Study on the Alkali-Activated Mechanism of Yellow River Sediment-Based Ecological Cementitious Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material and Mixed Proportion
2.1.1. Raw Material
2.1.2. Mixed Proportion
2.2. Experimental Method
2.2.1. Setting Time Test
2.2.2. Workability Test
2.2.3. Hydration Heat Test
2.2.4. Strength Test
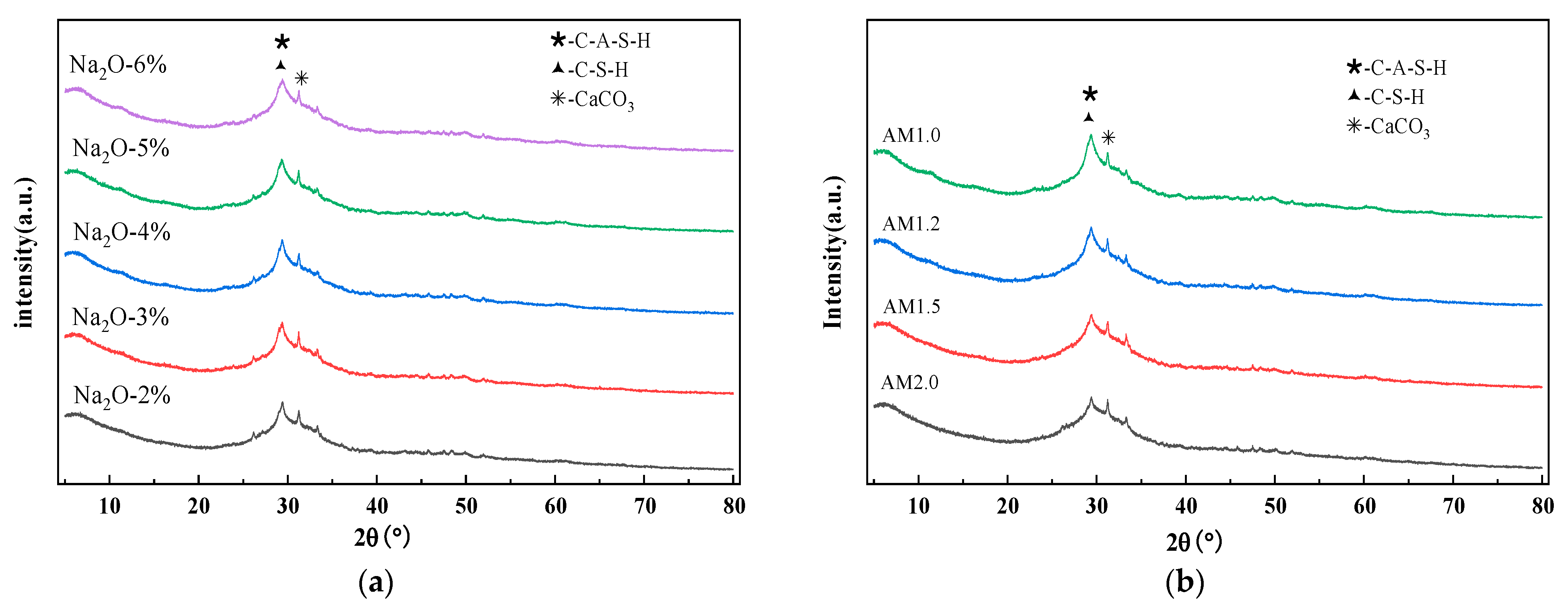
2.2.5. X-Ray Diffraction Analysis
2.2.6. Thermogravimetric Analysis
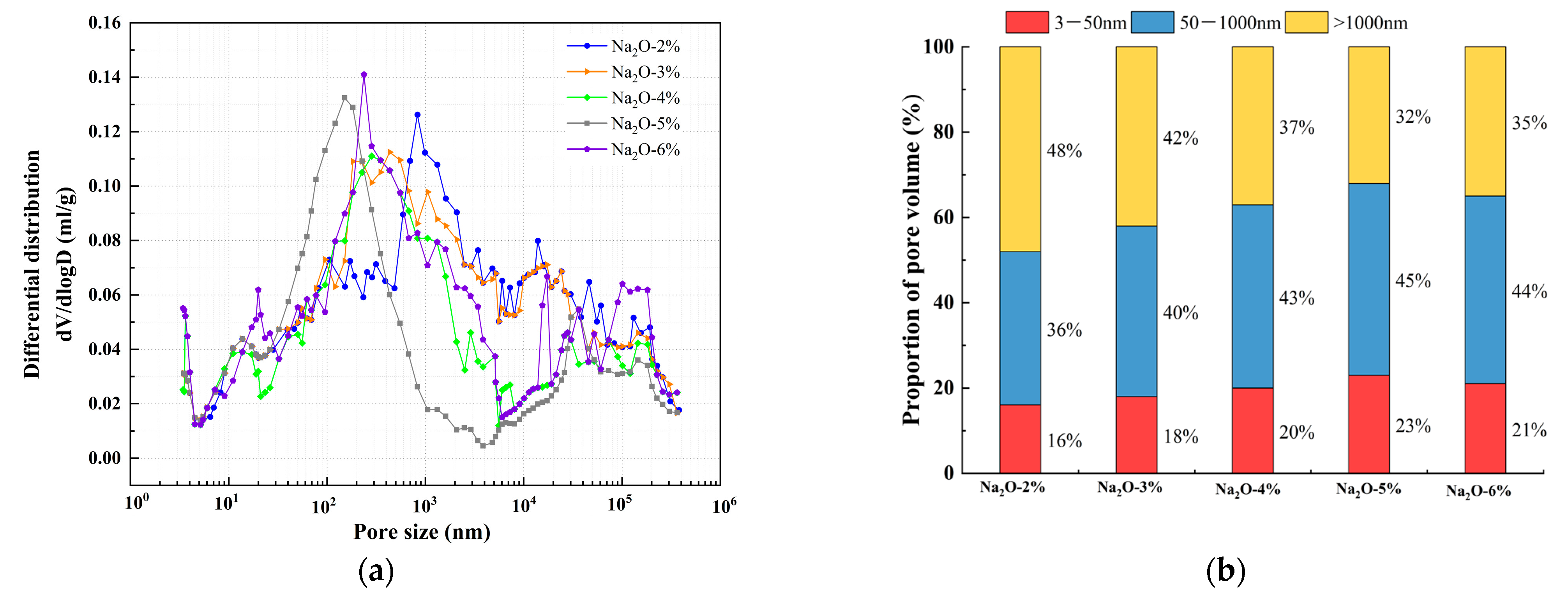
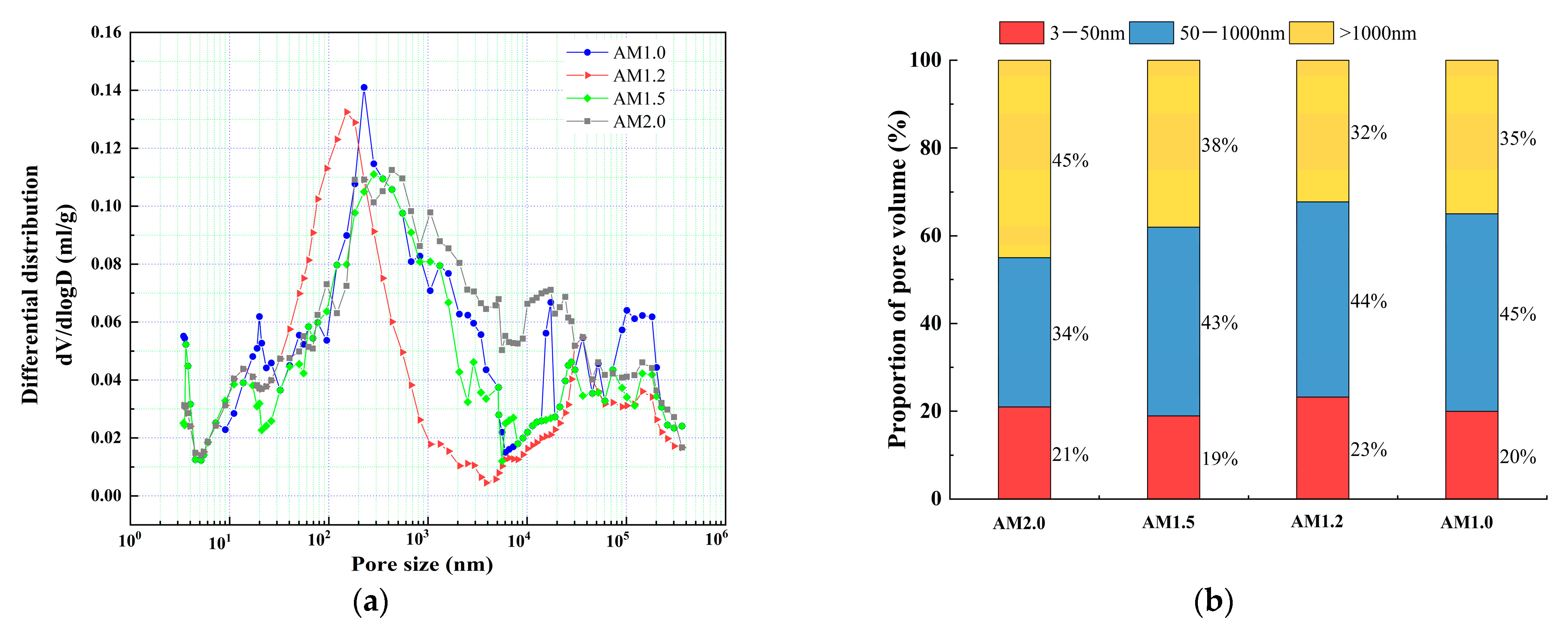
2.2.7. Porosity Test
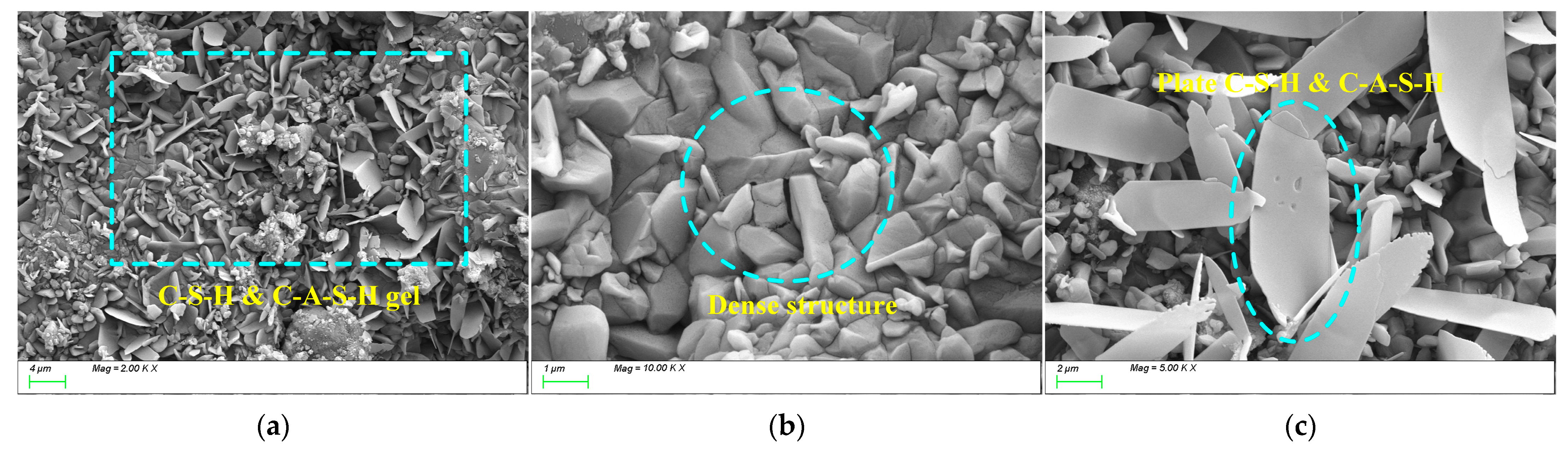
2.2.8. Scanning Electron Microscopy Test
3. Experiment Results and Analysis
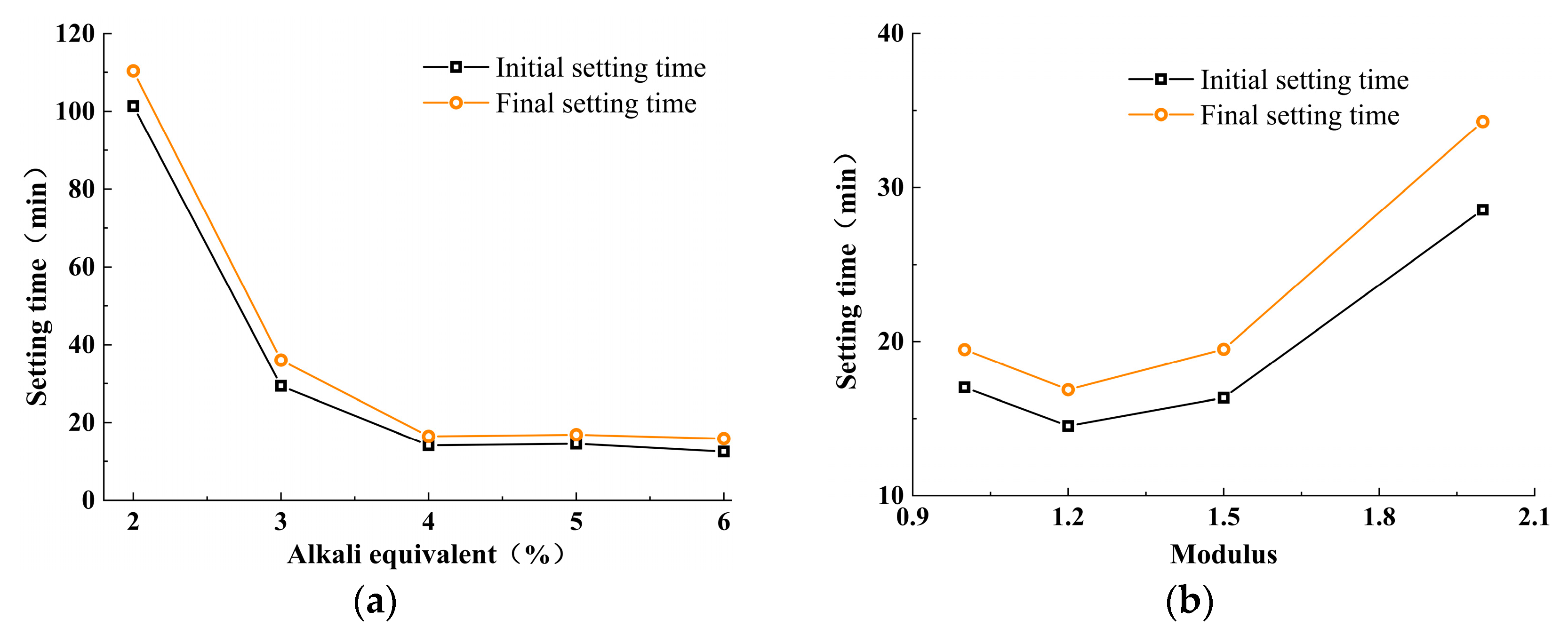
3.1. Effect of Alkali Activator Characteristic Parameters on Setting Time
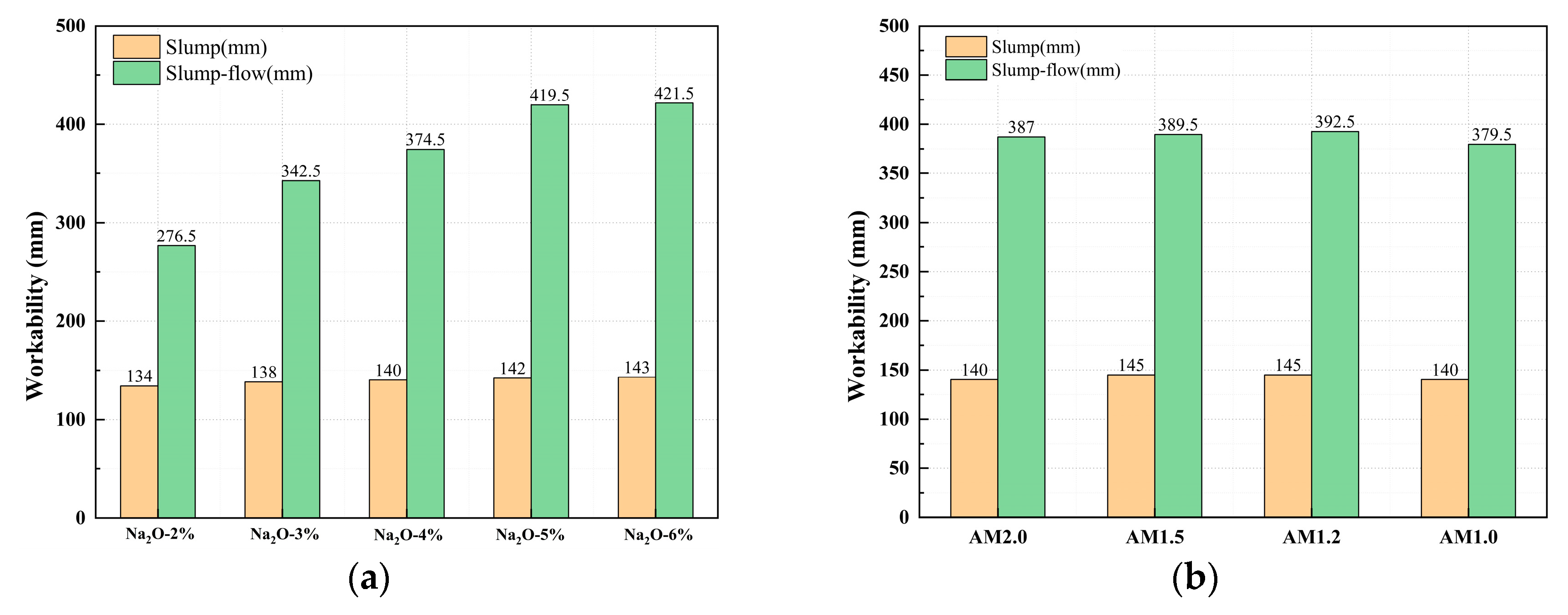
3.2. Effect of Alkali Activator Characteristic Parameters on Workability
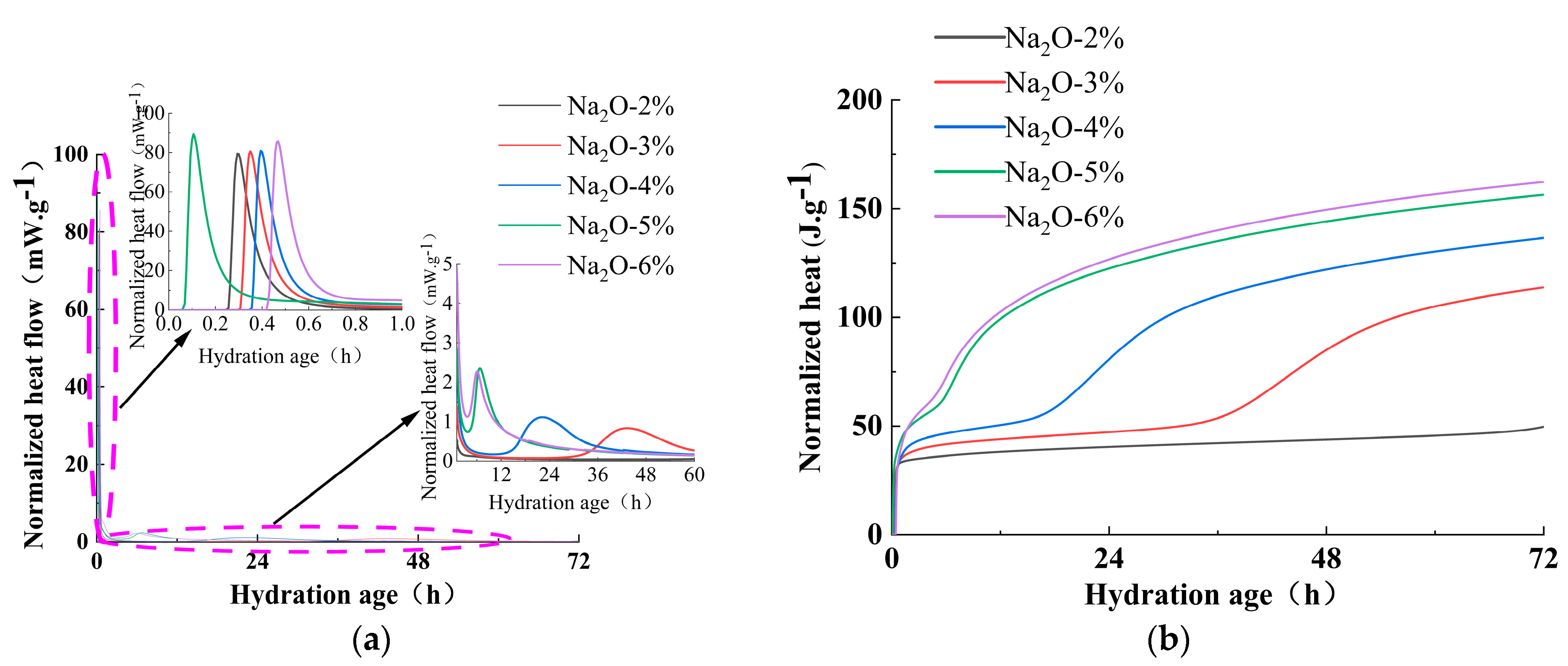
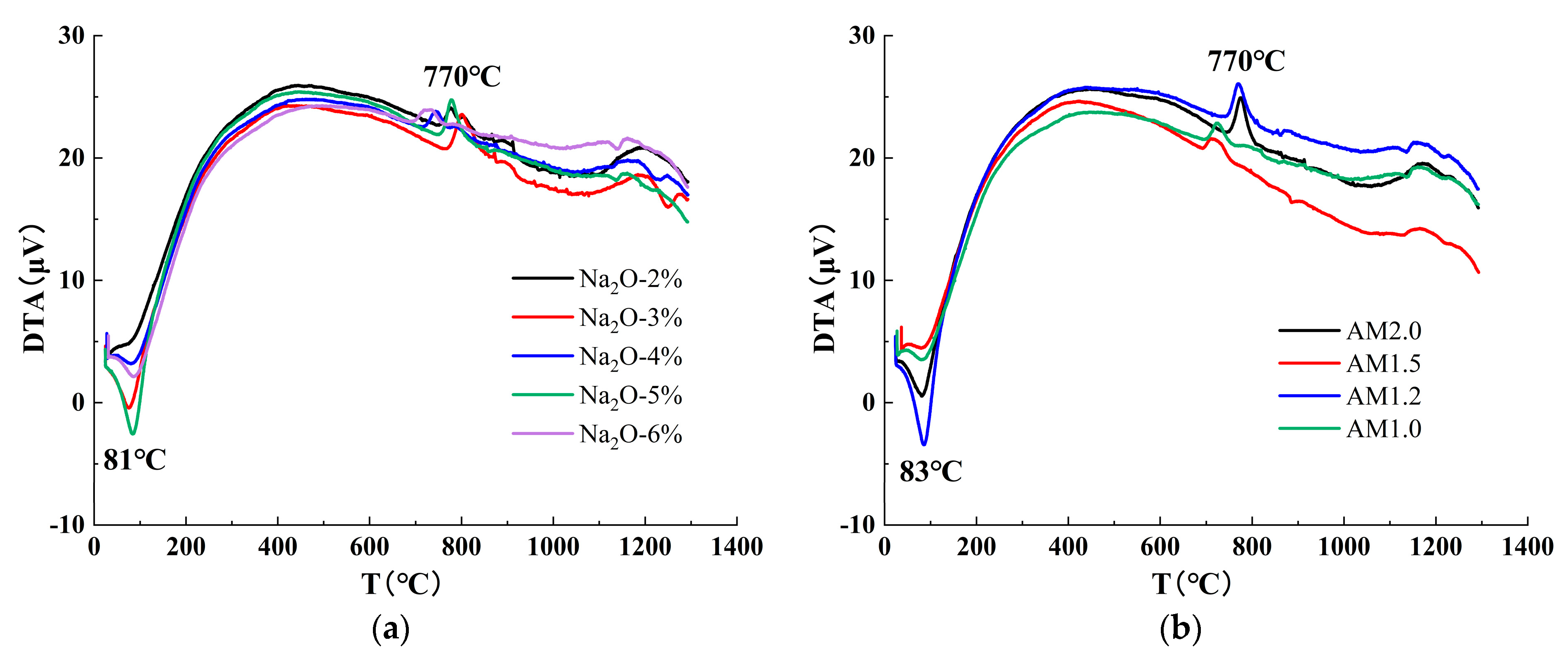
3.3. Effect of Alkali Activator Characteristic Parameters on the Early Reaction Process
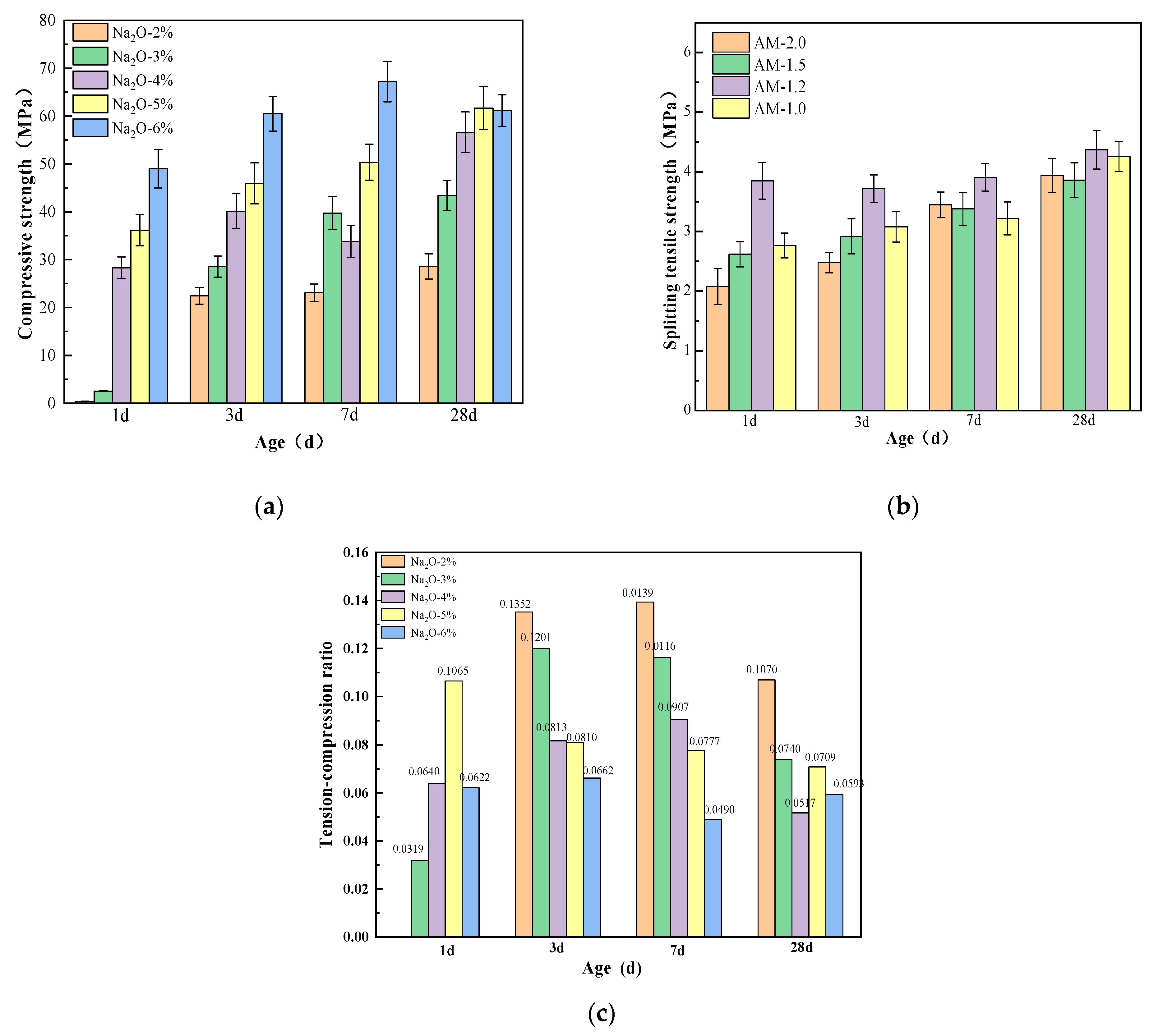
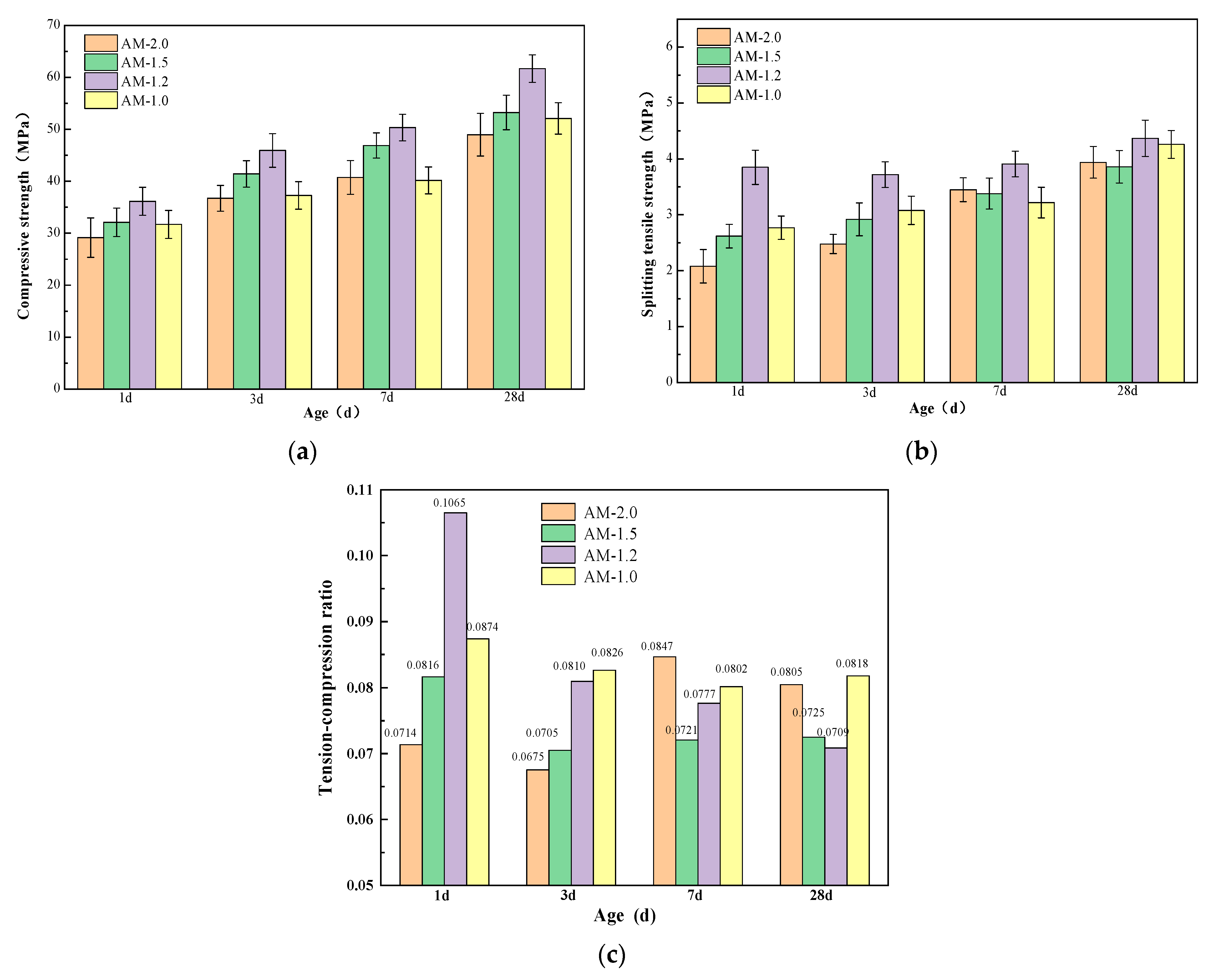
3.4. Effect of Alkali Activator Characteristic Parameters on Strength
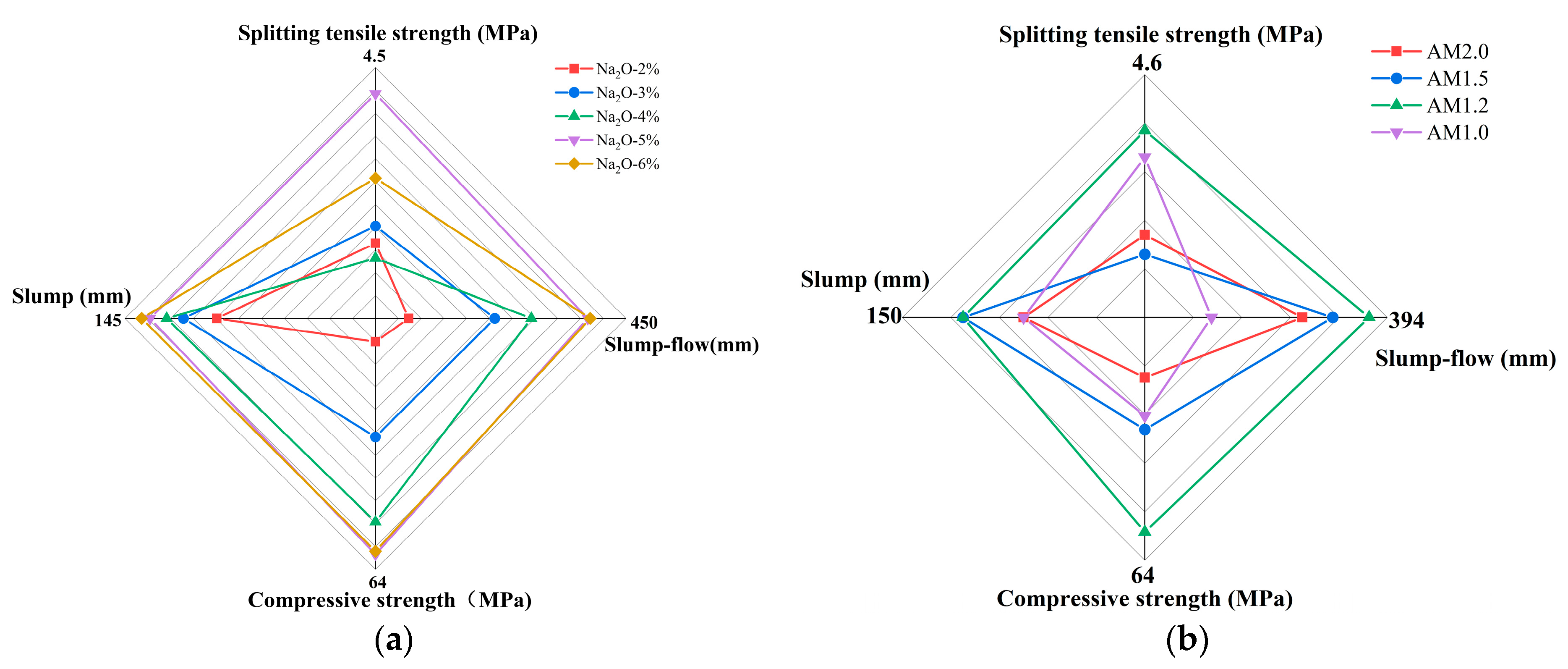
3.5. Four-Dimensional Evaluation
4. Influence Mechanism of Alkali Activator Characteristic Parameters on Characteristic Products and Microstructure
4.1. Characteristic Hydration Products
4.2. Pore Structure
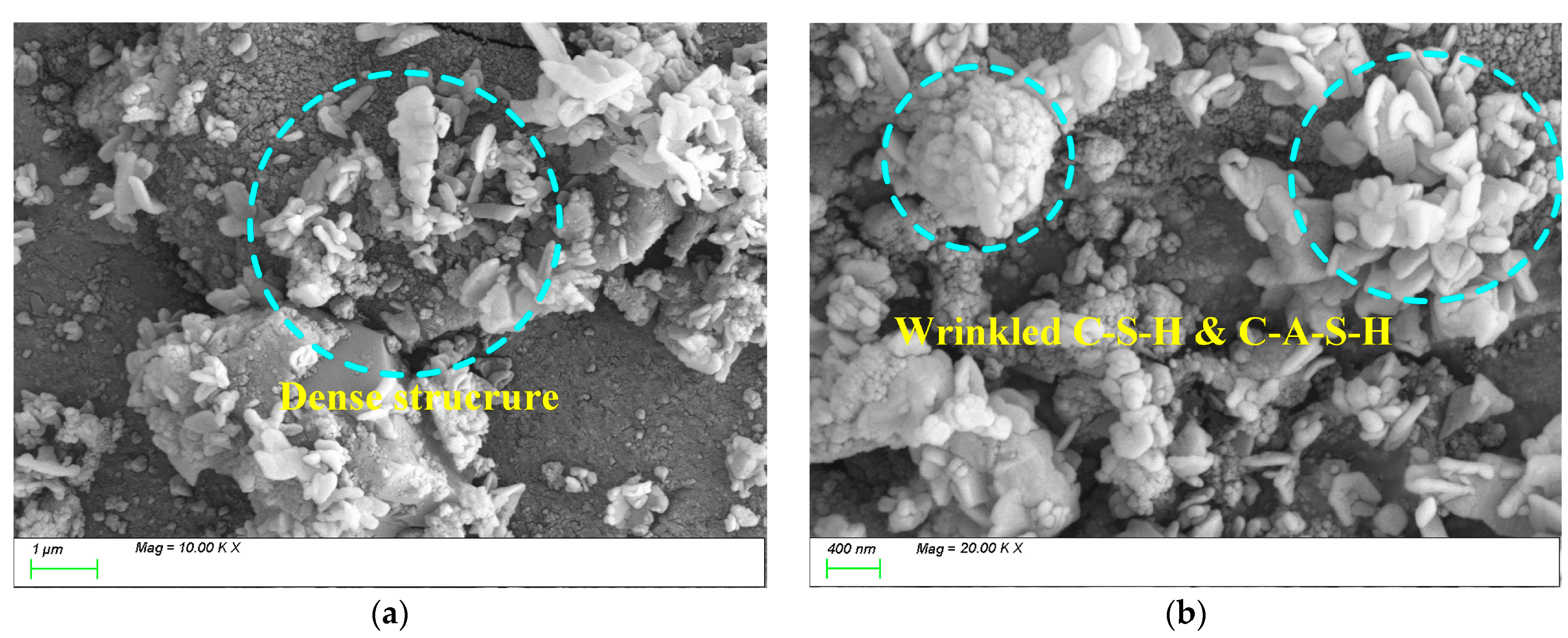
4.3. Matrix Microstructure
5. Conclusions
- (1)
- Both the alkali equivalent and the activator modulus influence the alkali-activation reaction process, leading to a faster setting time for paste. Alkali modulus accelerates the setting time up to 1.2, after which it stabilizes. An increase in alkali equivalent shows a faster increase in setting time compared to the alkali modulus. When the alkali equivalent increases to more than 5%, the initial and final setting times significantly decrease, dropping from 101 and 110 min to 13 and 16 min, respectively. Further increases in the alkali equivalent result in minimal changes to the setting times.
- (2)
- The workability of mixtures with different moduli is generally good. In comparison, the mixture with AM1.2 exhibits the best flowability. Compared to the activator modulus, the alkali equivalent has a more significant impact on the workability of the mixture. Increasing the alkali equivalent can effectively enhance the flowability of the mixture.
- (3)
- Increasing the alkali equivalent and reducing the activator modulus can improve the hydration heat release rate and total hydration heat during the initial reaction period, shorten the induction period, and accelerate the entry into the acceleration period. After 72 h of cumulative heat release, the hydration heat of 3%~6% Na2O pastes increased by 16.83%, 100%, 203.22%, and 213.86%, respectively, compared to 2% Na2O. The hydration heat of AM1.0~1.5 increased by 20.74%, 18.65%, and 11.40%, respectively, compared to AM2.0.
- (4)
- An appropriate alkali content and activator modulus can significantly accelerate the “dissolution–depolymerization–condensation” reaction process in the matrix, promoting rapid early strength development and stable long-term strength growth. An excessively high or low alkali content and activator modulus can lead to insufficient growth and uneven distribution of characteristic products, hindering the refinement of the matrix pore structure and resulting in weak strength development or even strength regression in later stages. Therefore, synergistic optimization and systematic control of the alkali equivalent and activator modulus are necessary.
- (5)
- When the alkali equivalent and activator modulus are 5% and 1.2, respectively, the matrix exhibits excellent flowability, balanced and sustained hydration heat release, high early strength, and stable long-term strength growth. The 28 day compressive and splitting tensile strengths of the specimens can reach 61.68 MPa and 4.37 MPa, respectively.
- (6)
- The determination of optimal alkali-activation parameters ensures steady progression of the “depolymerization–polycondensation” reaction and enables sustained strength development while preventing either insufficient reaction due to inadequate alkali activation or excessive pore coarsening and strength regression caused by over-activation. Although the current research focuses on alkali-activated slag Yellow River sediment composites due to experimental constraints, the parameter optimization methodology can be extended to the activation of other aluminosilicate solid wastes (such as red mud, metallurgical slag, steel slag, and tailings), providing a novel solution to the accumulation of aluminum industrial waste and facilitating the high-value utilization of solid waste resources.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| YRS | Yellow River sediment | nm | Nanometer |
| C-S-H | Calcium silicate hydrate | P | Pressure |
| C-A-S-H | Calcium aluminum silicate hydrate | XRD | X-ray diffraction |
| AM | Activator modulus | m | Meter |
| MIP | Mercury intrusion porosimetry | SiO2 | Silicon dioxide |
| AAM | Alkali-activated material | g | Gram |
| DTA | Differential thermal analysis | g.cm−3 | Grams per cubic centimeter |
| EDS | Energy dispersive spectroscopy | Sulfate ion | |
| OPC | Ordinary Portland cement | N | Newton |
| AA | Alkali activator | SiO2 | Silicon dioxide |
| AK | Alkali equivalent | SG | Specific gravity |
| mm | Millimeter | SEM | Scanning electron microscope |
| m2 | Square meters | μW | Microwatt |
| m2·kg−1 | Square meters per kilogram | NaOH | Sodium hydroxide |
| min | Minute | Vr | Pore diameter |
| MPa | Megapascal | % | Percentage |
References
- Yang, Z.X.; Zeng, L.Y. Research on the Current Situation of Mining Management of National Sandy and Stony soil Mines. Nat. Resour. Econ. China 2020, 33, 44–50. [Google Scholar]
- Zhang, J.J.; Raza, A.; Fu, W.C.; Yuan, C.F. Research on uniaxial compression performance and constitutive relationship of RBP-UHPC after high temperature. Sci. Eng. Compos. Mater. 2024, 31, 20240011. [Google Scholar]
- Yuan, C.F.; Feng, H.; Raza, A.; Shen, L.; Sun, Y.; Zhang, C.; Wang, B.; Wu, X.; Chen, N.; Sun, G.Z. Ultra-High-Performance Concrete with Waste Brick Powder and Preparation Method and Application Thereof. U.S. Patent No. 11,905,213, 20 February 2024. [Google Scholar]
- Wei, J.X.; Kuang, C. Empirical assessing cement CO2 emissions based on China’s economic and social development during 2001–2030. Sci. Total Environ. 2019, 653, 200–211. [Google Scholar] [CrossRef]
- Provis, J.L.; Palomo, A.; Shi, C. Advances in understanding alkali-Activated materials. Cem. Concr. Res. 2015, 78, 110–125. [Google Scholar] [CrossRef]
- Provis, J.L.; Bernal, S.A. Geopolymers and related alkali-activated materials. Annu. Rev. Mater. Res. 2014, 44, 299–327. [Google Scholar] [CrossRef]
- Mehta, A.; Siddique, R.; Ozbakkaloglu, T.; Shaikh, F.U.A.; Belarbi, R. Fly ash and ground granulated blast furnace slag-based alkali-activated concrete: Mechanical, transport and microstructural properties. Constr. Build. Mater. 2020, 257, 119548. [Google Scholar] [CrossRef]
- Mounika, G.; Ramesh, B.; Rama, J.S.K. Experimental investigation on physical and mechanical properties of alkali activated concrete using industrial and agro waste. Mater. Today Proc. 2020, 33, 4372–4376. [Google Scholar] [CrossRef]
- Sankar, K.; Stynoski, P.; Al-Chaar, G.K.; Kriven, W.M. Sodium silicate activated slag fly ash binders: Part I-Processing, microstructure, and mechanical properties. J. Am. Ceram. Soc. 2018, 101, 2228–2244. [Google Scholar] [CrossRef]
- Wang, Z.K.; Wang, K.; Zhao, J.; Wang, S.B.; Shumuye, E.D.; Yang, Z.H. The mechanical properties of GFRP bars embedded in geopolymer concrete after high temperature exposure. J. Build. Eng. 2022, 62, 105355. [Google Scholar] [CrossRef]
- Liu, T.L.; Gong, C.; Duan, L.C.; Qu, B. Effects of sodium citrate on compressive strength and microstructure of NaOH-activated fly ash/slag cement exposed to high temperature. Constr. Build. Mater. 2023, 363, 129852. [Google Scholar] [CrossRef]
- Shumuye, E.D.; Zhao, J.; Wang, Z.K. Effect of the curing condition and high temperature exposure on ground-granulated blast-furnace slag cement concrete. Int. J. Concr. Struct. Mater. 2021, 15, 15. [Google Scholar]
- Zabihi, S.M.; Tavakoli, H.R. Evaluation of monomer ratio on performance of GGBFS-RHA alkali-activated concretes. Concr. Struct. Mater. 2019, 208, 326–332. [Google Scholar]
- Ban, C.C.; Ee, T.L.; Ramli, M.; Akil, H.B.M.; Mo, K.H. Properties and microstructure of lime kiln dust activated slag-fly ash mortar. Concr. Struct. Mater. 2022, 347, 128518. [Google Scholar]
- Gopalakrishnan, R.; Chinnaraju, K. Durability of ambient cured alumina silicate concrete based on slag/fly ash blends against sulfate environment. Constr. Build. Mater. 2019, 204, 70–83. [Google Scholar]
- Ahmad, M.R.; Chen, B.; Shah, S.F.A. Influence of different admixtures on the mechanical and durability properties of one-part alkali-activated mortars. Constr. Build. Mater. 2020, 265, 120320. [Google Scholar]
- Xia, D.T.; Chen, R.L.; Cheng, J.J.; Tang, Y.J.; Xiao, C.Q.; Li, Z.X. Desert sand-high calcium fly ash-based alkali-activated mortar: Flowability, mechanical properties, and microscopic analysis. Constr. Build. Mater. 2023, 398, 131729. [Google Scholar] [CrossRef]
- Wang, B.M.; Li, G.N.; Han, J.N.; Zheng, Y.; Liu, H.; Song, W.Z. Study on the properties of artificial flood-prevention stone made by Yellow River silt. Constr. Build. Mater. 2017, 144, 484–492. [Google Scholar]
- He, H.T.; Yue, Q.Y.; Su, Y.; Gao, B.Y.; Gao, Y.; Wang, J.Z.; Yu, H. Preparation and mechanism of the sintered bricks produced from Yellow River silt and red mud. J. Hazard. Mater. 2012, 203, 53–61. [Google Scholar] [CrossRef]
- Raza, A.; Zhang, J.J.; Xu, S.W.; Umar, M.; Yuan, C.F. Experimental analysis of frost resistance and failure models in engineered cementitious composites with the integration of Yellow River sand. Sci. Eng. Compos. Mater. 2024, 31, 20240017. [Google Scholar]
- Jiang, S.; Xu, J.X.; Song, Y.B.; Xu, Y. Alkali-activated fly ash foam concrete with Yellow River silt: Physico-mechanical and structural properties. Constr. Build. Mater. 2023, 373, 130879. [Google Scholar]
- Jin, W.Z.; Chen, Y.M.; Lv, Y.J.; Bai, W.B.; Zhang, K.J.; Song, C.H.; Zhang, X.L. Mechanical Properties and Mechanism of Geopolymer Cementitious Materials Synergistically Prepared Using Red Mud and Yellow River Sand. Materials 2024, 17, 3810. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.F.; Raza, A.; Manan, A.; Ahmad, S.; Chao, W.; Umar, M. Numerical and experimental study of Yellow River sand in engineered cementitious composite. Proc. Inst. Civ. Eng.-Eng. Sustain. 2025, 178, 3–20. [Google Scholar]
- Li, G.N.; Wang, B.M.; Liu, H.; Song, W.Z.; Han, J.N. Mechanical property and microstructure of alkali-activated yellow river sediment-coal slime ash composites. J. Wuhan Univ. Technol. Mater. Sci. 2017, 32, 1080–1086. [Google Scholar]
- Li, G.N.; Wang, B.M.; Liu, H. Properties of Alkali-activated Yellow River Sediment-slag Composite Material. J. Wuhan Univ. Technol. Mater. Sci. 2019, 34, 114–121. [Google Scholar]
- Jing, X.Y.; Li, G.N.; Zhang, Y.; Han, J.N.; Wang, B.M. Experimental Research on the Modification of the Yellow River Sediment. Iran. J. Sci. Technol. Trans. Civ. Eng. 2021, 45, 1031–1037. [Google Scholar]
- Li, C.M.; Zhang, T.T.; Wang, L.J. Mechanical properties and microstructure of alkali activated Pisha sandstone geopolymer composites. Constr. Build. Mater. 2014, 68, 233–239. [Google Scholar]
- Bondar, D.; Lynsdale, C.J.; Milestone, N.B.; Hassani, N.; Ramezanianpour, A.A. Effect of type, form, and dosage of activators on strength of alkali-activated natural pozzolans. Cem. Concr. Compos. 2011, 33, 251–260. [Google Scholar]
- Aydin, S.; Baradan, B. Effect of activator type and content on properties of alkali-activated slag mortars. Compos. Part B Eng. 2014, 57, 166–172. [Google Scholar]
- Chi, M.; Huang, R. Binding mechanism and properties of alkali-activated fly ash/slag mortars. Constr. Build. Mater. 2013, 40, 291–298. [Google Scholar]
- Yuan, C.F.; Xu, S.W.; Raza, A.; Wang, C.; Wang, D. Influence and Mechanism of Curing Methods on Mechanical Properties of Manufactured Sand UHPC. Materials 2022, 15, 6183. [Google Scholar] [CrossRef]
- Raza, A.; Memon, B.A.; Oad, M. Effect of Curing Types on Compressive Strength of Recycled Aggregates Concrete. Quaid-E-Awam Univ. Res. J. Eng. 2019, 17, 7–12. [Google Scholar]
- GB/T 35159; Flash Setting Admixtures for Shotcrete. The Standardization Administration of the People’s Republic of China: Beijing, China, 2017. (In Chinese)
- Memon, B.A.; Oad, M.; Buller, A.H.; Raza, A. Effect of Curing Methods on Tensile Strength of Green Concrete Cylinders Made with Demolishing Coarse Aggregates. World J. Eng. Res. Technol. 2020, 6, 66–75. [Google Scholar]
- GB/T50081-2019; Standard for Test Methods of Concrete Physical and Mechanical Properties. China Architecture & Building Press: Beijing, China, 2019. (In Chinese)
- Zhang, Y.K.; Raza, A.; Umar, M.; Chen, Y.; Yuan, C.F. Study on Frost Resistance and Interface Bonding Performance through the Integration of Recycled Brick Powder in Ultra-High-Performance Concrete for Structural Reinforcement. Materials 2023, 16, 6999. [Google Scholar] [CrossRef]
- Razzak, A.; Memon, B.A.; Oad, M.; Raza, A. Effects of height to diameter ratio on compressive strength of recycled aggregate concrete. Quest Res. J. 2020, 18, 60–65. [Google Scholar]
- Yuan, C.F.; Zhang, J.J.; Raza, A.; Fu, W.C. Residual and damage properties of recycled brick powder-UHPFRC after high temperature. Proc. Inst. Civ. Eng-Constr. Mater. 2025, 178, 3–19. [Google Scholar]
- Wang, A.G.; Wang, X.Y.; Sun, D.S.; Zhu, Y.C.; Liu, K.W.; Jing, Y.; Guan, Y.M. Research Progress on Setting and Hardening of Geopolymers and Their Control. Mater. Rep. 2021, 35, 13001–13010. [Google Scholar]
- Nath, P.; Sarker, P.K. Effect of GGBFS on setting, workability and early strength properties of fly ash geopolymer concrete cured in ambient condition. Constr. Build. Mater. 2014, 66, 163. [Google Scholar]
- He, J.; Yang, C.H. Hydration Heat Evolution and Setting Performance of Alkali-slag Cement Activated with Water Glass. J. Civ. Environ. Eng. 2011, 33, 147–152. [Google Scholar]
- Chen, Y.L.; Zhang, Y.K.; Chen, T.J.; Chen, L.; Li, H.M.; Yang, Z.H.; Wang, Q. Preparation and Mechanism of Foaming Geopolymer with Alkali Activated High Calcium Fly Ash. Bull. Chin. Ceram. Soc. 2023, 42, 2787–2798. [Google Scholar]
- Su, L.J.; Fu, G.S.; Liang, B.; Sun, Q.; Zhang, X.D. Mechanical properties and microstructure evaluation of fly ash-Slag geopolymer foaming materials. Ceram. Int. 2022, 48, 18224–18237. [Google Scholar]
- Shekhovtsova, J.; Kearsley, E.P.; Kovtun, M. Effect of activator dosage, water-to-binder-solids ratio, temperature and duration of elevated temperature curing on the compressive strength of alkali-activated fly ash cement pastes. J. S. Afr. Inst. Civ. 2014, 56, 44–52. [Google Scholar]
- Heah, C.Y.; Kamarudin, H.; Al Bakri, A.M.; Bnhussain, M.; Nizar, I.K.; Ruazaidi, C.M.; Liew, Y.M. Kaolin-based geopolymers with various NaOH concentrations. Int. J. Miner. Metall. Mater. 2013, 20, 313–322. [Google Scholar]
- Zheng, W.Z.; Zou, M.G.; Wang, Y. Literature review of alkali-activated cementitious materials. J. Build. Struct. 2019, 40, 28–39. [Google Scholar]
- Zhang, D.W.; Wang, A.H. Review on Property of Geopolymer Binder and Its Engineering Application. J. Archit. Civ. Eng. 2020, 37, 13–38. [Google Scholar]
- Chen, X.; Wang, J.; Zhu, G.R.; Chen, Q.A. Review of the Main Control Factors on Mechanical Properties of Geopolymers. Bull. Chin. Ceram. Soc. 2017, 36, 2994–3002. [Google Scholar]
- Ghafoori, N.; Najimi, M.; Radke, B. Natural pozzolan-based geopolymers for sustainable construction. Environ. Earth Sci. 2016, 75, 1110. [Google Scholar]
- Görhan, G.; Kürklü, G. The influence of the NaOH solution on the properties of the fly ash-based geopolymer mortar cured at different temperatures. Compos. Part B Eng. 2014, 58, 371–377. [Google Scholar]
- Tao, X.; Xie, Z.L.; Hao, S.W.; Zhou, H.F. Acoustic emission behavior of fiber reinforced fly ash geopolymer under uniaxial compression. Acta Mater. Compos. Sin. 2014, 31, 1467–1475. [Google Scholar]
- Huang, B.T.; Wu, J.; Yu, J.; Dai, J.G.; Leung, C.K.; Li, V.C. Seawater sea-sand engineered/strain-hardening cementitious composites (ECC/SHCC): Assessment and modeling of crack characteristics. Cem. Concr. Res. 2021, 140, 106292. [Google Scholar]
- Ibrahim, M.; Johari, M.A.M.; Rahman, M.K.; Maslehuddin, M.K. Effect of alkaline activators and binder content on the properties of natural pozzolan-based alkali activated concrete. Constr. Build. Mater. 2017, 147, 648–660. [Google Scholar]
- Song, P.P.; Liu, Y.Z.; Kong, L.J.; Tang, Z.Y.; Sun, G.W. Research on design and optimization for compositions of ultra-high-performance geopolymer concrete. J. Build. Eng. 2025, 100, 111750. [Google Scholar]
- Yu, S.W.; Xia, M.; Sanjayan, J.; Yang, L.; Xiao, J.Z.; Du, H.J. Microstructural characterization of 3D printed concrete. J. Build. Eng. 2021, 44, 102948. [Google Scholar]
- Huang, H.F.; An, M.Z.; Wang, Y.; Yu, Z.R.; Ji, W.Y. Effect of environmental thermal fatigue on concrete performance based on mesostructural and microstructural analyses. Constr. Build. Mater. 2019, 207, 450–462. [Google Scholar]
- An, M.Z.; Huang, H.F.; Wang, Y.; Zhao, G.Y. Effect of thermal cycling on the properties of high-performance concrete: Microstructure and mechanism. Constr. Build. Mater. 2020, 243, 118310. [Google Scholar] [CrossRef]
- Shi, C.J.; Roy, D.; Krivenko, P. Alkali-Activated Cements and Concretes; CRC Press: London, UK, 2005. [Google Scholar]
- Cheng, T.W.; Chiu, J.P. Fire-resistant geopolymer produced by granulated blast furnace slag. Miner. Eng. 2003, 16, 205–210. [Google Scholar]
- Chindaprasirt, P.; Jaturapitakkul, C.; Chalee, W.; Rattanasak, U. Comparative study on the characteristics of fly ash and bottom ash geopolymers. Waste Manag. 2009, 29, 539–543. [Google Scholar] [PubMed]
- Ravikumar, D.; Neithalath, N. Effects of activator characteristics on the reaction product formation in slag binders activated using alkali silicate powder and NaOH. Cem. Concr. Compos. 2012, 34, 809–818. [Google Scholar] [CrossRef]
- Sakulich, A.R.; Miller, S.; Barsoum, M.W. Chemical and Microstructural Characterization of 20-Month-Old Alkali-Activated Slag Cements. J. Am. Ceram. Soc. 2010, 93, 1741–1748. [Google Scholar] [CrossRef]






| Minerals | SiO2 | CaO | Al2O3 | Fe2O3 | K2O | TiO2 | MgO | Other |
|---|---|---|---|---|---|---|---|---|
| YRS | 68.64 | 8.40 | 12.33 | 3.25 | 2.55 | 0.74 | 2.05 | 2.04 |
| GGBFS | 32.47 | 41.06 | 14.52 | 0.28 | 0.44 | 1.25 | 7.08 | 2.9 |
| SiO2/(%) | Na2O/(%) | H2O/(%) | Density/(g/cm3) | Modulus | Beaume |
|---|---|---|---|---|---|
| 30 | 13.5 | 56.5 | 1.51 | 2.3 | 50 |
| No. | Sand | NaOH | SS | GGBFS | Water |
| Na2O-2% | 1.000 | 0.008 | 0.051 | 0.660 | 0.235 |
| Na2O-3% | 1.000 | 0.012 | 0.077 | 0.660 | 0.221 |
| Na2O-4% | 1.000 | 0.016 | 0.102 | 0.660 | 0.206 |
| Na2O-5% | 1.000 | 0.020 | 0.128 | 0.660 | 0.192 |
| Na2O-6% | 1.000 | 0.024 | 0.153 | 0.660 | 0.177 |
| AM-2.0 | 1.000 | 0.006 | 0.208 | 0.646 | 0.140 |
| AM-1.5 | 1.000 | 0.015 | 0.158 | 0.655 | 0.173 |
| AM-1.2 | 1.000 | 0.020 | 0.128 | 0.660 | 0.192 |
| AM-1.0 | 1.000 | 0.024 | 0.107 | 0.665 | 0.205 |
| Properties | Performance Index | Specimen Size | Quantity |
|---|---|---|---|
| Setting time | initial setting time | — | — |
| final setting time | — | — | |
| Workability | slump | — | — |
| slump–flow | — | — | |
| Hydration heat | heat evolution rate | — | — |
| accumulated hydration heat | — | — | |
| Strength | compressive strength | 100 mm × 100 mm × 100 mm | 96 |
| splitting tensile strength | 100 mm × 100 mm × 100 mm | 96 | |
| Characteristic products | thermogravimetric analysis | 40 mm × 40 mm × 40 mm | 24 |
| X-ray diffraction analysis | 40 mm × 40 mm × 40 mm | 24 | |
| Microstructural properties | porosity | 40 mm × 40 mm × 40 mm | 24 |
| scanning electron microscopy | 40 mm × 40 mm × 40 mm | 24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.; Jiang, E.; Li, K.; Shi, H.; Chen, C.; Yuan, C. Study on the Alkali-Activated Mechanism of Yellow River Sediment-Based Ecological Cementitious Materials. Materials 2025, 18, 1559. https://doi.org/10.3390/ma18071559
Zhang G, Jiang E, Li K, Shi H, Chen C, Yuan C. Study on the Alkali-Activated Mechanism of Yellow River Sediment-Based Ecological Cementitious Materials. Materials. 2025; 18(7):1559. https://doi.org/10.3390/ma18071559
Chicago/Turabian StyleZhang, Ge, Enhui Jiang, Kunpeng Li, Huawei Shi, Chen Chen, and Chengfang Yuan. 2025. "Study on the Alkali-Activated Mechanism of Yellow River Sediment-Based Ecological Cementitious Materials" Materials 18, no. 7: 1559. https://doi.org/10.3390/ma18071559
APA StyleZhang, G., Jiang, E., Li, K., Shi, H., Chen, C., & Yuan, C. (2025). Study on the Alkali-Activated Mechanism of Yellow River Sediment-Based Ecological Cementitious Materials. Materials, 18(7), 1559. https://doi.org/10.3390/ma18071559





