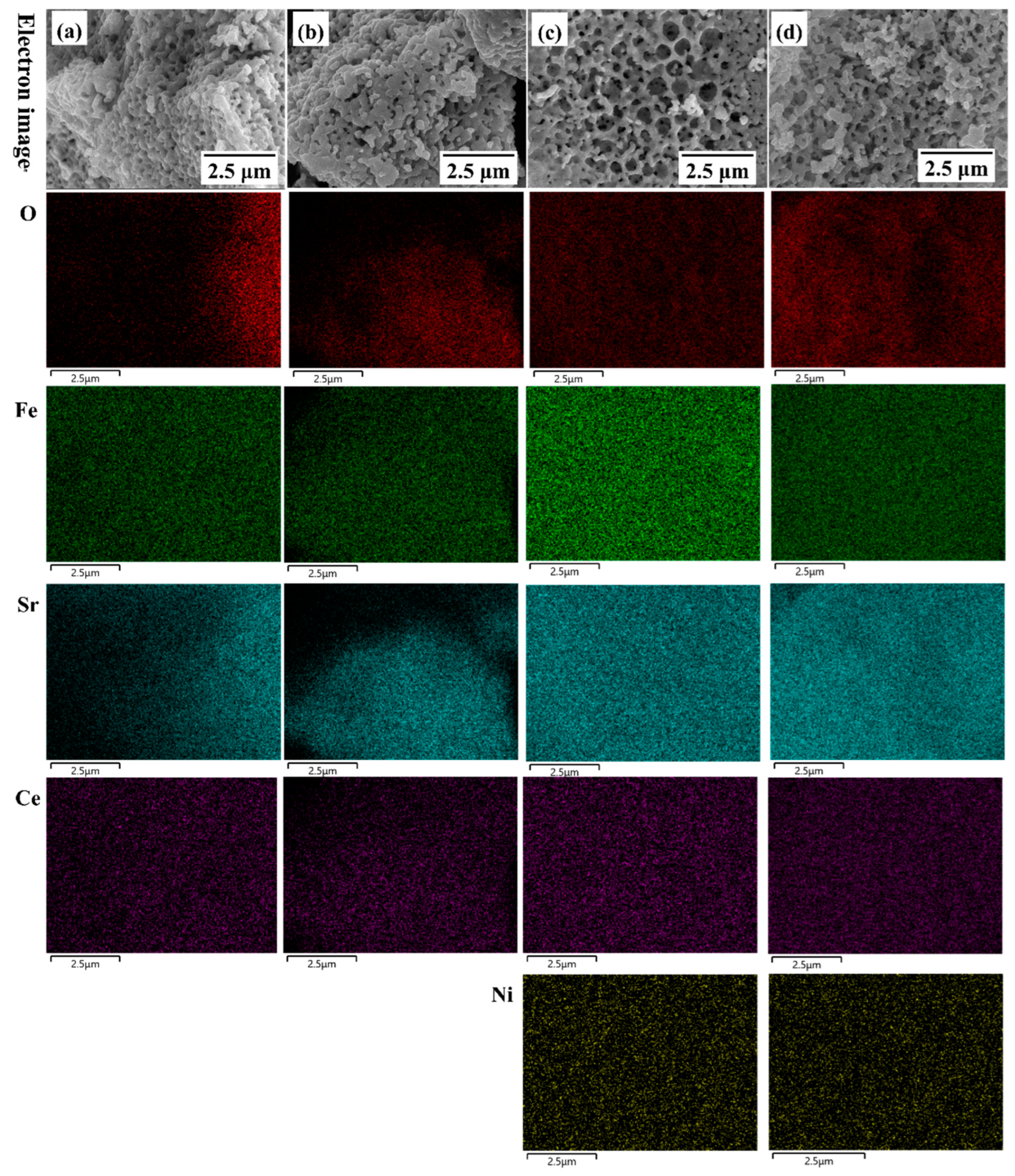
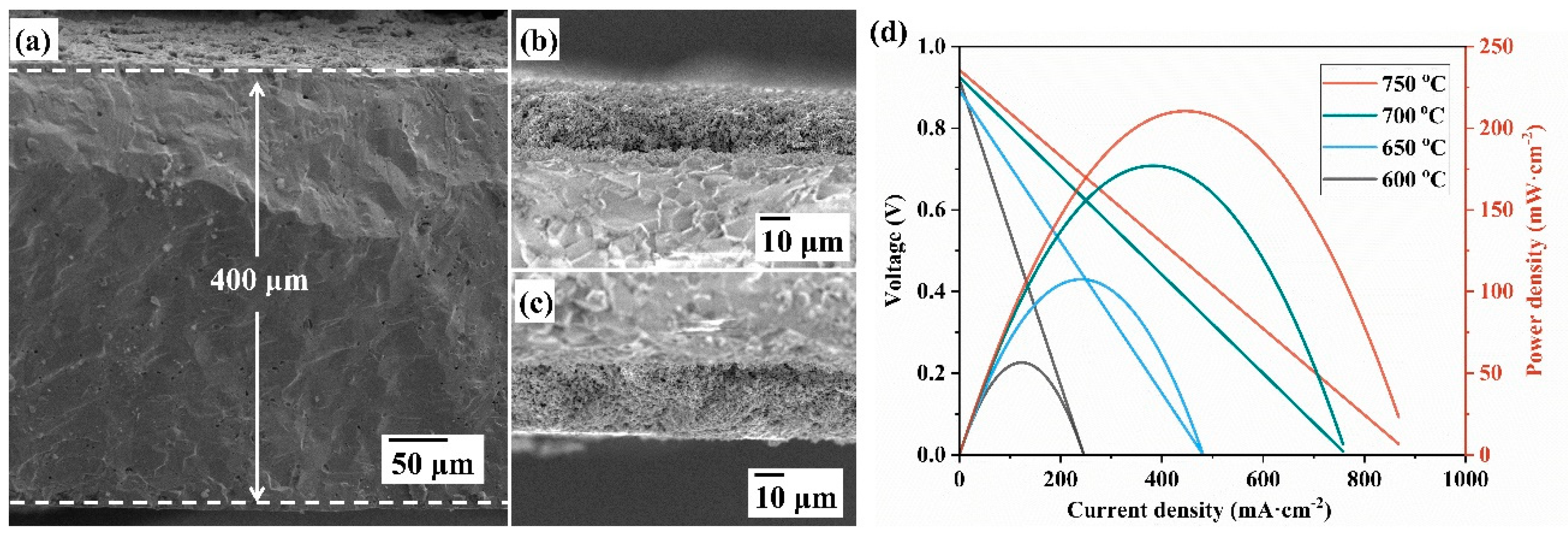
3. Result and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SOFCs | solid oxide fuel cells |
| PCFCs | proton ceramic fuel cells |
| XRD | X-ray diffraction |
| XPS | X-ray photoelectron spectroscopy |
| TGA | Thermogravimetric Analysis |
| TEC | thermal expansion coefficient |
| SEM | scanning electron microscopy |
| EDS | Energy Dispersive X-ray Spectroscopy |
| Rp | polarization resistance |
| Ro | Ohmic Resistance |
| T | temperature |
| Z′ | The real part of the impedance spectrum |
| −Z″ | The imaginary part of the impedance spectrum |
| Ea | activation energy |
References
- Albert, T.; Mónica, B.; José, S.; Skinner, S.J.; Kilner, J.A. Advances in layered oxide cathodes for intermediate temperature solid oxide fuel cells. J. Mater. Chem. 2010, 20, 3799. [Google Scholar]
- Wang, F.; Kishimoto, H.; Ishiyama, T.; Develos-Bagarinao, K.; Yokokawa, H. A review of sulfur poisoning of solid oxide fuel cell cathode materials for solid oxide fuel cells. J. Power Sources 2020, 478, 228763. [Google Scholar] [CrossRef]
- Aleksei, I.; Sergey, S.; Mariya, S.; Dmitrii, A.; Ekaterina, V.; Mikhail, V.; Irina, I.; Andrey, O.; Victor, V.; Vladislav, V. Oxygen Nonstoichiometry, Electrical Conductivity, Chemical Expansion and Electrode Properties of Perovskite-Type SrFe0.9V0.1O3−δ. Materials 2025, 18, 493. [Google Scholar]
- Lucía, S.; Romualdo, S.; José, L.; María, T.; Ainara, A.; José, A. Valence Variability Induced in SrMoO3 Perovskite by Mn Doping: Evaluation of a New Family of Anodes for Solid-Oxide Fuel Cells. Materials 2025, 18, 542. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, X.; Xu, Z.; Deng, H.; Dong, W.; Wang, B.; Feng, C.; Liu, X.; Wang, H. Semiconductor-Ionic Nanocomposite La0.1Sr0.9MnO3−δ-Ce0.8Sm0.2O2−δ Functional Layer for High Performance Low Temperature SOFC. Materials 2018, 11, 1549. [Google Scholar] [CrossRef]
- Schluckner, C.; Subotić, V.; Lawlor, V.; Hochenauer, C. CFD-simulation of effective carbon gasification strategies from high temperature SOFC Ni–YSZ cermet anodes. Int. J. Hydrogen Energy 2017, 42, 4434–4448. [Google Scholar] [CrossRef]
- Jia, C.; Wang, Y.; Molin, S.; Zhang, Y.; Chen, M.; Han, M. High temperature oxidation behavior of SUS430 SOFC interconnects with Mn-Co spinel coating in air. J. Alloys Compd. 2019, 787, 1327–1335. [Google Scholar] [CrossRef]
- Zhao, Q.; Geng, S.; Chen, G.; Wang, F. Influence of preoxidation on high temperature behavior of NiFe2 coated SOFC interconnect steel. Int. J. Hydrogen Energy 2019, 44, 13744–13756. [Google Scholar] [CrossRef]
- Shin, E.K.; Anggia, E.; Parveen, A.S.; Park, J.S. Optimization of the protonic ceramic composition in composite electrodes for high-performance protonic ceramic fuel cells. Int. J. Hydrogen Energy 2019, 44, 31323–31332. [Google Scholar] [CrossRef]
- Mather, G.C.; Muoz-Gil, D.; Javier, Z.; Porras-Vázquez, J.M.; Pérez-Coll, D. Perspectives on cathodes for protonic ceramic fuel cells. Appl. Sci. 2021, 11, 5363. [Google Scholar] [CrossRef]
- Mahadik, P.; Jain, D.; Shirsat, A.; Manoj, N.; Varma, S.; Wani, B.; Bharadwaj, S. Synthesis, stability and conductivity of SrCe0.8-xZrxY0.2O3−δ as electrolyte for proton conducting SOFC. Electrochim. Acta 2016, 219, 614–622. [Google Scholar] [CrossRef]
- Zhou, X.; Zheng, D.; Wang, Q.; Xia, C.; Wang, X.; Dong, W.; Ganesh, K.; Wang, H.; Wang, B. In situ formation of Ba3CoNb2O9/Ba5Nb4O15 heterostructure in electrolytes for enhancing proton conductivity and SOFC performance. ACS Appl. Mater. Interfaces 2023, 15, 41525–41536. [Google Scholar] [CrossRef] [PubMed]
- Park, I.; Kim, J.; Lee, H.; Park, J.; Shin, D. BaCeO3–BaCe0.8Sm0.2O3−δ bi-layer electrolyte-based protonic ceramic fuel cell. Solid State Ion. 2013, 252, 152–156. [Google Scholar] [CrossRef]
- Zhang, Q.; Guo, Y.; Ding, J.; Xia, S. Hole conductivity in the electrolyte of proton-conducting SOFC: Mathematical model and experimental investigation. J. Alloys Compd. 2019, 801, 343–351. [Google Scholar] [CrossRef]
- Mojave, P.; Chitsaz, A.; Sadeghi, M.; Khalilarya, S. Comprehensive comparison of SOFCs with proton-conducting electrolyte and oxygen ion-conducting electrolyte: Thermo-economic analysis and multi-objective optimization. Energy Convers. Manag. 2020, 205, 112455. [Google Scholar] [CrossRef]
- He, S.; Dai, H.; Bi, L. Protonic SOFCs with a novel La0.4K0.1Ca0.5MnO3−δ cathode. Ceram. Int. 2022, 48, 35599–35605. [Google Scholar] [CrossRef]
- Cao, J.; Ji, Y.; Shao, Z. Perovskites for protonic ceramic fuel cells: A review. Energy Environ. Sci. 2022, 15, 2200–2232. [Google Scholar] [CrossRef]
- Bello, I.T.; Zhai, S.; He, Q.; Cheng, C.; Dai, Y.; Chen, B.; Zhang, Y.; Ni, M. Materials development and prospective for protonic ceramic fuel cells. Int. J. Energy Res. 2022, 46, 3. [Google Scholar] [CrossRef]
- Magdalena, D.; Bartłomiej, L.; Radosław, L.; Salius, D.; Tomas, Š.; Algimantas, K.; Michał, M.; Maciej, S.; Alicja, R.; Przemysław, G. Samples of Ba1−xSrxCe0.9Y0.1O3−δ, 0 < x < 0.1, with improved chemical stability in CO2-H2 gas-involving atmospheres as potential electrolytes for a proton ceramic fuel cell. Materials 2020, 13, 1874. [Google Scholar] [CrossRef]
- Hua, B.; Yan, N.; Li, M.; Sun, Y.; Luo, J. Anode-engineered protonic ceramic fuel cell with excellent performance and fuel compatibility. Adv. Mater. 2016, 28, 8921. [Google Scholar] [CrossRef][Green Version]
- Yang, X.; Wang, Z.; Li, G.; Zhou, Y.; Sun, C.; Bi, L. Sc-doped Ba0.5Sr0.5Co0.8Fe0.2O3−δ cathodes for protonic ceramic fuel cells. Ceram. Int. 2024, 50, 40375–40383. [Google Scholar] [CrossRef]
- Choi, J.; Kim, B.; Song, S.; Jong-Sung, P. A composite cathode with undoped LaFeO3 for protonic ceramic fuel cells. Int. J. Hydrogen Energy 2016, 41, 9619–9626. [Google Scholar] [CrossRef]
- Yao, P.; Zhang, J.; Qiu, Q.; Li, G.; Zhao, Y.; Yu, F.; Li, Y. Design of a perovskite oxide cathode for a protonic ceramic fuel cell. Ceram. Int. 2024, 50, 2373–2382. [Google Scholar] [CrossRef]
- Tao, H.; Xie, J.; Wu, Y.; Wang, S. Evaluation of PrNi0.4Fe0.6O3−δ as a symmetrical SOFC electrode material. Int. J. Hydrogen Energy 2018, 43, 15423–15432. [Google Scholar] [CrossRef]
- Cai, H.; Zhang, L.; Xu, J.; Huang, J.; Long, W. Cobalt–free La0.5Sr0.5Fe0.9Mo0.1O3−δ electrode for symmetrical SOFC running on H2 and CO fuels. Electrochim. Acta 2019, 320, 134642. [Google Scholar] [CrossRef]
- Santos-Gómez, L.D.; Porras-Vázquez, J.M.; Losilla, E.R.; Marrero-López, D.; Slater, P.R. Investigation of PO43- oxyanion-doping on the properties of CaFe0.4Ti0.6O3−δ for potential application as symmetrical electrodes for SOFCs. J. Alloys Compd. 2020, 835, 155437. [Google Scholar] [CrossRef]
- Chang, S.; Kim, S.; Kim, Y.; Dong, Y.; Folkman, C.; Jeong, D.; Choi, W.; Borisevich, A.; Eastman, J.; Bhattacharya, A.; et al. Confined polaronic transport in (LaFeO3)n/(SrFeO3)1 superlattices. APL Mater. 2019, 7, 071117. [Google Scholar] [CrossRef]
- Santana, J.; Krogel, J.; Kent, P.; Reboredo, F. Diffusion quantum Monte Carlo calculations of SrFeO3 and LaFeO3. J. Chem. Phys. 2017, 147, 034701. [Google Scholar] [CrossRef]
- Andriushin, N.; Grumbach, J.; Kulbakov, A.; Tymoshenko, Y.; Onykiienko, Y.; Cheng, R.; Granovsky, S.; Skourski, Y.; Ollivier, J.; Walker, H.; et al. Anomalous Quasielastic Scattering Contribution in the Centrosymmetric Multi-q Helimagnet SrFeO3. Phys. Rev. X 2025, 15, 011038. [Google Scholar] [CrossRef]
- Østergaard, M.; Strunck, A.; Boffa, V.; Jørgensen, M. Kinetics of strontium carbonate formation on a Ce-doped SrFeO3 perovskite. Catalysts 2022, 12, 265. [Google Scholar] [CrossRef]
- Bulfin, B.; Vieten, J.; Richter, S.; Naik, J.; Patzke, G.; Roeb, M.; Sattler, C.; Steinfeld, A. Isothermal relaxation kinetics for the reduction and oxidation of SrFeO3 based perovskites. Phys. Chem. Chem. Phys. 2020, 22, 2466–2474. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Yang, J.; Zhang, H.; Meng, J.; Meng, F. Cobalt-free perovskite SrTa0.1Mo0.1Fe0.8O3−δ as cathode for intermediate-temperature solid oxide fuel cells. Int. J. Energy Res. 2020, 44, 925–933. [Google Scholar] [CrossRef]
- Cao, Y.; Zhu, Z.; Zhao, Y.; Zhao, W.; Wei, Z.; Liu, T. Development of tungsten stabilized SrFe0.8W0.2O3−δ material as novel symmetrical electrode for solid oxide fuel cells. J. Power Sources 2020, 455, 227951. [Google Scholar] [CrossRef]
- Li, B.; He, S.; Li, J.; Yue, X.; Irvine, J.T.S.; Xie, D.; Ni, J.; Ni, C. A Ce/Ru co-doped SrFeO3−δ perovskite for a coke-resistant anode of a symmetrical solid oxide fuel cell. ACS Catal. 2020, 10, 14398–14409. [Google Scholar] [CrossRef]
- Balci, M.; Payveren, M.; Saatci, B.; Ari, M. Synthesis and characterization of cubic δ-phase stabilized Bi2O3 electrolytes by triple rare earth cation doping for intermediate temperatures SOFCs. Micro Nanostruct. 2024, 194, 207944. [Google Scholar] [CrossRef]
- Chen, F.; Hao, S.; Huang, S.; Lu, Y. Nanoscale SiO2-coated superhydrophobic meshes via electro-spray deposition for oil-water separation. Powder Technol. 2020, 373, 82–92. [Google Scholar] [CrossRef]
- He, X.; Meng, B.; Sun, Y.; Liu, B.; Li, M. Electron beam physical vapor deposition of YSZ electrolyte coatings for SOFCs. Appl. Surf. Sci. 2008, 254, 7159–7164. [Google Scholar] [CrossRef]
- Shin, T.; Shin, M.; Park, G.; Lee, S.; Woo, S.; Yu, J. Fabrication and characterization of oxide ion conducting films, Zr1−xMxO2−δ (M = Y, Sc) on porous SOFC anodes, prepared by electron beam physical vapor deposition. Sustain. Energy Fuels 2017, 1, 103–111. [Google Scholar] [CrossRef]
- Anthony, S.; Küngas, R.; Vohs, J.; Gorte, R. Modification of SOFC Cathodes by Atomic Layer Deposition. J. Electrochem. Soc. 2013, 160, F1225–F1231. [Google Scholar]
- Marizy, A.; Dsaunay, T.; Chery, D.; Roussel, P.; Ringued, A.; Cassir, M. Atomic Layer Deposition, a Key Technique for Processing Thin-Layered SOFC Materials—Case of Epitaxial Thin Layers of CeO2 Catalyst. ECS Trans. 2013, 57, 983–990. [Google Scholar] [CrossRef]



| Samples | Cell Parameters (Å) | Cell Volume (Å3) | Reliability Factor (%) | |||
|---|---|---|---|---|---|---|
| a | b | c | wRp | Rp | ||
| SCF | 5.505 | 5.502 | 7.787 | 235.9 | 9.32 | 7.99 |
| SCFN | 5.491 | 5.502 | 7.768 | 234.7 | 9.89 | 8.11 |
| O | Fe | Sr | Ce | Ni | ||
|---|---|---|---|---|---|---|
| SCF | Before | 53.3 | 24.0 | 18.0 | 7.3 | - |
| After | 48.8 | 29.2 | 14.3 | 5.4 | - | |
| SCFN | Before | 53.7 | 21.1 | 18.3 | 4.5 | 2.5 |
| After | 50.6 | 23.4 | 18.4 | 5.0 | 2.6 | |
| Percentage Composition | SCF | SCFN | |||
|---|---|---|---|---|---|
| Before Reduction | After Reduction | Before Reduction | After Reduction | ||
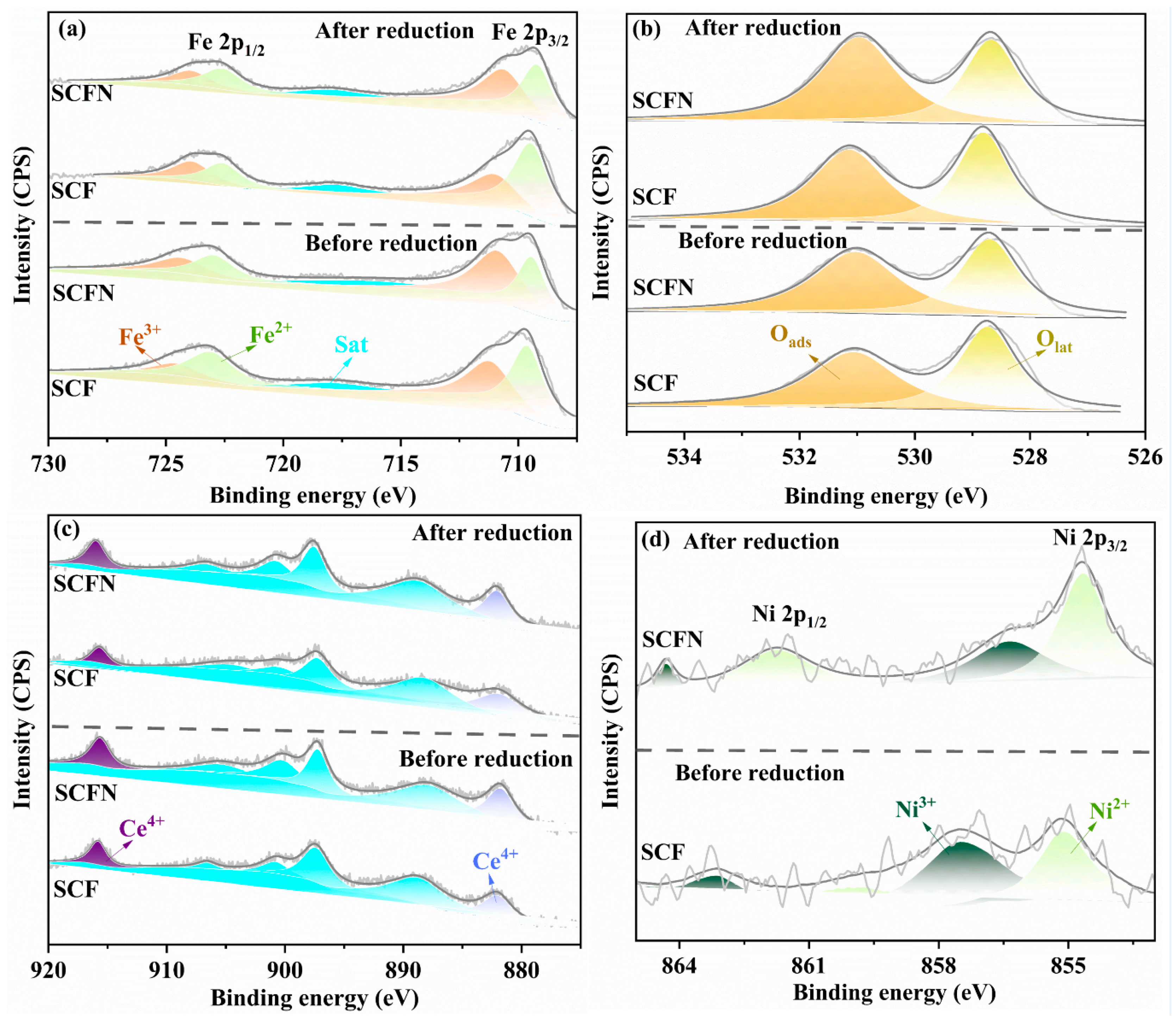
| Fe | Fe3+ | 40.27 | 38.66 | 55.37 | 46.54 |
| Fe2+ | 59.73 | 61.34 | 44.63 | 53.46 | |
| O | Olat | 49.59 | 51.48 | 53.80 | 55.14 |
| Oads | 50.41 | 48.52 | 46.20 | 44.86 | |
| Ce | Ce4+ | 34.22 | 24.65 | 34.80 | 33.55 |
| Ce3+ | 65.78 | 75.35 | 65.20 | 66.45 | |
| Ni | Ni3+ | - | - | 48.81 | 32.08 |
| Ni2+ | - | - | 51.19 | 67.92 | |
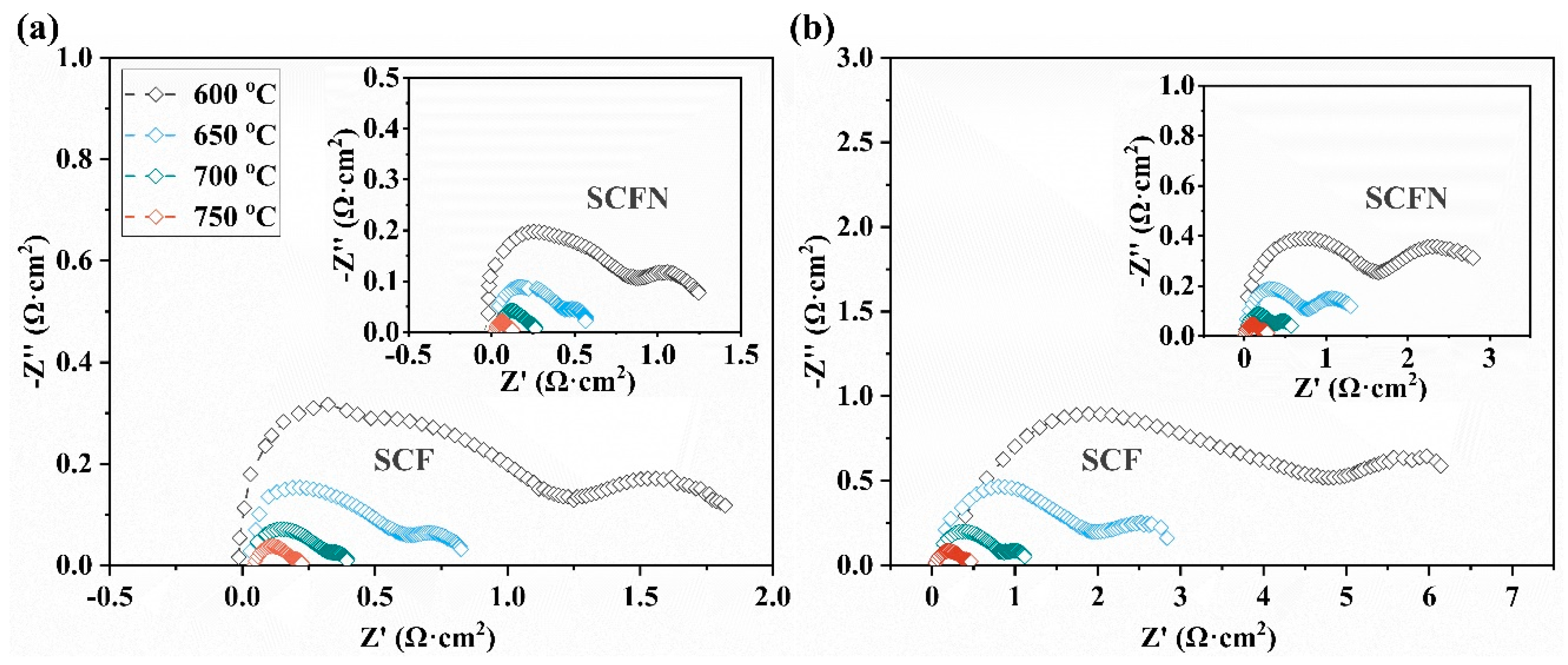
| T (°C) | SCF | SCFN | ||
|---|---|---|---|---|
| Rp in Air (Ω·cm2) | Rp in 5%H2 Rp (Ω·cm2) | Rp in Air (Ω·cm2) | Rp in 5%H2 Rp (Ω·cm2) | |
| 600 | 2.00 | 7.20 | 1.32 | 3.40 |
| 650 | 0.83 | 2.95 | 0.60 | 1.50 |
| 700 | 0.39 | 1.25 | 0.26 | 0.58 |
| 750 | 0.22 | 0.50 | 0.13 | 0.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, J.; Sun, Y.; Yin, C.; Wang, H.; Liu, Z.; Zhou, Z.; Wu, K.; Zhou, J. The Investigation of Ni-Doped SrFeO3−δ Perovskite for a Symmetrical Electrode in Proton Ceramic Fuel Cells. Materials 2025, 18, 1460. https://doi.org/10.3390/ma18071460
Cui J, Sun Y, Yin C, Wang H, Liu Z, Zhou Z, Wu K, Zhou J. The Investigation of Ni-Doped SrFeO3−δ Perovskite for a Symmetrical Electrode in Proton Ceramic Fuel Cells. Materials. 2025; 18(7):1460. https://doi.org/10.3390/ma18071460
Chicago/Turabian StyleCui, Jiajia, Yueyue Sun, Chaofan Yin, Hao Wang, Zhengrong Liu, Zilin Zhou, Kai Wu, and Jun Zhou. 2025. "The Investigation of Ni-Doped SrFeO3−δ Perovskite for a Symmetrical Electrode in Proton Ceramic Fuel Cells" Materials 18, no. 7: 1460. https://doi.org/10.3390/ma18071460
APA StyleCui, J., Sun, Y., Yin, C., Wang, H., Liu, Z., Zhou, Z., Wu, K., & Zhou, J. (2025). The Investigation of Ni-Doped SrFeO3−δ Perovskite for a Symmetrical Electrode in Proton Ceramic Fuel Cells. Materials, 18(7), 1460. https://doi.org/10.3390/ma18071460






