Effect of Ni Content on the Dissolution Behavior of Hot-Dip Tin-Coated Copper Wire and the Evolution of a Cu–Sn Intermetallic Compound Layer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material Preparation
2.2. Experimental Procedure
2.3. Specimen Preparation and Characterization
3. Results
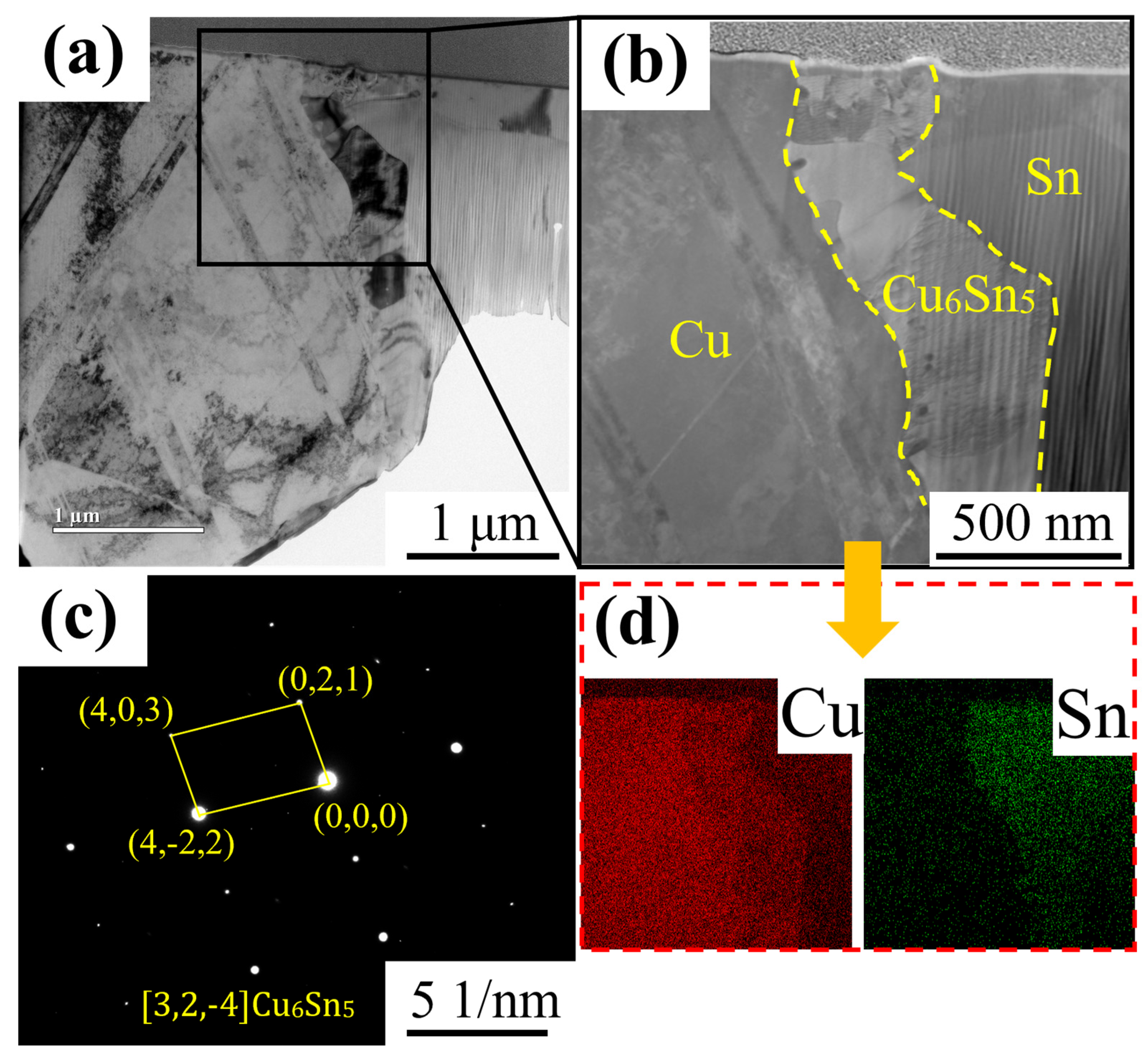
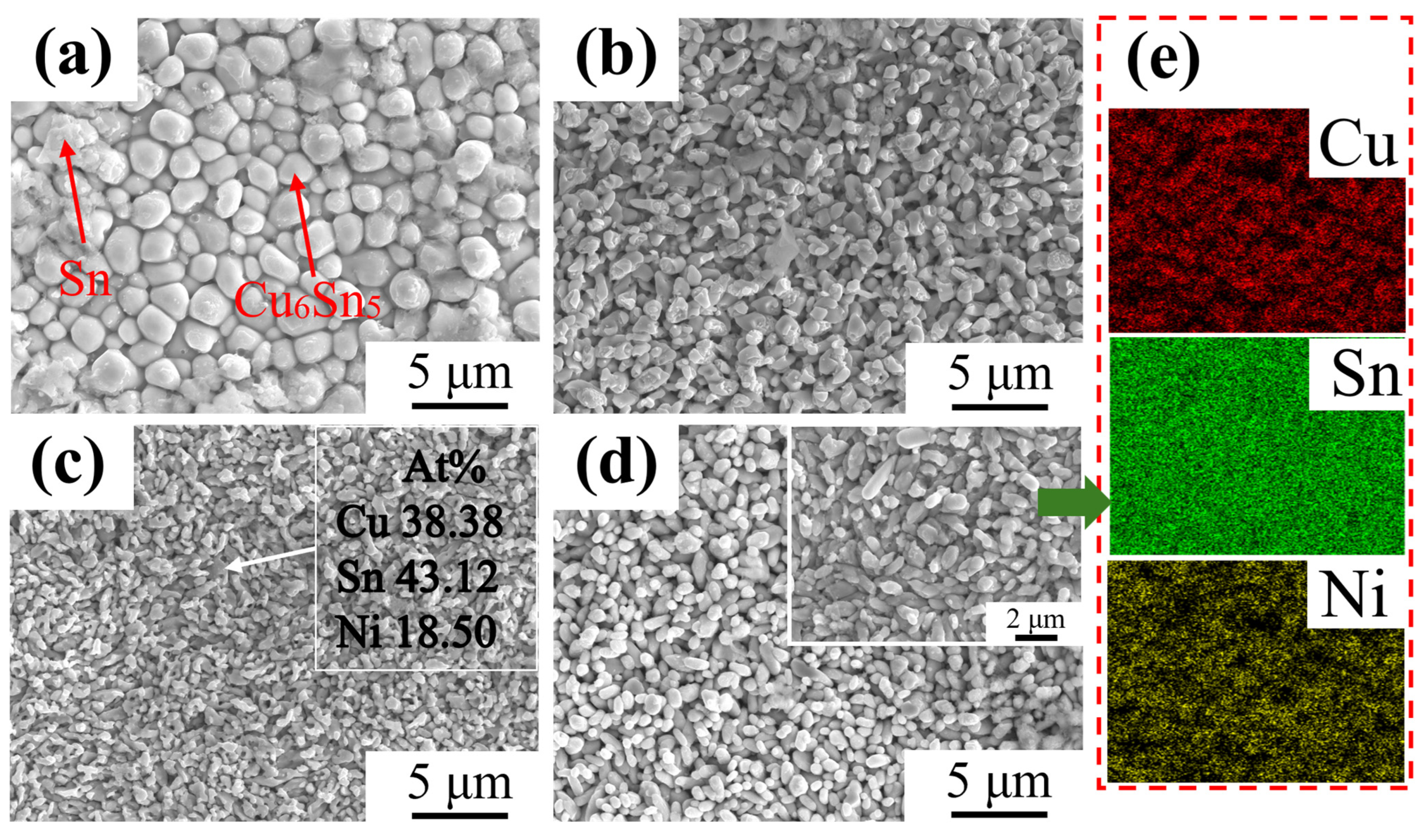
3.1. Phase Composition and Microstructure of IMC Layer
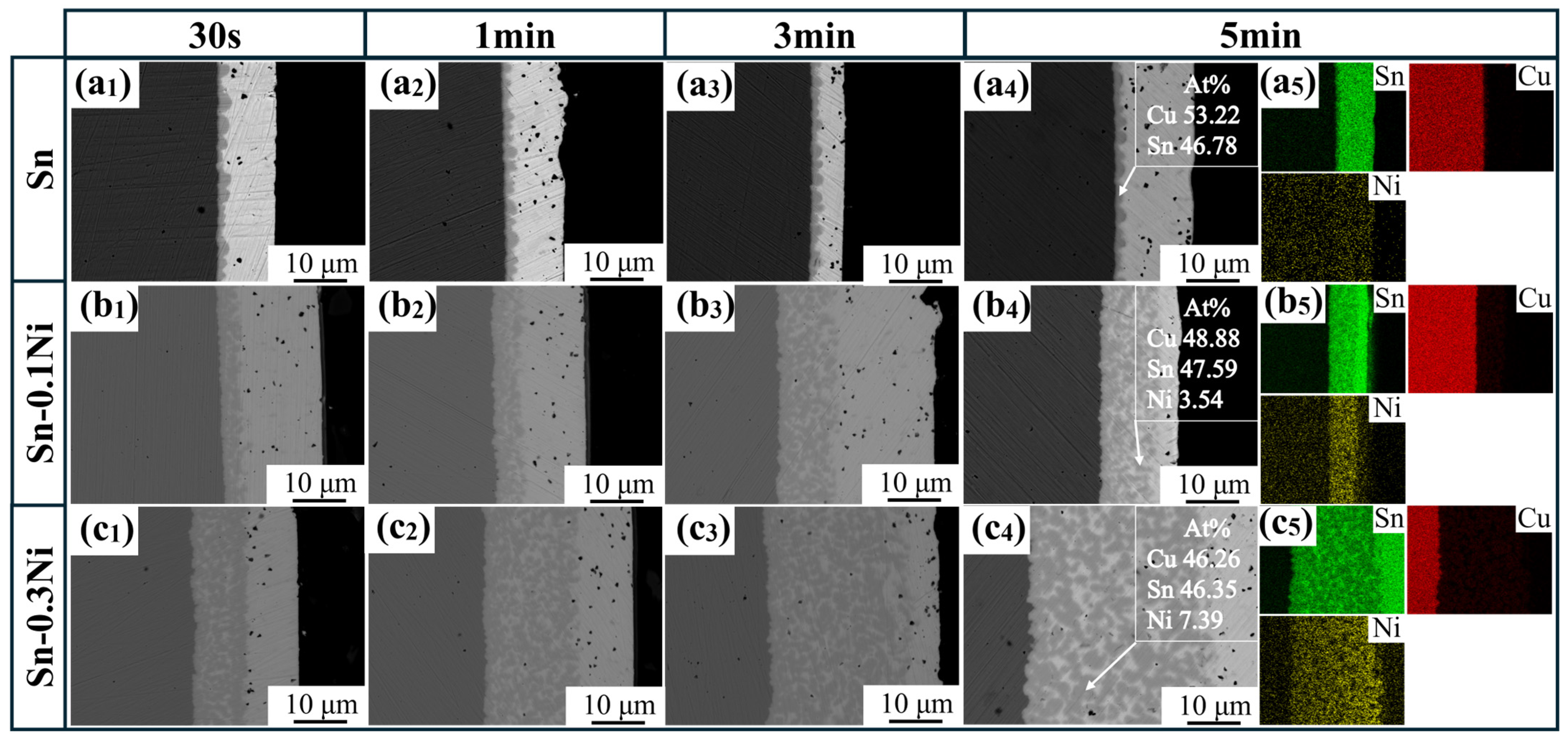
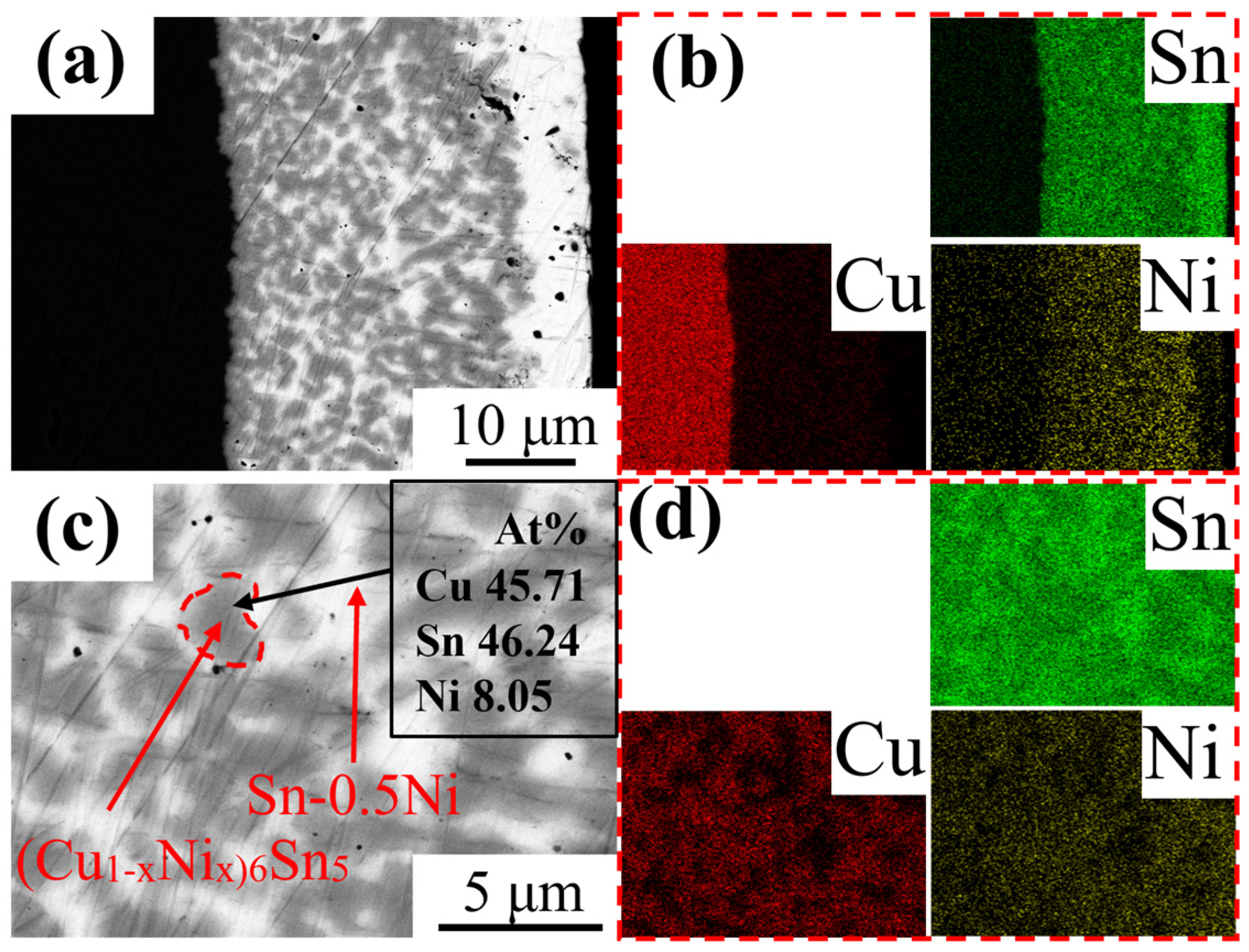
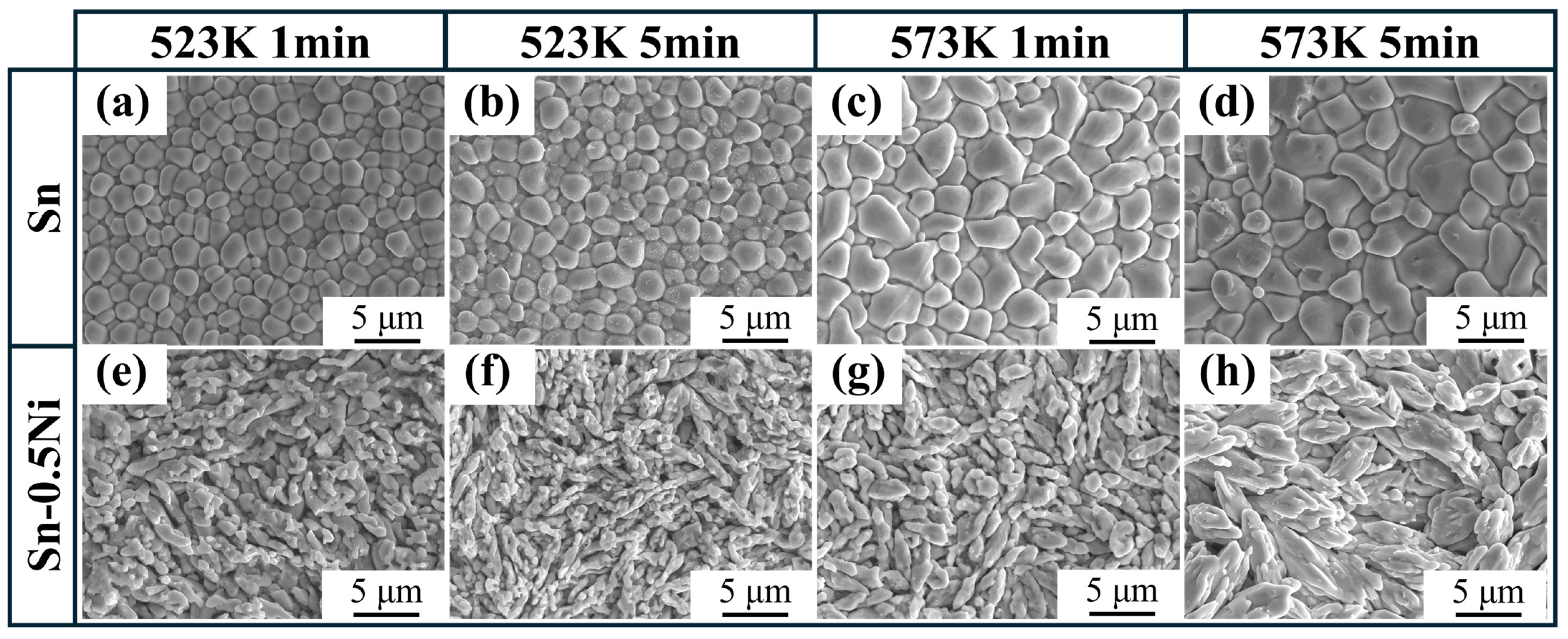
3.2. Microstructure Evolution of IMC Layer
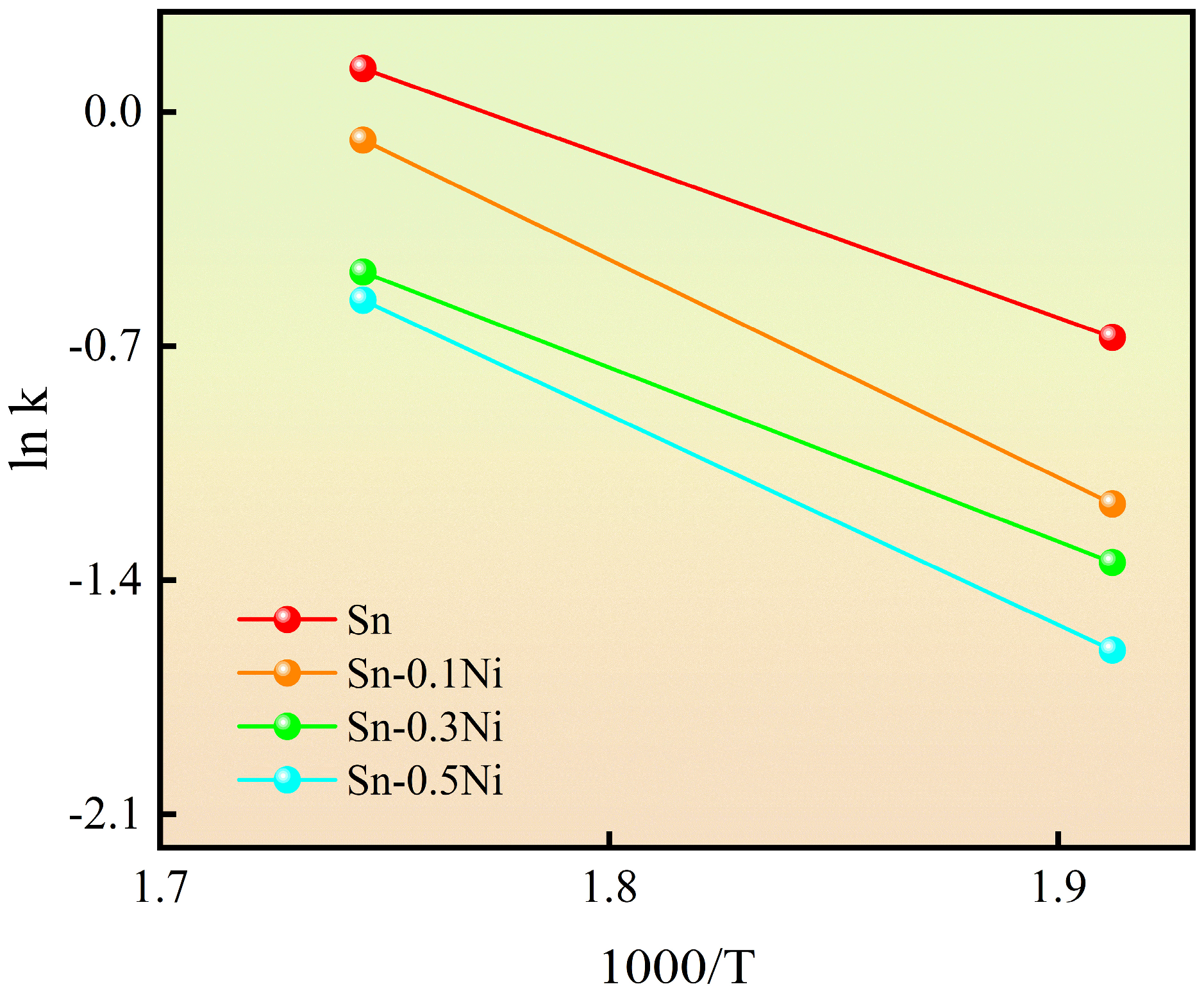
3.3. Dissolution Kinetics of Cu
4. Discussion
5. Conclusions
- (1)
- Kinetic analysis revealed that Ni introduction significantly increased the effective activation energy (Q) of Cu dissolution (from 40.84 kJ/mol in pure Sn to 54.21 kJ/mol in Sn-0.5Ni), indicating that Ni inhibits the kinetic process of Cu atoms escaping the lattice by enhancing diffusion barriers. This mechanism extended the complete dissolution time of copper wire at 573 K from 20 min in pure Sn to 60 min in Sn-0.5Ni, with a threefold reduction in dissolution rate;
- (2)
- In pure Sn systems, Cu6Sn5 grains at the interface exhibited regular equiaxed morphology with uniform size (~2 μm diameter at 523 K). With Ni addition, the IMC layer transformed into irregular fibrous grains with refined dimensions (aspect ratio of 3:1 in Sn-0.5Ni at 523 K). EDS elemental mapping confirmed that Ni atoms substitute Cu in the Cu6Sn5 lattice to form (Cu1−xNix)6Sn5 ternary phases, with Ni-rich regions overlapping Cu distribution. This demonstrates that Ni alters IMC growth patterns through solid-solution strengthening mechanisms;
- (3)
- In pure Sn systems, Cu6Sn5 layer thickness was solely temperature-dependent (increasing from 1 μm at 523 K to 3 μm at 573 K) and independent of dissolution time, indicating diffusion-limited growth. In contrast, Ni-containing systems displayed time–temperature synergistic effects: at 573 K in Sn-0.5Ni, the IMC layer thickness increased from 3 μm (1 min) to 5 μm (5 min), with growth kinetics following an interface reaction-dominated mechanism (time exponent n ≈ 1), deviating significantly from the traditional diffusion-controlled model (n = 0.5, parabolic law). This discovery provides new theoretical insights for designing high-reliability tinned copper interfaces.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ouyang, Y.L.; Liu, Y.H.; Zhu, Q.Q.; Zhang, G.S.; Song, K.X.; Huang, T.; Lu, W.W.; Liu, D.; Liu, A.K.; Wang, B.B.; et al. Study on microstructure control of copper-tin biphase interface in hot-dip tin-plated electronic copper strip. J. Mater. Res. Technol. 2024, 33, 7169–7181. [Google Scholar] [CrossRef]
- Xiong, R.L.; Xiao, K.; Yi, P.; Hu, Y.T.; Dong, C.F.; Wu, J.S.; Li, X.G. The influence of Bacillus subtilis on tin-coated copper in an aqueous environment. RSC Adv. 2018, 8, 4671–4679. [Google Scholar] [CrossRef]
- Suzuki, T.; Inoue, M. Cu Plating with Sn and Subsequent Bronze Formation under Mild Conditions. J. Chem. Educ. 2021, 98, 946–950. [Google Scholar] [CrossRef]
- Knight, A.; Aglan, H.; Burdick, D. Identification of Tin Whisker Growth on Tin Plated Copper Substrate. Microsc. Microanal. 2017, 23, 1772–1773. [Google Scholar] [CrossRef]
- Tsai, S.T.; Chiang, P.C.; Liu, C.; Feng, S.P.; Chen, C.M. Suppression of Void Formation at Sn/Cu Joint Due to Twin Formation in Cu Electrodeposit. JOM 2019, 71, 3012–3022. [Google Scholar] [CrossRef]
- Hsu, S.Y.; Chen, C.M.; Song, J.M.; Nishikawa, H. Surface modification of Cu electroplated layers for Cu–Sn transient liquid phase bonding. Mater. Chem. Phys. 2022, 277, 125621. [Google Scholar] [CrossRef]
- Liu, Z.J.; Xi, M.R. Review-Revisiting the Electroplating Process for Zn-Metal Anodes: New Application of Traditional Electroplating Additive in ZIBs. J. Electrochem. Soc. 2022, 169, 120508. [Google Scholar] [CrossRef]
- Zhang, X.D.; Yang, C.L.; Sun, M.L.; Hu, A.M.; Li, M.; Gao, L.M.; Hang, T.; Ling, H.Q. Inhibition of tin whisker by electroplating ultra-thin Co-W amorphous barrier layer. Mater. Charact. 2020, 162, 110221. [Google Scholar] [CrossRef]
- Gunji, T.; Umehashi, Y.; Tsunoi, H.; Yokoi, K.; Kawai, A.; Matsumoto, F. Preparation of chemical-resistant atomically ordered Sn-Ni alloy films by electroless plating. J. Alloys Compd. 2021, 877, 160100. [Google Scholar] [CrossRef]
- Hwang, H.S.; Yoon, T.; Jang, J.; Kim, J.J.; Ryu, J.H.; Oh, S.M. Carbon fabric as a current collector for electroless-plated Cu6Sn5 negative electrode for lithium-ion batteries. J. Alloys Compd. 2017, 692, 583–588. [Google Scholar] [CrossRef]
- Muench, F. Electroless Plating of Metal Nanomaterials. Chemelectrochem 2021, 8, 2993–3012. [Google Scholar] [CrossRef]
- Yin, T.; Xiang, N.; Wang, G.X.; Tian, B.H.; Sun, W.T.; Zhang, X.Y. Optimal Hot-Dipped Tinning Process Routine for the Fabrication of Solderable Sn Coatings on Circuit Lead Frames. Materials 2020, 13, 1191. [Google Scholar] [CrossRef]
- Yao, X.F.; Xia, S.F.; Lv, Y.K.; Yang, W.; Chen, J. Effects of hot dipping temperature on microstructure and mechanical properties of Pb40Sn60 alloy coating on copper wires. Mater. Sci. Eng. A Struct. Mater. Prop. Microstruct. Process. 2021, 810, 140991. [Google Scholar] [CrossRef]
- Chen, K.J.; Hung, F.Y.; Lui, T.S.; Hsu, L. Studies of Interfacial Microstructures and Series Resistance on Electroplated and Hot-Dipped Sn-xCu Photovoltaic Modules. J. Electron. Mater. 2018, 47, 6028–6035. [Google Scholar] [CrossRef]
- Abu Bakar, M.; Sunar, M.S.M.; Jalar, A.; Atiqah, A.; Ani, F.C.; Ahmad, I.; Zolkefli, Z.E. Effect of Sn Plating Thickness on Wettability, Solderability, and Electrical Connections of Electronic Lead Connectors for Surface Mount Technology Applications. Sains Malays. 2023, 52, 3261–3272. [Google Scholar] [CrossRef]
- Shang, P.J.; Tian, F.F.; Liu, Z.Q. Identification and Evolution of Intermetallic Compounds Formed at the Interface between In-48Sn and Cu during Liquid Soldering Reactions. Metals 2024, 14, 139. [Google Scholar] [CrossRef]
- Oliveira, R.; Cruz, C.; Barros, A.; Bertelli, F.; Spinelli, J.E.; Garcia, A.; Cheung, N. Thermal conductance at Sn-0.5mass%Al solder alloy/substrate interface as a factor for tailoring cellular/dendritic growth. J. Therm. Anal. Calorim. 2022, 147, 4945–4958. [Google Scholar] [CrossRef]
- Liang, J.; Dariavach, N.; Callahan, P.; Shangguan, D. Metallurgy and kinetics of liquid-solid interfacial reaction during lead-free soldering. Mater. Trans. 2006, 47, 317–325. [Google Scholar] [CrossRef]
- Chen, S.W.; Wang, C.H. Interfacial reactions of Sn-Cu/Ni couples at 250 °C. J. Mater. Res. 2006, 21, 2270–2277. [Google Scholar] [CrossRef]
- Harcuba, P.; Janecek, M. Microstructure Changes and Physical Properties of the Intermetallic Compounds Formed at the Interface Between Sn-Cu Solders and a Cu Substrate Due to a Minor Addition of Ni. J. Electron. Mater. 2010, 39, 2553–2557. [Google Scholar] [CrossRef]
- Zeng, G.; McDonald, S.D.; Mu, D.K.; Terada, Y.; Yasuda, H.; Gu, Q.F.; Nogita, K. Ni segregation in the interfacial (Cu,Ni)6Sn5 intermetallic layer of Sn-0.7Cu-0.05Ni/Cu ball grid array (BGA) joints. Intermetallics 2014, 54, 20–27. [Google Scholar] [CrossRef]
- Wang, Y.W.; Chang, C.C.; Kao, C.R. Minimum effective Ni addition to SnAgCu solders for retarding Cu3Sn growth. J. Alloys Compd. 2009, 478, L1–L4. [Google Scholar] [CrossRef]
- Tay, S.L.; Haseeb, A.; Johan, M.R.; Munroe, P.R.; Quadir, M.Z. Influence of Ni nanoparticle on the morphology and growth of interfacial intermetallic compounds between Sn-3.8Ag-0.7Cu lead-free solder and copper substrate. Intermetallics 2013, 33, 8–15. [Google Scholar] [CrossRef]
- Hu, X.W.; Ke, Z.R. Growth behavior of interfacial Cu–Sn intermetallic compounds of Sn/Cu reaction couples during dip soldering and aging. J. Mater. Sci. Mater. Electron. 2014, 25, 936–945. [Google Scholar] [CrossRef]
- Yen, Y.W.; Chou, W.T.; Tseng, Y.; Lee, C.; Hsu, C.L. Investigation of dissolution behavior of metallic substrates and intermetallic compound in molten lead-free solders. J. Electron. Mater. 2008, 37, 73–83. [Google Scholar] [CrossRef]
- Sui, Y.W.; Sun, R.; Qi, J.Q.; He, Y.Z.; Wei, F.X.; Meng, Q.K.; Sun, Z. Morphologies and evolution of intermetallic compounds formed between Sn1.0Ag0.7Cu composite solder and Cu substrate. Rare Met. 2023, 42, 1043–1049. [Google Scholar] [CrossRef]
- Xiang, N.; Yin, T.; Tian, B.H.; Tang, S.W.; Chen, E. Evaluation on the Manufacturability of Solderable Sn Coatings Obtained by Employing Hot-Dipped Tinning Process. JOM 2019, 71, 4284–4295. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Wei, C.W.; Han, J.J.; Cao, H.J.; Chen, H.T.; Li, M.Y. Growth evolution and formation mechanism of η′-Cu6Sn5 whiskers on η-Cu6Sn5 intermetallics during room-temperature ageing. Acta Mater. 2020, 183, 340–349. [Google Scholar] [CrossRef]
- Salleh, M.; McDonald, S.D.; Nogita, K. Effects of Ni and TiO2 additions in as-reflowed and annealed Sn0.7Cu solders on Cu substrates. J. Mater. Process. Technol. 2017, 242, 235–245. [Google Scholar] [CrossRef]
- Chia, P.Y.; Haseeb, A.; Mannan, S.H. Reactions in Electrodeposited Cu/Sn and Cu/Ni/Sn Nanoscale Multilayers for Interconnects. Materials 2016, 9, 430. [Google Scholar] [CrossRef]
- Luu, T.T.; Duan, A.; Aasmundtveit, K.E.; Hoivik, N. Optimized Cu–Sn Wafer-Level Bonding Using Intermetallic Phase Characterization. J. Electron. Mater. 2013, 42, 3582–3592. [Google Scholar] [CrossRef]
- Pal, M.K.; Bajaj, V. Nucleation and Location of Kirkendall Voids at the Tin-Based Solder/Copper Joint: A Review. Adv. Eng. Mater. 2023, 25, 2300671. [Google Scholar] [CrossRef]
- Yuan, Y.; Guan, Y.Y.; Li, D.J.; Moelans, N. Investigation of diffusion behavior in Cu–Sn solid state diffusion couples. J. Alloys Compd. 2016, 661, 282–293. [Google Scholar] [CrossRef]
- Gu, X.; Chan, Y.C. Electromigration in line-type Cu/Sn-Bi/Cu solder joints. J. Electron. Mater. 2008, 37, 1721–1726. [Google Scholar] [CrossRef]
- Huang, J.R.; Tsai, C.M.; Lin, Y.W.; Kao, C.R. Pronounced electromigration of Cu in molten Sn-based solders. J. Mater. Res. 2008, 23, 250–257. [Google Scholar] [CrossRef]
- Oh, S.H.; Jeong, Y.J.; Na, S.H.; Kim, J.; Lee, B.J. Formation of columnar grains during diffusional growth of Nb3Sn layer and its suppression. Acta Mater. 2024, 263, 119542. [Google Scholar] [CrossRef]



| Alloy Designation | Sn | Ni |
|---|---|---|
| Sn | 100 | 0 |
| Sn-0.1Ni | 99.9 | 0.1 |
| Sn-0.3Ni | 99.7 | 0.3 |
| Sn-0.5Ni | 99.5 | 0.5 |
| Temperature | Sn | Sn-0.1Ni | Sn-0.3Ni | Sn-0.5Ni |
|---|---|---|---|---|
| 523 K | 40 | 75 | 110 | 150 |
| 573 K | 20 | 35 | 50 | 60 |
| Effective Activation Energy | Sn | Sn-0.1Ni | Sn-0.3Ni | Sn-0.5Ni |
|---|---|---|---|---|
| Q | 40.84 | 43.67 | 52.14 | 54.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Zhang, J.; Niu, S.; Fan, J.; Tang, S.; Tang, S.; Yin, N.; Liu, J.; Li, M. Effect of Ni Content on the Dissolution Behavior of Hot-Dip Tin-Coated Copper Wire and the Evolution of a Cu–Sn Intermetallic Compound Layer. Materials 2025, 18, 1714. https://doi.org/10.3390/ma18081714
Wang Q, Zhang J, Niu S, Fan J, Tang S, Tang S, Yin N, Liu J, Li M. Effect of Ni Content on the Dissolution Behavior of Hot-Dip Tin-Coated Copper Wire and the Evolution of a Cu–Sn Intermetallic Compound Layer. Materials. 2025; 18(8):1714. https://doi.org/10.3390/ma18081714
Chicago/Turabian StyleWang, Qi, Jinhan Zhang, Song Niu, Jinjin Fan, Shijun Tang, Shihong Tang, Ningkang Yin, Jingxuan Liu, and Mingmao Li. 2025. "Effect of Ni Content on the Dissolution Behavior of Hot-Dip Tin-Coated Copper Wire and the Evolution of a Cu–Sn Intermetallic Compound Layer" Materials 18, no. 8: 1714. https://doi.org/10.3390/ma18081714
APA StyleWang, Q., Zhang, J., Niu, S., Fan, J., Tang, S., Tang, S., Yin, N., Liu, J., & Li, M. (2025). Effect of Ni Content on the Dissolution Behavior of Hot-Dip Tin-Coated Copper Wire and the Evolution of a Cu–Sn Intermetallic Compound Layer. Materials, 18(8), 1714. https://doi.org/10.3390/ma18081714






