Silver-Exchanged Clinoptilolite-Rich Natural Zeolite for Radon Removal from Air
Highlights
- Clinoptilolite-type zeolite was chemically modified with Ag+ ions.
- Rn adsorption experiments were conducted using a closed-circuit system.
- Ag+ modification improved the Rn removal efficiency by up to 50%.
- Ag+-exchanged natural zeolite is a promising material for Rn mitigation in air.
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Natural and Silver-Exchanged Zeolite Characterization
2.3. Preparation of Silver-Exchanged Zeolite
2.3.1. Zeolite Treatment with Sodium Salt
2.3.2. Zeolite Treatment with AgNO3
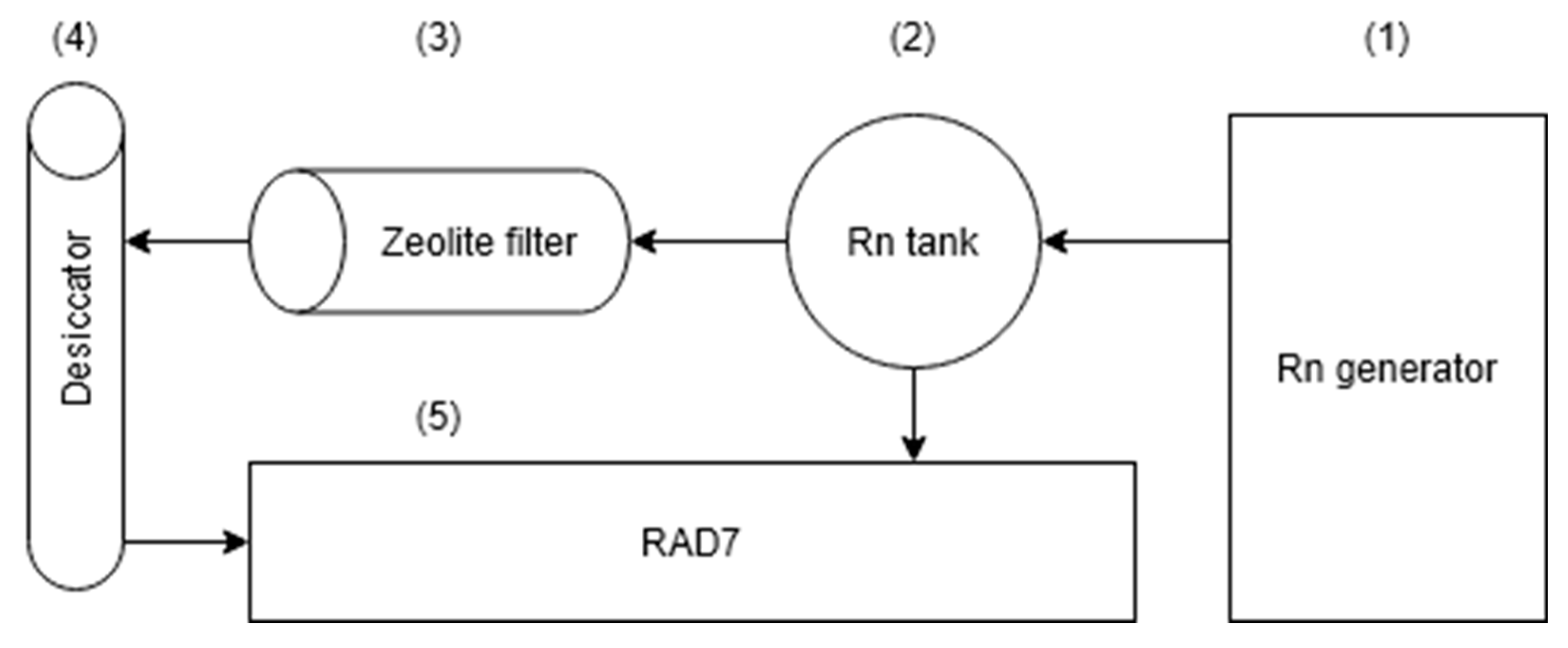
2.4. Experimental Setup for 222Rn Adsorption
3. Results and Discussion
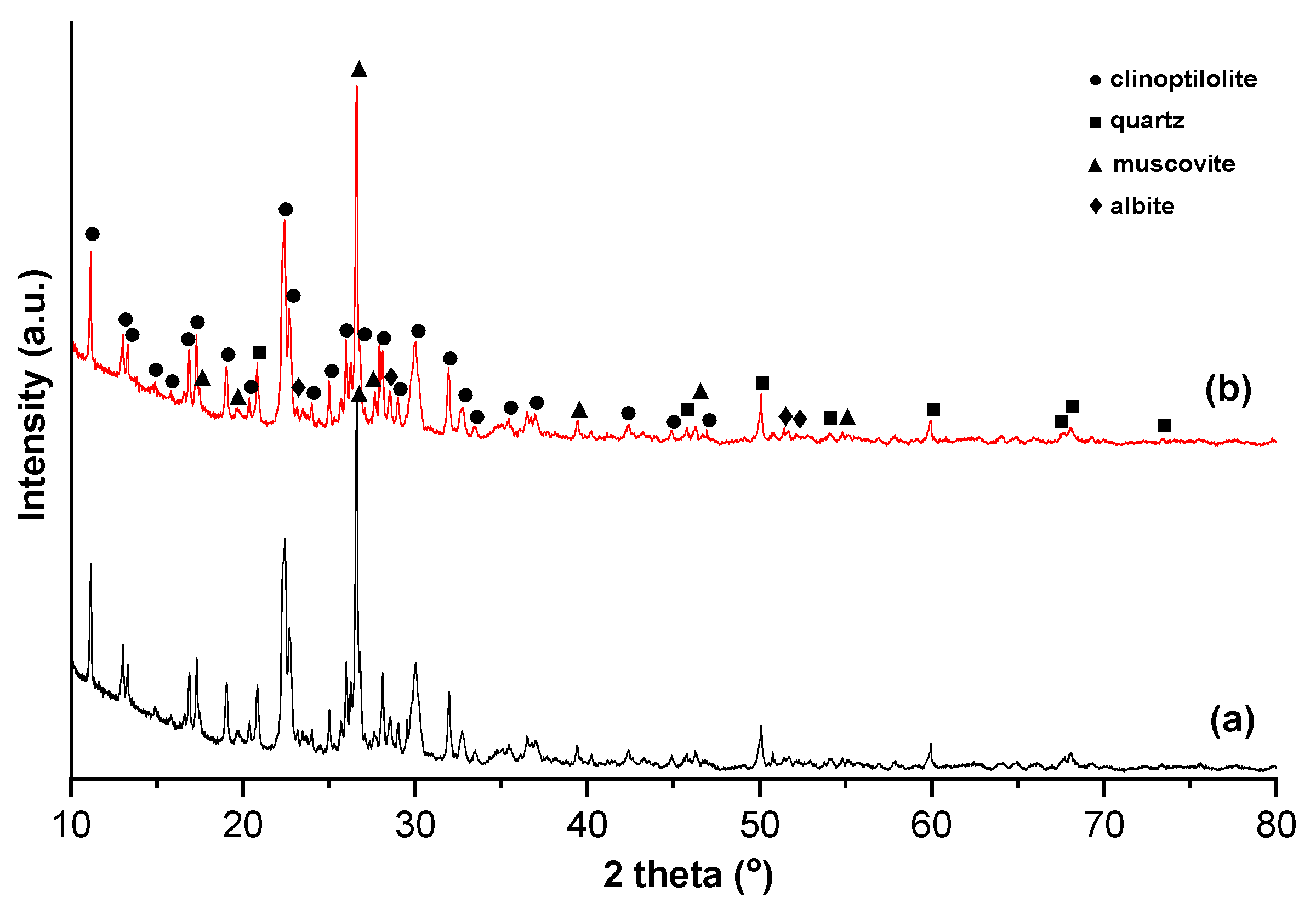
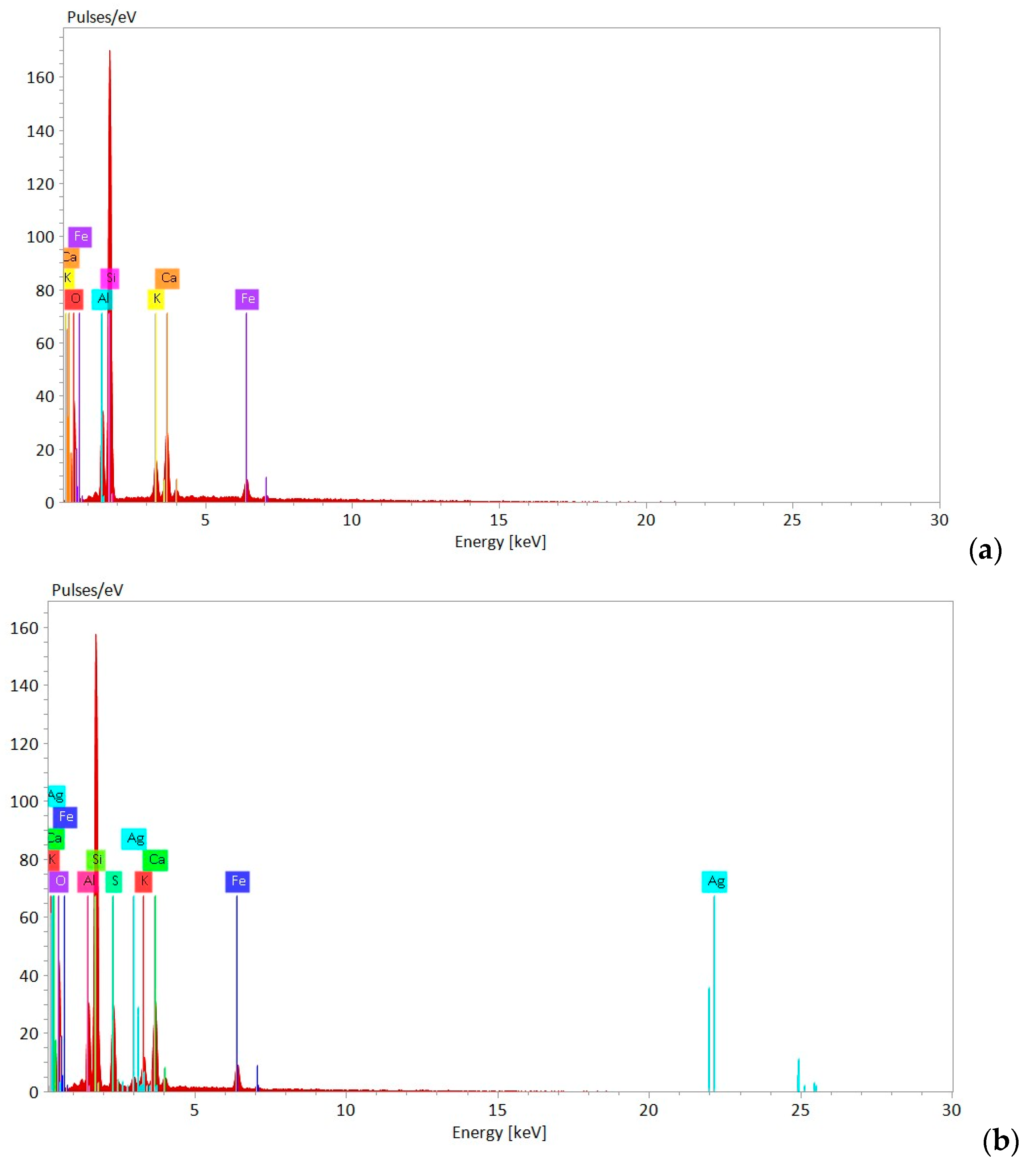
3.1. Characteristics of Zeolite Samples
3.1.1. Physical–Chemical and Mineralogical Characteristics of NZ and NZ-Ag+
3.1.2. Physical–Chemical Characteristics of Natural Zeolite
3.2. Radon Adsorption Experiments
3.2.1. System Leak Tests
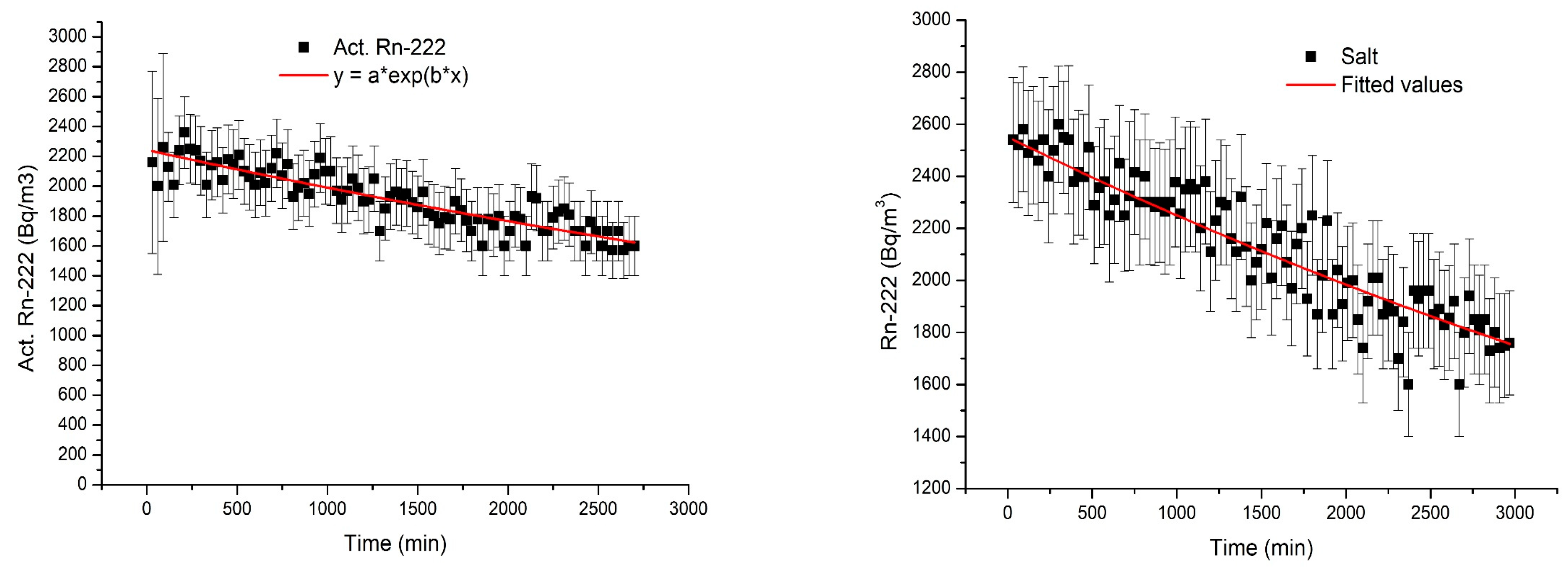
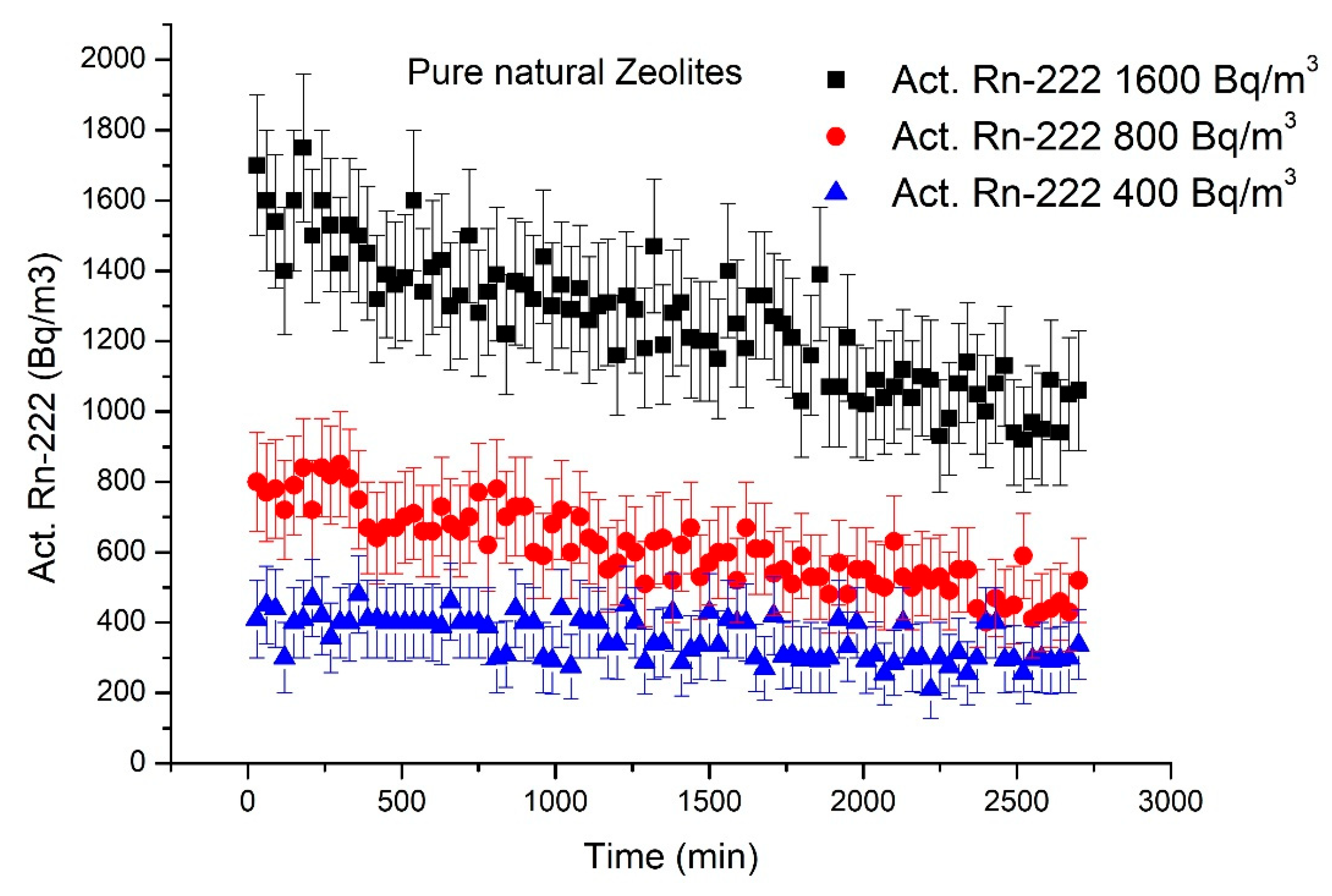
3.2.2. Radon Adsorption by NZ
3.2.3. Radon Adsorption by NZ-Ag+
3.2.4. 222Rn Removal Performance of Each Adsorbent
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nayak, T.; Basak, S.; Deb, A.; Dhal, P.K. A Systematic Review on Groundwater Radon Distribution with Human Health Consequences and Probable Mitigation Strategy. J. Environ. Radioact. 2022, 247, 106852. [Google Scholar] [CrossRef] [PubMed]
- Darby, S.; Hill, D.; Auvinen, A.; Barros-Dios, J.M.; Baysson, H.; Bochicchio, F.; Deo, H.; Falk, R.; Forastiere, F.; Hakama, M.; et al. Radon in Homes and Risk of Lung Cancer: Collaborative Analysis of Individual Data from 13 European Case-Control Studies. BMJ 2004, 330, 223. [Google Scholar] [CrossRef] [PubMed]
- Begy, R.-C.; Savin, C.-F.; Timar-Gabor, A. Correction of the Effects of Carbon Dioxide and Hydrogen Sulfide on Electrostatic Cell Monitors Measurements of Radon in Water. J. Environ. Chem. Eng. 2021, 10, 107040. [Google Scholar] [CrossRef]
- World Health Organization. Handbook on Indoor Radon. A Public Health Perspective. 2019. Available online: https://www.who.int/publications/i/item/9789241547673 (accessed on 24 February 2025).
- Florică, Ş.; Burghele, B.-D.; Bican-Brişan, N.; Begy, R.; Codrea, V.; Cucoş, A.; Catalina, T.; Dicu, T.; Dobrei, G.; Istrate, A.; et al. The Path from Geology to Indoor Radon. Environ. Geochem. Health 2020, 42, 2655–2665. [Google Scholar] [CrossRef]
- Colenghi, V.; Lepore, L.; Di Carlo, C.; Bochicchio, F.; Remetti, R. Development of an Electrostatic Precipitator Prototype to Reduce Exposure to Radon Progeny in Poorly Ventilated Workplaces. J. Radiat. Res. Appl. Sci. 2020, 13, 747–757. [Google Scholar] [CrossRef]
- Bruenner, S.; Cichon, D.; Eurin, G.; Gómez, P.H.; Jörg, F.; Undagoitia, T.M.; Simgen, H.; Rupp, N. Radon Daughter Removal from PTFE Surfaces and Its Application in Liquid Xenon Detectors. Eur. Phys. J. C 2021, 81, 343. [Google Scholar] [CrossRef]
- Kang, J.; Singh, B.K.; Um, W. Efficient Radon Removal Using Fluorine-Functionalized Natural Zeolite. J. Environ. Radioact. 2021, 233, 106607. [Google Scholar] [CrossRef]
- Yuan, L.; Geng, S.; Mao, J.; Wang, Q. Investigating the Mitigation Effects of Radon Progeny by Composite Radon Removal Device. J. Radioanal. Nucl. Chem. 2018, 319, 205–211. [Google Scholar] [CrossRef]
- Garcia-Fayos, B.; Juste, B.; Sancho, M.; Arnal, J.M.; Noverques, A.; Verdú, G. Study of Potential Capacity as Adsorbent of Moringa Oleifera Substrates for Treatment of Radon Contaminated Air in Indoor Spaces: Preliminary Test. Radiat. Phys. Chem. 2019, 167, 108262. [Google Scholar] [CrossRef]
- Karunakara, N.; Kumara, K.S.; Yashodhara, I.; Sahoo, B.K.; Gaware, J.J.; Sapra, B.K.; Mayya, Y.S. Evaluation of Radon Adsorption Characteristics of a Coconut Shell-Based Activated Charcoal System for Radon and Thoron Removal Applications. J. Environ. Radioact. 2015, 142, 87–95. [Google Scholar] [CrossRef]
- Hong, M.; Yu, L.; Wang, Y.; Zhang, J.; Chen, Z.; Dong, L.; Zan, Q.; Li, R. Heavy Metal Adsorption with Zeolites: The Role of Hierarchical Pore Architecture. Chem. Eng. J. 2018, 359, 363–372. [Google Scholar] [CrossRef]
- Senila, M.; Cadar, O.; Senila, L.; Hoaghia, A.; Miu, I. Mercury Determination in Natural Zeolites by Thermal Decomposition Atomic Absorption Spectrometry: Method Validation in Compliance with Requirements for Use as Dietary Supplements. Molecules 2019, 24, 4023. [Google Scholar] [CrossRef]
- Ambrosino, F.; La Verde, G.; Gagliardo, G.; Mottareale, R.; Della Peruta, G.; Imparato, C.; D’Elia, A.; Pugliese, M. Radon Exhalation Rate: A Metrological Approach for Radiation Protection. Sensors 2024, 24, 3633. [Google Scholar] [CrossRef]
- Pavelić, S.K.; Medica, J.S.; Gumbarević, D.; Filošević, A.; Pržulj, N.; Pavelić, K. Critical Review on Zeolite Clinoptilolite Safety and Medical Applications in Vivo. Front. Pharmacol. 2018, 9, 1350. [Google Scholar] [CrossRef]
- Senila, M.; Cadar, O. Modification of Natural Zeolites and Their Applications for Heavy Metal Removal from Polluted Environments: Challenges, Recent Advances, and Perspectives. Heliyon 2024, 10, e25303. [Google Scholar] [CrossRef]
- Dyer, A.; Las, T.; Zubair, M. The Use of Natural Zeolites for Radioactive Waste Treatment: Studies on Leaching from Zeolite/Cement Composites. J. Radioanal. Nucl. Chem. 2000, 243, 839–841. [Google Scholar] [CrossRef]
- Gagliardo, G.; Hanfi, M.Y.; La Verde, G.; Pugliese, M.; Gargiulo, N.; Caputo, D.; Ambrosino, F. Efficacy of Zeolites in Radon Adsorption: State of The Art and Development of an Optimized Approach. Isot. Environ. Health Stud. 2024, 60, 471–484. [Google Scholar] [CrossRef]
- Bikit, I.; Mrdja, D.; Bikit, K.; Grujic, S.; Knezevic, D.; Forkapic, S.; Kozmidis-Luburic, U. Radon Adsorption by Zeolite. Radiat. Meas. 2014, 72, 70–74. [Google Scholar] [CrossRef]
- Osmanlioglu, A. Removal of Radioactive Gas by Zeolite Filter from Nuclear Power Plants. Nat. Appl. Sci. J. 2022, 5, 14–20. [Google Scholar] [CrossRef]
- He, D.-L.; Yin, G.-F.; Dong, F.-Q.; Liu, L.-B.; Luo, Y.-J. Research on the Additives to Reduce Radioactive Pollutants in the Building Materials Containing Fly Ash. J. Hazard. Mater. 2010, 177, 573–581. [Google Scholar] [CrossRef]
- Ansón, A.; Kuznicki, S.M.; Kuznicki, T.; Haastrup, T.; Wang, Y.; Lin, C.C.H.; Sawada, J.A.; Eyring, E.M.; Hunter, D. Adsorption of Argon, Oxygen, and Nitrogen on Silver Exchanged ETS-10 Molecular Sieve. Microporous Mesoporous Mater. 2007, 109, 577–580. [Google Scholar] [CrossRef]
- Paschalides, J.S.; Marinakis, G.S.; Petropoulos, N.P. Passive, Integrated Measurement of Radon Using 5A Synthetic Zeolite and Blue Silica Gel. Appl. Radiat. Isot. 2009, 68, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Hedström, H.; Foreman, M.; Ekberg, C.; Ramebäck, H. Radon Capture with Silver Exchanged Zeolites. Radiochim. Acta 2012, 100, 395–399. [Google Scholar] [CrossRef]
- Heinitz, S.; Mermans, J.; Maertens, D.; Skliarova, H.; Aerts, A.; Cardinaels, T.; Gueibe, C.; Rutten, J.; Ireland, N.; Kuznicki, D.; et al. Adsorption of Radon on Silver Exchanged Zeolites at Ambient Temperatures. Sci. Rep. 2023, 13, 6811. [Google Scholar] [CrossRef]
- Veselska, O.; Llido, O.; Piro, M.-C.; Vaidya, S.; Kuznicki, S.; Busto, J. Exploring the Potential Use of Silver-Exchanged Zeolites for Adsorption of Radon Traces in Low Background Experiments. Prog. Theor. Exp. Phys. 2023, 2024, 023C01. [Google Scholar] [CrossRef]
- Sebastian, J.; Jasra, R.V. Anomalous Adsorption of Nitrogen and Argon in Silver Exchanged Zeolite A. Chem. Commun. 2003, 2, 268–269. [Google Scholar] [CrossRef]
- Kuznicki, S.M.; Anson, A.; Koenig, A.; Kuznicki, T.M.; Haastrup, T.; Eyring, E.M.; Hunter, D. Xenon Adsorption on Modified ETS-10. J. Phys. Chem. C 2007, 111, 1560–1562. [Google Scholar] [CrossRef]
- Sone, T.; Takeuchi, Y.; Matsukura, M.; Nakano, Y.; Ogawa, H.; Sekiya, H.; Wakihara, T.; Hirano, S.; Taniguchi, A. Study of Radon Removal Performance of Silver-Ion Exchanged Zeolite from Air for Underground Experiments. Prog. Theor. Exp. Phys. 2025, 2025, 013H01. [Google Scholar] [CrossRef]
- Pehlivan, H.; Balköse, D.; Ülkü, S.; Tihminlioglu, F. Characterization of Pure and Silver Exchanged Natural Zeolite Filled Polypropylene Composite Films. Compos. Sci. Technol. 2005, 65, 2049–2058. [Google Scholar] [CrossRef]
- Copcia, V.E.; Luchian, C.; Dunca, S.; Bilba, N.; Hristodor, C.M. Antibacterial Activity of Silver-Modified Natural Clinoptilolite. J. Mater. Sci. 2011, 46, 7121–7128. [Google Scholar] [CrossRef]
- Aparicio-Vázquez, S.; Fall, C.; Islas-Espinoza, M.; Alcántara, D.; Petranovskii, V.; Olguín, M.T. Influence of Experimental Conditions to Obtain Silver-Modified Zeolite-Rich Tuffs on the Antimicrobial Activity for Escherichia coli Suspended in Aqueous Media. Environ. Technol. Innov. 2021, 23, 101707. [Google Scholar] [CrossRef]
- Znak, Z.; Zin, O.; Mashtaler, A.; Korniy, S.; Sukhatskiy, Y.; Gogate, P.R.; Mnykh, R.; Thanekar, P. Improved Modification of Clinoptilolite with Silver Using Ultrasonic Radiation. Ultrason. Sonochem. 2021, 73, 105496. [Google Scholar] [CrossRef] [PubMed]
- Șenilă, M.; Neag, E.; Tănăselia, C.; Șenilă, L. Removal of Cesium and Strontium Ions from Aqueous Solutions by Thermally Treated Natural Zeolite. Materials 2023, 16, 2965. [Google Scholar] [CrossRef] [PubMed]
- US EPA. Cation-Exchange Capacity of Soils (Ammonium Acetate): Test Methods for Evaluating Solid Waste; SW-846, Method 9080; US EPA, Office of Solid Waste and Emergency Response: Washington, DC, USA, 1986.
- Senila, M.; Cadar, O.; Senila, L.; Angyus, B.S. Simulated Bioavailability of Heavy Metals (Cd, Cr, Cu, Pb, Zn) in Contaminated Soil Amended with Natural Zeolite Using Diffusive Gradients in Thin-Films (DGT) Technique. Agriculture 2022, 12, 321. [Google Scholar] [CrossRef]
- Akkoca, D.B.; Yilgin, M.; Ural, M.; Alcin, H.; Mergen, A. Hydrothermal and Thermal Treatment of Natural Clinoptilolite Zeolite from Bigadic, Turkey: An Experimental Study. Geochem. Int. 2013, 51, 495–504. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, D.; Bu, H.; Deng, L.; Liu, H.; Yuan, P.; Du, P.; Song, H. XRD-based quantitative analysis of clay minerals using reference intensity ratios, mineral intensity factors, Rietveld, and full pattern summation methods: A critical review. Solid Earth Sci. 2018, 3, 16–29. [Google Scholar] [CrossRef]
- Akhigbe, L.; Ouki, S.; Saroj, D.; Lim, X.M. Silver-Modified Clinoptilolite for the Removal of Escherichia coli and Heavy Metals from Aqueous Solutions. Environ. Sci. Pollut. Res. 2014, 21, 10940–10948. [Google Scholar] [CrossRef]
- Hanim, S.A.M.; Malek, N.A.N.N.; Ibrahim, Z. Amine-Functionalized, Silver-Exchanged Zeolite NaY: Preparation, Characterization and Antibacterial Activity. Appl. Surf. Sci. 2015, 360, 121–130. [Google Scholar] [CrossRef]
- Cerrillo, J.L.; Palomares, A.E.; Rey, F. Silver Exchanged Zeolites as Bactericidal Additives in Polymeric Materials. Microporous Mesoporous Mater. 2020, 305, 110367. [Google Scholar] [CrossRef]
- Faryad, S.; Ghorbanpour, M.; Safajou-Jahankhanemlou, M. Effect of Silver Salt Type on the Physicochemical Properties and Antimicrobial Activity of Solid-State Ag-Exchanged Zeolites. Int. J. Ceram. Eng. Sci. 2024, 6, e10241. [Google Scholar] [CrossRef]
- Mansouri, N.; Rikhtegar, N.; Panahi, H.A.; Atabi, F.; Shahraki, B.K. Porosity, characterization and structural properties of natural zeolite—Clinoptilolite—As a sorbent. Environ. Prot. Eng. 2013, 39, 139–152. [Google Scholar]
- Kulawong, S.; Artkla, R.; Sriprapakhan, P.; Maneechot, P. Biogas Purification by Adsorption of Hydrogen Sulphide on NaX and Ag-Exchanged NaX Zeolites. Biomass Bioenergy 2022, 159, 106417. [Google Scholar] [CrossRef]
- Munakata, K.; Kanjo, S.; Yamatsuki, S.; Koga, A.; Ianovski, D. Adsorption of Noble Gases on Silver-Mordenite. J. Nucl. Sci. Technol. 2003, 40, 695–697. [Google Scholar] [CrossRef]
- Busto, R.N.J.; Fierro, V. Measurements and understanding of radon adsorption in nanoporous materials. In Proceedings of the Low Radioactivity Techniques 2015 (LRT), Seattle, WA, USA, 18–20 March 2015; Available online: http://hal.in2p3.fr/in2p3-01150614 (accessed on 22 February 2025).
- Nguyen, H.G.; Konya, G.; Eyring, E.M.; Hunter, D.B.; Truong, T.N. Theoretical Study on the Interaction between Xenon and Positively Charged Silver Clusters in Gas Phase and on the (001) Chabazite Surface. J. Phys. Chem. C 2009, 113, 12818–12825. [Google Scholar] [CrossRef]
- Mortazavi, S.M.J.; Mehdizadeh, S.; Zehtabian, M.; Sina, S. Development of an Economical Radon-Resistant Construction Technique That is Applicable in National Radon-Reduction Programmes. Int. J. Low Radiat. 2009, 6, 113–118. [Google Scholar] [CrossRef]
- Srilakshmi, C. Nanoadsorbents for the Separation of Noble Gases. In Adsorption Through Advanced Nanoscale Materials; Elsevier: Amsterdam, The Netherlands, 2023; pp. 325–342. [Google Scholar]



| Tests | NZ (×10−4 min−1) | Tests | NZ-Ag+ (×10−4 min−1) |
|---|---|---|---|
| Test 1 (1600 Bq/m3) | 0.484 | Test 1 (1200 Bq/m3) | 0.855 |
| Test 2 (800 Bq/m3) | 0.846 | Test 2 (500 Bq/m3) | 0.836 |
| Test 3 (400 Bq/m3) | 0.160 | Test 3 (400 Bq/m3) | 0.549 |
| Mean | 0.496 | Mean | 0.746 |
| Geometrical mean | 0.403 | Geometrical mean | 0.732 |
| NZ | NZ-Ag+ | |
|---|---|---|
| %Ads/h | 1.192 | 1.792 |
| %Ads/H·g | 0.037 | 0.056 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senila, M.; Cadar, O.; Begy, R.-C.; Tanaselia, C.; Simedru, D.; Roman, C. Silver-Exchanged Clinoptilolite-Rich Natural Zeolite for Radon Removal from Air. Materials 2025, 18, 1465. https://doi.org/10.3390/ma18071465
Senila M, Cadar O, Begy R-C, Tanaselia C, Simedru D, Roman C. Silver-Exchanged Clinoptilolite-Rich Natural Zeolite for Radon Removal from Air. Materials. 2025; 18(7):1465. https://doi.org/10.3390/ma18071465
Chicago/Turabian StyleSenila, Marin, Oana Cadar, Robert-Csaba Begy, Claudiu Tanaselia, Dorina Simedru, and Cecilia Roman. 2025. "Silver-Exchanged Clinoptilolite-Rich Natural Zeolite for Radon Removal from Air" Materials 18, no. 7: 1465. https://doi.org/10.3390/ma18071465
APA StyleSenila, M., Cadar, O., Begy, R.-C., Tanaselia, C., Simedru, D., & Roman, C. (2025). Silver-Exchanged Clinoptilolite-Rich Natural Zeolite for Radon Removal from Air. Materials, 18(7), 1465. https://doi.org/10.3390/ma18071465









