Impact of NaHCO3/Na2CO3 Buffer Reagent on Mitigating the Corrosion of C110 Steel in Water-Based Annulus Protection Fluid at Ultrahigh Temperature
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Corrosion Performance in Deaerated Solution
3.2. Corrosion Performance in Aerated Solution
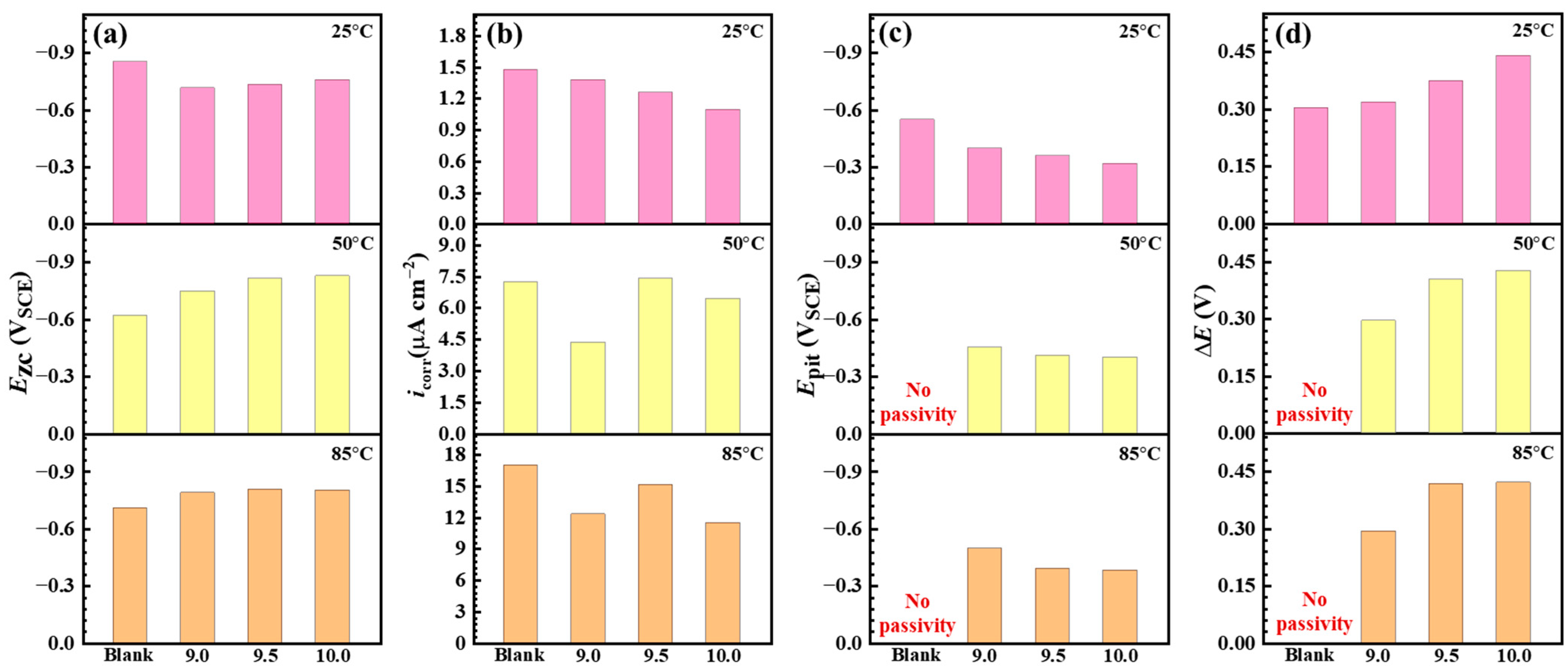
3.3. Electrochemical Corrosion Measurements
4. Discussion
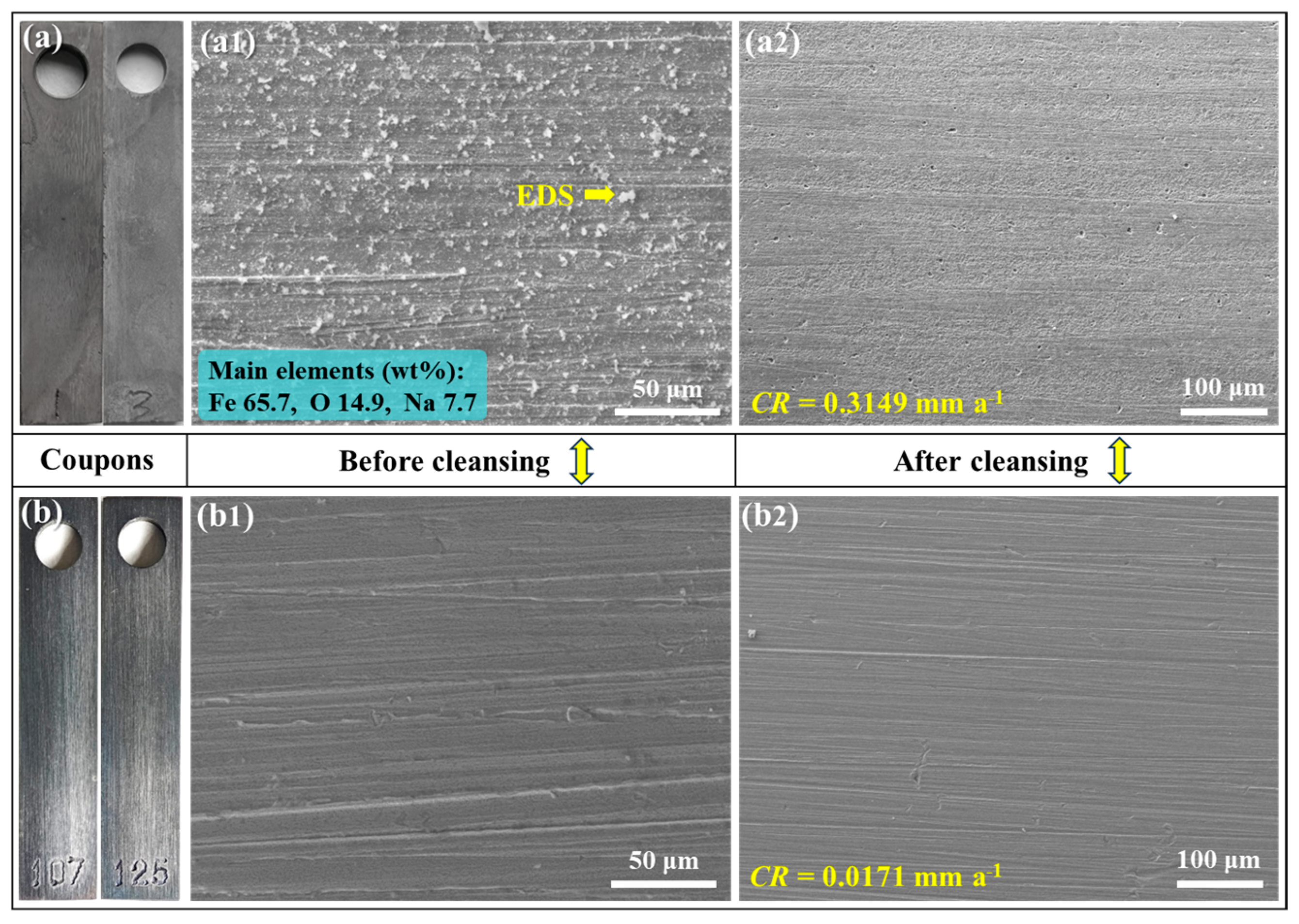
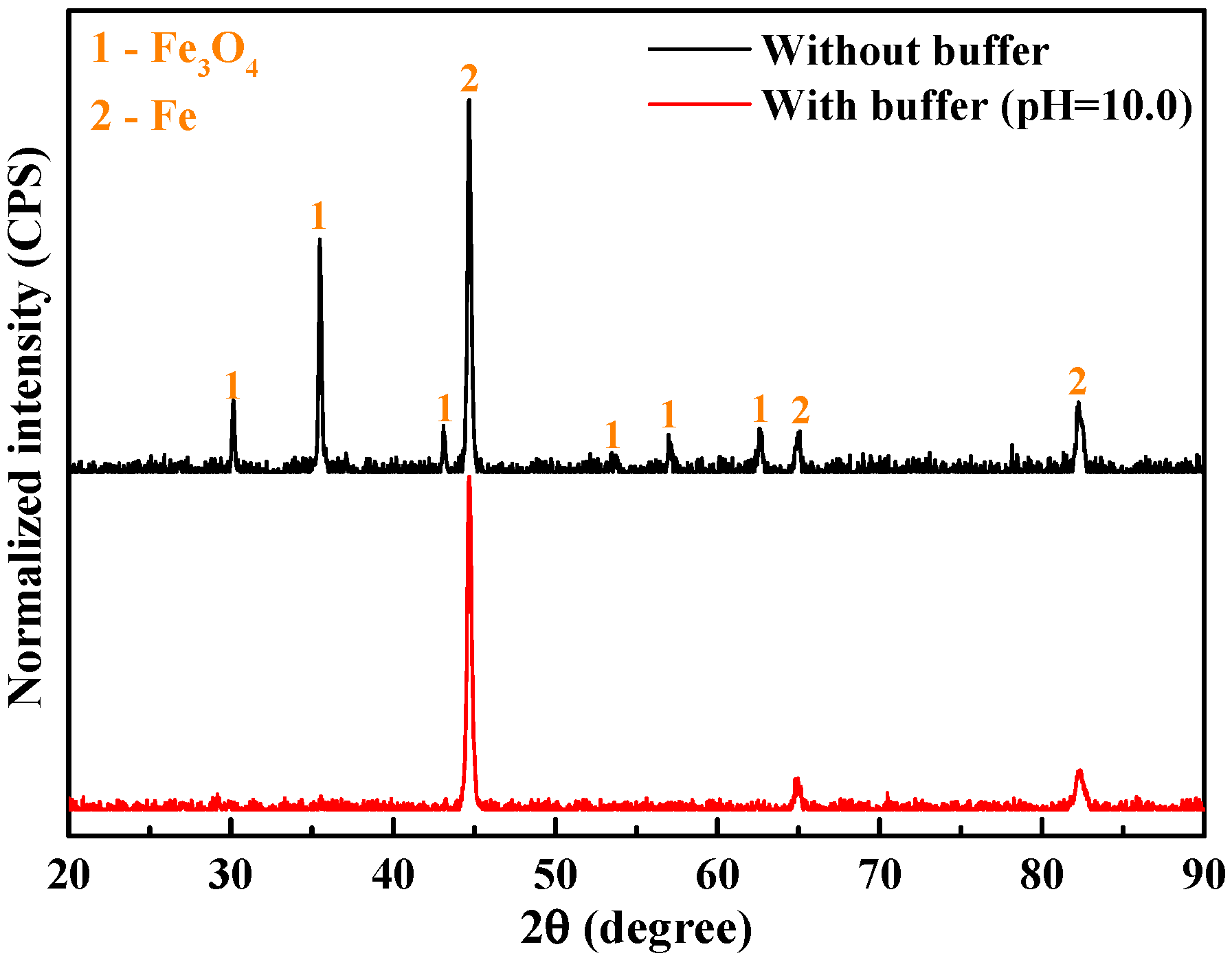
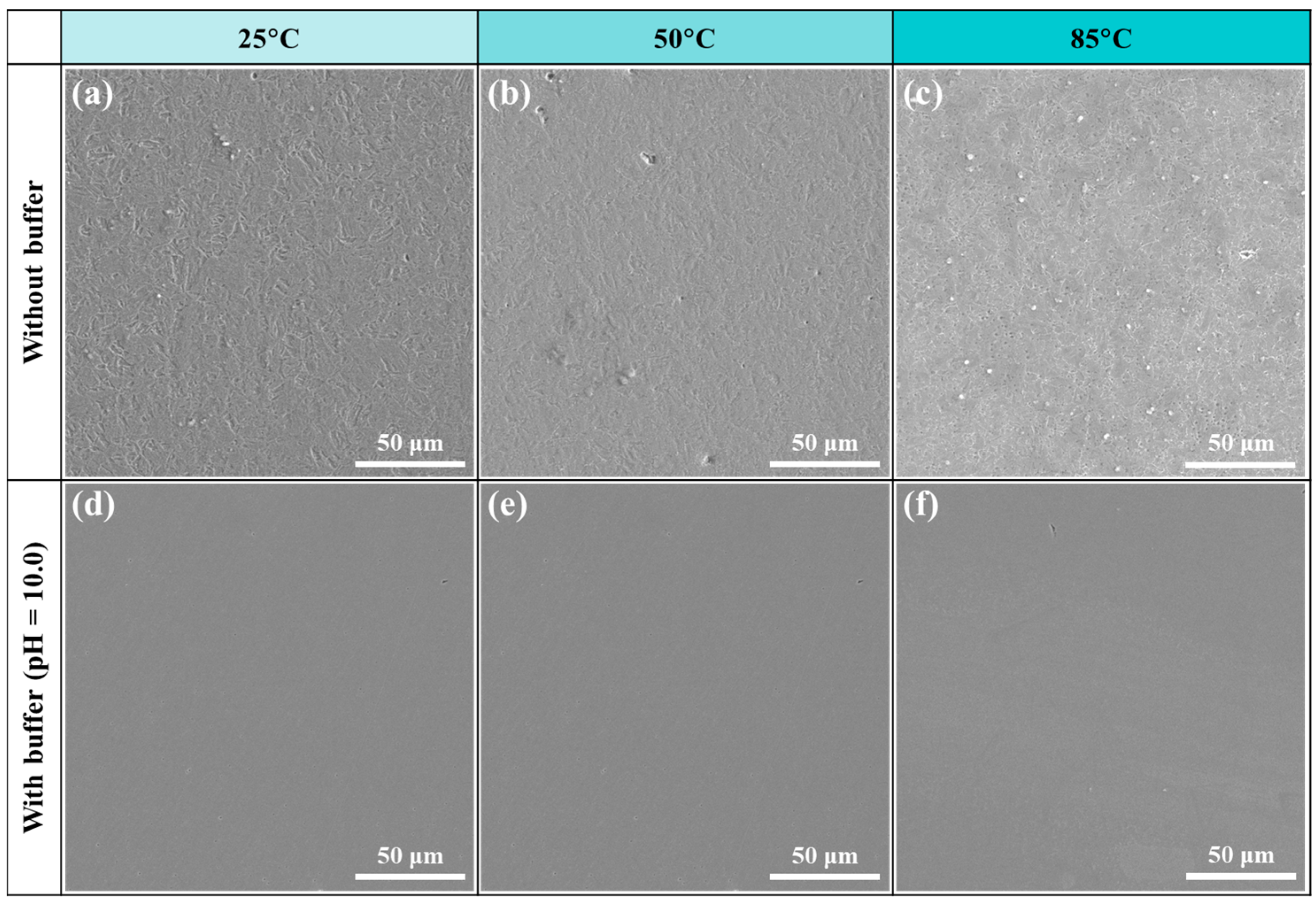
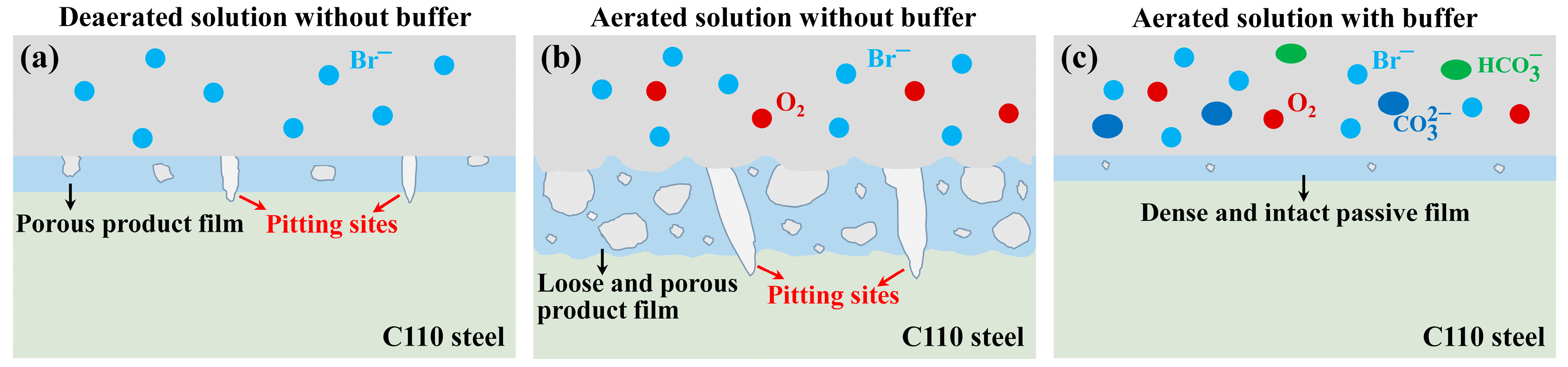
4.1. Corrosion in the Absence of Buffer Reagents
4.2. Na2CO3/NaHCO3 Induced Passivation
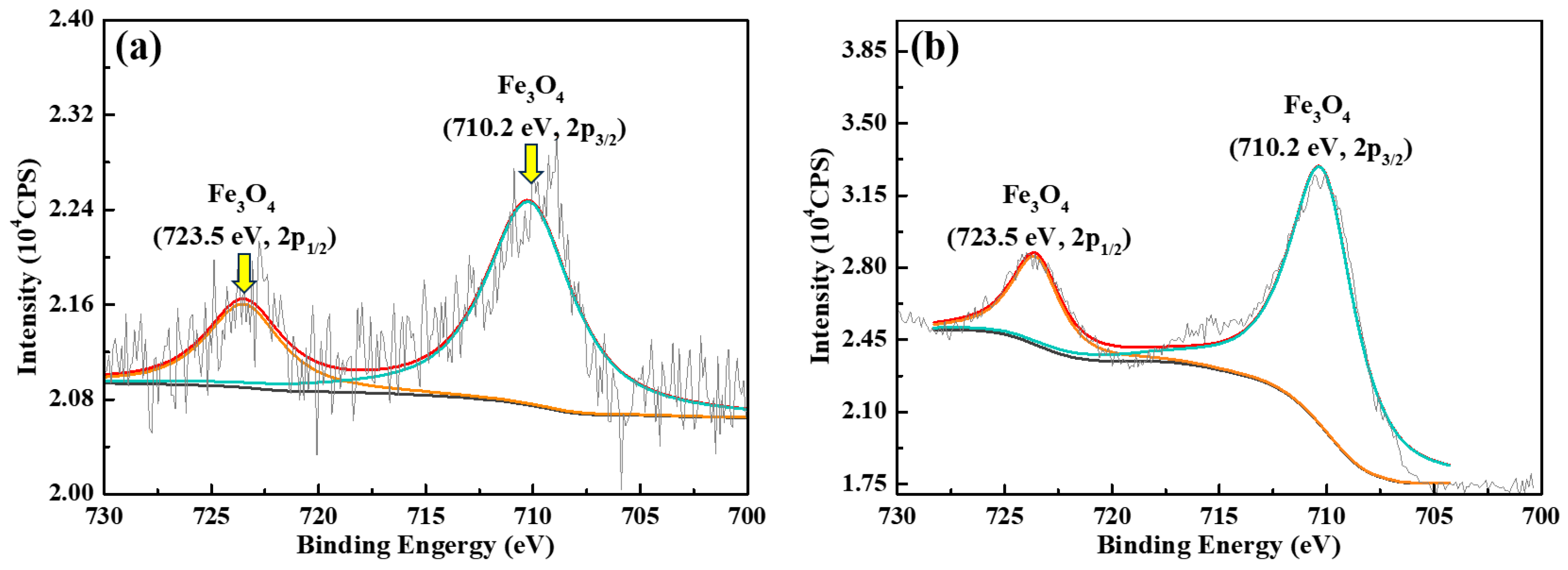
4.3. Corrosion Product vs. Passive Film
5. Conclusions
- (1)
- The Na2CO3/NaHCO3 buffer reagents effectively inhibit the corrosion of C110 steel at 220 °C. Compared with the deaerated solution, the aerated condition is more aggressive and induces severe corrosion, and the highest corrosion rate can reach 0.6746 mm a−1. However, once the buffer system is introduced, the corrosion rate is notably decreased, and the highest inhibition effect is obtained in aerated solution with CO2/H2S.
- (2)
- Under the buffered condition, a higher pH leads to better inhibition performance. Meanwhile, the mitigation effect of the Na2CO3/NaHCO3 buffer system is more pronounced in the corrosion medium with higher aggressivity, i.e., the inhibition efficiency lies in the order of deaerated solution (82.4%) < aerated solution (94.6%) < aerated solution with CO2/H2S (96.1%).
- (3)
- In the absence of buffer reagents, loose and porous corrosion products (mainly Fe3O4) are generated with insufficient protection to the steel substrate, resulting in uniform etching and pitting corrosion. The addition of buffer reagents facilitates the formation of thinner but denser and more protective Fe3O4 passive film, which is the primary reason for the high inhibition efficiency.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Askari, M.; Aliofkhazraei, M.; Jafari, R.; Hamghalam, P.; Hajizadeh, A. Downhole corrosion inhibitors for oil and gas production—A review. Appl. Surf. Sci. Adv. 2021, 6, 100128. [Google Scholar] [CrossRef]
- Yang, T.; Xu, L.; Wang, J.; Zhang, M.; Yao, Y.; Gao, G.; Xu, W.; Li, C. Research progress on CO2 corrosion and protective countermeasures for oil casing. J. Chin. Soc. Corros. Prot. 2024, 44, 1134–1144. (In Chinese) [Google Scholar]
- Long, Y.; Song, W.W.; Fu, A.Q.; Xie, J.F.; Feng, Y.R.; Bai, Z.Q.; Yin, C.X.; Ma, Q.W.; Ji, N.; Kuang, X.R. Combined effect of hydrogen embrittlement and corrosion on the cracking behaviour of C110 low alloy steel in O2-contaminated H2S environment. Corros. Sci. 2022, 194, 109926. [Google Scholar] [CrossRef]
- Yuan, Y.; Xiang, Y.; Li, C.; Zhao, X.; Yan, W.; Yao, E. Research progress on corrosion of CO2 injection well tubing in CCUS system. J. Chin. Soc. Corros. Prot. 2024, 44, 15–26. (In Chinese) [Google Scholar]
- Chen, Q.; Tang, Q.; Qin, Z.; Li, Y.; Li, L.; Li, X.; Yuan, J.; Su, H.; Fu, A. Corrosion behavior of hot-dip aluminum coating in “high temperature-salt deposited-CO2/O2” multi-degree coupling environment. J. Chin. Soc. Corros. Prot. 2023, 43, 569–577. (In Chinese) [Google Scholar]
- Zeng, D.Z.; Dong, B.J.; Zhang, S.S.; Yi, Y.G.; Huang, Z.Y.; Tian, G.; Yu, H.Y.; Sun, Y.C. Annular corrosion risk analysis of gas injection in CO2 flooding and development of oil-based annulus protection fluid. J. Pet. Sci. Eng. 2022, 208, 109526. [Google Scholar] [CrossRef]
- Zeng, D.Z.; Dong, B.J.; Yu, Z.M.; Huang, Z.Y.; Yi, Y.G.; Yu, H.Y.; Li, J.; Tian, G. Design of water-based annulus protection fluid for CO2 flooding injection well. J. Pet. Sci. Eng. 2021, 205, 108726. [Google Scholar] [CrossRef]
- Jain, R.; Mahto, V. Evaluation of polyacrylamide/clay composite as a potential drilling fluid additive in inhibitive water based drilling fluid system. J. Pet. Sci. Eng. 2015, 133, 612–621. [Google Scholar] [CrossRef]
- Ismail, A.R.; Aftab, A.; Ibupoto, Z.H.; Zolkifile, N. The novel approach for the enhancement of rheological properties of water-based drilling fluids by using multi-walled carbon nanotube, nanosilica and glass beads. J. Pet. Sci. Eng. 2016, 139, 264–275. [Google Scholar] [CrossRef]
- Elgaddafi, R.; Ahmed, R.; Hassani, S.; Shah, S.; Osisanya, S.O. Corrosion of C110 carbon steel in high-pressure aqueous environment with mixed hydrocarbon and CO2 gas. J. Pet. Sci. Eng. 2016, 146, 777–787. [Google Scholar] [CrossRef]
- Zhang, Q.; Ren, L.; Lei, X.; Wei, W.; Zhang, J. Two fatigue fracture modes at different temperatures of 10%Cr heat-resistant steel weldment filled with Ni-based alloy. Eng. Fail. Anal. 2024, 161, 108336. [Google Scholar] [CrossRef]
- Liu, Q.L.; Han, X.; Cao, J.; Du, L.; Jia, N.; Zheng, R.; Chen, W.; Zeng, D.Z. Design of multifunctional and efficient water-based annulus protection fluid for HTHP sour gas wells. Processes 2023, 11, 171. [Google Scholar] [CrossRef]
- Fu, A.; Long, Y.; Liu, H.; Zhao, M.; Xie, J.; Su, H.; Li, X.; Yuan, J.; Lei, X.; Yin, C.; et al. Stress corrosion cracking behavior of super 13Cr tubing in phosphate packer fluid of high pressure high temperature gas well. Eng. Fail. Anal. 2022, 139, 106478. [Google Scholar] [CrossRef]
- Aftab, A.; Ismail, A.R.; Khokhar, S.; Ibupoto, Z.H. Novel zinc oxide nanoparticles deposited acrylamide composite used for enhancing the performance of water-based drilling fluids at elevated temperature conditions. J. Pet. Sci. Eng. 2016, 146, 1142–1157. [Google Scholar] [CrossRef]
- Liu, W.Y.; Shi, T.H.; Li, S.; Lu, Q.; Zhang, Z.; Feng, S.B.; Ming, C.Z.; Wu, K. Failure analysis of a fracture tubing used in the formate annulus protection fluid. Eng. Fail. Anal. 2019, 95, 248–262. [Google Scholar] [CrossRef]
- Ramasamy, J.; Amanullah, M. Nanocellulose for oil and gas field drilling and cementing applications. J. Pet. Sci. Eng. 2020, 184, 106292. [Google Scholar] [CrossRef]
- Aftab, A.; Ali, M.; Sahito, M.F.; Mohanty, U.S.; Jha, N.K.; Akhondzadeh, H.; Azhar, M.R.; Ismail, A.R.; Keshavarz, A.; Iglauer, S. Environmental friendliness and high performance of multifunctional tween 80/ZnO-nanoparticles-added water-based drilling fluid: An experimental approach. ACS Sustain. Chem. Eng. 2020, 8, 11224–11243. [Google Scholar] [CrossRef]
- Wang, J.D.; Yin, T.F.; Qi, W.L.; Li, X.P.; Zhao, Y.; Zhang, T.; Wang, F.H. Investigation of the corrosion and passive behavior of HP-13Cr stainless steel in formate annulus protection fluid. Acta Metall. Sin. Engl. Lett. 2024, 37, 1087–1103. [Google Scholar] [CrossRef]
- Obot, I.B.; Onyeachu, I.B.; Umoren, S.A.; Quraishi, M.A.; Sorour, A.A.; Chen, T.; Aljeaban, N.; Wang, Q.W. High temperature sweet corrosion and inhibition in the oil and gas industry: Progress, challenges and future perspectives. J. Pet. Sci. Eng. 2020, 185, 106469. [Google Scholar] [CrossRef]
- Olivo, J.D.; Brown, B.; Young, D.; Nesic, S. Effect of corrosion inhibitor alkyl tail length on the electrochemical process governing CO2 corrosion of mild steel. Corrosion 2019, 75, 137–139. [Google Scholar] [CrossRef]
- Beverskog, B.; Puigdomenech, I. Revised pourbaix diagrams for iron at 25–300 °C. Corros. Sci. 1996, 38, 2121–2135. [Google Scholar] [CrossRef]
- Pourbaix, M.J.N. Atlas of Electrochemical Equilibria in Aqueous Solutions; Pergamon Press: Oxford, UK, 1966. [Google Scholar]
- AMPP NACE SP0775-2023 Standard; Preparation, Installation, Analysis, and Interpretation of Corrosion Coupons in Hydrocarbon Operations. Internal Corrosion Management: Houston, TX, USA, 2023.
- Song, Y.; Jiang, G.; Chen, Y.; Zhao, P.; Tian, Y. Effects of chloride ions on corrosion of ductile iron and carbon steel in soil environments. Sci. Rep. 2017, 7, 6865. [Google Scholar] [CrossRef]
- Wagner, C.D. NIST X-Ray Photoelectron Spectroscopy Database, NIST Standard Reference Database Number 20; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Lei, X.; Dong, X.; Hao, L.; Wang, J.; Macdonald, D.D.; Wang, N. Investigation of the ordered and disordered corrosion morphologies on Ni-based alloy in the passive state. Corros. Sci. 2023, 224, 111490. [Google Scholar] [CrossRef]
- Song, L.; Liu, Z.; Li, X.; Du, C. Characteristics of hydrogen embrittlement in high-pH stress corrosion cracking of X100 pipeline steel in carbonate/ bicarbonate solution. Constr. Build. Mater. 2020, 263, 120124. [Google Scholar] [CrossRef]
- Marcus, P. Corrosion Mechanisms in Theory and Practice, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Fu, A.Q.; Tang, X.; Cheng, Y.F. Characterization of corrosion of X70 pipeline steel in thin electrolyte layer under disbonded coating by scanning Kelvin probe. Corros. Sci. 2009, 51, 186–190. [Google Scholar] [CrossRef]
- Barker, R.; Burkle, D.; Charpentier, T.; Thompson, H.; Neville, A. A review of iron carbonate (FeCO3) formation in the oil and gas industry. Corros. Sci. 2018, 142, 312–341. [Google Scholar] [CrossRef]
- Lei, X.; Wang, H.; Mao, F.; Zhang, J.; Zhao, M.; Fu, A.; Feng, Y.; Macdonald, D.D. Electrochemical behaviour of martensitic stainless steel after immersion in a H2S-saturated solution. Corros. Sci. 2018, 131, 164–173. [Google Scholar] [CrossRef]
- Tanupabrungsun, T. Thermodynamics and Kinetics of Carbon Dioxide Corrosion of Mild Steel at Elevated Temperatures. Ph.D. Thesis, Ohio University, Athens, OH, USA, 2012. [Google Scholar]
- Macdonald, D.D.; Lei, X. Theoretical Interpretation of Anion Size Effects in Passivity Breakdown. J. Electrochem. Soc. 2016, 163, C738–C744. [Google Scholar] [CrossRef]
- Macdonald, D.D. The Point Defect Model for the Passive State. J. Electrochem. Soc. 1992, 139, 3434–3449. [Google Scholar] [CrossRef]
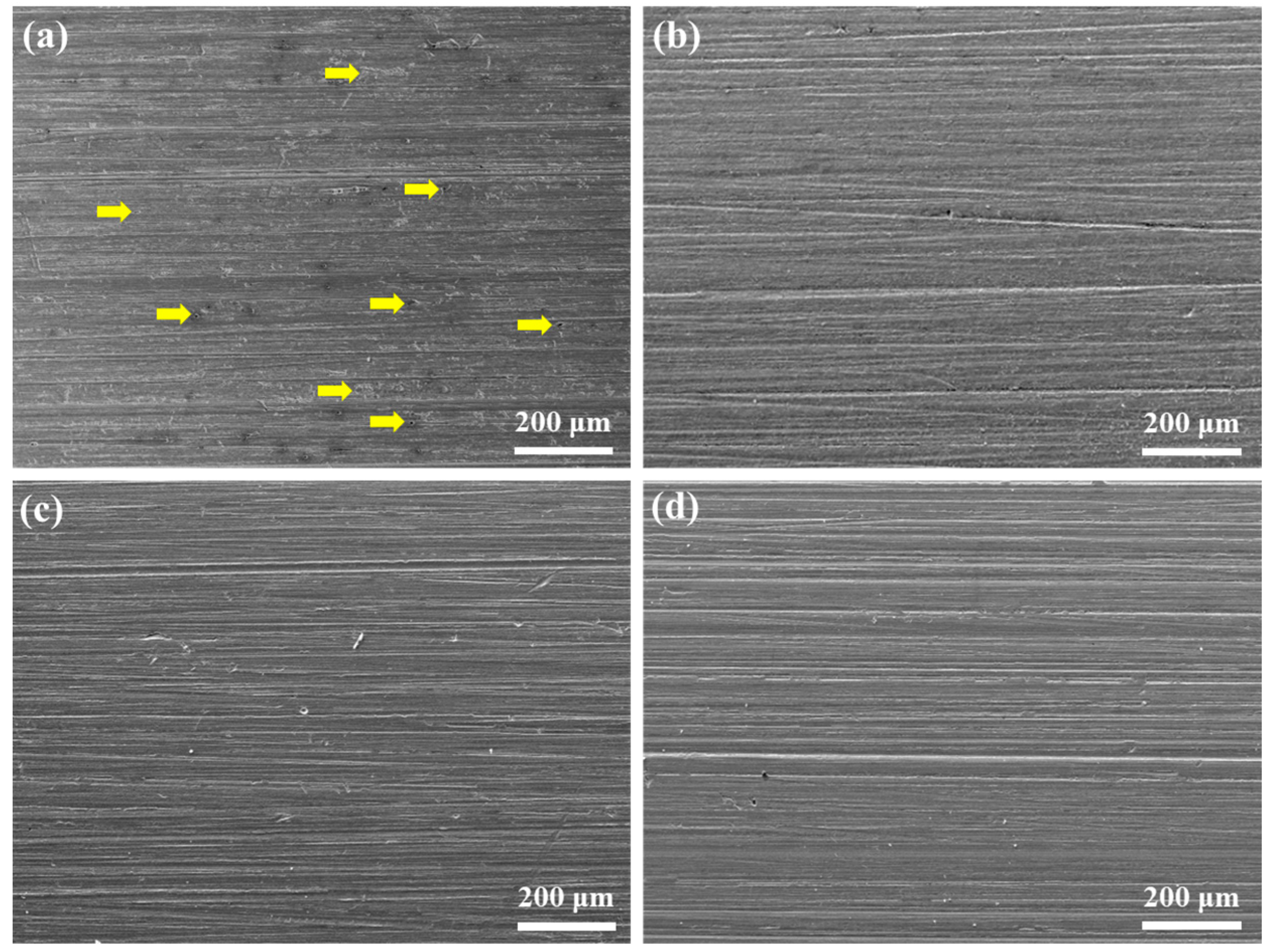

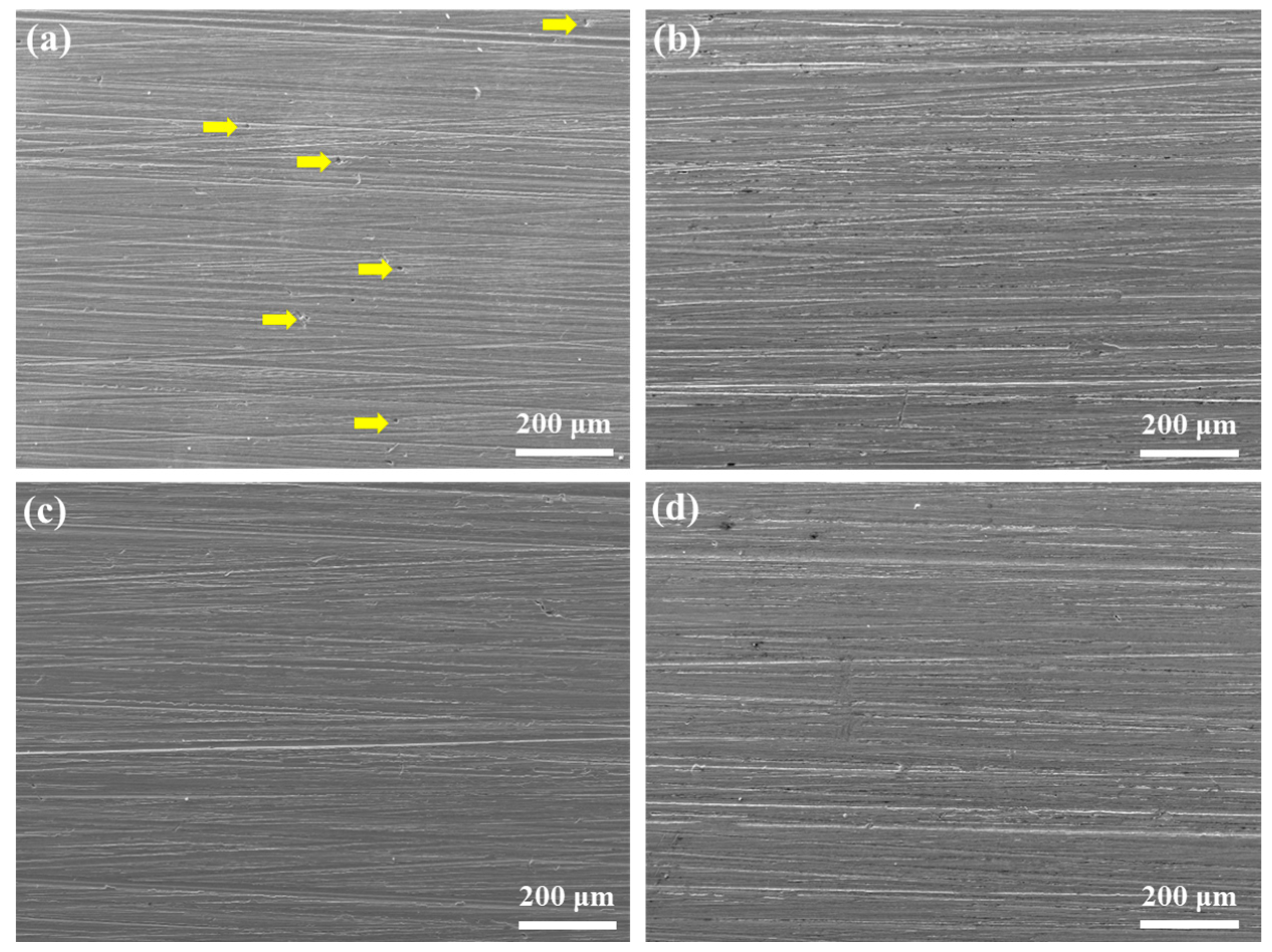



| Element | C | Si | Mn | Ni | Cr | Mo | Cu | P | S | Fe |
|---|---|---|---|---|---|---|---|---|---|---|
| Content | 0.27 | 0.17 | 0.44 | 0.06 | 0.47 | 0.82 | 0.04 | 0.007 | 0.001 | Bal. |
| Temperature | Condition | Ezc (VSCE) | icorr (µA cm−2) | Epit (VSCE) | ΔE (V) |
|---|---|---|---|---|---|
| 25 °C | Blank | −0.855 | 1.479 | −0.551 | 0.304 |
| 9.0 | −0.717 | 1.380 | −0.398 | 0.319 | |
| 9.5 | −0.733 | 1.259 | −0.359 | 0.374 | |
| 10.0 | −0.759 | 1.096 | −0.319 | 0.440 | |
| 50 °C | Blank | −0.621 | 7.244 | No passivity | No passivity |
| 9.0 | −0.748 | 4.365 | −0.452 | 0.296 | |
| 9.5 | −0.814 | 7.413 | −0.410 | 0.404 | |
| 10.0 | −0.829 | 6.457 | −0.402 | 0.427 | |
| 85 °C | Blank | −0.710 | 16.982 | No passivity | No passivity |
| 9.0 | −0.789 | 12.303 | −0.496 | 0.293 | |
| 9.5 | −0.808 | 15.136 | −0.390 | 0.418 | |
| 10.0 | −0.802 | 11.482 | −0.381 | 0.421 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Zhao, M.; Li, Y.; Xie, J.; Song, W.; Zhang, J.; Wang, M.; Zhou, J.; Wang, Y.; Lei, X.; et al. Impact of NaHCO3/Na2CO3 Buffer Reagent on Mitigating the Corrosion of C110 Steel in Water-Based Annulus Protection Fluid at Ultrahigh Temperature. Materials 2025, 18, 1668. https://doi.org/10.3390/ma18071668
Zhang Z, Zhao M, Li Y, Xie J, Song W, Zhang J, Wang M, Zhou J, Wang Y, Lei X, et al. Impact of NaHCO3/Na2CO3 Buffer Reagent on Mitigating the Corrosion of C110 Steel in Water-Based Annulus Protection Fluid at Ultrahigh Temperature. Materials. 2025; 18(7):1668. https://doi.org/10.3390/ma18071668
Chicago/Turabian StyleZhang, Zhi, Mifeng Zhao, Yan Li, Junfeng Xie, Wenwen Song, Juantao Zhang, Mengkai Wang, Jie Zhou, Yuan Wang, Xiaowei Lei, and et al. 2025. "Impact of NaHCO3/Na2CO3 Buffer Reagent on Mitigating the Corrosion of C110 Steel in Water-Based Annulus Protection Fluid at Ultrahigh Temperature" Materials 18, no. 7: 1668. https://doi.org/10.3390/ma18071668
APA StyleZhang, Z., Zhao, M., Li, Y., Xie, J., Song, W., Zhang, J., Wang, M., Zhou, J., Wang, Y., Lei, X., & Li, D. (2025). Impact of NaHCO3/Na2CO3 Buffer Reagent on Mitigating the Corrosion of C110 Steel in Water-Based Annulus Protection Fluid at Ultrahigh Temperature. Materials, 18(7), 1668. https://doi.org/10.3390/ma18071668







