A Study on the Corrosion Behavior of Fe/Ni-Based Structural Materials in Unpurified Molten Chloride Salt
Abstract
1. Introduction
2. Materials and Methods
2.1. Structural Alloy
2.2. Preparation of Eutectic Salts and Structural Alloy Corrosion Test
2.3. Surface and Cross-Section Treatment for Characterization
3. Results
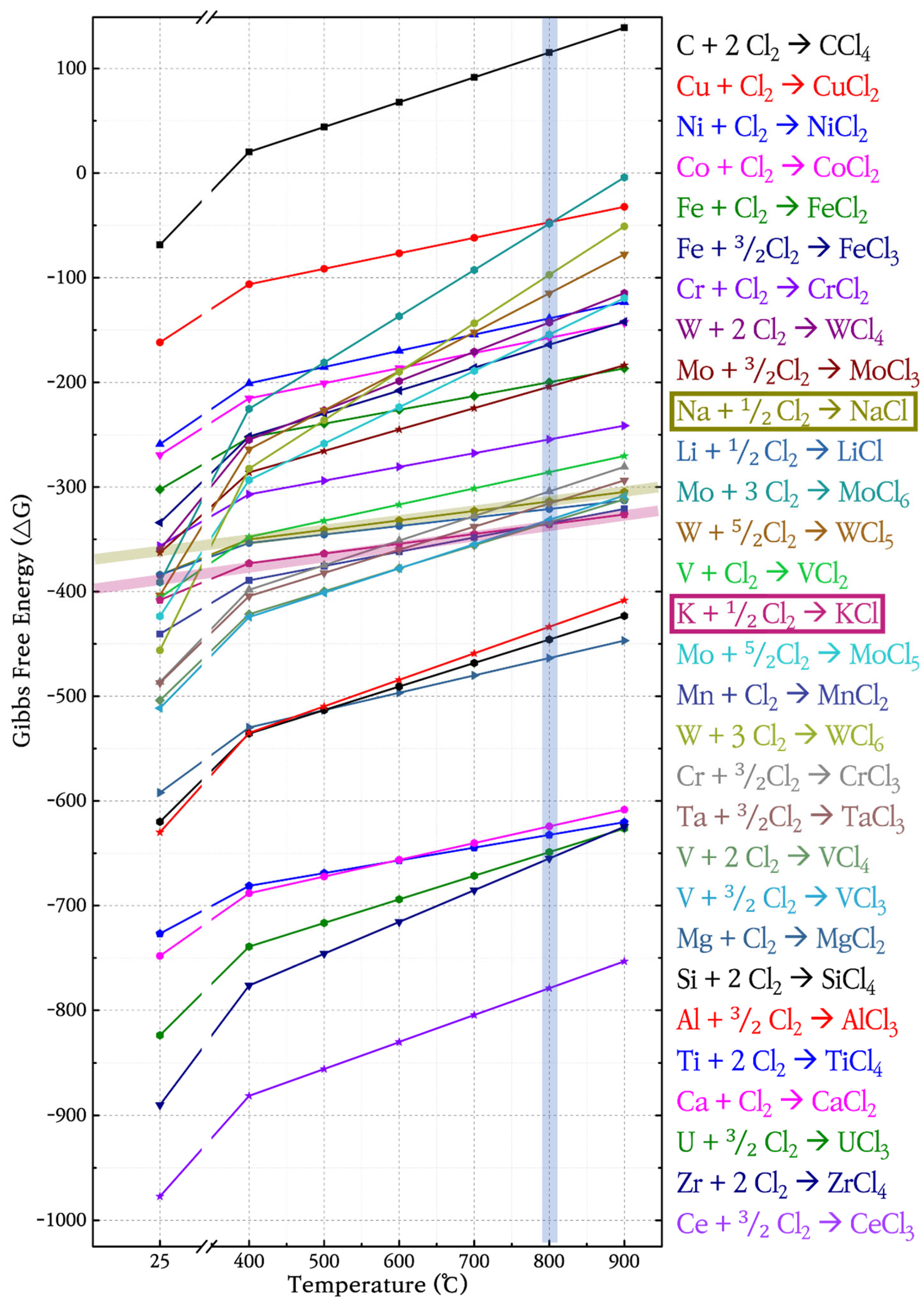
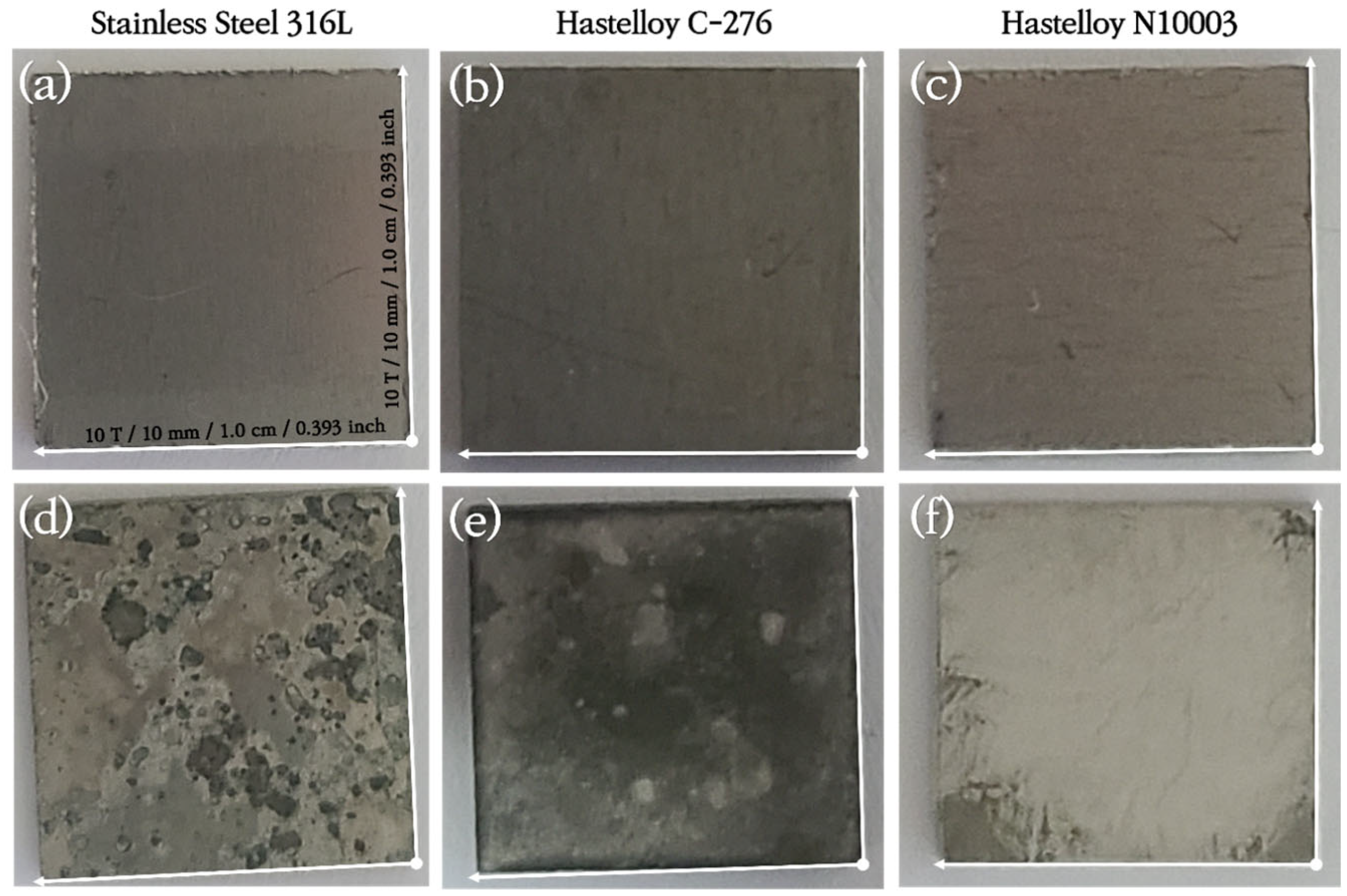
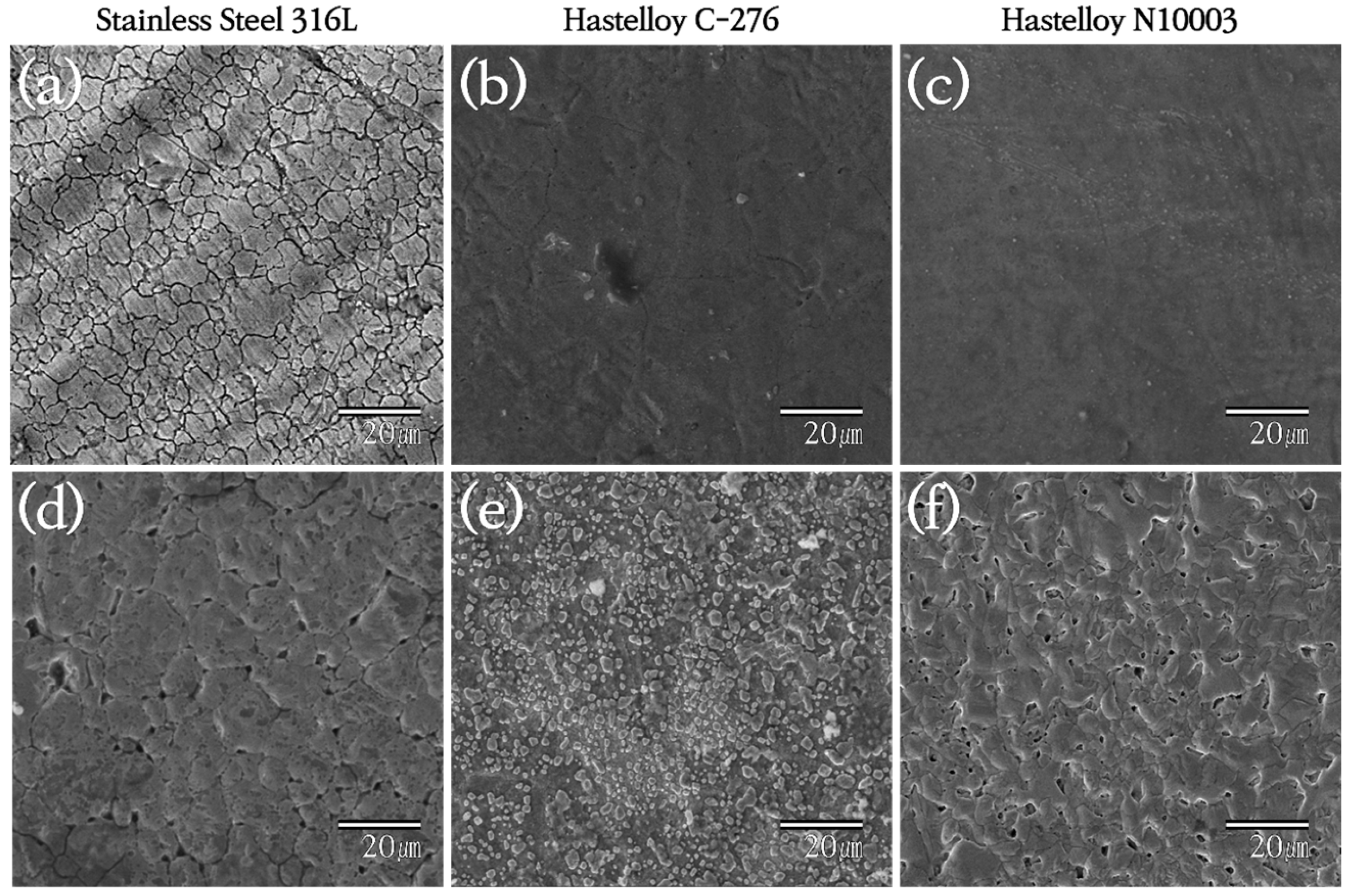
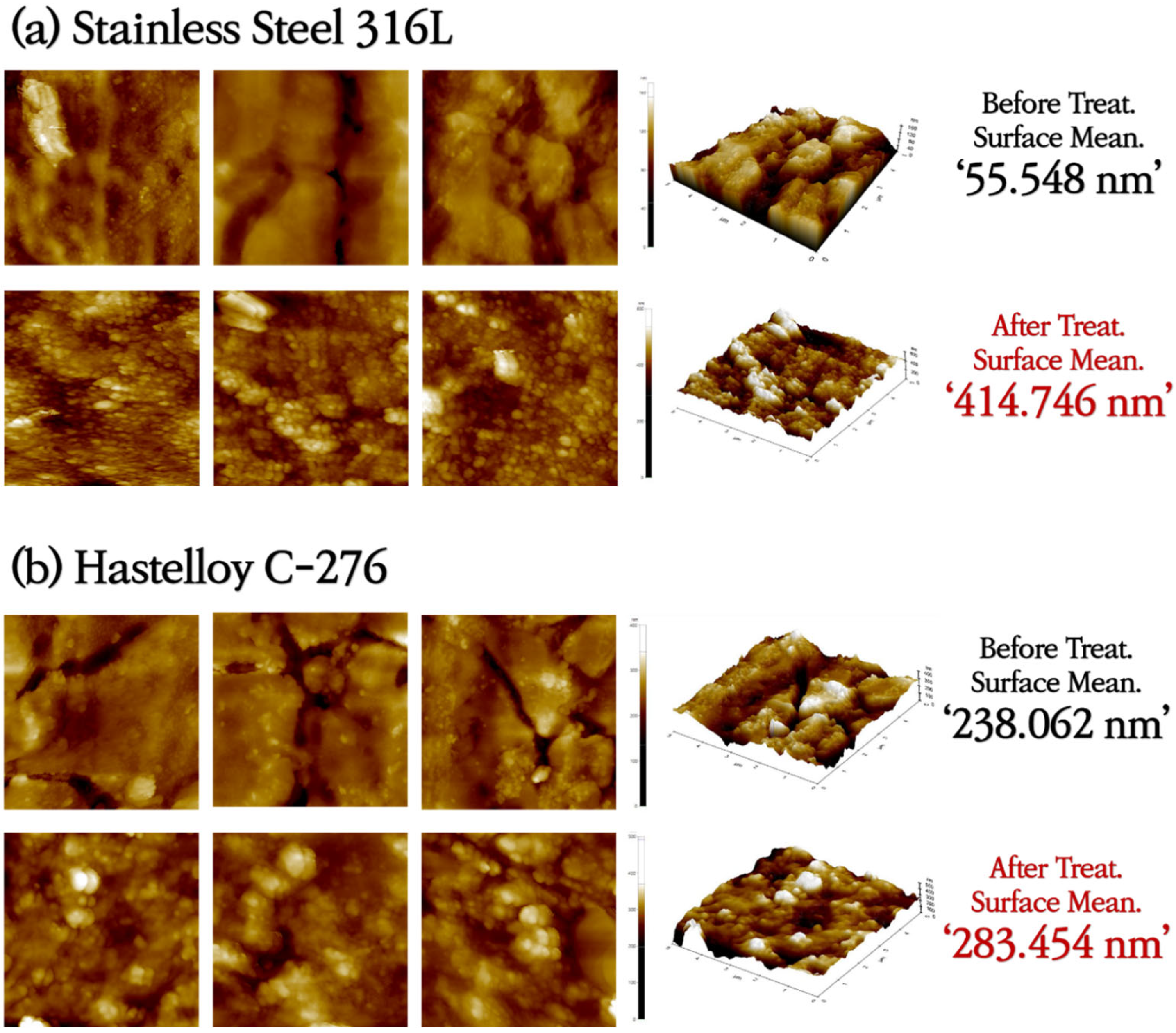
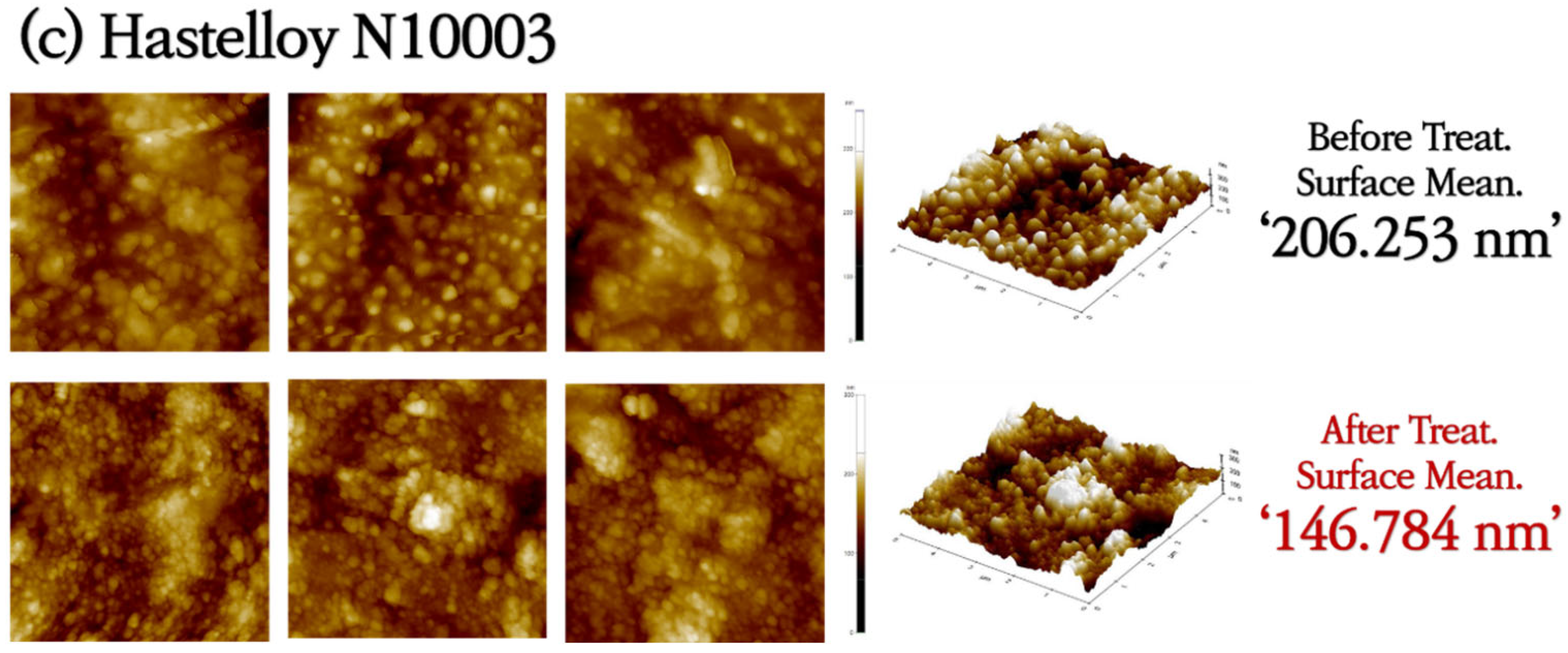
3.1. Analysis of Surface Morphologies and Corrosion Behavior
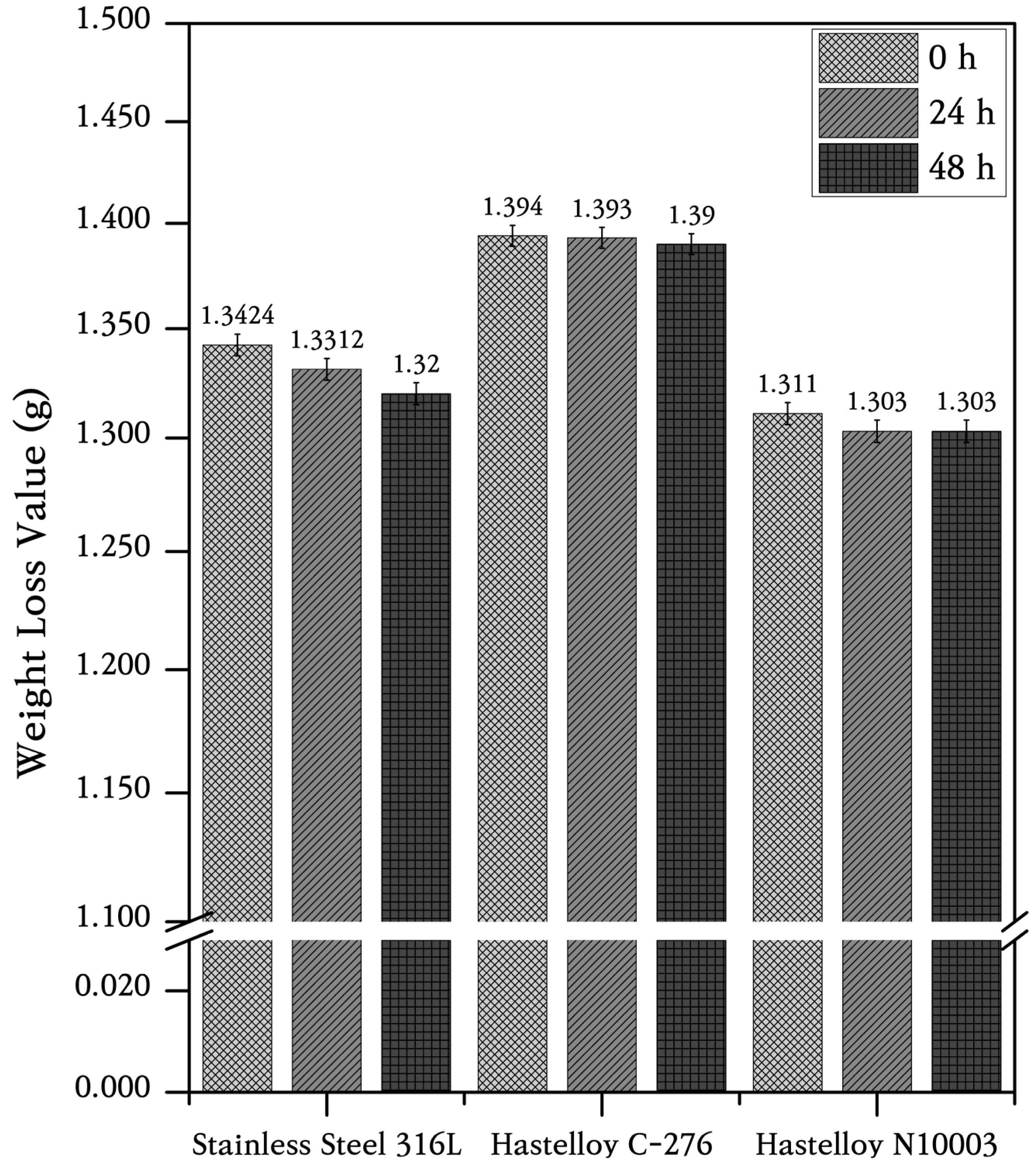
3.2. Corrosion Rate of Structural Materials in ClNaK Salt
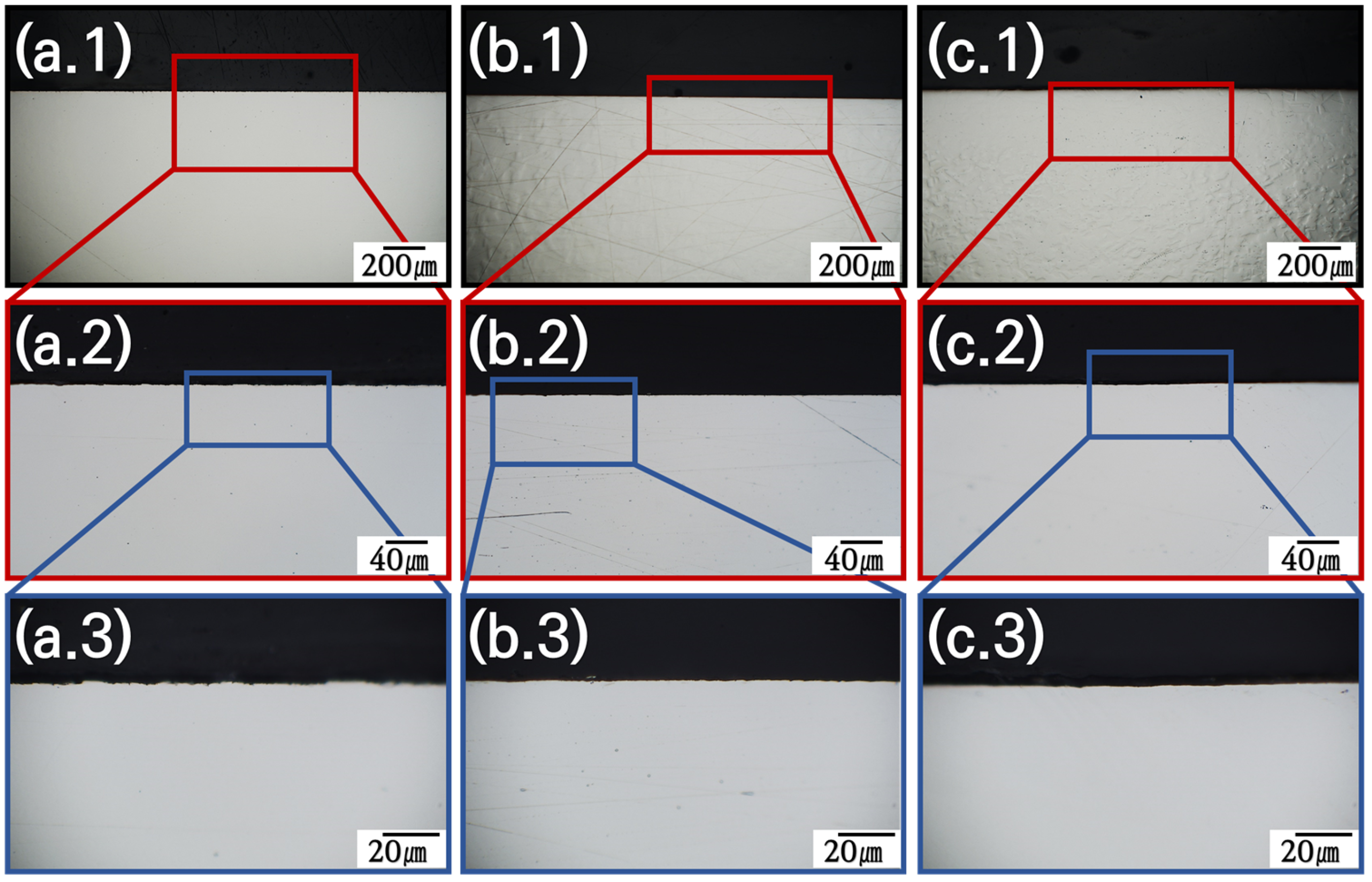
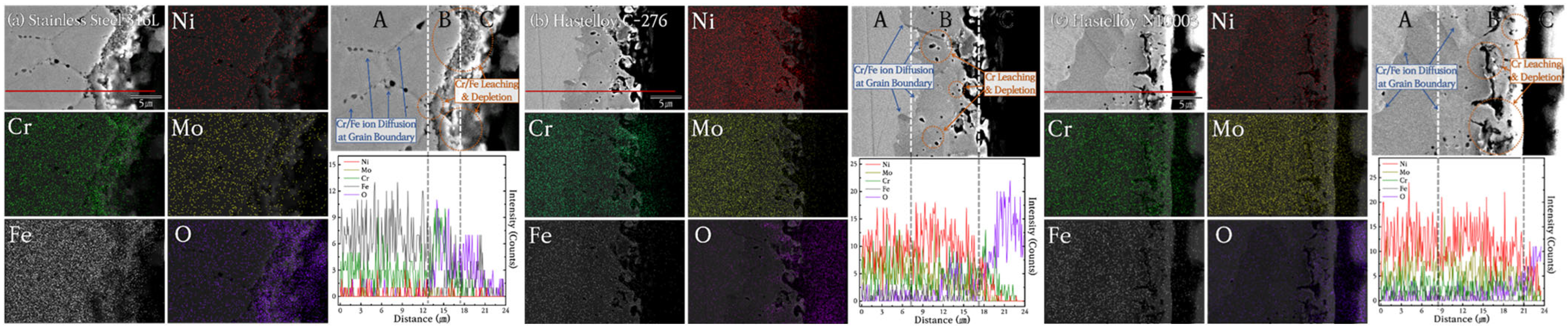
3.3. Cross-Section Invasion of Cl-Based Salt
3.4. Selection of Structural Materials for Cl-Based MSRs
4. Discussion
4.1. Fe-Balance vs. Ni-Balance Alloys
4.2. Cr Structural Materials 16.0 wt% Cr vs. 7.0 wt%
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lenzen, M. Life cycle energy and greenhouse gas emissions of nuclear energy: A review. Energy Convers. Manag. 2008, 49, 2178–2199. [Google Scholar] [CrossRef]
- Buongiorno, J.; Corradini, M.; Parsons, J.; Petti, D. Nuclear energy in a carbon-constrained world: Big challenges and big opportunities. IEEE Power Energy Mag. 2019, 17, 69–77. [Google Scholar] [CrossRef]
- Jo, H.J.; Yeom, H.; Gutierrez, E.; Sridharan, K.; Corradini, M. Evaluation of critical heat flux of ATF candidate coating materials in pool boiling. Nucl. Eng. Des. 2019, 354, 110166. [Google Scholar] [CrossRef]
- Kim, H.G.; Kim, I.H.; Jung, Y.I.; Park, D.J.; Park, J.H.; Yang, J.H.; Koo, Y.H. Progress of surface modified Zr cladding development for ATF at KAERI. In Proceedings of the 2017 Water Reactor Fuel Performance Meeting, Jeju Island, Republic of Korea, 10–14 September 2017; pp. 10–14. [Google Scholar]
- Kim, H.G.; Kim, I.H.; Park, J.Y.; Koo, Y.H. Application of coating technology on zirconium-based alloy to decrease high-temperature oxidation. In Zirconium in the Nuclear Industry: 17th International Symposium; STP 1543; ASTM International: West Conshohocken, PA, USA, 2015; pp. 346–369. [Google Scholar] [CrossRef]
- Kim, H.G.; Yang, J.H.; Kim, W.J.; Koo, Y.H. Development Status of Accident-Tolerant Fuel for Light Water Reactors in Korea. Nucl. Eng. Technol. 2016, 48, 1–15. [Google Scholar] [CrossRef]
- Kim, D.Y.; Lee, Y.N.; Kim, J.H.; Kim, Y.; Yoon, Y.S. Applicability of swaging as an alternative for the fabrication of accident-tolerant fuel cladding. Energies 2020, 13, 3182. [Google Scholar] [CrossRef]
- Kim, J.-W.; Min, H.-W.; Ko, J.; Kim, Y.; Yoon, Y.S. Study of structural stability at high temperature of pseudo-single tube with double layer as an alternative method for accident-tolerant fuel cladding. J. Nucl. Mater. 2022, 566, 153800. [Google Scholar] [CrossRef]
- Xia, S.Q.; Wang, Z.; Yang, T.F.; Zhang, Y. Irradiation Behavior in High Entropy Alloys. J. Iron Steel Res. Int. 2015, 22, 879–884. [Google Scholar] [CrossRef]
- Kim, M.S.; Lee, S.H.; Jung, J.G.; Eah, K. Prediction of grain structure in direct-chill cast Al–Zn–Mg–Cu billets using cellular automaton-finite element method. Prog. Nat. Sci. Mater. Int. 2021, 31, 434–441. [Google Scholar] [CrossRef]
- Tan, L.; Allen, T.R.; Busby, J.T. Grain boundary engineering for structure materials of nuclear reactors. J. Nucl. Mater. 2013, 441, 661–666. [Google Scholar] [CrossRef]
- Lee, H.G.; Kim, D.; Kim, W.J.; Park, J.Y. Reaction–diffusion bonding of CVD SiC using CrAl thin coating layer. J. Korean Ceram. Soc. 2022, 59, 113–123. [Google Scholar] [CrossRef]
- Romatoski, R.R.; Hu, L.W. Fluoride salt coolant properties for nuclear reactor applications: A review. Ann. Nucl. Energy 2017, 109, 635–647. [Google Scholar] [CrossRef]
- Rosenthal, M. An Account of Oak Ridge National Laboratory’s Thirteen Nuclear Reactors. 2010. Available online: http://info.ornl.gov/sites/publications/files/Pub20808.pdf (accessed on 23 March 2020).
- McFarlane, J.; Taylor, P.; Holcomb, D.; Poore, W.P. Review of Hazards Associated with Molten Salt Reactor Fuel Processing Operations. 2019. Available online: www.osti.gov (accessed on 14 June 2020).
- Galashev, A.Y. Molecular dynamics study of ionic diffusion and the FLiNaK salt melt structure. Nucl. Eng. Technol. 2022, 55, 1324–1331. [Google Scholar] [CrossRef]
- Karfidov, E.; Nikitina, E.; Erzhenkov, M.; Seliverstov, K.; Chernenky, P.; Mullabaev, A.; Tsvetov, V.; Mushnikov, P.; Karimov, K.; Molchanova, N.; et al. Corrosion Behavior of Candidate Functional Materials for Molten Salts Reactors in LiF–NaF–KF Containing Actinide Fluoride Imitators. Materials 2022, 15, 761. [Google Scholar] [CrossRef]
- Creasman, S.E.; Pathirana, V.; Chvala, O. Sensitivity study of parameters important to Molten Salt Reactor Safety. Nucl. Eng. Technology 2023, 55, 1687–1707. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, J.; Wu, W.; Zhou, W. Corrosion in the molten fluoride and chloride salts and materials development for nuclear applications. Prog. Mater. Sci. 2018, 97, 448–487. [Google Scholar] [CrossRef]
- Qu, L.; Wang, Q.; Mao, J.; Xu, S.; Zhang, H.; Shi, Z.; Li, X. Study of anti-chlorine corrosion of anion exchange resin based superhydrophobic cement mortar in chloride salt environment. Constr. Build. Mater. 2021, 313, 125540. [Google Scholar] [CrossRef]
- He, Z.; Zhao, H.; Song, J.; Guo, X.; Liu, Z.; Zhong, Y.; Marrow, T.J. Densification of matrix graphite for spherical fuel elements used in molten salt reactor via addition of green pitch coke. Nucl. Eng. Technol. 2022, 54, 1161–1166. [Google Scholar] [CrossRef]
- Sadiq, M.; Wen, F.; Dagestani, A.A. Environmental footprint impacts of nuclear energy consumption: The role of environmental technology and globalization in ten largest ecological footprint countries. Nucl. Eng. Technol. 2022, 54, 3672–3681. [Google Scholar] [CrossRef]
- Capelli, E.; Beneš, O.; Konings, R.J.M. Thermodynamic assessment of the LiF-ThF4-PuF3-UF4 system. J. Nucl. Mater. 2015, 462, 43–53. [Google Scholar] [CrossRef]
- Wang, Y.; Goh, B.; Nelaturu, P.; Duong, T.; Hassan, N.; David, R.; Moorehead, M.; Chaudhuri, S.; Creuziger, A.; Hattrick-Simpers, J.; et al. Accelerated Discovery of Molten Salt Corrosion-Resistant Alloy by High-Throughput Experimental and Modeling Methods Coupled to Data Analytics. arXiv 2021. [Google Scholar] [CrossRef]
- Persdotter, A.; Eklund, J.; Liske, J.; Jonsson, T. Beyond breakaway corrosion—Influence of chromium, nickel and aluminum on corrosion of iron-based alloys at 600 °C. Corros. Sci. 2020, 177, 108961. [Google Scholar] [CrossRef]
- Logan, S.R. The Origin and Status of the Arrhenius Equation. 1982. Available online: https://pubs.acs.org/sharingguidelines (accessed on 23 March 2020).
- Kang, M.J.; Yoon, D.H. Effects of impurities on the slip viscosity and sintered properties of low-soda easy-sintered α-alumina. J. Korean Ceram. Soc. 2022, 59, 595–603. [Google Scholar] [CrossRef]
- Yang, G. Life Cycle Reliability Engineering; Wiley: Hoboken, NJ, USA, 2007. [Google Scholar]
- Sopher, R.; Nixon, J.; Gorecki, C.; Gefen, A. Effects of intramuscular fat infiltration, scarring, and spasticity on the risk for sitting-acquired deep tissue injury in spinal cord injury patients. J. Biomech. Eng. 2011, 133, 021011. [Google Scholar] [CrossRef]
- Peleg, M.; Normand, M.D.; Corradini, M.G. The Arrhenius equation revisited. Crit. Rev. Food Sci. Nutr. 2012, 52, 830–851. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Yue, B.; Yan, L.; Jiang, T.; Peng, S. Unifying the diffusion coefficients of lanthanides and actinides in binary molten salt mixtures: A data review. J. Mol. Liq. 2020, 297, 112106. [Google Scholar] [CrossRef]
- Phan, T.T.T.; Nguyen, T.D.; Lee, J.S. Vacuum plasma treatment on carbon nanoparticles for highly sensitive square wave voltammetric sensor of heavy metal ions. Synth. Met. 2022, 291, 117203. [Google Scholar] [CrossRef]
- Lee, S.R.; Bae, K.M.; Baek, J.J.; Kang, M.C.; Lee, T.I. Adhesion enhancement between aluminum and butyl rubber by (3-mercaptopropyl) trimethoxy silane for vibration damping plate. J. Adhes. Sci. Technol. 2021, 35, 1114–1124. [Google Scholar] [CrossRef]
- Kondo, M.; Nagasaka, T.; Xu, Q.; Muroga, T.; Sagara, A.; Noda, N.; Ninomiya, D.; Nagura, M.; Suzuki, A.; Terai, T.; et al. Corrosion characteristics of reduced activation ferritic steel, JLF-1 (8.92Cr-2W) in molten salts Flibe and Flinak. Fusion Eng. Des. 2009, 84, 1081–1085. [Google Scholar] [CrossRef]
- Raiman, S.S.; Lee, S. Aggregation and data analysis of corrosion studies in molten chloride and fluoride salts. J. Nucl. Mater. 2018, 511, 523–535. [Google Scholar] [CrossRef]
- Lei, Y.B.; Wang, Z.B.; Zhang, B.; Luo, Z.P.; Lu, J.; Lu, K. Enhanced mechanical properties and corrosion resistance of 316L stainless steel by pre-forming a gradient nanostructured surface layer and annealing. Acta Mater. 2021, 208, 116773. [Google Scholar] [CrossRef]
- Li, X.; Yang, J.; Feng, X.; Hu, Y.; Zou, H.; Zhang, C.; Xiong, L.; Zheng, X.; Liu, Y. Electrochemical performance of porous Ni-Cr-Mo-Cu alloys for hydrogen evolution reactions in alkali solution. Mater. Res. Express 2020, 7, 095505. [Google Scholar] [CrossRef]
- Luo, Y.; Jiang, W.; Zhang, Y.; Hao, M.; Tu, S.T. Creep rupture behavior of Hastelloy C276-BNi2 brazed joint. Mater. Sci. Eng. A 2018, 711, 223–232. [Google Scholar] [CrossRef]
- Patel, N.S.; Pavlík, V.; Boča, M. High-Temperature Corrosion Behavior of Superalloys in Molten Salts—A Review. Crit. Rev. Solid State Mater. Sci. 2017, 42, 83–97. [Google Scholar] [CrossRef]
- Zhu, H.; Muránsky, O.; Wei, T.; Davis, J.; Budzakoska-Testone, E.; Huang, H.; Drew, M. The effect of applied stress on the high-temperature creep behaviour and microstructure of NiMoCr Hastelloy-N® alloy. Materialia 2021, 16, 101069. [Google Scholar] [CrossRef]
- Danon, A.E.; Muránsky, O.; Karatchevtseva, I.; Zhang, Z.; Li, Z.J.; Scales, N.; Kruzic, J.J.; Edwards, L. Molten salt corrosion (FLiNaK) of a Ni–Mo–Cr alloy and its welds for application in energy-generation and energy-storage systems. Corros. Sci. 2020, 164, 108306. [Google Scholar] [CrossRef]
- Muránsky, O.; Yang, C.; Zhu, H.; Karatchevtseva, I.; Sláma, P.; Nový, Z.; Edwards, L. Molten salt corrosion of Ni-Mo-Cr candidate structural materials for Molten Salt Reactor (MSR) systems. Corros. Sci. 2019, 159, 108087. [Google Scholar] [CrossRef]
- Olson, L.C.; Ambrosek, J.W.; Sridharan, K.; Anderson, M.H.; Allen, T.R. Materials corrosion in molten LiF-NaF-KF salt. J. Fluor. Chem. 2009, 130, 67–73. [Google Scholar] [CrossRef]
- Qiu, J.; Zou, Y.; Yu, G.; Liu, H.; Jia, Y.; Li, Z.; Huai, P.; Zhou, X.; Xu, H. Compatibility of container materials with Cr in molten FLiNaK salt. J. Fluor. Chem. 2014, 168, 69–74. [Google Scholar] [CrossRef]
- D’Souza, B.; Zhuo, W.; Yang, Q.; Leong, A.; Zhang, J. Impurity driven corrosion behavior of HAYNES® 230® alloy in molten chloride Salt. Corros. Sci. 2021, 187, 109483. [Google Scholar] [CrossRef]
- Ding, W.; Gomez-Vidal, J.; Bonk, A.; Bauer, T. Molten chloride salts for next generation CSP plants: Electrolytical salt purification for reducing corrosive impurity level. Sol. Energy Mater. Sol. Cells 2019, 199, 8–15. [Google Scholar] [CrossRef]
- Ouyang, F.Y.; Chang, C.H.; You, B.C.; Yeh, T.K.; Kai, J.J. Effect of moisture on corrosion of Ni-based alloys in molten alkali fluoride FLiNaK salt environments. J. Nucl. Mater. 2013, 437, 201–207. [Google Scholar] [CrossRef]
- Yang, X.; Liu, M.; Gao, Y.; Zhang, D.; Feng, S.; Liu, H.; Yu, G.; Wu, G.; Wang, M.; Zhou, X.; et al. Effect of oxygen on the corrosion of SiC in LiF-NaF-KF molten salt. Corros. Sci. 2016, 103, 165–172. [Google Scholar] [CrossRef]
- Bae, J.H.; Yu, J.M.; Dao, V.H.; Lok, V.; Yoon, K.B. Effects of processing parameters on creep behavior of 316L stainless steel produced using selective laser melting. J. Mech. Sci. Technol. 2021, 35, 3803–3812. [Google Scholar] [CrossRef]
- Yu, J.M.; Dao, V.H.; Yoon, K.B. Investigation of creep behavior of 316L stainless steel produced by selective laser melting with various processing parameters. J. Mech. Sci. Technol. 2020, 34, 3249–3259. [Google Scholar] [CrossRef]
- Cheng, W.J.; Chen, D.J.; Wang, C.J. High-temperature corrosion of Cr-Mo steel in molten LiNO3-NaNO3-KNO3 eutectic salt for thermal energy storage. Sol. Energy Mater. Sol. Cells 2015, 132, 563–569. [Google Scholar] [CrossRef]
- Vignarooban, K.; Pugazhendhi, P.; Tucker, C.; Gervasio, D.; Kannan, A.M. Corrosion resistance of Hastelloys in molten metal-chloride heat-transfer fluids for concentrating solar power applications. Sol. Energy 2014, 103, 62–69. [Google Scholar] [CrossRef]




| Structural Material/ Element Ratio (wt%) | Stainless Steel 316L (Fe-Based Alloy) | Hastelloy C-276 (Ni-Based Alloy) | Hastelloy N10003
(Ni-Based Alloy) |
|---|---|---|---|
| Fe | Balance | 5.0 | 4.0 |
| Ni | 10.0 | Balance | Balance |
| Cr | 16.0 | 16.0 | 7.0 |
| Mo | 2.0 | 16.0 | 16.0 |
| Mn | 2.0 | 1.0 | 0.8 |
| Si | 0.75 | 0.08 | 1.0 |
| C | 0.03 | 0.01 | 0.06 |
| Cu | - | 0.5 | 0.35 |
| Co | - | 2.5 | 0.02 |
| W | - | 4.0 | 0.5 |
| V | - | 0.35 | 0.5 |
| N | 0.1 | - | - |
| P | 0.045 | - | - |
| S | 0.03 | - | - |
| Ni 2p1 (870 eV) | Cr 2p1 (584 eV) | Mo 3d3 (231 eV) | Fe 2p1 (720 eV) | O 1s (531 eV) | ||
|---|---|---|---|---|---|---|
| Ni 2p3 (853 eV) | Cr 2p3 (575 eV) | Mo 3d5 (228 eV) | Fe 2p3 (707 eV) | |||
| SS316L | Surface (at%) | 11.22 | - | 0.03 | 87.39 | - |
| 0.80 | 0.30 | 0.27 | 0 | |||
| * Bulk (at%) | 4.01 | 0.18 | - | 7.15 | 65.69 | |
| 9.69 | 0.43 | - | 12.86 | |||
| Hastelloy C | Surface (at%) | 3.89 | - | 0.58 | 0.20 | 87.74 |
| 7.16 | 0.21 | - | 0.23 | |||
| Bulk (at%) | 38.86 | 1.15 | 0.28 | - | 18.86 | |
| 36.31 | 1.66 | 0.18 | 2.70 | |||
| Hastelloy N | Surface (at%) | - | 0.06 | - | 7.44 | 84.38 |
| 7.59 | 0.46 | - | 0.08 | |||
| Bulk (at%) | 38.46 | 0.18 | 0.69 | - | 17.22 | |
| 39.22 | 0.27 | 0.19 | 3.76 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, U.; Kim, M.W.; Na, J.; Lee, M.; Kim, S.J.; Kim, D.-J.; Yoon, Y.S. A Study on the Corrosion Behavior of Fe/Ni-Based Structural Materials in Unpurified Molten Chloride Salt. Materials 2025, 18, 1653. https://doi.org/10.3390/ma18071653
Lee U, Kim MW, Na J, Lee M, Kim SJ, Kim D-J, Yoon YS. A Study on the Corrosion Behavior of Fe/Ni-Based Structural Materials in Unpurified Molten Chloride Salt. Materials. 2025; 18(7):1653. https://doi.org/10.3390/ma18071653
Chicago/Turabian StyleLee, Unho, Min Wook Kim, Jisu Na, Mingyu Lee, Sung Joong Kim, Dong-Joo Kim, and Young Soo Yoon. 2025. "A Study on the Corrosion Behavior of Fe/Ni-Based Structural Materials in Unpurified Molten Chloride Salt" Materials 18, no. 7: 1653. https://doi.org/10.3390/ma18071653
APA StyleLee, U., Kim, M. W., Na, J., Lee, M., Kim, S. J., Kim, D.-J., & Yoon, Y. S. (2025). A Study on the Corrosion Behavior of Fe/Ni-Based Structural Materials in Unpurified Molten Chloride Salt. Materials, 18(7), 1653. https://doi.org/10.3390/ma18071653









