The Recent Developments of Thermomechanical Processing for Biomedical Mg Alloys and Their Clinical Applications
Abstract
:1. Introduction
1.1. Background of Biomedical Mg Alloys
1.2. Design of Chemical Compositions for Biomedical Mg Alloys
1.3. Performance of Biomedical Mg Alloys by Alloying Composition
2. Deformation for Biomedical Mg Alloy
2.1. Processing Technologies for Biomedical Mg Alloys
2.1.1. Hot Extrusion
Conventional Extrusion (Direct Extrusion)
Equal-Channel Angular Pressing
Cyclic Extrusion Compression
2.1.2. Hot Rolling
Conventional Rolling
Cross-Rolling
Accumulative Roll Bonding
2.1.3. Hot Forging
Radial Forging
Multi-Directional Forging
2.2. Mechanical Properties of Biomedical Mg Alloys
2.3. Biological and Corrosion Performance of Biomedical Mg Alloys
3. Applications of Biomedical Mg Alloy
3.1. Hemostatic Clip
3.2. Bone Screw
3.3. Bone Plate
3.4. Intramedullary Nail
3.5. Cardiovascular Stent
3.6. Oral Implant
3.7. Tumor Treatment
3.8. Biliary Stent
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Du, B.N.; Hu, Z.Y.; Sheng, L.Y.; Xu, D.K.; Zheng, Y.F.; Xi, T.F. Influence of Zn Content on Microstructure and Tensile Properties of Mg-Zn-Y-Nd Alloy. Acta Metall. Sin. (Engl. Lett.) 2018, 31, 351–361. [Google Scholar]
- He, M.; Chen, L.; Yin, M.; Xu, S.; Liang, Z. Review on magnesium and magnesium-based alloys as biomaterials for bone immobilization. J. Mater. Res. Technol. 2023, 23, 4396–4419. [Google Scholar]
- Thomas, K.K.; Zafar, M.N.; Pitt, W.G.; Husseini, G.A. Biodegradable Magnesium Alloys for Biomedical Implants: Properties, Challenges, and Surface Modifications with a Focus on Orthopedic Fixation Repair. Appl. Sci. 2023, 14, 10. [Google Scholar] [CrossRef]
- Khodaei, T.; Schmitzer, E.; Suresh, A.P.; Acharya, A.P. Immune response differences in degradable and non-degradable alloy implants. Bioact. Mater. 2023, 24, 153–170. [Google Scholar]
- Zhao, C.; Wen, M.; Wang, Q.; Ouyang, W.; Xu, D.; Jia, Z.; Zheng, Y.; Xi, T.; Sheng, L. Tailoring the corrosion resistance and biological performance of Mg-Zn-Y-Nd bioimplants with multiphasic, pore-sealed cerium-doped ceramic coatings via facile one-pot plasma electrolytic oxidation. J. Mater. Sci. Technol. 2025, 230, 60–79. [Google Scholar]
- Kang, Y.Y.; Du, B.N.; Li, Y.M.; Wang, B.J.; Sheng, L.Y.; Shao, L.Q.; Zheng, Y.F.; Xi, T.F. Optimizing mechanical property and cytocompatibility of the biodegradable Mg-Zn-Y-Nd alloy by hot extrusion and heat treatment. J. Mater. Sci. Technol. 2019, 35, 6–18. [Google Scholar]
- Kumar, A.; Choudhari, A.; Gupta, A.K.; Kumar, A. Rare-Earth based magnesium alloys as a potential biomaterial for the future. J. Magnes. Alloys 2024, 12, 3841–3897. [Google Scholar]
- Tsakiris, V.; Tardei, C.; Clicinschi, F.M. Biodegradable Mg alloys for orthopedic implants—A review. J. Magnes. Alloys 2021, 9, 1884–1905. [Google Scholar]
- Niranjan, C.A.; Raghavendra, T.; Rao, M.P.; Siddaraju, C.; Gupta, M.; Jain, V.K.S.; Aishwarya, R. Magnesium alloys as extremely promising alternatives for temporary orthopedic implants—A review. J. Magnes. Alloys 2023, 11, 2688–2718. [Google Scholar]
- Verma, A.; Ogata, S. Magnesium based alloys for reinforcing biopolymer composites and coatings: A critical overview on biomedical materials. Adv. Ind. Eng. Polym. Res. 2023, 6, 341–355. [Google Scholar]
- Zhao, C.C.; Ouyang, W.T.; Wen, M.; Yang, C.; Xu, D.K.; Zheng, Y.F.; Xi, T.F.; Sheng, L.Y. Optimizing corrosion resistance of the Mg-4Zn-0.5Y-0.5Nd alloy by regulation of secondary phase and grain structure. J. Mater. Res. Technol. 2025, 35, 435–450. [Google Scholar]
- Sheng, L.; Wei, L.; Lai, C.; Xi, T. Comparative study the effect of magnesium and magnesium alloy on the activity of nerve cell. Basic Clin. Pharmacol. 2019, 125, 17. [Google Scholar]
- Wang, J.; Xu, J.; Wang, X.; Sheng, L.; Zheng, L.; Song, B.; Wu, G.; Zhang, R.; Yao, H.; Zheng, N.; et al. Magnesium-pretreated periosteum for promoting bone-tendon healing after anterior cruciate ligament reconstruction. Biomaterials. 2021, 268, 120576. [Google Scholar]
- Saha, S.; Lestari, W.; Dini, C.; Sarian, M.N.; Hermawan, H.; Barão, V.A.R.; Sukotjo, C.; Takoudis, C. Corrosion in Mg-alloy biomedical implants- the strategies to reduce the impact of the corrosion inflammatory reaction and microbial activity. J. Magnes. Alloys 2022, 10, 3306–3326. [Google Scholar]
- Felten, M.; Chaineux, V.; Zhang, S.; Tehranchi, A.; Hickel, T.; Scheu, C.; Spille, J.; Lipińska-Chwałek, M.; Mayer, J.; Berkels, B.; et al. The effect of Laves phases and nano-precipitates on the electrochemical corrosion resistance of Mg-Al-Ca alloys under alkaline conditions. J. Magnes. Alloys 2024, 12, 2447–2461. [Google Scholar]
- Li, C.; Xu, D.; Wang, B.; Sheng, L.; Wu, R.; Han, E. Effects of icosahedral phase on mechanical anisotropy of as-extruded Mg-14Li (in wt%) based alloys. J. Mater. Sci. Technol. 2019, 35, 2477–2484. [Google Scholar]
- Yang, C.; Cui, S.; Fu, R.K.; Sheng, L.; Wen, M.; Xu, D.; Zhao, Y.; Zheng, Y.; Chu, P.K.; Wu, Z. Optimization of the in vitro biodegradability, cytocompatibility, and wear resistance of the AZ31B alloy by micro-arc oxidation coatings doped with zinc phosphate. J. Mater. Sci. Technol. 2024, 179, 224–239. [Google Scholar]
- Behera, M.; Denys, A.; Shabadi, R.; Allain, F.; Gruescu, C. Micro-alloying of Zn and Ca in vacuum induction casted bioresorbable Mg system: Perspectives on corrosion resistance, cytocompatibility, and inflammatory response. J. Magnes. Alloys 2024, 12, 2812–2825. [Google Scholar]
- Zhao, C.; Wen, M.; Wang, J.; Xu, D.; Zheng, Y.; Sheng, L. Regulating microstructure and mechanical properties of the as-cast Mg-4Zn-0.5Y-0.5 Nd alloy by heat treatment. J. Alloys Compd. 2025, 1010, 177232. [Google Scholar]
- Chang, C.; Yao, G.; Cox, S.C.; Zhang, X.; Sheng, L.; Liu, M.; Cheng, W.; Lu, Y.; Yan, X. From macro-, through meso- to micro-scale: Densification behavior, deformation response and microstructural evolution of selective laser melted Mg-RE alloy. J. Magnes. Alloys, 2025; in press. [Google Scholar] [CrossRef]
- Zohrevand, M.; Alizadeh, R.; Mahmudi, R. Using different strategies to improve properties of the biodegradable Mg-4Li-4Zn alloy. J. Mater. Res. Technol. 2023, 27, 2066–2079. [Google Scholar]
- Ansari, N.; Alabtah, F.G.; Albakri, M.I.; Khraisheh, M. Post processing of additive manufactured Mg alloys: Current status, challenges, and opportunities. J. Magnes. Alloys 2024, 12, 1283–1310. [Google Scholar]
- Hernández, L.; Ramón-Sierra, J.; Soria-Castro, M.; Bacelis, Á.; Rodríguez-Gattorno, G.; Ortiz-Vázquez, E.; Acosta, G. Assessment of Mg(OH)2/TiO2 coating in the Mg-Ca-Zn alloy for improved corrosion resistance and antibacterial performance. J. Magnes. Alloys 2023, 11, 361–378. [Google Scholar]
- Yang, C.; Wang, C.; Shen, Z.; Zhou, L.; Sheng, L.; Xu, D.; Zheng, Y.; Chu, P.K.; Xiao, S.; Ying, T.; et al. Simultaneous improvement of wear and corrosion resistance of microarc oxidation coatings on ZK61 Mg alloy by doping with ZrO2 nanoparticles. J. Mater. Sci. Technol. 2025, 224, 312–327. [Google Scholar]
- Bazhenov, V.; Koltygin, A.; Komissarov, A.; Li, A.; Bautin, V.; Khasenova, R.; Anishchenko, A.; Seferyan, A.; Komissarova, J.; Estrin, Y. Gallium-containing magnesium alloy for potential use as temporary implants in osteosynthesis. J. Magnes. Alloys 2020, 8, 352–363. [Google Scholar]
- Abazari, S.; Shamsipur, A.; Bakhsheshi-Rad, H.R.; Keshavarz, M.; Kehtari, M.; Ramakrishna, S.; Berto, F. MgO-incorporated carbon nanotubes-reinforced Mg-based composites to improve mechanical, corrosion, and biological properties targeting biomedical applications. J. Mater. Res. Technol. 2022, 20, 976–990. [Google Scholar]
- Iskhakova, K.; Cwieka, H.; Meers, S.; Helmholz, H.; Davydok, A.; Storm, M.; Baltruschat, I.M.; Galli, S.; Profrock, D.; Will, O.; et al. Multi-modal investigation of the bone micro- and ultrastructure, and elemental distribution in the presence of Mg-xGd screws at mid-term healing stages. Bioact. Mater. 2024, 41, 657–671. [Google Scholar]
- Bai, J.; Yang, Y.; Wen, C.; Chen, J.; Zhou, G.; Jiang, B.; Peng, X.; Pan, F. Applications of magnesium alloys for aerospace: A review. J. Magnes. Alloys 2023, 11, 3609–3619. [Google Scholar]
- Poblano-Salas, C.A.; Henao, J.; Giraldo-Betancur, A.L.; Forero-Sossa, P.; Espinosa-Arbelaez, D.G.; González-Sánchez, J.A.; Dzib-Pérez, L.R.; Estrada-Moo, S.T.; Pech-Pech, I.E. HVOF-sprayed HAp/S53P4 BG composite coatings on an AZ31 alloy for potential applications in temporary implants. J. Magnes. Alloys 2024, 12, 345–360. [Google Scholar]
- Wang, J.; Meng, L.; Xie, W.; Ji, C.; Wang, R.; Zhang, P.; Jin, L.; Sheng, L.; Zheng, Y. Corrosion and in vitro cytocompatibility investigation on the designed Mg-Zn-Ag metallic glasses for biomedical application. J. Magnes. Alloys 2024, 12, 1566–1580. [Google Scholar]
- Wang, J.; Wang, Y.; Wang, R.; Wang, Q.; Wen, M.; Wang, J.; Sheng, L.; Zheng, Y.; Xi, T. A Review on 3D Printing Processes in Pharmaceutical Engineering and Tissue Engineering: Applications, Trends and Challenges. Adv. Mater. Technol. 2024, 10, 2400620. [Google Scholar] [CrossRef]
- Prosolov, K.A.; Luginin, N.A.; Litvinova, L.S.; Fedorov, M.A.; Anisenya, I.I.; Mushtovatova, L.S.; Snetkov, A.A.; Bukharov, A.V.; Khlusov, I.A.; Sharkeev, Y.P. Antibacterial and biocompatible Zn and Cu containing CaP magnetron coatings for MgCa alloy functionalization. J. Mater. Res. Technol. 2023, 25, 2177–2203. [Google Scholar]
- Kaseem, M.; Alluhayb, A.H.; Thanaa, T.T.; Fattah-alhosseini, A.; Alkaseem, M. Enhancing the photocatalytic performance and chemical durability of AZ31 magnesium alloy by incorporating two types of nanoparticles through plasma electrolytic oxidation. J. Magnes. Alloys 2024, 12, 4521–4537. [Google Scholar] [CrossRef]
- Mashtalyar, D.V.; Imshinetskiy, I.M.; Nadaraia, K.V.; Gnedenkov, A.S.; Suchkov, S.N.; Opra, D.P.; Pustovalov, E.V.; Yu Ustinov, A.; Sinebryukhov, S.L.; Gnedenkov, S.V. Effect of TiO2 nanoparticles on the photocatalytic properties of PEO coatings on Mg alloy. J. Magnes. Alloys 2023, 11, 735–752. [Google Scholar]
- Pacheco, M.; Aroso, I.M.; Silva, J.M.; Lamaka, S.V.; Bohlen, J.; Nienaber, M.; Letzig, D.; Lima, E.; Barros, A.A.; Reis, R.L. Understanding the corrosion of Mg alloys in in vitro urinary tract conditions: A step forward towards a biodegradable metallic ureteral stent. J. Magnes. Alloys 2023, 11, 4301–4324. [Google Scholar] [CrossRef]
- Wang, B.; Xu, D.; Zhao, T.; Sheng, L. Effect of CaCl2 and NaHCO3 in Physiological Saline Solution on the Corrosion Behavior of an As-Extruded Mg-Zn-Y-Nd alloy. Acta Metall. Sin. (Engl. Lett.) 2020, 34, 239–247. [Google Scholar] [CrossRef]
- Delavar, H.; Mostahsan, A.J.; Ibrahim, H. Corrosion and corrosion-fatigue behavior of magnesium metal matrix composites for bio-implant applications: A review. J. Magnes. Alloys 2023, 11, 1125–1161. [Google Scholar]
- Fattah-alhosseini, A.; Chaharmahali, R.; Alizad, S.; Kaseem, M. Corrosion behavior of composite coatings containing hydroxyapatite particles on Mg alloys by plasma electrolytic oxidation: A review. J. Magnes. Alloys 2023, 11, 2999–3011. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, S.; Bai, J.; Yang, Y.; Wang, Y.; Zhou, Y.; Jiang, W.; Wang, J.; Zhu, J.; Zhu, C.; et al. Magnesium-based biomaterials for coordinated tissue repair: A comprehensive overview of design strategies, advantages, and challenges. J. Magnes. Alloys 2024, 12, 3025–3061. [Google Scholar]
- Dvorský, D.; Inoue, S.-I.; Yoshida, A.; Kubásek, J.; Duchoň, J.; de Prado, E.; Školáková, A.; Hosová, K.; Svora, P.; Kawamura, Y. Advantages of rapid solidification over casting of Mg-0.4Zn-1Y alloy. J. Magnes. Alloys 2024, 12, 2847–2862. [Google Scholar] [CrossRef]
- Badkoobeh, F.; Mostaan, H.; Rafiei, M.; Bakhsheshi-Rad, H.R.; RamaKrishna, S.; Chen, X. Additive manufacturing of biodegradable magnesium-based materials: Design strategies, properties, and biomedical applications. J. Magnes. Alloys 2023, 11, 801–839. [Google Scholar] [CrossRef]
- Gnedenkov, A.S.; Sinebryukhov, S.L.; Nomerovskii, A.D.; Marchenko, V.S.; Ustinov, A.Y.; Gnedenkov, S.V. Carboxylates as green corrosion inhibitors of magnesium alloy for biomedical application. J. Magnes. Alloys 2024, 12, 2909–2936. [Google Scholar] [CrossRef]
- Berger, L.; Dolert, S.; Akhmetshina, T.; Burkhard, J.P.; Tegelkamp, M.; Rich, A.M.; Rubin, W.; Darwiche, S.; Kuhn, G.; Schäublin, R.E.; et al. In vivo performance of lean bioabsorbable Mg-Ca alloy X0 and comparison to WE43: Influence of surface modification and alloying content. Bioact. Mater. 2025, 44, 501–515. [Google Scholar] [CrossRef]
- Chen, L.; Lin, Z.; Wang, M.; Huang, W.; Ke, J.; Zhao, D.; Yin, Q.; Zhang, Y. Treatment of trauma-induced femoral head necrosis with biodegradable pure Mg screw-fixed pedicle iliac bone flap. J. Orthop. Translat. 2019, 17, 133–137. [Google Scholar] [CrossRef]
- Foster, A.L.; Moriarty, T.F.; Zalavras, C.; Morgenstern, M.; Jaiprakash, A.; Crawford, R.; Burch, M.A.; Boot, W.; Tetsworth, K.; Miclau, T.; et al. The influence of biomechanical stability on bone healing and fracture-related infection: The legacy of Stephan Perren. Injury 2021, 52, 43–52. [Google Scholar] [CrossRef]
- Redondo-Trasobares, B.; Sarasa-Roca, M.; Rosell-Pradas, J.; Calvo-Tapies, J.; Gracia-Villa, L.; Albareda-Albareda, J. Comparative clinical and biomechanical study of different types of osteosynthesis in the treatment of distal femur fractures. Rev. Esp. Cir. Ortop. Traumatol. 2023, 67, T216–T225. [Google Scholar] [CrossRef]
- Wu, Y.C.; Hsieh, M.W.; Wang, W.T.; Chang, Y.H.; Lee, S.S.; Huang, S.H.; Hou, M.F.; Tseng, C.C.; Kuo, Y.R. A novel biodegradable magnesium skin staple: A safety and functional evaluation. Asian J. Surg. 2024, 47, 3048–3055. [Google Scholar] [CrossRef]
- Yanagisawa, Y.; Shimizu, Y.; Mukai, T.; Sano, Y.; Odashima, K.; Ikeo, N.; Saito, H.; Yamauchi, K.; Takahashi, T.; Kumamoto, H. Biodegradation behaviors of magnesium(Mg)-based alloy nails in autologous bone grafts: In vivo study in rabbit skulls. J. Appl. Biomater. Funct. Mater. 2022, 20, 22808000221095230. [Google Scholar] [CrossRef]
- Yu, X.; Li, D.; Liu, Y.; Ding, P.; He, X.; Zhao, Y.; Chen, M.; Liu, D. In vitro and in vivo studies on the degradation and biosafety of Mg-Zn-Ca-Y alloy hemostatic clip with the carotid artery of SD rat model. Mater. Sci. Eng. C 2020, 115, 111093. [Google Scholar] [CrossRef]
- Bai, H.; He, X.; Ding, P.; Liu, D.; Chen, M. Fabrication, microstructure, and properties of a biodegradable Mg-Zn-Ca clip. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 1741–1749. [Google Scholar] [CrossRef]
- Li, J.; Qin, L.; Yang, K.; Ma, Z.; Wang, Y.; Cheng, L.; Zhao, D. Materials evolution of bone plates for internal fixation of bone fractures: A review. J. Mater. Sci. Technol. 2020, 36, 190–208. [Google Scholar]
- Wang, S.; Du, C.; Shen, X.; Wu, X.; Ouyang, S.; Tan, J.; She, J.; Tang, A.; Chen, X.; Pan, F. Rational design, synthesis and prospect of biodegradable magnesium alloy vascular stents. J. Magnes. Alloys 2023, 11, 3012–3037. [Google Scholar]
- Vautrin, A.; Wesseling, M.; Wirix-Speetjens, R.; Gomez-Benito, M.J. Time-dependent in silico modelling of orthognathic surgery to support the design of biodegradable bone plates. J. Mech. Behav. Biomed. Mater. 2021, 121, 104641. [Google Scholar]
- Huang, Y.T.; Hung, F.Y.; Yen, C.W. Microstructure and fracture toughness of hot-rolling biomedical degradable ZKX500 magnesium bone plates. Micron 2023, 172, 103500. [Google Scholar]
- Gerashi, E.; Alizadeh, R.; Mahmudi, R. Improved corrosion resistance and mechanical properties of biodegradable Mg-4Zn-xSr alloys: Effects of heat treatment, Sr additions, and multi-directional forging. J. Mater. Res. Technol. 2022, 20, 3363–3380. [Google Scholar]
- Raguraman, S.; Priyadarshini, M.S.; Nguyen, T.; McGovern, R.; Kim, A.; Griebel, A.J.; Clancy, P.; Weihs, T.P. Machine learning-guided accelerated discovery of structure-property correlations in lean magnesium alloys for biomedical applications. J. Magnes. Alloys 2024, 12, 2267–2283. [Google Scholar]
- Liu, Y.; Zheng, Y.; Chen, X.H.; Yang, J.A.; Pan, H.; Chen, D.; Wang, L.; Zhang, J.; Zhu, D.; Wu, S.; et al. Fundamental Theory of Biodegradable Metals—Definition, Criteria, and Design. Adv. Funct. Mater. 2019, 29, 1805402. [Google Scholar]
- Yang, C.; Sheng, L.; Zhao, C.; Wu, D.; Zheng, Y. Regulating the ablation of nanoparticle-doped MAO coating on Mg alloy by MgF2 passivation layer construction. Mater. Lett. 2024, 355, 135559. [Google Scholar]
- Thekkepat, K.; Han, H.-S.; Choi, J.-W.; Lee, S.-C.; Yoon, E.S.; Li, G.; Seok, H.-K.; Kim, Y.-C.; Kim, J.-H.; Cha, P.-R. Computational design of Mg alloys with minimal galvanic corrosion. J. Magnes. Alloys 2022, 10, 1972–1980. [Google Scholar]
- Gungor, A.; Incesu, A. Effects of alloying elements and thermomechanical process on the mechanical and corrosion properties of biodegradable Mg alloys. J. Magnes. Alloys 2021, 9, 241–253. [Google Scholar]
- Fattah-Alhosseini, A.; Karbasi, M.; Chaharmahali, R.; Fardosi, A.; Kaseem, M. An overview of electrochemical, non-electrochemical and analytical approaches for studying corrosion in magnesium and its alloys. J. Magnes. Alloys 2024, 12, 3516–3542. [Google Scholar] [CrossRef]
- Wang, B.; Xu, D.; Zhuang, X.; Sheng, L. Inhibiting effect of I-phase formation on the plastic instability of the duplex structured Mg-8Li-6Zn-1.2 Y (in wt.%) alloy. J. Magnes. Alloys 2023, 11, 2196–2204. [Google Scholar] [CrossRef]
- Wang, B.; Xu, D.; Jiang, C.; Sheng, L.; Han, E. Relationship between the fatigue behavior and grain structures of an as-extruded Mg-6.2% Zn-0.6% Zr (in wt.%) alloy. J. Mater. Sci. Technol. 2023, 149, 119–126. [Google Scholar] [CrossRef]
- Sheng, L.Y.; Guo, J.T.; Tian, Y.X.; Zhou, L.Z.; Ye, H.Q. Microstructure and mechanical properties of rapidly solidified NiAl-Cr(Mo) eutectic alloy doped with trace Dy. J. Alloys Compd. 2009, 475, 730–734. [Google Scholar] [CrossRef]
- Ramesh, S.; Anne, G.; Nayaka, H.S.; Sahu, S.; Ramesh, M.R. Influence of Multidirectional Forging on Microstructural, Mechanical, and Corrosion Behavior of Mg-Zn Alloy. J. Mater. Eng. Perform. 2019, 28, 2053–2062. [Google Scholar]
- Bazhenov, V.; Li, A.; Tavolzhanskii, S.; Bazlov, A.; Tabachkova, N.; Koltygin, A.; Komissarov, A.; Shin, K.S. Microstructure and Mechanical Properties of Hot-Extruded Mg-Zn-Ga-(Y) Biodegradable Alloys. Materials 2022, 15, 6849. [Google Scholar] [CrossRef]
- Yurchenko, N.Y.; Stepanov, N.D.; Salishchev, G.A.; Serebryany, V.N.; Martynenko, N.S.; Lukyanova, E.A.; Rokhlin, L.L.; Birbilis, N.; Dobatkin, S.V.; Estrin, Y.Z. Effect of multiaxial deformation on structure, mechanical properties, and corrosion resistance of a Mg-Ca alloy. J. Magnes. Alloys 2022, 10, 266–280. [Google Scholar] [CrossRef]
- Chen, K.; Xie, X.; Tang, H.; Sun, H.; Qin, L.; Zheng, Y.; Gu, X.; Fan, Y. In vitro and in vivo degradation behavior of Mg-2Sr-Ca and Mg-2Sr-Zn alloys. Bioact. Mater. 2020, 5, 275–285. [Google Scholar] [CrossRef]
- Wang, C.; Zeng, L.; Zhang, W.; Tang, F.; Ding, W.; Xiao, S.; Liang, T. Enhanced mechanical properties and corrosion resistance of rolled Mg-1.5Sn-0.5Ca alloy by Ce microalloying. Mater. Charact. 2021, 179, 111325. [Google Scholar] [CrossRef]
- Zemková, M.; Minárik, P.; Dittrich, J.; Bohlen, J.; Král, R. Individual effect of Y and Nd on the microstructure formation of Mg-Y-Nd alloys processed by severe plastic deformation and their effect on the subsequent mechanical and corrosion properties. J. Magnes. Alloys 2023, 11, 509–521. [Google Scholar] [CrossRef]
- Chai, Y.; He, C.; Jiang, B.; Fu, J.; Jiang, Z.; Yang, Q.; Sheng, H.; Huang, G.; Zhang, D.; Pan, F. Influence of minor Ce additions on the microstructure and mechanical properties of Mg-1.0Sn-0.6Ca alloy. J. Mater. Sci. Technol. 2020, 37, 26–37. [Google Scholar] [CrossRef]
- Shi, L.L.; Huang, Y.; Yang, L.; Feyerabend, F.; Mendis, C.; Willumeit, R.; Ulrich Kainer, K.; Hort, N. Mechanical properties and corrosion behavior of Mg-Gd-Ca-Zr alloys for medical applications. J. Mech. Behav. Biomed. Mater. 2015, 47, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Huang, Y.; Peng, Q.; Feyerabend, F.; Kainer, K.U.; Willumeit, R.; Hort, N. Mechanical and corrosion properties of binary Mg-Dy alloys for medical applications. Mater. Sci. Eng. B 2011, 176, 1827–1834. [Google Scholar] [CrossRef]
- Wang, J.; Meng, L.; Zhang, Z.; Sa, B.; Fu, X.; Sheng, L.; Xu, D.; Zheng, Y. Investigation on the crystal structure and mechanical properties of the ternary compound Mg11-xZnxSr combined with experimental measurements and first-principles calculations. J. Magnes. Alloys 2023, 11, 1074–1082. [Google Scholar] [CrossRef]
- Li, C.Q.; Xu, D.K.; Yu, S.; Sheng, L.Y.; Han, E.H. Effect of Icosahedral Phase on Crystallographic Texture and Mechanical Anisotropy of Mg-4%Li Based Alloys. J. Mater. Sci. Technol. 2017, 33, 475–480. [Google Scholar] [CrossRef]
- Zhou, T.S.; Li, Y.Q.; Guo, F.F.; Li, Q.L.; Jia, Z.; Liu, D.X. Achieving high strength-ductility synergy in Mg-6Sn-3Zn-0.3Zr (wt.%) alloy via a combination of casting, pre-treatment and hot extrusion. J. Magnes. Alloys 2023, 12, 4622–4645. [Google Scholar] [CrossRef]
- Kasaeian-Naeini, M.; Sedighi, M.; Hashemi, R. Severe plastic deformation (SPD) of biodegradable magnesium alloys and composites: A review of developments and prospects. J. Magnes. Alloys 2022, 10, 938–955. [Google Scholar]
- Baral, S.K.; Thawre, M.M.; Ratna Sunil, B.; Dumpala, R. A review on developing high-performance ZE41 magnesium alloy by using bulk deformation and surface modification methods. J. Magnes. Alloys 2023, 11, 776–800. [Google Scholar]
- Li, C.; Xu, D.; Wang, B.; Sheng, L.; Han, E. Suppressing effect of heat treatment on the Portevin–Le Chatelier phenomenon of Mg-4% Li-6% Zn-1.2% Y alloy. J. Mater. Sci. Technol. 2016, 32, 1232–1238. [Google Scholar] [CrossRef]
- Kiani, F.; Lin, J.; Munir, K.; Wen, C.; Li, Y. Improvements in mechanical, corrosion, and biocompatibility properties of Mg-Zr-Sr-Dy alloys via extrusion for biodegradable implant applications. J. Magnes. Alloys 2023, 11, 3840–3865. [Google Scholar]
- Deng, J.F.; Tian, J.; Zhou, Y.C.; Chang, Y.Y.; Liang, W.; Ma, J.Y. Plastic deformation and fracture mechanisms of rolled Mg-8Gd-4Y-Zn and AZ31 magnesium alloys. Mater. Des. 2022, 223, 111179. [Google Scholar] [CrossRef]
- Zou, J.F.; Ma, L.F.; Jia, W.T.; Le, Q.C.; Qin, G.W.; Yuan, Y. Microstructural and mechanical response of ZK60 magnesium alloy subjected to radial forging. J. Mater. Sci. Technol. 2021, 83, 228–238. [Google Scholar] [CrossRef]
- Deng, J.F.; Tian, J.; Zhou, Y.C.; Chang, Y.Y.; Liang, W.; Ma, J.Y. Plastic deformation mechanism and hardening mechanism of rolled Rare-Earth magnesium alloy thin sheet. Mater. Des. 2022, 218, 110678. [Google Scholar] [CrossRef]
- Peng, X.; Liu, W.; Wu, G.; Ji, H.; Ding, W. Plastic deformation and heat treatment of Mg-Li alloys: A review. J. Mater. Sci. Technol. 2022, 99, 193–206. [Google Scholar] [CrossRef]
- Papenberg, N.P.; Gneiger, S.; Uggowitzer, P.J.; Pogatscher, S. Lean Wrought Magnesium Alloys. Materials 2021, 14, 4282. [Google Scholar] [CrossRef]
- Liao, Q.; Hu, W.; Chen, R.; Le, Q. Effect of Zn/Y atomic ratio on precipitation behavior and dynamic recrystallization behavior of Mg-Zn-Y alloy under different extrusion temperature. J. Mater. Res. Technol. 2023, 27, 48–62. [Google Scholar] [CrossRef]
- Wang, B.; Xu, D.; Cai, X.; Qiao, Y.; Sheng, L. Effect of rolling ratios on the microstructural evolution and corrosion performance of an as-rolled Mg-8 wt.% Li alloy. J. Magnes. Alloys 2021, 9, 560–568. [Google Scholar] [CrossRef]
- Ahmadi, S.; Alimirzaloo, V.; Faraji, G.; Doniavi, A. Properties inhomogeneity of AM60 magnesium alloy processed by cyclic extrusion compression angular pressing followed by extrusion. Trans. Nonferrous Met. Soc. China 2021, 31, 655–665. [Google Scholar] [CrossRef]
- Horky, J.; Bryła, K.; Krystian, M.; Mozdzen, G.; Mingler, B.; Sajti, L. Improving mechanical properties of lean Mg-Zn-Ca alloy for absorbable implants via Double Equal Channel Angular Pressing (D-ECAP). Mater. Sci. Eng. A 2021, 826, 142002. [Google Scholar] [CrossRef]
- Martynenko, N.; Lukyanova, E.; Serebryany, V.; Prosvirnin, D.; Terentiev, V.; Raab, G.; Dobatkin, S.; Estrin, Y. Effect of equal channel angular pressing on structure, texture, mechanical and in-service properties of a biodegradable magnesium alloy. Mater. Lett. 2019, 238, 218–221. [Google Scholar] [CrossRef]
- Sheng, K.; Li, W.; Du, P.; Mei, D.; Zhu, S.; Wang, L.; Guan, S. Shortening the manufacturing process of degradable magnesium alloy minitube for vascular stents by introducing cyclic extrusion compression. J. Magnes. Alloys 2024, 12, 3204–3215. [Google Scholar] [CrossRef]
- Wang, L.P.; Chen, T.; Jiang, W.Y.; Feng, Y.C.; Cao, G.J.; Zhu, Y. Microstructure and mechanical properties of AM60B magnesium alloy prepared by cyclic extrusion compression. Trans. Nonferrous Met. Soc. China 2013, 23, 3200–3205. [Google Scholar] [CrossRef]
- Wu, Q.; Zhu, S.; Wang, L.; Liu, Q.; Yue, G.; Wang, J.; Guan, S. The microstructure and properties of cyclic extrusion compression treated Mg-Zn-Y-Nd alloy for vascular stent application. J. Mech. Behav. Biomed. Mater. 2012, 8, 1–7. [Google Scholar] [CrossRef]
- Sułkowski, B.; Janoska, M.; Boczkal, G.; Chulist, R.; Mroczkowski, M.; Pałka, P. The effect of severe plastic deformation on the Mg properties after CEC deformation. J. Magnes. Alloys 2020, 8, 761–768. [Google Scholar] [CrossRef]
- Tian, J.; Deng, J.; Zhou, Y.; Chang, Y.; Liang, W.; Ma, J. Slip behavior during tension of rare earth magnesium alloys processed by different rolling methods. J. Mater. Res. Technol. 2023, 22, 473–488. [Google Scholar] [CrossRef]
- Zhi, C.; Wu, Z.; Ma, L.; Huang, Z.; Zheng, Z.; Xv, H.; Jia, W.; Lei, J. Effect of thickness ratio on interfacial structure and mechanical properties of Mg/Al composite plates in differential temperature asymmetrical rolling. J. Mater. Res. Technol. 2023, 24, 8332–8347. [Google Scholar] [CrossRef]
- Ren, X.; Huang, Y.; Zhang, X.; Li, H.; Zhao, Y. Influence of shear deformation during asymmetric rolling on the microstructure, texture, and mechanical properties of the AZ31B magnesium alloy sheet. Mater. Sci. Eng. A 2021, 800, 140306. [Google Scholar] [CrossRef]
- Ji, Y.F.; Duan, J.R.; Yuan, H.; Li, H.Y.; Sun, J.; Ma, L.F. Effect of variable thickness cross rolling on edge crack and microstructure gradient of AZ31 magnesium alloy. J. Cent. South Univ. 2022, 29, 1124–1132. [Google Scholar] [CrossRef]
- Zou, Y.; Guo, S.; Tang, S.; Assari, A.H.; Azimi, M.; Ghaderi, S.; Mahmoodi, M. Enhancing grain refinement and mechanical properties of AA1100/MgAZ31/AA1100 composites by using different roll bonding techniques. Mater. Today Commun. 2024, 38, 108000. [Google Scholar] [CrossRef]
- Zhang, A.X.; Li, F.; Huo, P.D.; Niu, W.T.; Gao, R.H. Response mechanism of matrix microstructure evolution and mechanical behavior to Mg/Al composite plate by hard-plate accumulative roll bonding. J. Mater. Res. Technol. 2023, 23, 3312–3321. [Google Scholar] [CrossRef]
- Zhang, W.; Su, Z.; Hu, X.; Ju, D.; Zhao, H. The Evolution of Microstructure and Electromagnetic Interference Shielding Effectiveness of Mg-2Mn-1Ce Alloy Prepared by Accumulative Roll Bonding Process. J. Mater. Eng. Perform. 2023, 33, 1672–1684. [Google Scholar] [CrossRef]
- Bencherifa, I.; Alili, B.; Baudin, T.; Brisset, F.; Thiaudière, D.; Mocuta, C.; Bradai, D. On the microstructure and texture of intermetallics in Al/Mg/Al multi-layer composite fabricated by Accumulative Roll Bonding. Micron 2023, 173, 103507. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Pan, H.; He, Z.; Tang, W.; Li, C.; Yang, C.; Xie, D.; Qin, G. Mechanical anisotropy and microstructural heterogeneity of a free-forged ME20 alloy with a large cross section. Mater. Sci. Eng. A 2023, 863, 144551. [Google Scholar] [CrossRef]
- Li, Z.J.; Wang, J.G.; Ni, T.; Zhang, W.; Tong, G.D.; Chen, X.G.; Li, J.; Yan, R.F. Microstructure evolution and deformation mechanism of AZ80 alloy during die forging. Mater. Sci. Eng. A 2023, 869, 144789. [Google Scholar] [CrossRef]
- Xia, X.; Guo, Y.; Li, M.; Gao, S.; Xiang, L. Influence of temperature and cumulative strain on microstructure and mechanical properties of multi-directional forged Mg-Gd-Y-Zn-Zr alloy. J. Mater. Res. Technol. 2024, 30, 2458–2466. [Google Scholar] [CrossRef]
- Cao, F.F.; Deng, K.K.; Nie, K.B.; Kang, J.W.; Niu, H.Y. Microstructure and corrosion properties of Mg-4Zn-2Gd-0.5Ca alloy influenced by multidirectional forging. J. Alloys Compd. 2019, 770, 1208–1220. [Google Scholar] [CrossRef]
- Zeng, J.; Wang, F.; Wei, X.; Dong, S.; Zhang, Z.; Dong, J. A New Constitutive Model for Thermal Deformation of Magnesium Alloys. Metall. Mater. Trans. A 2020, 51, 497–512. [Google Scholar] [CrossRef]
- Liu, Z.; Nie, J.F.; Zhao, Y.h. Effect of deformation processing on microstructure evolution and mechanical properties of Mg−Li alloys: A review. Trans. Nonferrous Met. Soc. China 2024, 34, 1–25. [Google Scholar] [CrossRef]
- Li, L.; Liu, W.; Qi, F.; Wu, D.; Zhang, Z. Effects of deformation twins on microstructure evolution, mechanical properties and corrosion behaviors in magnesium alloys—A review. J. Magnes. Alloys 2022, 10, 2334–2353. [Google Scholar] [CrossRef]
- Sheng, L.; Yang, F.; Xi, T.; Guo, J.; Ye, H. Microstructure evolution and mechanical properties of Ni3Al/Al2O3 composite during self-propagation high-temperature synthesis and hot extrusion. Mater. Sci. Eng. A 2012, 555, 131–138. [Google Scholar] [CrossRef]
- Rakshith, M.; Seenuvasaperumal, P. Review on the effect of different processing techniques on the microstructure and mechanical behaviour of AZ31 Magnesium alloy. J. Magnes. Alloys 2021, 9, 1692–1714. [Google Scholar]
- Guan, R.G.; Cipriano, A.F.; Zhao, Z.Y.; Lock, J.; Tie, D.; Zhao, T.; Cui, T.; Liu, H. Development and evaluation of a magnesium-zinc-strontium alloy for biomedical applications—Alloy processing, microstructure, mechanical properties, and biodegradation. Mater. Sci. Eng. C 2013, 33, 3661–3669. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Xu, D.; Sheng, L.; Han, E.; Sun, J. Deformation and fracture mechanisms of an annealing-tailored “bimodal” grain-structured Mg alloy. J. Mater. Sci. Technol. 2019, 35, 2423–2429. [Google Scholar] [CrossRef]
- Sheng, L.; Yang, F.; Xi, T.; Lai, C.; Ye, H. Influence of heat treatment on interface of Cu/Al bimetal composite fabricated by cold rolling. Compos. Part B Eng. 2011, 42, 1468–1473. [Google Scholar] [CrossRef]
- Wang, S.; Xu, D.; Wang, B.; Sheng, L.; Han, E.; Dong, C. Effect of solution treatment on stress corrosion cracking behavior of an as-forged Mg-Zn-Y-Zr alloy. Sci. Rep. 2016, 6, 29471. [Google Scholar] [CrossRef]
- Ouyang, W.; Zhang, L.; Wu, H.; Wu, D.; Zhang, S.; Qin, X.; Jiang, S.; Li, S.; Zhang, W.; Sheng, L. Optimized mechanical properties of the hot forged Ti-6Al-4V alloy by regulating multiscale microstructure via laser shock peening. Int. J. Mach. Tools Manuf. 2024, 201, 104192. [Google Scholar] [CrossRef]
- Martynenko, N.; Anisimova, N.; Kiselevskiy, M.; Tabachkova, N.; Temralieva, D.; Prosvirnin, D.; Terentiev, V.; Koltygin, A.; Belov, V.; Morosov, M.; et al. Structure, mechanical characteristics, biodegradation, and in vitro cytotoxicity of magnesium alloy ZX11 processed by rotary swaging. J. Magnes. Alloys 2020, 8, 1038–1046. [Google Scholar] [CrossRef]
- Wang, H.; Luo, X.C.; Zhang, D.T.; Qiu, C.; Chen, D.L. High-strength extruded magnesium alloys: A critical review. J. Mater. Sci. Technol. 2024, 199, 27–52. [Google Scholar] [CrossRef]
- Wang, J.; Long, Y.; Yang, C.; Liu, J.; Tang, A.; Yu, Z.; Pan, F. Hot deformation behavior and extrusion temperature-dependent microstructure, texture and mechanical properties of Mg-1Mn alloy. J. Mater. Res. Technol. 2024, 30, 1662–1676. [Google Scholar] [CrossRef]
- Gui, Z.; Jiang, F.; Kang, Z.; Zhang, F.; Li, Z.; Zhang, J. Microstructure and Properties of Micro-Alloyed Mg-2.0Nd-0.2Sr by Heat Treatment and Extrusion. Acta Metall. Sin. (Engl. Lett.) 2022, 36, 323–334. [Google Scholar] [CrossRef]
- Cai, C.; Alves, M.M.; Song, R.; Wang, Y.; Li, J.; Montemor, M.F. Non-destructive corrosion study on a magnesium alloy with mechanical properties tailored for biodegradable cardiovascular stent applications. J. Mater. Sci. Technol. 2021, 66, 128–138. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, J.; Weiyang, J.; Qiao, B.; Wang, Y.; Li, Y.; Jiang, D. A novel biodegradable Mg-1Zn-0.5Sn alloy: Mechanical properties, corrosion behavior, biocompatibility, and antibacterial activity. J. Magnes. Alloys 2020, 8, 374–386. [Google Scholar] [CrossRef]
- Du, B.N.; Hu, Z.Y.; Sheng, L.Y.; Xu, D.K.; Qiao, Y.X.; Wang, B.J.; Wang, J.; Zheng, Y.F.; Xi, T.F. Microstructural characteristics and mechanical properties of the hot extruded Mg-Zn-Y-Nd alloys. J. Mater. Sci. Technol. 2021, 60, 44–55. [Google Scholar]
- Dai, Y.; Xiao, M.; Hu, Y.; Yang, Y.; Jiang, B.; Zheng, T.; Dong, L.; Yang, B.; Zheng, C. Effect of extrusion parameters on microstructure and mechanical properties of Mg-0.12Ca-0.08Ba alloy. J. Mater. Res. Technol. 2022, 20, 1570–1579. [Google Scholar] [CrossRef]
- Sheng, L.; Zhang, X.; Zhao, H.; Du, B.; Zheng, Y.; Xi, T. Influence of Multi-Pass Hot Extrusion on Microstructure and Mechanical Properties of the Mg-4Zn-1.2Y-0.8Nd Alloy. Crystals 2021, 11, 425. [Google Scholar] [CrossRef]
- Sheng, L.Y.; Du, B.N.; Hu, Z.Y.; Qiao, Y.X.; Xiao, Z.P.; Wang, B.J.; Xu, D.K.; Zheng, Y.F.; Xi, T.F. Effects of annealing treatment on microstructure and tensile behavior of the Mg-Zn-Y-Nd alloy. J. Magnes. Alloys 2020, 8, 601–613. [Google Scholar] [CrossRef]
- Du, B.; Hu, Z.; Wang, J.; Sheng, L.; Zhao, H.; Zheng, Y.; Xi, T. Effect of extrusion process on the mechanical and in vitro degradation performance of a biomedical Mg-Zn-Y-Nd alloy. Bioact. Mater. 2020, 5, 219–227. [Google Scholar] [CrossRef]
- Alateyah, A.I.; Alrumayh, A.; Alhabib, O.; AlSulaim, S.K.; Aljouie, M.A.S.; Alqatuimy, M.; Altoaimi, S.A.; El-Garaihy, W.H. Effect of microstructural and texture evolution of ECAP-processed Mg-Zn-Zr alloy on the corrosion and wear behaviours for bone repair applications. J. Eng. Res. 2024; in press. [Google Scholar] [CrossRef]
- Alateyah, A.I.; Alawad, M.O.; Aljohani, T.A.; El-Garaihy, W.H. Influence of Ultrafine-Grained Microstructure and Texture Evolution of ECAPed ZK30 Magnesium Alloy on the Corrosion Behavior in Different Corrosive Agents. Materials 2022, 15, 5515. [Google Scholar] [CrossRef]
- Gautam, P.C.; Al-Samman, T.; Thangaraju, S.; Aich, S.; Biswas, S. Effect of ECAP strain path on deformation behaviour, microstructure, texture evolution, and mechanical properties in magnesium. Mater. Sci. Eng. A 2024, 913, 147077. [Google Scholar] [CrossRef]
- Tong, L.B.; Chu, J.H.; Sun, W.T.; Jiang, Z.H.; Zou, D.N.; Liu, S.F.; Kamado, S.; Zheng, M.Y. Development of a high-strength Mg alloy with superior ductility through a unique texture modification from equal channel angular pressing. J. Magnes. Alloys 2021, 9, 1007–1018. [Google Scholar] [CrossRef]
- Prithivirajan, S.; Nyahale, M.B.; Naik, G.M.; Narendranath, S.; Prabhu, A.; Rekha, P.D. Bio-corrosion impacts on mechanical integrity of ZM21 Mg for orthopaedic implant application processed by equal channel angular pressing. J. Mater. Sci. Mater. Med. 2021, 32, 65. [Google Scholar] [CrossRef] [PubMed]
- Lei, W.; Zhang, H. Analysis of microstructural evolution and compressive properties for pure Mg after room-temperature ECAP. Mater. Lett. 2020, 271, 127781. [Google Scholar] [CrossRef]
- Gopi, K.R.; Anil Kumar, K.S.; Madhu, H.C. Effect of equal channel angular pressing on magnesium alloys—A review. Mater. Today Proc. 2024; in press. [Google Scholar] [CrossRef]
- Alateyah, A.I.; BaQais, A.; Ahmed, M.M.Z.; Zedan, Y.; Alawad, M.O.; El-Asfoury, M.S.; El-Garaihy, W.H. Improved corrosion resistance and mechanical properties of severely deformed ZM31 alloy. Heliyon 2024, 10, e26400. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y. Dynamic recrystallization mechanism, texture evolution development and mechanical characteristics of a Mg-8.7Gd-4.18Y-0.42Zr magnesium alloy by ECAP. Prog. Nat. Sci. Mater. Int. 2024, 34, 376–388. [Google Scholar] [CrossRef]
- Zhou, X.; Xiao, S.; Li, M.; Wang, Y.; Lu, X.; Chen, Z.; Guo, Z.; Xiao, H.; Guo, J. Effect of rotational-die ECAP parameters on microstructure and mechanical properties of Mg97Y2Zn alloys. J. Mater. Res. Technol. 2024, 29, 3832–3841. [Google Scholar] [CrossRef]
- Arhin, G.; Ma, A.B.; Jiang, J.H.; Taylor, E.K.; Song, D. Microstructure evolution and mechanical properties of Mg-Mn-RE alloy processed by equal channel angular pressing. Mater. Today Commun. 2024, 38, 107744. [Google Scholar] [CrossRef]
- Li, M.; Yao, M.; Ning, Y.; Yu, J.; Xing, Z.; Gao, S.; Zhang, F.; Xia, X.; Zhao, G.; Liu, P.; et al. The effects of ECAP, Mn and Ca elements on the microstructure and corrosion properties of magnesium alloys were investigated. Int. J. Electrochem. Sci. 2024, 19, 100850. [Google Scholar] [CrossRef]
- El-Sanabary, S.; Kouta, H.; Shaban, M.; Alrumayh, A.; Alateyah, A.I.; Alsunaydih, F.N.; Alawad, M.O.; El-Taybany, Y.; El-Asfoury, M.S.; El-Garaihy, W.H. A comparative study of machine learning and response surface methodologies for optimizing wear parameters of ECAP-processed ZX30 alloy. Heliyon 2024, 10, e33967. [Google Scholar] [CrossRef]
- Li, B.; Duan, Y.; Li, M.; Qi, H.; Zheng, S.; Peng, M. Effects of broken β-Mg17Al12 and non-uniform Mg2Pb particles introduced by EX-ECAP on microstructure and mechanical properties of Mg-Pb-9.2Al-0.8B alloys. J. Mater. Res. Technol. 2024, 31, 908–926. [Google Scholar] [CrossRef]
- Sun, Z.; Li, Y.; Ma, M.; Li, X.; Shi, G.; Yuan, J.; Chen, D.; Zhang, K. Study on the effect of the ECAP deformation on the organization and properties of the extruded Mg-Sn-Al alloys. Mater. Lett. 2024, 357, 135775. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, B.; Li, Y.; Hu, S.; Li, X.; Liu, D. Preparation of ultra-high ductility and high strength Mg-Sn-Zn-Zr alloy by differential thermal ECAP (DT-ECAP) induced heterogeneous structure. J. Mater. Sci. Technol. 2024, 199, 222–245. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, B.; Zhang, M.; Li, Y.; Hu, S.; Li, X.; Liu, D. Excellent strength-ductility synergy properties of Mg-Sn-Zn-Zr alloy mediated by a novel differential thermal ECAP (DT-ECAP). Mater. Sci. Eng. A 2024, 899, 146469. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Zhang, L.; Wang, Q.; Zhou, H.; Li, W. Damping performance of SiC nanoparticles reinforced magnesium matrix composites processed by cyclic extrusion and compression. J. Magnes. Alloys 2023, 11, 1608–1617. [Google Scholar] [CrossRef]
- Marheb, A.; Subhi, A. Microstructure and Mechanical Properties of ZK60 Mg Alloy Processed by Cyclic Expansion-Extrusion (CEE) at Different Temperatures. Eng. Technol. J. 2022, 40, 1624–1630. [Google Scholar] [CrossRef]
- Shi, Y.; Zheng, J.; Ji, J.; Zhang, H.; Zhang, Z.; Wang, Q.; Xue, Y. The improvement of grain refinement, texture modification and mechanical properties of pure Mg prepared by cyclic expansion extrusion with an asymmetric extrusion cavity. Mater. Res. Express. 2021, 8, 046530. [Google Scholar] [CrossRef]
- Mashoufi, K.; Garmroodi, P.; Mirzakhani, A.; Assempour, A. Cyclic contraction-expansion extrusion (CCEE): An innovate severe plastic deformation method for tailoring the microstructure and mechanical properties of magnesium AZ91 alloy. J. Mater. Res. Technol. 2023, 26, 8541–8554. [Google Scholar] [CrossRef]
- Zhang, Y.; Kent, D.; Wang, G.; StJohn, D.; Dargusch, M.S. The cold-rolling behaviour of AZ31 tubes for fabrication of biodegradable stents. J. Mech. Behav. Biomed. Mater. 2014, 39, 292–303. [Google Scholar] [CrossRef]
- Rahim, S.A.; Mohan, K.S.S.; Rabeeh, V.P.M.; Joseph, M.A.; Mubarak Ali, M.; Hanas, T. Hot rolled Mg-Ca/nHA composite for biodegradable implant material—A novel approach. Mater. Today Commun. 2023, 35, 106235. [Google Scholar] [CrossRef]
- Li, Q.K.; Yan, H.; Chen, R.S. Effect of rolling reduction on deformation mechanism and twinning behavior of WE43 magnesium alloy. Trans. Nonferrous Met. Soc. China 2022, 32, 3901–3913. [Google Scholar] [CrossRef]
- Ma, C.; Ma, X.; Pei, X.; Xu, Y.; Peng, P.; Wang, N. Effect of rolling reduction on the microstructure and mechanical properties of hot-rolled Mg-Li-Al-Ca alloys. Mater. Today Commun. 2023, 37, 107469. [Google Scholar] [CrossRef]
- Rao, X.; Wu, Y.; Pei, X.; Jing, Y.; Luo, L.; Liu, Y.; Lu, J. Influence of rolling temperature on microstructural evolution and mechanical behavior of AZ31 alloy with accumulative roll bonding. Mater. Sci. Eng. A 2019, 754, 112–120. [Google Scholar] [CrossRef]
- Tu, J.; Zhou, T.; Liu, L.; Shi, L.; Hu, L.; Song, D.; Song, B.; Yang, M.; Chen, Q.; Pan, F. Effect of rolling speeds on texture modification and mechanical properties of the AZ31 sheet by a combination of equal channel angular rolling and continuous bending at high temperature. J. Alloys Compd. 2018, 768, 598–607. [Google Scholar] [CrossRef]
- Kong, F.; Yang, Y.; Chen, H.; Liu, H.; Fan, C.; Xie, W.; Wei, G. Dynamic recrystallization and deformation constitutive analysis of Mg-Zn-Nd-Zr alloys during hot rolling. Heliyon 2022, 8, e09995. [Google Scholar] [CrossRef]
- Li, X.; Guo, F.; Ma, Y.; Jiang, L.; Lai, H.; Liu, H.; Zhang, D.; Pei, R. Rolling texture development in a dual-phase Mg-Li alloy: The role of temperature. J. Magnes. Alloys 2023, 11, 2980–2990. [Google Scholar] [CrossRef]
- Liu, K.; Hu, D.; Lou, F.; Yu, Z.; Li, S.; Du, X.; Du, W. Effects of deformation temperatures on microstructures, aging behaviors and mechanical properties of Mg-Gd-Er-Zr alloys fabricated by hard-plate rolling. J. Magnes. Alloys 2024, 12, 2345–2359. [Google Scholar] [CrossRef]
- Niu, W.; Wang, D.; Wang, G.; Li, J. Recrystallization and Anisotropy of AZ31 Magnesium Alloy by Asynchronous Rolling. Metals 2023, 13, 1631. [Google Scholar] [CrossRef]
- Wang, D.; Jing, Y.; Gao, Y.; Li, J.; Shi, Y.; Misra, R.D.K. Enhanced mechanical properties of AZ91 magnesium alloy by asynchronously large-strain high-efficiency rolling with bimodal grain structure. J. Mater. Res. Technol. 2023, 27, 4430–4439. [Google Scholar] [CrossRef]
- Wang, K.; Wang, X.; Dang, C.; Zhao, P.; Wang, C.; Zhang, Z.; Liu, M.; Wang, J. Microstructure evolution and mechanical properties of high-strength Mg-Gd-Y-Zn-Mn alloy processed by asymmetric hot rolling. J. Mater. Res. Technol. 2023, 24, 2907–2917. [Google Scholar] [CrossRef]
- Gerashi, E.; Alizadeh, R.; Langdon, T.G. Effect of crystallographic texture and twinning on the corrosion behavior of Mg alloys: A review. J. Magnes. Alloys 2022, 10, 313–325. [Google Scholar] [CrossRef]
- Chen, T.; Chen, Z.; Yi, L.; Xiong, J.; Liu, C. Effects of texture on anisotropy of mechanical properties in annealed Mg-0.6%Zr-1.0%Cd sheets by unidirectional and cross rolling. Mater. Sci. Eng. A 2014, 615, 324–330. [Google Scholar] [CrossRef]
- Han, Y.; Chen, S.; Wan, Y.; Liu, C.; Chen, Z. Microstructure, texture evolution and mechanical anisotropy of Mg-Gd-Y-Zn-Zr alloy sheets produced by unidirectional and cross rolling. Mater. Sci. Eng. A 2024, 893, 146127. [Google Scholar] [CrossRef]
- Lin, X.; Chen, Z.; Shao, J.; Xiong, J.; Hu, Z.; Liu, C. Deformation mechanism, orientation evolution and mechanical properties of annealed cross-rolled Mg-Zn-Zr-Y-Gd sheet during tension. J. Magnes. Alloys 2023, 11, 2340–2350. [Google Scholar] [CrossRef]
- Vignesh, P.; Abraham, A.; Kumaran, S. Texture-governed electrochemical behaviour of cross rolled Mg-5Al-5Sn (AT55) magnesium alloy. Mater. Chem. Phys. 2022, 290, 126622. [Google Scholar] [CrossRef]
- Wang, D.; Xu, D.; Wang, B.; Yan, C.; Wang, S.; Xu, X.; Zhang, L.; Lu, C. Effect of cross rolling on the microstructure and mechanical performance of a dual-phase structured Mg-8Li-6Zn-1Y (in wt.%) alloy. J. Mater. Sci. Technol. 2024, 176, 132–144. [Google Scholar] [CrossRef]
- Wang, Q.; Jiang, B.; Liu, L.; Yang, Q.; Xia, D.; Zhang, D.; Huang, G.; Pan, F. Reduction per pass effect on texture traits and mechanical anisotropy of Mg-Al-Zn-Mn-Ca alloy subjected to unidirectional and cross rolling. J. Mater. Res. Technol. 2020, 9, 9607–9619. [Google Scholar] [CrossRef]
- Zhang, P.; Xin, Y.; Zhang, L.; Pan, S.; Liu, Q. On the texture memory effect of a cross-rolled Mg-2Zn-2Gd plate after unidirectional rolling. J. Mater. Sci. Technol. 2020, 41, 98–104. [Google Scholar] [CrossRef]
- Trojanova, Z.; Dzugan, J.; Halmesova, K.; Nemeth, G.; Minarik, P.; Lukac, P.; Bohlen, J. Influence of Accumulative Roll Bonding on the Texture and Tensile Properties of an AZ31 Magnesium Alloy Sheets. Materials 2018, 11, 73. [Google Scholar] [CrossRef]
- Li, Z.G.; Li, W.Q.; Liu, F.N.; Ma, P.K.; Liu, P.L.; Jia, H.L. Microstructure and mechanical properties of AT31/ATX3105 magnesium alloy composite sheets fabricated by accumulative roll bonding. J. Mater. Res. Technol. 2024, 31, 1596–1606. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, D.; Tong, X.; Lin, J.; Li, Y.; Wen, C. Mechanical properties, corrosion behavior, and cytotoxicity of biodegradable Zn/Mg multilayered composites prepared by accumulative roll bonding process. Acta Biomater. 2024, 173, 509–525. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.T.; Ostrom, T. Mechanical behavior of cast and forged magnesium alloys and their microstructures. Mater. Sci. Eng. A 2008, 490, 52–56. [Google Scholar] [CrossRef]
- Papenberg, N.P.; Gneiger, S.; Weissensteiner, I.; Uggowitzer, P.J.; Pogatscher, S. Mg-Alloys for Forging Applications-A Review. Materials 2020, 13, 985. [Google Scholar] [CrossRef]
- Mandal, S.; Nigamananda Sahoo, S.; Balla, V.K.; Das, M.; Roy, M. Synergistic improvement of antibacterial, mechanical and degradation properties of Cu added Mg-Zn-Zr alloy. Mater. Lett. 2023, 339, 134115. [Google Scholar] [CrossRef]
- Jiang, Y.; Le, Q.; Zhu, Y.; Liao, Q.; Wang, T.; Bao, L.; Wang, P. Review on forming process of magnesium alloy characteristic forgings. J. Alloys Compd. 2024, 970, 172666. [Google Scholar] [CrossRef]
- Rogachev, S.O.; Bazhenov, V.E.; Komissarov, A.A.; Ten, D.V.; Li, A.V.; Andreev, V.A.; Statnik, E.S.; Sadykova, I.A.; Plegunova, S.V.; Yushchuk, V.V.; et al. High strength and ductility in a new Mg-Zn-Ga biocompatible alloy by drawing and rotary forging. Results Mater. 2024, 21, 100524. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, X.; Nie, J.; Wei, K.; Mao, Q.; Lu, F.; Zhao, Y. Achieving ultra-strong Magnesium–lithium alloys by low-strain rotary swaging. Mater. Res. Lett. 2021, 9, 255–262. [Google Scholar] [CrossRef]
- Dong, B.; Zhang, Z.; Yu, J.; Che, X.; Meng, M.; Zhang, J. Microstructure, texture evolution and mechanical properties of multi-directional forged Mg-13Gd-4Y-2Zn-0.5Zr alloy under decreasing temperature. J. Alloys Compd. 2020, 823, 153776. [Google Scholar] [CrossRef]
- Huang, Y.; Wan, Y.; Liu, C.; Jiang, S.; Gao, Y.; Chen, Z. Effect of forging temperature on the microstructure, subsequent aging precipitation behavior, and mechanical properties of Mg-Gd-Y-Zr-Ag alloy. J. Mater. Sci. Technol. 2024, 181, 41–57. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, X.; Li, S.; Yu, J. Strengthening mechanism based on dislocation-twin interaction under room temperature multi-directional forging of AZ80 Mg alloy. J. Mater. Res. Technol. 2024, 29, 3656–3672. [Google Scholar] [CrossRef]
- Wang, H.; Xiao, X.; Qiao, C.; Wu, D.; Wang, Y.; Liu, Z.; Hao, L. Insight into multidirectional forging induced corrosion resistance evolution of Mg-Zn-Zr alloy. Mater. Lett. 2024, 355, 135587. [Google Scholar] [CrossRef]
- Li, S.; Wang, Z.; Zhao, X.; Wang, X.; Yu, J. Study on the plasticity improvement mechanism and grain refinement of AZ80 Mg alloy under cryogenic multi-directional forging. J. Mater. Res. Technol. 2024, 33, 384–397. [Google Scholar] [CrossRef]
- Wang, D.; Li, S.; Xin, Y.; Zhao, X. Simultaneously improving the deformation tolerance and mechanical properties of AZ80 magnesium alloy through the combination of pre-cryogenic and Multi-directional forging. Mater. Lett. 2024, 363, 136233. [Google Scholar] [CrossRef]
- Deng, Y.; Yan, H.; Li, Q.; Chen, J.; Xia, W.; Su, B.; Wu, M.; Yu, Y.; Song, M. Enhancing strength and ductility of low RE content Mg-Gd-Y-Zr alloy via a novel thermomechanical treatment based on multi-directional forging. J. Alloys Compd. 2023, 958, 170535. [Google Scholar] [CrossRef]
- Zhang, Z.; Yuan, L.; Zheng, M.; Wei, Q.; Shan, D.; Guo, B. Achievement of high strength and good ductility in the large-size AZ80 Mg alloy using a designed multi-directional forging process and aging treatment. J. Mater. Process. Technol. 2023, 311, 117828. [Google Scholar] [CrossRef]
- Guo, K.; Liu, M.; Wang, J.; Sun, Y.; Li, W.; Zhu, S.; Wang, L.; Guan, S. Microstructure and texture evolution of fine-grained Mg-Zn-Y-Nd alloy micro-tubes for biodegradable vascular stents processed by hot extrusion and rapid cooling. J. Magnes. Alloys 2020, 8, 873–882. [Google Scholar] [CrossRef]
- Hou, C.; Cao, H.; Qi, F.; Wang, Q.; Li, L.; Zhao, N.; Zhang, D.; Ouyang, X. Investigation on microstructures and mechanical properties of Mg-6Zn-0.5 Ce-xMn (x = 0 and 1) wrought magnesium alloys. J. Magnes. Alloys 2022, 10, 993–1003. [Google Scholar] [CrossRef]
- Tian, Y.; Miao, H.W.; Niu, J.L.; Huang, H.; Kang, B.; Zeng, H.; Ding, W.J.; Yuan, G.Y. Effects of annealing on mechanical properties and degradation behavior of biodegradable JDBM magnesium alloy wires. Trans. Nonferrous Met. Soc. China 2021, 31, 2615–2625. [Google Scholar] [CrossRef]
- Song, Y.; Yuan, K.; Li, X.; Qiao, Y. Microstructure and properties of biomedical Mg-Zn-Ca alloy at different extrusion temperatures. Mater. Today Commun. 2023, 35, 105578. [Google Scholar] [CrossRef]
- Fan, J.; Qiu, X.; Niu, X.; Tian, Z.; Sun, W.; Liu, X.; Li, Y.; Li, W.; Meng, J. Microstructure, mechanical properties, in vitro degradation and cytotoxicity evaluations of Mg-1.5Y-1.2Zn-0.44Zr alloys for biodegradable metallic implants. Mater. Sci. Eng. C 2013, 33, 2345–2352. [Google Scholar] [CrossRef]
- Du, B.N.; Xiao, Z.P.; Qiao, Y.X.; Zheng, L.; Yu, B.Y.; Xu, D.K.; Sheng, L.Y. Optimization of microstructure and mechanical property of a Mg-Zn-Y-Nd alloy by extrusion process. J. Alloys Compd. 2019, 775, 990–1001. [Google Scholar]
- Kiani, F.; Lin, J.; Vahid, A.; Munir, K.; Wen, C.; Li, Y. Mechanical and corrosion properties of extruded Mg-Zr-Sr alloys for biodegradable implant applications. Mater. Sci. Eng. A 2022, 831, 142192. [Google Scholar]
- Kubásek, J.; Dvorský, D.; Veselý, J.; Minárik, P.; Zemková, M.; Vojtěch, D. Characterization of the High-Strength Mg-3Nd-0.5Zn Alloy Prepared by Thermomechanical Processing. Acta Metall. Sin. (Engl. Lett.) 2018, 32, 321–331. [Google Scholar]
- Zhang, Y.; Peng, W.; Guo, T.; Wang, Y.; Li, S. Effect of Ca Content on Properties of Extruded Mg-3Zn-0.5Sr-xCa Alloys for Medical Applications. Mater. Res. 2019, 22, e20180013. [Google Scholar]
- Torkian, A.; Faraji, G.; Pedram, M.S. Mechanical properties and in vivo biodegradability of Mg-Zr-Y-Nd-La magnesium alloy produced by a combined severe plastic deformation. Rare Met. 2019, 40, 651–662. [Google Scholar]
- El-Mahallawy, N.; Palkowski, H.; Klingner, A.; Diaa, A.; Shoeib, M. Effect of 1.0 wt. % Zn addition on the microstructure, mechanical properties, and bio-corrosion behaviour of micro alloyed Mg-0.24Sn-0.04Mn alloy as biodegradable material. Mater. Today Commun. 2020, 24, 100999. [Google Scholar]
- Merson, D.; Brilevsky, A.; Myagkikh, P.; Tarkova, A.; Prokhorikhin, A.; Kretov, E.; Frolova, T.; Vinogradov, A. The Functional Properties of Mg-Zn-X Biodegradable Magnesium Alloys. Materials 2020, 13, 544. [Google Scholar] [CrossRef]
- Wang, X.; Huang, Y.; Yang, L.; Liu, Y.; Wang, C.; Guo, E. Effects of Ga/Ag addition on corrosion behavior, cytotoxicity, and antibacterial activity of biodegradable Mg-2Zn alloy. J. Mater. Res. Technol. 2024, 29, 3144–3155. [Google Scholar]
- Kiani, F.; Lin, J.; Vahid, A.; Munir, K.; Wen, C.; Li, Y. Microstructures, mechanical properties, corrosion, and biocompatibility of extruded Mg-Zr-Sr-Ho alloys for biodegradable implant applications. J. Magnes. Alloys 2023, 11, 110–136. [Google Scholar]
- Kaviani, M.; Ebrahimi, G.R.; Ezatpour, H.R. Improving the mechanical properties and biocorrosion resistance of extruded Mg-Zn-Ca-Mn alloy through hot deformation. Mater. Chem. Phys. 2019, 234, 245–258. [Google Scholar]
- Martinez, D.C.; Dobkowska, A.; Marek, R.; Cwieka, H.; Jaroszewicz, J.; Plocinski, T.; Donik, C.; Helmholz, H.; Luthringer-Feyerabend, B.; Zeller-Plumhoff, B.; et al. In vitro and in vivo degradation behavior of Mg-0.45Zn-0.45Ca (ZX00) screws for orthopedic applications. Bioact. Mater. 2023, 28, 132–154. [Google Scholar] [PubMed]
- Marek, R.; Eichler, J.; Schwarze, U.Y.; Fischerauer, S.; Suljevic, O.; Berger, L.; Loffler, J.F.; Uggowitzer, P.J.; Weinberg, A.M. Long-term in vivo degradation of Mg-Zn-Ca elastic stable intramedullary nails and their influence on the physis of juvenile sheep. Biomater. Adv. 2023, 150, 213417. [Google Scholar] [CrossRef] [PubMed]
- Bazhenov, V.E.; Li, A.V.; Komissarov, A.A.; Koltygin, A.V.; Tavolzhanskii, S.A.; Bautin, V.A.; Voropaeva, O.O.; Mukhametshina, A.M.; Tokar, A.A. Microstructure and mechanical and corrosion properties of hot-extruded Mg-Zn-Ca-(Mn) biodegradable alloys. J. Magnes. Alloys 2021, 9, 1428–1442. [Google Scholar] [CrossRef]
- Cihova, M.; Martinelli, E.; Schmutz, P.; Myrissa, A.; Schaublin, R.; Weinberg, A.M.; Uggowitzer, P.J.; Loffler, J.F. The role of zinc in the biocorrosion behavior of resorbable Mg-Zn-Ca alloys. Acta Biomater. 2019, 100, 398–414. [Google Scholar]
- Gui, Z.; Kang, Z.; Li, Y. Corrosion mechanism of the as-cast and as-extruded biodegradable Mg-3.0Gd-2.7Zn-0.4Zr-0.1Mn alloys. Mater. Sci. Eng. C 2019, 96, 831–840. [Google Scholar] [CrossRef]
- Panemangalore, D.B.; Shabadi, R.; Gupta, M. Corrosion Behavior, Microstructure and Mechanical Properties of Novel Mg-Zn-Ca-Er Alloy for Bio-Medical Applications. Metals 2021, 11, 519. [Google Scholar] [CrossRef]
- Munir, K.; Lin, J.; Tong, X.; Biesiekierski, A.; Li, Y.; Wen, C. Impact of scandium and terbium on the mechanical properties, corrosion behavior, and biocompatibility of biodegradable Mg-Zn-Zr-Mn alloys. J. Magnes. Alloys 2024, 12, 546–572. [Google Scholar] [CrossRef]
- Jiang, J.; Cui, H.; Geng, F.; Guo, X. Research progress on vascular clips for minimally invasive surgery. Prog. Med. Dev. 2023, 1, 75–83. [Google Scholar] [CrossRef]
- Urade, T.; Yoshida, T.; Ikeo, N.; Naka, K.; Kido, M.; Toyama, H.; Ueno, K.; Tanaka, M.; Mukai, T.; Fukumoto, T. Novel biodegradable magnesium alloy clips compared with titanium clips for hepatectomy in a rat model. BMC Surg. 2019, 19, 130. [Google Scholar] [CrossRef]
- Yoshida, T.; Fukumoto, T.; Urade, T.; Kido, M.; Toyama, H.; Asari, S.; Ajiki, T.; Ikeo, N.; Mukai, T.; Ku, Y. Development of a new biodegradable operative clip made of a magnesium alloy: Evaluation of its safety and tolerability for canine cholecystectomy. Surgery 2017, 161, 1553–1560. [Google Scholar] [CrossRef]
- Chang, Y.H.; Tseng, C.C.; Chao, C.Y.; Chen, C.H.; Lin, S.Y.; Du, J.K. Mg-Zn-Ca Alloys for Hemostasis Clips for Vessel Ligation: In Vitro and In Vivo Studies of Their Degradation and Response. Materials 2020, 13, 3039. [Google Scholar] [CrossRef] [PubMed]
- Ding, P.; Liu, Y.; He, X.; Liu, D.; Chen, M. In vitro and in vivo biocompatibility of Mg-Zn-Ca alloy operative clip. Bioact. Mater. 2019, 4, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Gao, M.; Tian, X.; Cao, D.; Tan, L. Study on Mechanical Properties and Degradation Behavior of Magnesium Alloy Vascular Clip. J. Funct. Biomater. 2023, 14, 501. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Zheng, X.; Tian, Y.; Shi, Y.; Zhang, X.; Song, C. Structural Optimization, Fabrication, and Corrosion Behaviors of Biodegradable Mg-Nd-Zn-Zr Alloy Hemostatic Clip. Metals 2022, 12, 1979. [Google Scholar] [CrossRef]
- Mao, L.; Zhou, Y.; Zheng, X.; Cai, X.; Chen, Y.; Yang, W.; Wang, J.; Zhang, J.; Song, C. Structural optimization and in vitro corrosion analysis of biodegradable Mg-Nd-Zn-Zr alloy clip. J. Mech. Behav. Biomed. Mater. 2024, 161, 106790. [Google Scholar] [CrossRef]
- Sefa, S.; Wieland, D.C.F.; Helmholz, H.; Zeller-Plumhoff, B.; Wennerberg, A.; Moosmann, J.; Willumeit-Römer, R.; Galli, S. Assessing the long-term in vivo degradation behavior of magnesium alloys—A high resolution synchrotron radiation micro computed tomography study. Front. Biomater. Sci. 2022, 1, 925471. [Google Scholar] [CrossRef]
- Xie, K.; Wang, L.; Guo, Y.; Zhao, S.; Yang, Y.; Dong, D.; Ding, W.; Dai, K.; Gong, W.; Yuan, G.; et al. Effectiveness and safety of biodegradable Mg-Nd-Zn-Zr alloy screws for the treatment of medial malleolar fractures. J. Orthop. Transl. 2021, 27, 96–100. [Google Scholar] [CrossRef]
- Jiao, Z.; Lyu, S.; Wang, L.; You, C.; Chen, M. Improving the mechanical properties and corrosion resistance of biodegradable Mg-Zn-Ca-Mn alloy bone screw through structural optimization. J. Mater. Res. Technol. 2022, 21, 1442–1453. [Google Scholar] [CrossRef]
- Sontgen, S.; Keilig, L.; Kabir, K.; Weber, A.; Reimann, S.; Welle, K.; Bourauel, C. Mechanical and numerical investigations of biodegradable magnesium alloy screws for fracture treatment. J. Biomed. Mater. Res. B Appl. Biomater. 2023, 111, 7–15. [Google Scholar] [CrossRef]
- Lee, C.H.; Woo, S.; Choi, H.D. Results of the Use of Bioabsorbable Magnesium Screws for Surgical Treatment of Mason Type II Radial Head Fractures. Clin. Orthop. Surg. 2023, 15, 1013–1021. [Google Scholar] [CrossRef]
- Lam, W.-H.; Tso, C.-Y.; Tang, N.; Cheung, W.-H.; Qin, L.; Wong, R.M.-Y. Biodegradable magnesium screws in elbow fracture fixation: Clinical case series. J. Orthop. Trauma Rehabil. 2021, 29, 2210491720986983. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, H.; Wang, W.; Zan, R.; Peng, H.; Zhang, S.; Zhang, X. Translational status of biomedical Mg devices in China. Bioact. Mater. 2019, 4, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, J.; Liu, W.; Li, Y.; Qin, L. Biodegradable magnesium (Mg) implantation does not impose related metabolic disorders in rats with chronic renal failure. Sci. Rep. 2016, 6, 26341. [Google Scholar] [CrossRef] [PubMed]
- Cianni, L.; Vitiello, R.; Greco, T.; Sirgiovanni, M.; Ragonesi, G.; Maccauro, G.; Perisano, C. Predictive Factors of Poor Outcome in Sanders Type III and IV Calcaneal Fractures Treated with an Open Reduction and Internal Fixation with Plate: A Medium-Term Follow-Up. J. Clin. Med. 2022, 11, 5660. [Google Scholar] [CrossRef]
- Verma, V.; Pal, K. Experimental and numerical investigation on ZnP coated Mg-Mn based composite materials for internal fixation of tibia midshaft fracture: A comprehensive study. Surf. Coat. Technol. 2022, 447, 128785. [Google Scholar] [CrossRef]
- Wang, Y.; Liang, W.; Liu, X.; Li, Q.; Xie, Y.; Jiang, Y. Osteogenesis and degradation behavior of magnesium alloy plate in vivo. Eur. J. Inflamm. 2021, 19, 20587392211034078. [Google Scholar] [CrossRef]
- Rich, A.M.; Rubin, W.; Rickli, S.; Akhmetshina, T.; Cossu, J.; Berger, L.; Magno, M.; Nuss, K.M.; Schaller, B.; Loffler, J.F. Development of an implantable sensor system for in vivo strain, temperature, and pH monitoring: Comparative evaluation of titanium and resorbable magnesium plates. Bioact. Mater. 2025, 43, 603–618. [Google Scholar] [CrossRef]
- Rendenbach, C.; Fischer, H.; Kopp, A.; Schmidt-Bleek, K.; Kreiker, H.; Stumpp, S.; Thiele, M.; Duda, G.; Hanken, H.; Beck-Broichsitter, B.; et al. Improved in vivo osseointegration and degradation behavior of PEO surface-modified WE43 magnesium plates and screws after 6 and 12 months. Mater. Sci. Eng. C 2021, 129, 112380. [Google Scholar] [CrossRef]
- Haslhofer, D.J.; Gotterbarm, T.; Klasan, A. High Complication Rate and High Percentage of Regressing Radiolucency in Magnesium Screw Fixation in 18 Consecutive Patients. J. Pers. Med. 2023, 13, 357. [Google Scholar] [CrossRef]
- Backer, H.C.; Heyland, M.; Wu, C.H.; Perka, C.; Stockle, U.; Braun, K.F. Breakage of intramedullary femoral nailing or femoral plating: How to prevent implant failure. Eur. J. Med. Res. 2022, 27, 7. [Google Scholar] [CrossRef]
- Sun, M.; Shao, H.; Xu, H.; Yang, X.; Dong, M.; Gong, J.; Yu, M.; Gou, Z.; He, Y.; Liu, A. Biodegradable intramedullary nail (BIN) with high-strength bioceramics for bone fracture. J. Mater. Chem. B 2021, 9, 969–982. [Google Scholar] [PubMed]
- Ye, Y.; Shao, H.; Jing, Z.; Nian, Z.; Gong, Y. Magnesium-Containing Silicate Bioceramic Degradable Intramedullary Nail for Bone Fractures. Crystals 2022, 12, 974. [Google Scholar] [CrossRef]
- Sisk, M.R.; Yang, L.C.; Paul, K.D.; Elphingstone, J.W.; Brabston, E.W.; Ponce, B.A.; Martin, E.C.; Corriveau, K.M. Biomechanical principles of intramedullary nails in veterinary and human medicine. Vet. Comp. Orthop. Traumatol. 2024, 37, 257–262. [Google Scholar]
- Bekos, A.; Sioutis, S.; Kostroglou, A.; Saranteas, T.; Mavrogenis, A.F. The history of intramedullary nailing. Int. Orthop. 2021, 45, 1355–1361. [Google Scholar]
- Chen, P.; Fan, Z.; Xu, N.; Wang, H. A biomechanical investigation of a novel intramedullary nail used to salvage failed internal fixations in intertrochanteric fractures. J. Orthop. Surg. Res. 2023, 18, 632. [Google Scholar]
- Salman, L.A.; Al-Ani, A.; Radi, M.F.A.; Abudalou, A.F.; Baroudi, O.M.; Ajaj, A.A.; Alkhayarin, M.; Ahmed, G. Open versus closed intramedullary nailing of femur shaft fractures in adults: A systematic review and meta-analysis. Int. Orthop. 2023, 47, 3031–3041. [Google Scholar]
- Ye, L.; Xu, J.; Mi, J.; He, X.; Pan, Q.; Zheng, L.; Zu, H.; Chen, Z.; Dai, B.; Li, X.; et al. Biodegradable magnesium combined with distraction osteogenesis synergistically stimulates bone tissue regeneration via CGRP-FAK-VEGF signaling axis. Biomaterials 2021, 275, 120984. [Google Scholar]
- Zheng, N.; Xu, J.; Ruan, Y.C.; Chang, L.; Wang, X.; Yao, H.; Wang, J.; Zhang, R.; Xue, Q.; Tang, N.; et al. Magnesium facilitates the healing of atypical femoral fractures: A single-cell transcriptomic study. Mater. Today 2022, 52, 43–62. [Google Scholar]
- Sun, Y.; Helmholz, H.; Will, O.; Damm, T.; Wiese, B.; Luczak, M.; Peschke, E.; Luthringer-Feyerabend, B.; Ebel, T.; Hövener, J.-B. Dynamic in vivo monitoring of fracture healing process in response to magnesium implant with multimodal imaging: Pilot longitudinal study in a rat external fixation model. Biomater. Sci. 2022, 10, 1532–1543. [Google Scholar]
- Adam, R.; Antoniac, I.; Avram, G.M.; Niculescu, M. 1Ca alloy intramedullary nailing influence on bone callus formation and on vital organs functions, as an alternative to bioplastics. Rom. J. Milit. Med. 2022, 125, 15–23. [Google Scholar]
- Chow, D.H.K.; Wang, J.; Wan, P.; Zheng, L.; Ong, M.T.Y.; Huang, L.; Tong, W.; Tan, L.; Yang, K.; Qin, L. Biodegradable magnesium pins enhanced the healing of transverse patellar fracture in rabbits. Bioact. Mater. 2021, 6, 4176–4185. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, A.; Wu, J.; Guo, S.; Sun, Q. Application and Perspectives: Magnesium Materials in Bone Regeneration. ACS Biomater. Sci. Eng. 2024, 10, 3514–3527. [Google Scholar] [CrossRef] [PubMed]
- Arias-Blanco, A.; Marco, M.; Giner, E.; Larraínzar-Garijo, R.; Miguélez, M.H. Experimental and numerical analysis of the influence of intramedullary nail position on the cut-out phenomenon. Comput. Methods Programs Biomed. 2023, 240, 107734. [Google Scholar] [CrossRef]
- Walter, N.; Rupp, M.; Kruckel, J.; Alt, V. Individual and commercially available antimicrobial coatings for intramedullary nails for the treatment of infected long bone non-unions—A systematic review. Injury 2022, 53 (Suppl. S3), S74–S80. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yan, C.; Mei, D.; Li, Y.; Sheng, K.; Wang, J.; Wang, L.; Zhu, S.; Guan, S. Optimized structure design of asymmetrical Mg alloy cerebrovascular stent with high flexibility. Smart Mater. Manuf. 2024, 2, 100040. [Google Scholar] [CrossRef]
- Wen, Y.; Li, Y.; Yang, R.; Chen, Y.; Shen, Y.; Liu, Y.; Liu, X.; Zhang, B.; Li, H. Biofunctional coatings and drug-coated stents for restenosis therapy. Mater. Today Bio 2024, 29, 101259. [Google Scholar] [CrossRef]
- Anderson, D.E.J.; Le, H.H.; Vu, H.; Johnson, J.; Aslan, J.E.; Goldman, J.; Hinds, M.T. Thrombogenicity of biodegradable metals. Bioact. Mater. 2024, 38, 411–421. [Google Scholar]
- Liu, K.P.; Cheng, A.Y.; You, J.L.; Chang, Y.H.; Tseng, C.C.; Ger, M.D. Biocompatibility and corrosion resistance of drug coatings with different polymers for magnesium alloy cardiovascular stents. Colloids Surf. B 2025, 245, 114202. [Google Scholar] [CrossRef]
- Bian, D.; Zhou, X.; Liu, J.; Li, W.; Shen, D.; Zheng, Y.; Gu, W.; Jiang, J.; Li, M.; Chu, X.; et al. Degradation behaviors and in-vivo biocompatibility of a rare earth- and aluminum-free magnesium-based stent. Acta Biomater. 2021, 124, 382–397. [Google Scholar] [CrossRef]
- Sun, X.; Li, H.; Qi, L.; Wang, F.; Hou, Y.; Li, J.; Guan, S. Construction and biocompatibility evaluation of MOF/S-HA composite coating on the surface of magnesium alloy vascular stent. Prog. Org. Coat. 2024, 189, 108177. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Y.; Wang, X.; Xie, Y.; Bai, L.; Guan, S. Fucoidan/collagen composite coating on magnesium alloy for better corrosion resistance and pro-endothelialization potential. Int. J. Biol. Macromol. 2024, 255, 128044. [Google Scholar] [CrossRef] [PubMed]
- Qiu, T.; Miao, Y.; Wu, Y.; Pang, S.; Guo, W. Micro milling of WE43 magnesium alloy cardiovascular stent and its mechanical property enhancement by ultrasonic vibration assisted micro deburring. Mater. Lett. 2024, 377, 137459. [Google Scholar] [CrossRef]
- Pan, C.; Yang, N.; Chen, J.; Hong, Q.; Zhu, L.; Zhang, B. Constructing NO/CO gases-releasing bioactive coating to synergistically enhance corrosion resistance and biocompatibility of magnesium alloy for cardiovascular stents. Colloids Surf. A 2025, 704, 135521. [Google Scholar] [CrossRef]
- Hou, Y.; Zhang, X.; Li, J.; Wang, L.; Guan, S. A multi-functional MgF2/polydopamine/hyaluronan-astaxanthin coating on the biodegradable ZE21B alloy with better corrosion resistance and biocompatibility for cardiovascular application. J. Magnes. Alloys 2024, 12, 1102–1116. [Google Scholar] [CrossRef]
- Jia, Q.; Jia, Q.; Zhu, S.; Zheng, Y.; Guan, S. A Cu(II)-eluting coating through silk fibroin film on ZE21B alloy designed for in situ endotheliazation biofunction. Colloids Surf. B 2024, 236, 113808. [Google Scholar] [CrossRef]
- Mousavizadeh, S.M.; Yu, M.; Gilchrist, M.D.; Zhang, N. Preparation of a polycaprolactone coating on WE43 for biodegradable stent applications using dual solvents, ultrasonic atomization spray, and anodization. Prog. Org. Coat. 2024, 193, 108528. [Google Scholar] [CrossRef]
- Zhang, J.; Xiang, Z.; Ren, X.; Zhang, B.; Fu, D.; Wang, Y. Superior toughness Anticorrosion-Bioactive integrated multilayer coating with excellent adhesion for biodegradable Magnesium-Based stents. Chem. Eng. J. 2024, 481, 148400. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.; Chen, L.; Bai, L.; Guan, S. Co-immobilization of natural marine polysaccharides and bioactive peptides on ZE21B magnesium alloy to enhance hemocompatibility and cytocompatibility. Int. J. Biol. Macromol. 2024, 272, 132747. [Google Scholar] [CrossRef]
- Ma, Y.; Li, J.; Zhou, Y. Construction and biocompatibility evaluation of CS-FA/VA composite coating on the surface of ZE21B vascular stent. Appl. Surf. Sci. 2025, 681, 161413. [Google Scholar] [CrossRef]
- Chen, J.; Xu, R.; Meng, L.; Yan, F.; Wang, L.; Xu, Y.; Zhang, Q.; Zhai, W.; Pan, C. Biomimetic hydrogel coatings for improving the corrosion resistance, hemocompatibility, and endothelial cell growth of the magnesium alloy. Colloids Surf. B 2025, 245, 114204. [Google Scholar] [CrossRef]
- Vishnu, J.; Praveenkumar, K.; Anil Kumar, A.; Nair, A.; Arjun, R.; Gopakumar Pillai, V.; Shankar, B.; V Shankar, K. Multifunctional zinc oxide loaded stearic acid surfaces on biodegradable magnesium WE43 alloy with hydrophobic, self-cleaning and biocompatible attributes. Appl. Surf. Sci. 2025, 680, 161455. [Google Scholar] [CrossRef]
- De Hemptinne, Q.; Xaplanteris, P.; Guedes, A.; Demeure, F.; Vandeloo, B.; Dugauquier, C.; Picard, F.; Warne, D.W.; Pilgrim, T.; Iglesias, J.F.; et al. Magmaris Resorbable Magnesium Scaffold Versus Conventional Drug-Eluting Stent in ST-Segment Elevation Myocardial Infarction: 1-Year Results of a Propensity-Score-Matching Comparison. Cardiovasc. Revasc. Med. 2022, 43, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Garcia, H.M.; Haude, M.; Kuku, K.; Hideo-Kajita, A.; Ince, H.; Abizaid, A.; Tölg, R.; Lemos, P.A.; von Birgelen, C.; Christiansen, E.H.; et al. In vivo serial invasive imaging of the second-generation drug-eluting absorbable metal scaffold (Magmaris—DREAMS 2G) in de novo coronary lesions: Insights from the BIOSOLVE-II First-In-Man Trial. Int. J. Cardiol. 2018, 255, 22–28. [Google Scholar] [CrossRef]
- Haude, M.; Wlodarczak, A.; Van Der Schaaf, R.; Torzewski, J.; Ferdinande, B.; Escaned, J.; Iglesias, J.; Bennett, J.; Toth, G.; Joner, M. First in human study BIOMAG-I: 12 months results of the sirolimus eluting resorbable coronary magnesium scaffold system (DREAMS 3G) in the treatment of subjects with de novo coronary artery lesions. Eur. Heart J. 2023, 44, ehad655.1501. [Google Scholar] [CrossRef]
- Seguchi, M.; Aytekin, A.; Xhepa, E.; Haude, M.; Wlodarczak, A.; van der Schaaf, R.J.; Torzewski, J.; Ferdinande, B.; Escaned, J.; Iglesias, J.F. CRT-600.03 Optical Coherence Tomography Characteristics of the Novel Resorbable Magnesium Scaffold at 6 Months: An Intravascular Imaging Analysis of the First-in-Human Trial on DREAMS 3G. JACC Cardiovasc. Interv. 2023, 16, S75. [Google Scholar] [CrossRef]
- Chen, K.; Zhao, L.; Huang, C.; Yin, X.; Zhang, X.; Li, P.; Gu, X.; Fan, Y. Recent Advances in the Development of Magnesium-Based Alloy Guided Bone Regeneration (GBR) Membrane. Metals 2022, 12, 2074. [Google Scholar] [CrossRef]
- Lin, D.J.; Hung, F.Y.; Lee, H.P.; Yeh, M.L. Development of a Novel Degradation-Controlled Magnesium-Based Regeneration Membrane for Future Guided Bone Regeneration (GBR) Therapy. Metals 2017, 7, 481. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, J.; Tong, H.; Lu, J.; Li, W.; Liu, F.; Gong, H.; Zhang, Z.; Li, Y. Effect of fluoride ion concentration on the corrosion behaviour of WE43 alloy in artificial saliva for dental applications. Corros. Sci. 2024, 226, 111672. [Google Scholar] [CrossRef]
- Rider, P.; Kacarevic, Z.P.; Elad, A.; Tadic, D.; Rothamel, D.; Sauer, G.; Bornert, F.; Windisch, P.; Hangyasi, D.B.; Molnar, B.; et al. Biodegradable magnesium barrier membrane used for guided bone regeneration in dental surgery. Bioact. Mater. 2022, 14, 152–168. [Google Scholar] [CrossRef]
- Shan, Y.; Qiao, B.; Ouyang, S.; Du, C.; Zhao, L.; Wang, G.; Ye, J.; Xiong, Y.; Wei, Y.; Song, J.; et al. Biodegradable Mg-Ca/Mg-Cu bilayer membranes with enhanced mechanical, osteogenesis and antibacterial performances for GBR applications. J. Magnes. Alloys 2024, 13, 792–809. [Google Scholar] [CrossRef]
- Chen, K.; Zhao, Y.; Liu, C.; Li, Q.; Bai, Y.; Li, P.; Wang, C.; Gu, X.; Fan, Y. Novel Mg-Ca-La alloys for guided bone regeneration: Mechanical performance, stress corrosion behavior and biocompatibility. Mater. Today Commun. 2022, 32, 103949. [Google Scholar] [CrossRef]
- Ouyang, S.; Wu, X.; Meng, L.; Jing, X.; Qiao, L.; She, J.; Zheng, K.; Chen, X.; Pan, F. More than the barrier effect: Biodegradable Mg-Ag alloy membranes for guided bone/tissue regeneration. J. Magnes. Alloys 2024, 12, 4454–4467. [Google Scholar] [CrossRef]
- Si, J.; Shen, H.; Miao, H.; Tian, Y.; Huang, H.; Shi, J.; Yuan, G.; Shen, G. In vitro and in vivo evaluations of Mg-Zn-Gd alloy membrane on guided bone regeneration for rabbit calvarial defect. J. Magnes. Alloys 2021, 9, 281–291. [Google Scholar] [CrossRef]
- Yan, Z.; Zhu, J.; Liu, G.; Liu, Z.; Guo, C.; Cui, N.; Han, J. Feasibility and Efficacy of a Degradable Magnesium-Alloy GBR Membrane for Bone Augmentation in a Distal Bone-Defect Model in Beagle Dogs. Bioinorg. Chem. Appl. 2022, 2022, 4941635. [Google Scholar] [CrossRef]
- Contuzzi, N.; Casalino, G.; Boccaccio, A.; Ballini, A.; Charitos, I.A.; Bottalico, L.; Santacroce, L. Metals Biotribology and Oral Microbiota Biocorrosion Mechanisms. J. Funct. Biomater. 2022, 14, 14. [Google Scholar] [CrossRef]
- Herber, V.; Okutan, B.; Antonoglou, G.; Sommer, N.G.; Payer, M. Bioresorbable Magnesium-Based Alloys as Novel Biomaterials in Oral Bone Regeneration: General Review and Clinical Perspectives. J. Clin. Med. 2021, 10, 1842. [Google Scholar] [CrossRef]
- Dini, C.; Yamashita, K.M.; Sacramento, C.M.; Borges, M.H.R.; Takeda, T.T.S.; Silva, J.P.S.; Nagay, B.E.; Costa, R.C.; Cruz, N.C.; Range, E.C.; et al. Tailoring magnesium-doped coatings for improving surface and biological properties of titanium-based dental implants. Colloid. Surf. B 2025, 246, 114382. [Google Scholar] [CrossRef]
- Dargusch, M.S.; Balasubramani, N.; Yang, N.; Johnston, S.; Ali, Y.; Wang, G.; Venezuela, J.; Carluccio, J.; Lau, C.; Allavena, R.; et al. In vivo performance of a rare earth free Mg-Zn-Ca alloy manufactured using twin roll casting for potential applications in the cranial and maxillofacial fixation devices. Bioact. Mater. 2022, 12, 85–96. [Google Scholar] [CrossRef]
- Tang, H.; Li, Q.; Li, M.; Gu, X.; Cheng, C.; Fan, Y. In vitro and in vivo evaluation of micro-alloyed magnesium for potential application in alveolar bone fixation screws. J. Mater. Sci. Technol. 2023, 144, 62–69. [Google Scholar] [CrossRef]
- Jung, O.; Hesse, B.; Stojanovic, S.; Seim, C.; Weitkamp, T.; Batinic, M.; Goerke, O.; Kacarevic, Z.P.; Rider, P.; Najman, S.; et al. Biocompatibility Analyses of HF-Passivated Magnesium Screws for Guided Bone Regeneration (GBR). Int. J. Mol. Sci. 2021, 22, 12567. [Google Scholar] [CrossRef]
- Kacarevic, Z.P.; Rider, P.; Elad, A.; Tadic, D.; Rothamel, D.; Sauer, G.; Bornert, F.; Windisch, P.; Hangyasi, D.B.; Molnar, B.; et al. Biodegradable magnesium fixation screw for barrier membranes used in guided bone regeneration. Bioact. Mater. 2022, 14, 15–30. [Google Scholar] [PubMed]
- Laiteerapong, A.; Lochaiwatana, Y.; Hirata, I.; Okazaki, M.; Mori, K.; Murakami, S.; Poolthong, S. A novel glass ionomer cement containing MgCO3 apatite induced the increased proliferation and differentiation of human pulp cells in vitro. Dent. Mater. J. 2012, 31, 772–778. [Google Scholar] [CrossRef] [PubMed]
- Vujovic, S.; Desnica, J.; Stanisic, D.; Ognjanovic, I.; Stevanovic, M.; Rosic, G. Applications of Biodegradable Magnesium-Based Materials in Reconstructive Oral and Maxillofacial Surgery: A Review. Molecules 2022, 27, 5529. [Google Scholar] [CrossRef] [PubMed]
- Patel, A. Benign vs malignant tumors. JAMA Oncol. 2020, 6, 1488. [Google Scholar]
- Tiwari, A.; Trivedi, R.; Lin, S.Y. Tumor microenvironment: Barrier or opportunity towards effective cancer therapy. J. Biomed. Sci. 2022, 29, 83. [Google Scholar] [CrossRef]
- Wang, B.; Hu, S.; Teng, Y.; Chen, J.; Wang, H.; Xu, Y.; Wang, K.; Xu, J.; Cheng, Y.; Gao, X. Current advance of nanotechnology in diagnosis and treatment for malignant tumors. Signal Transduct. Target. Ther. 2024, 9, 200. [Google Scholar]
- Chen, Q.; Fan, Y.; Dong, S.; Han, P.; Xie, T.; Wang, C.; Zeng, X.; Ding, W.; Meng, Z.; Wang, L. A highly degradable Mg-Al-Ca alloy with superior anti-tumor efficacy. J. Magnes. Alloys 2023, 11, 4206–4217. [Google Scholar] [CrossRef]
- Lu, X.; Zuo, R.; Chen, J.; Hu, Y.; Wei, C.; Guo, Y.; Xiong, S.; Wang, S.; Zhang, S.; Cui, Y.; et al. Antitumor property of WE43 magnesium alloy subjected to anodic oxidation plus heat treatment. Colloids Surf. A 2024, 680, 132723. [Google Scholar] [CrossRef]
- Peng, H.; Fan, K.; Zan, R.; Gong, Z.J.; Sun, W.; Sun, Y.; Wang, W.; Jiang, H.; Lou, J.; Ni, J.; et al. Degradable magnesium implants inhibit gallbladder cancer. Acta Biomater. 2021, 128, 514–522. [Google Scholar]
- Li, H.; Feng, X.; Li, H.; Ma, S.; Song, W.; Yang, B.; Jiang, T.; Yang, C. The Supplement of Magnesium Element to Inhibit Colorectal Tumor Cells. Biol. Trace Elem. Res. 2023, 201, 2895–2903. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, Y.; Xiong, X.; Qian, K.; Gao, Z.; Ye, Y.; Chu, C.; Xue, F.; Bai, J. The antitumor effect of biodegradable metals (Mg, Zn, and Fe) on colon cancer. Mater. Lett. 2024, 360, 136049. [Google Scholar]
- Cheng, S.; Shao, H.; Yin, D.; Zhou, J.; Jian, L.; Xie, J.; Zhang, Y.; Wang, D.; Peng, F. Molecular mechanism underlying the action of a celastrol-loaded layered double hydroxide-coated magnesium alloy in osteosarcoma inhibition and bone regeneration. ACS Biomater. Sci. Eng. 2023, 9, 4940–4952. [Google Scholar] [PubMed]
- Globig, P.; Madurawala, R.; Willumeit-Romer, R.; Martini, F.; Mazzoni, E.; Luthringer-Feyerabend, B.J.C. Mg-based materials diminish tumor spreading and cancer metastases. Bioact. Mater. 2023, 19, 594–610. [Google Scholar] [PubMed]
- Xu, B.; Song, Y.; Yang, K.; Li, Y.; Chen, B.; Liao, X.; Jia, Q. Magnesium metal and its corrosion products: Promising materials for tumor interventional therapy. J. Magnes. Alloys 2023, 11, 763–775. [Google Scholar]
- Zan, R.; Wang, H.; Cai, W.; Ni, J.; Luthringer-Feyerabend, B.J.C.; Wang, W.; Peng, H.; Ji, W.; Yan, J.; Xia, J.; et al. Controlled release of hydrogen by implantation of magnesium induces P53-mediated tumor cells apoptosis. Bioact. Mater. 2022, 9, 385–396. [Google Scholar]
- Globig, P.; Willumeit-Romer, R.; Martini, F.; Mazzoni, E.; Luthringer-Feyerabend, B.J.C. Slow degrading Mg-based materials induce tumor cell dormancy on an osteosarcoma-fibroblast coculture model. Bioact. Mater. 2022, 16, 320–333. [Google Scholar]
- Dai, Y.; Tang, Y.; Xu, X.; Luo, Z.; Zhang, Y.; Li, Z.; Lin, Z.; Zhao, S.; Zeng, M.; Sun, B.; et al. Evaluation of the mechanisms and effects of Mg-Ag-Y alloy on the tumor growth and metastasis of the MG63 osteosarcoma cell line. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 2537–2548. [Google Scholar]
- Ge, J.; Yang, N.; Yang, Y.; Yu, H.; Yang, X.; Wang, Y.; Wang, T.; Cheng, S.; Wang, Y.; Han, Z.; et al. The combination of eddy thermal effect of biodegradable magnesium with immune checkpoint blockade shows enhanced efficacy against osteosarcoma. Bioact. Mater. 2023, 25, 73–85. [Google Scholar]
- Li, M.; Yao, M.; Wang, W.; Wan, P.; Chu, X.; Zheng, Y.; Yang, K.; Zhang, Y. Nitrogen-containing bisphosphonate-loaded micro-arc oxidation coating for biodegradable magnesium alloy pellets inhibits osteosarcoma through targeting of the mevalonate pathway. Acta Biomater. 2021, 121, 682–694. [Google Scholar]
- Ahmad, N.; Alomar, S.Y.; Albalawi, F.; Khan, M.R.; Farshori, N.N.; Wahab, R.; Shaik, M.R. Anticancer potentiality of green synthesized Mg-Co ferrites nanoparticles against human breast cancer MCF-7 cells. J. King Saud Univ. Sci. 2023, 35, 102708. [Google Scholar]
- Liu, L.; Wu, Y.; Ye, J.; Fu, Q.; Su, L.; Wu, Z.; Feng, J.; Chen, Z.; Song, J. Synthesis of magnesium nanoparticle for NIR-II-photoacoustic-imaging-guided synergistic burst-like and H2 cancer therapy. Chem 2022, 8, 2990–3007. [Google Scholar]
- Anisimova, N.; Kiselevskiy, M.; Martynenko, N.; Willumeit-Romer, R.; Kornyushenkov, E.; Rodionov, M.; Dobatkin, S.; Estrin, Y. Anti-tumour activity of Mg-6%Ag and Mg-10%Gd alloys in mice with inoculated melanoma. Mater. Sci. Eng. C 2021, 130, 112464. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, K.; Deng, C.; Tang, H.; Wang, J.; Dai, X.; Zhang, B.; Sun, Z.; Ren, G.; Zhang, H.; et al. Biliary stents for active materials and surface modification: Recent advances and future perspectives. Bioact. Mater. 2024, 42, 587–612. [Google Scholar] [CrossRef]
- Yan, J.; Ye, Z.; Wang, X.; Zhong, D.; Wang, Z.; Yan, T.; Li, T.; Yuan, Y.; Liu, Y.; Wang, Y.; et al. Recent research progresses of bioengineered biliary stents. Mater. Today Bio 2024, 29, 101290. [Google Scholar]
- Choudhury, S.; Asthana, S.; Homer-Vanniasinkam, S.; Chatterjee, K. Emerging trends in biliary stents: A materials and manufacturing perspective. Biomater. Sci. 2022, 10, 3716–3729. [Google Scholar]
- Li, T.; Xu, W.; Liu, C.; He, J.; Wang, Q.; Zhang, D.; Sui, K.; Zhang, Z.; Sun, H.; Yang, K.; et al. Anticancer Effect of Biodegradable Magnesium on Hepatobiliary Carcinoma: An In Vitro and In Vivo Study. ACS Biomater. Sci. Eng. 2021, 7, 2774–2782. [Google Scholar]
- Zuo, M.; Wang, W.; Wu, H.; Yang, L.; Yan, J.; Ni, J.; Zhang, S.; Song, Y.; Wang, J.; Zhang, X. In vitro degradation and mineralization of high-purity magnesium in three physiological fluids. Mater. Lett. 2019, 240, 279–283. [Google Scholar] [CrossRef]
- Peng, H.; Gong, Z.; Zan, R.; Wang, W.; Yu, H.; Sun, Y.; Ma, C.; Wang, W.; Suo, T.; Zhang, X. Research on the degradation behaviors of biomedical Mg-2 wt.% Zn alloy under a biliary environment in vitro and in vivo. J. Magnes. Alloys 2022, 13, 1066–1077. [Google Scholar]
- Kim, H.W.; Lee, W.-Y.; Song, K.C. Effect of Biodegradable Polymer Coating on the Corrosion Rates and Mechanical Properties of Biliary Magnesium Alloy Stents. Korean Chem. Eng. Res. 2020, 58, 36–43. [Google Scholar]
- Song, G.; Zhao, H.Q.; Liu, Q.; Fan, Z. A review on biodegradable biliary stents: Materials and future trends. Bioact. Mater. 2022, 17, 488–495. [Google Scholar]
- Liu, Y.; Zheng, S.; Li, N.; Guo, H.; Zheng, Y.; Peng, J. Study on the in vitro degradation behavior of pure Mg and WE43 in human bile for 60 days for future usage in biliary. Mater. Lett. 2016, 179, 100–103. [Google Scholar] [CrossRef]
- Song, Y.; Qin, G.; Du, L.; Hu, H.; Han, Y. In vitro and in vivo assessment of biocompatibility of AZ31 alloy as biliary stents: A preclinical approach. Arch. Med. Sci. 2022, 18, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zheng, S.; Li, N.; Guo, H.; Zheng, Y.; Peng, J. In vivo response of AZ31 alloy as biliary stents: A 6 months evaluation in rabbits. Sci. Rep. 2017, 7, 40184. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, K.; Liu, H.; Shao, Y.; Chu, C.; Xue, F.; Bai, J. A study of a biodegradable braided Mg stent for biliary reconstruction. J. Mater. Sci. 2020, 55, 17170–17182. [Google Scholar] [CrossRef]
- Marra, P.; Carbone, F.S.; Dulcetta, L.; Bonaffini, P.A.; Muglia, R.; D’Antiga, L.; Sironi, S. A new biodegradable stent to improve the management of biliary strictures in pediatric split liver transplantation. Cardiovasc. Intervent. Radiol. 2022, 45, 867–872. [Google Scholar] [CrossRef]
- Chen, X.; Xia, Y.; Shen, S.; Wang, C.; Zan, R.; Yu, H.; Yang, S.; Zheng, X.; Yang, J.; Suo, T.; et al. Research on the Current Application Status of Magnesium Metal Stents in Human Luminal Cavities. J. Funct. Biomater. 2023, 14, 462. [Google Scholar] [CrossRef]
- Yang, K.; Sun, W.; Cui, L.; Zou, Y.; Wen, C.; Zeng, R. Advances in functional coatings on biliary stents. Regener. Biomater. 2024, 11, rbae001. [Google Scholar] [CrossRef]
- Xin, H.; Liu, Y.; Xiao, Y.; Wen, M.; Sheng, L.; Jia, Z. Design and Nanoengineering of Photoactive Antimicrobials for Bioapplications: From Fundamentals to Advanced Strategies. Adv. Funct. Mater. 2024, 34, 2402607. [Google Scholar] [CrossRef]
- Benzarti, Z.; Itani, S.; Castro, J.D.; Carvalho, S.; Ramos, A.S. Design multifunctional Mg-Zr coatings regulating Mg alloy bioabsorption. J. Magnes. Alloys 2024, 12, 1461–1478. [Google Scholar] [CrossRef]
- Kalaiyarasan, M.; Pugalmani, S.; Rajendran, N. Fabrication of chitosan/silica hybrid coating on AZ31 Mg alloy for orthopaedic applications. J. Magnes. Alloys 2023, 11, 614–628. [Google Scholar] [CrossRef]
- Sheng, L.; Xiao, Y.; Jiao, C.; Du, B.; Li, Y.; Wu, Z.; Shao, L. Influence of layer number on microstructure, mechanical properties and wear behavior of the TiN/Ti multilayer coatings fabricated by high-power magnetron sputtering deposition. J. Manuf. Process 2021, 70, 529–542. [Google Scholar]
- Wang, S.D.; Xu, D.K.; Wang, B.J.; Sheng, L.Y.; Qiao, Y.X.; Han, E.H.; Dong, C. Influence of phase dissolution and hydrogen absorption on the stress corrosion cracking behavior of Mg-7%Gd-5%Y-1%Nd-0.5%Zr alloy in 3.5 wt.% NaCl solution. Corros. Sci. 2018, 142, 185–200. [Google Scholar]

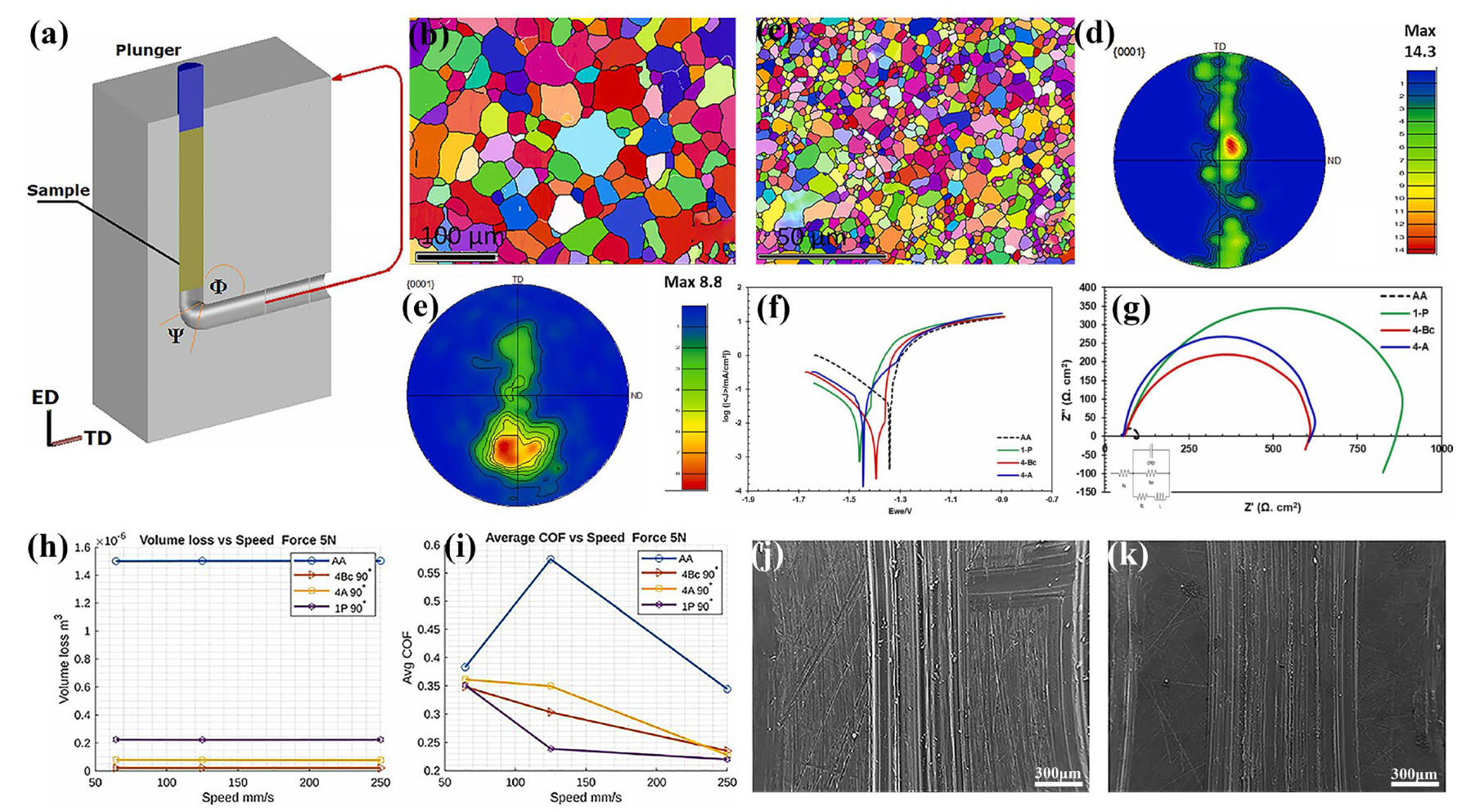
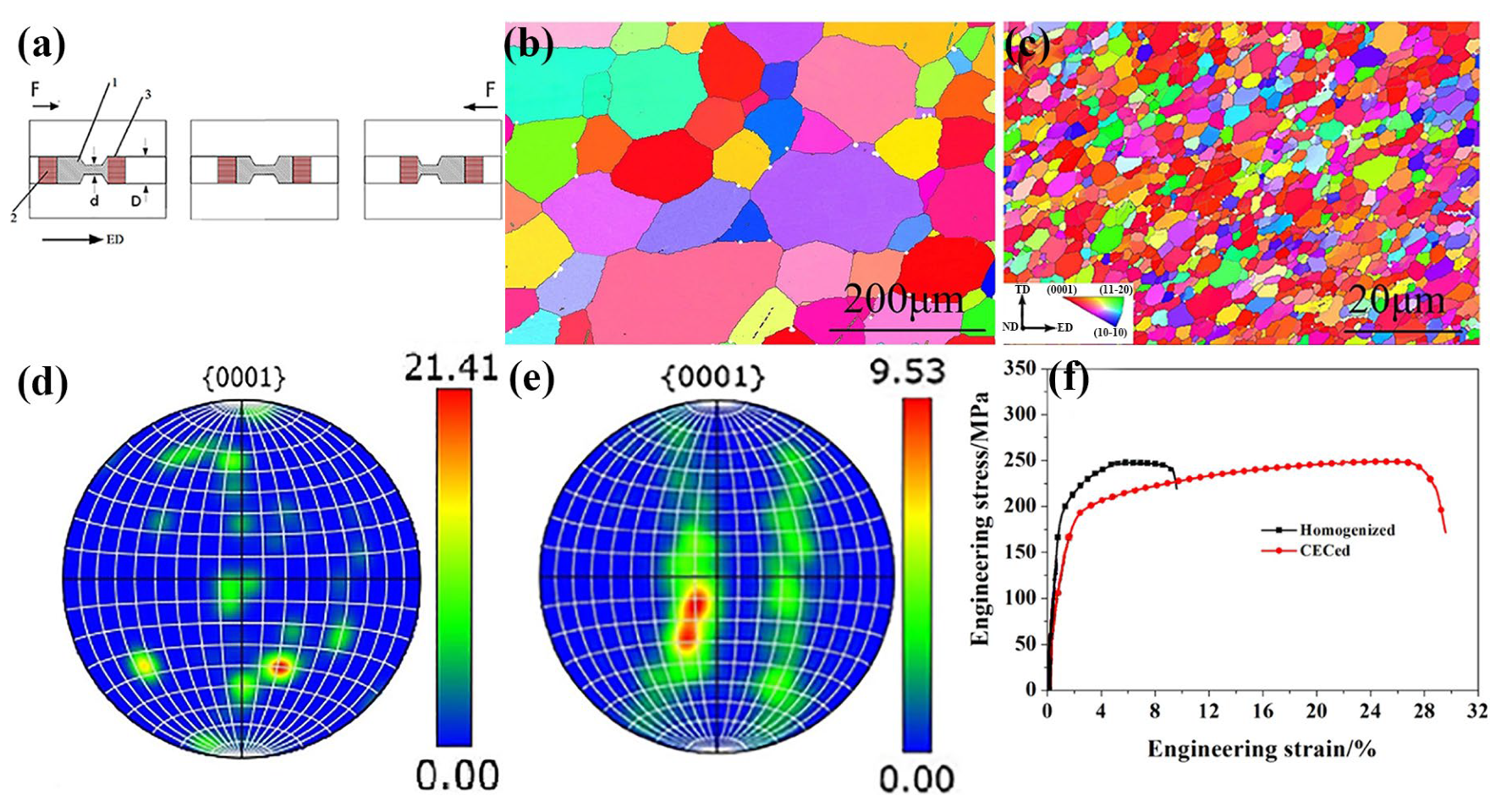

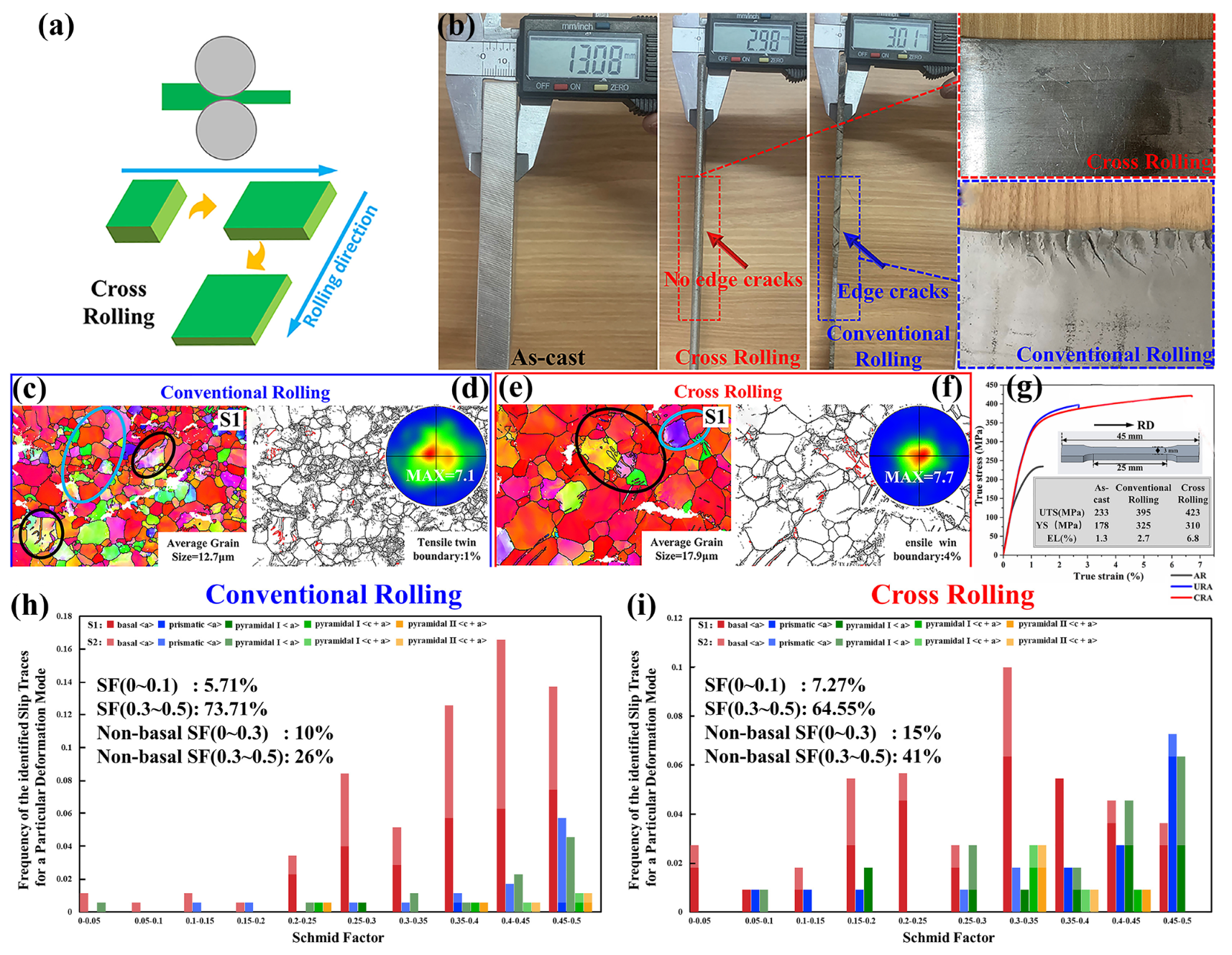
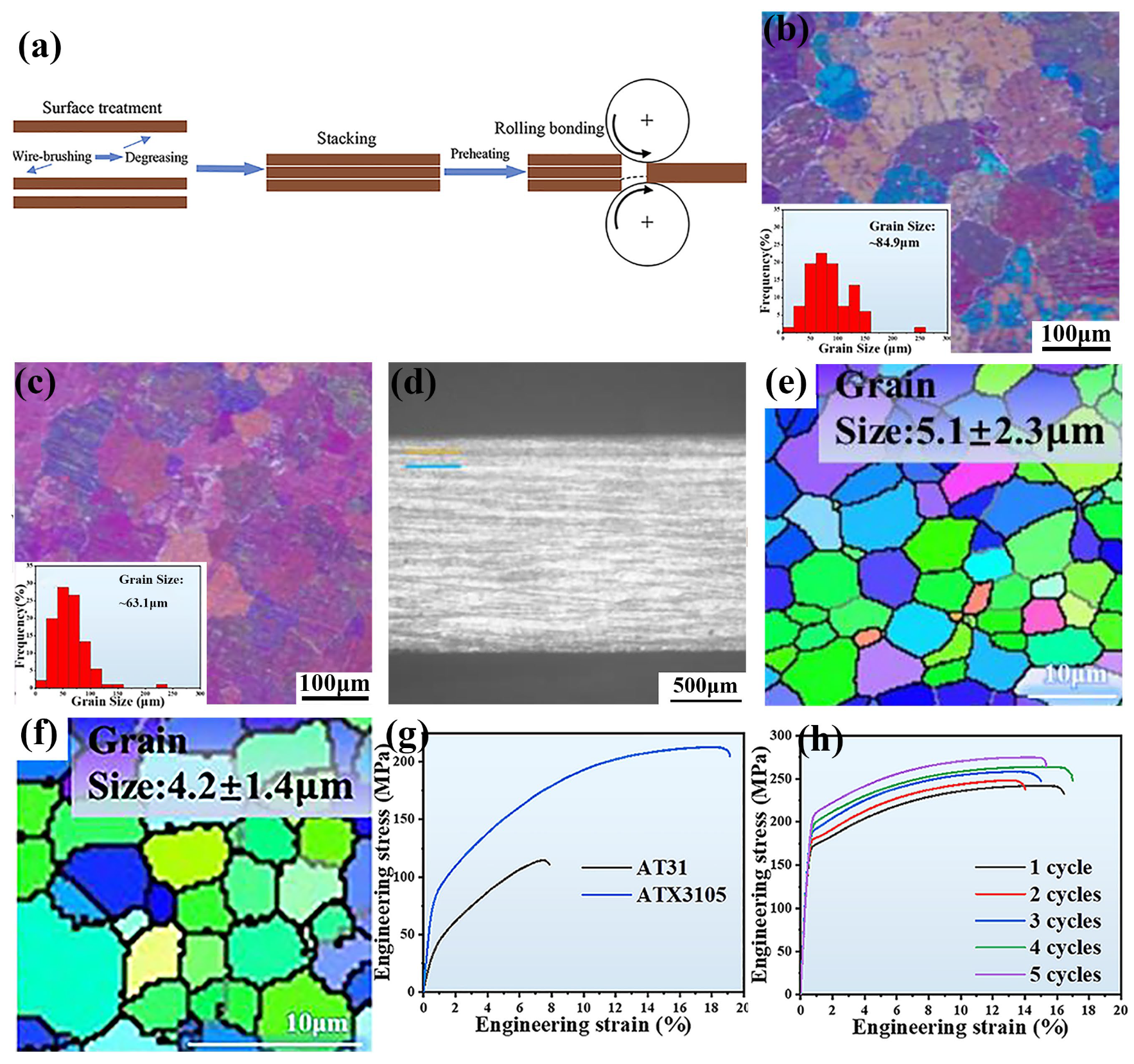

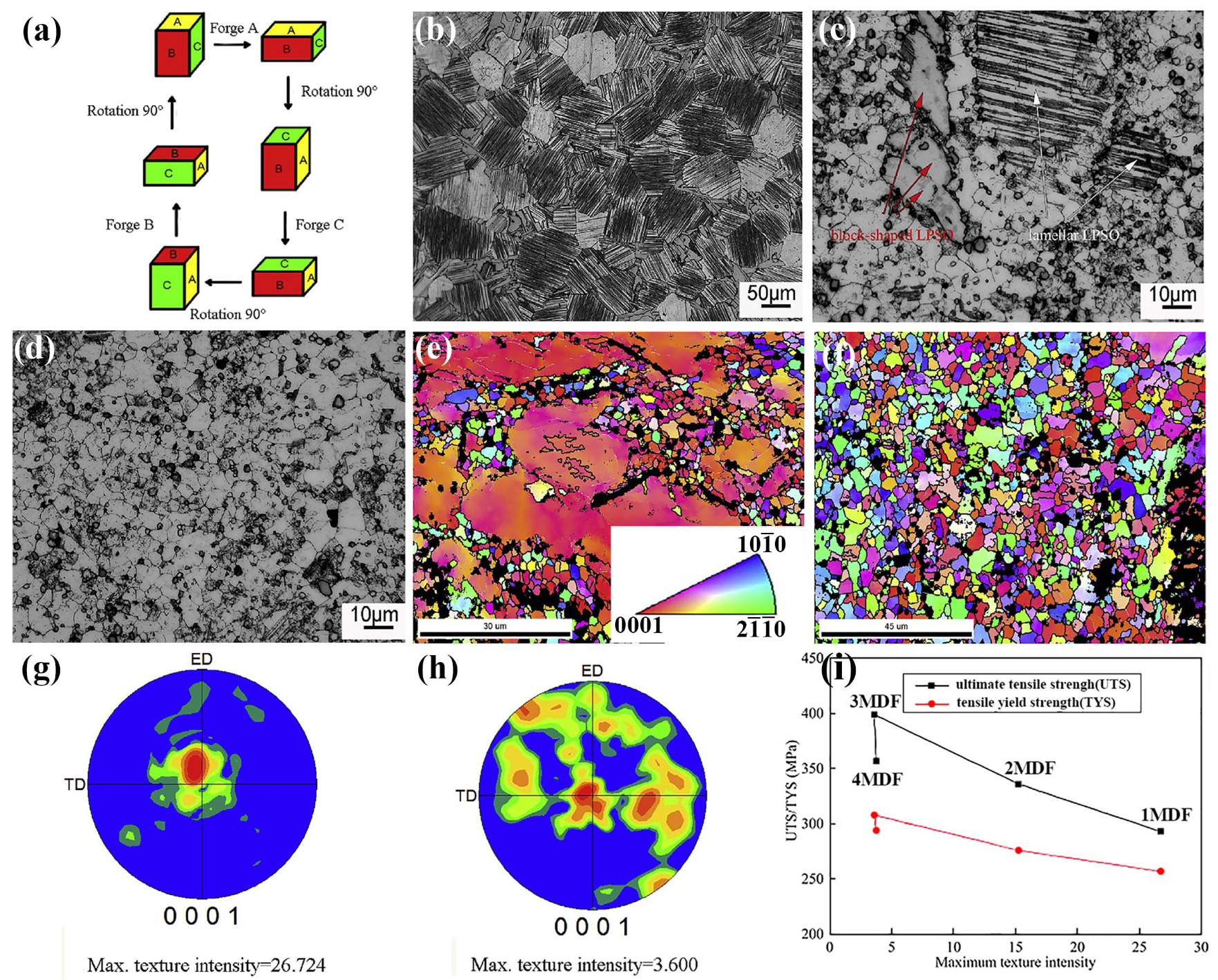
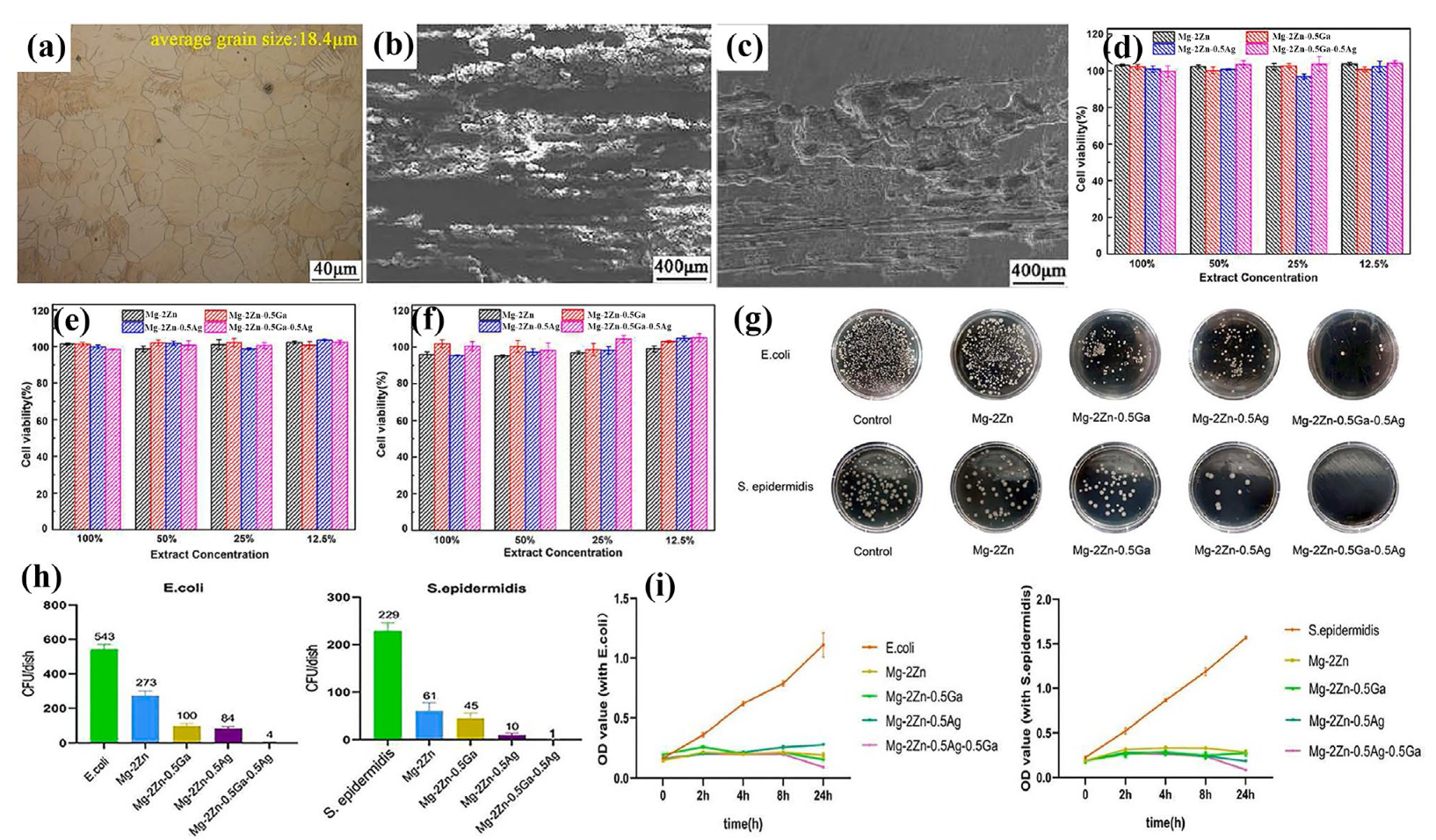
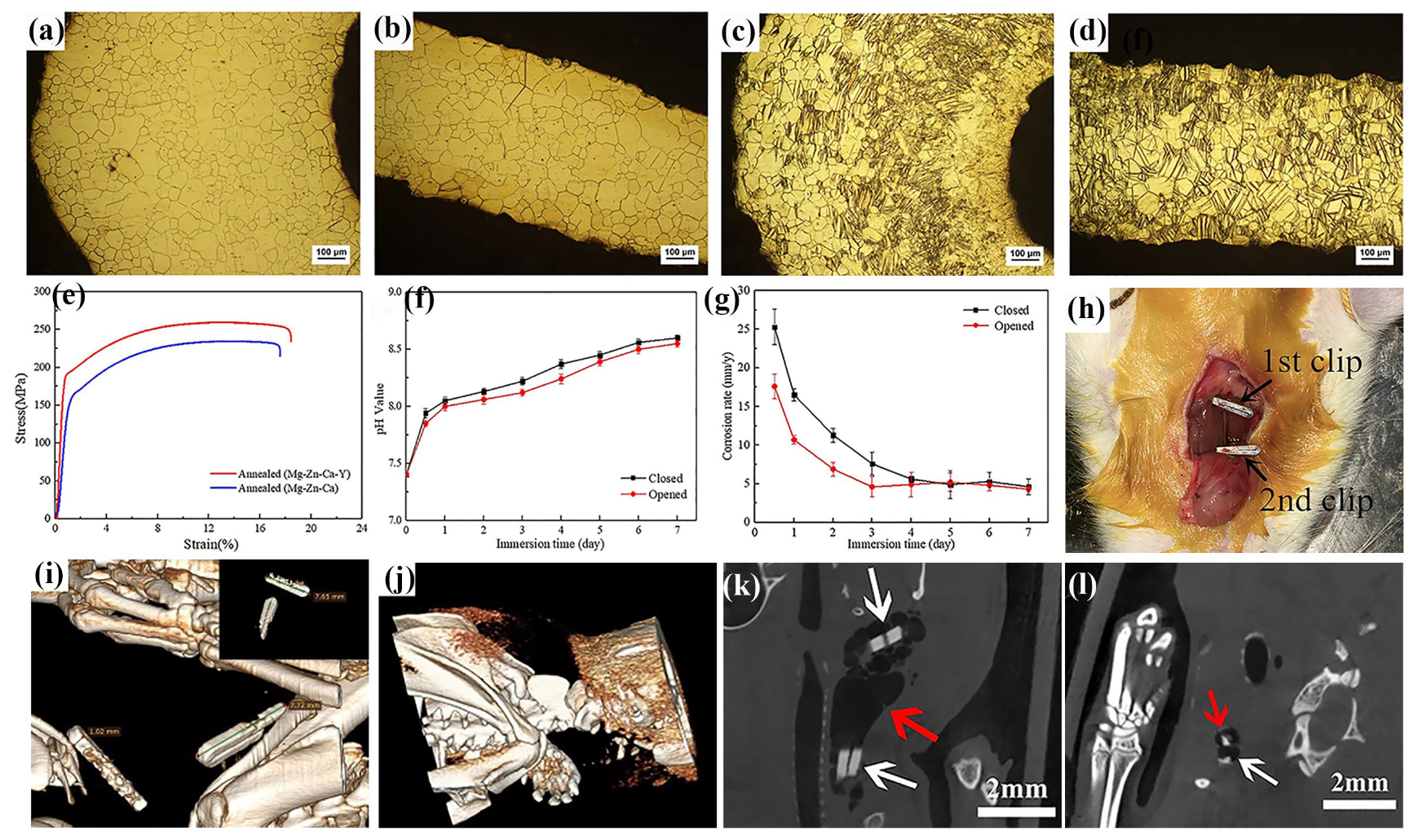
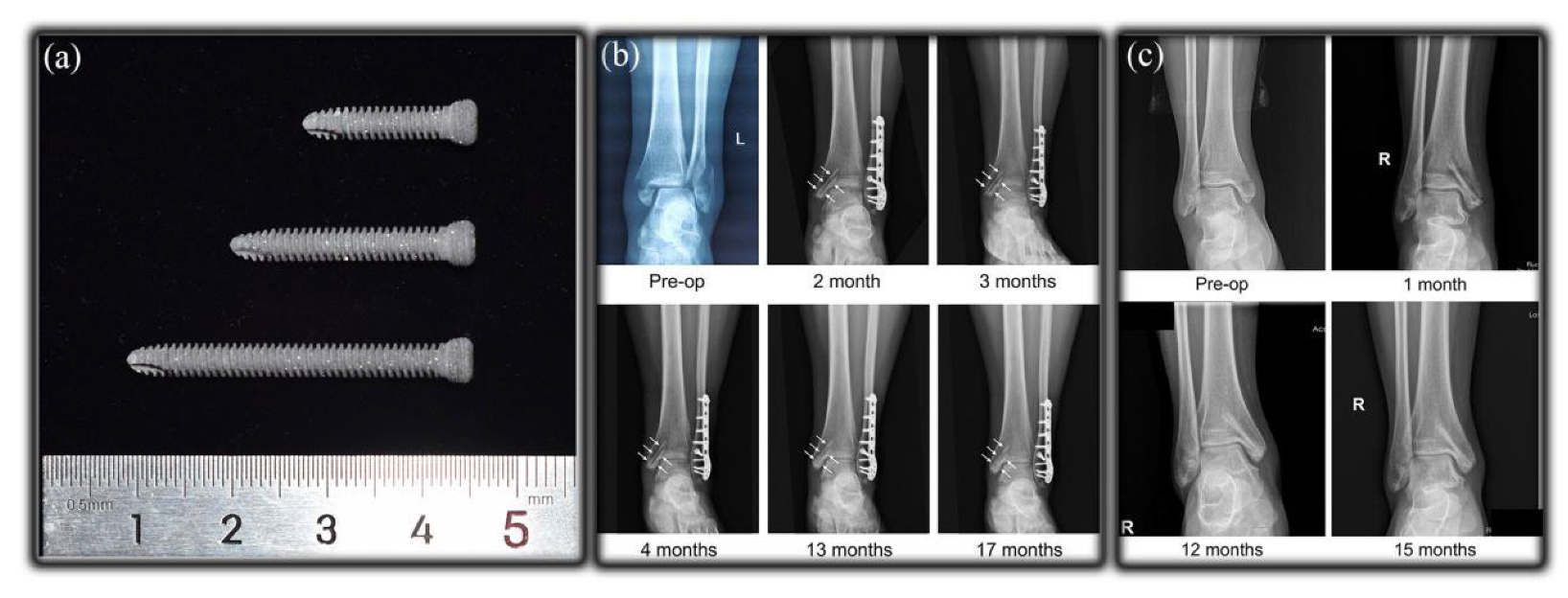
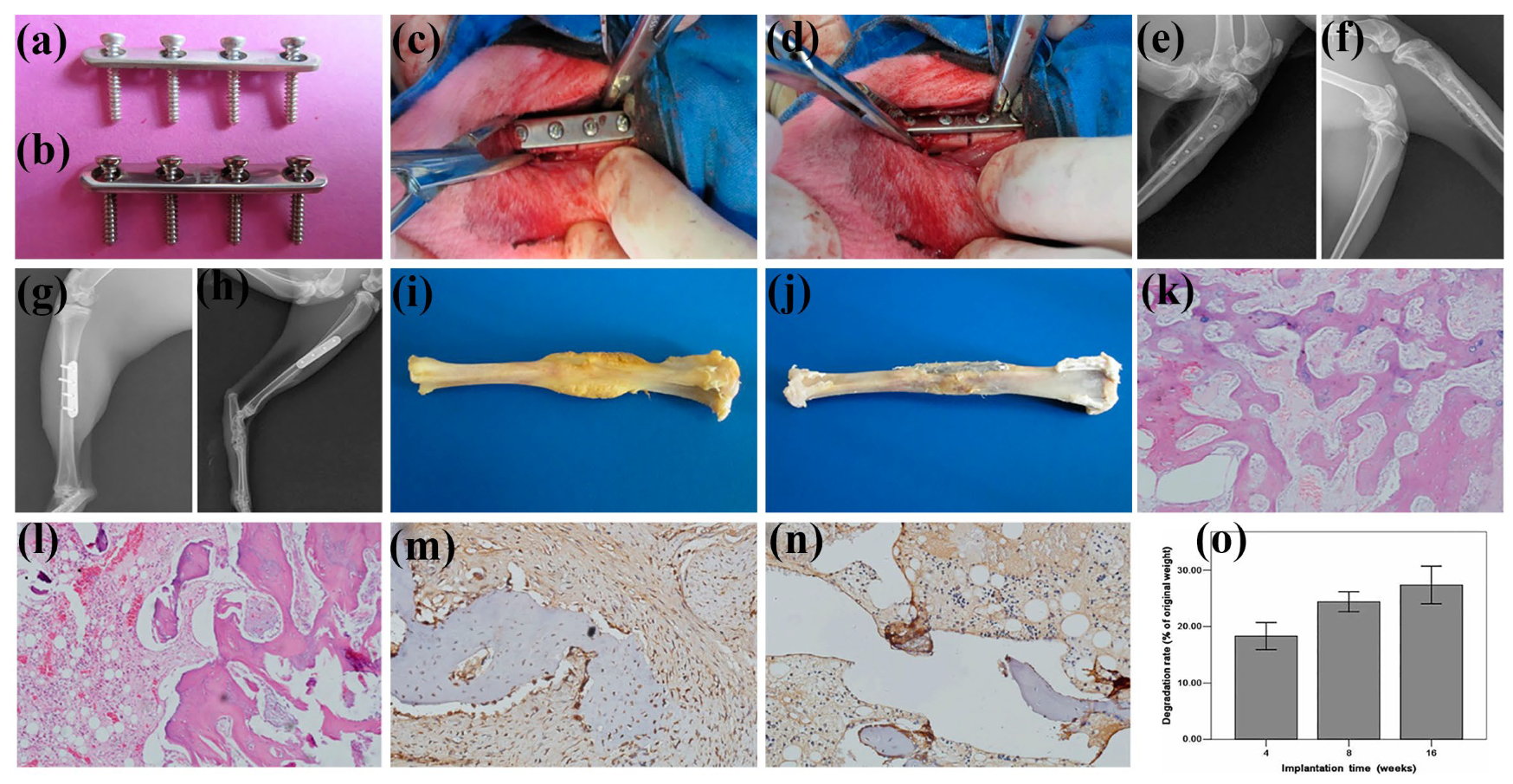
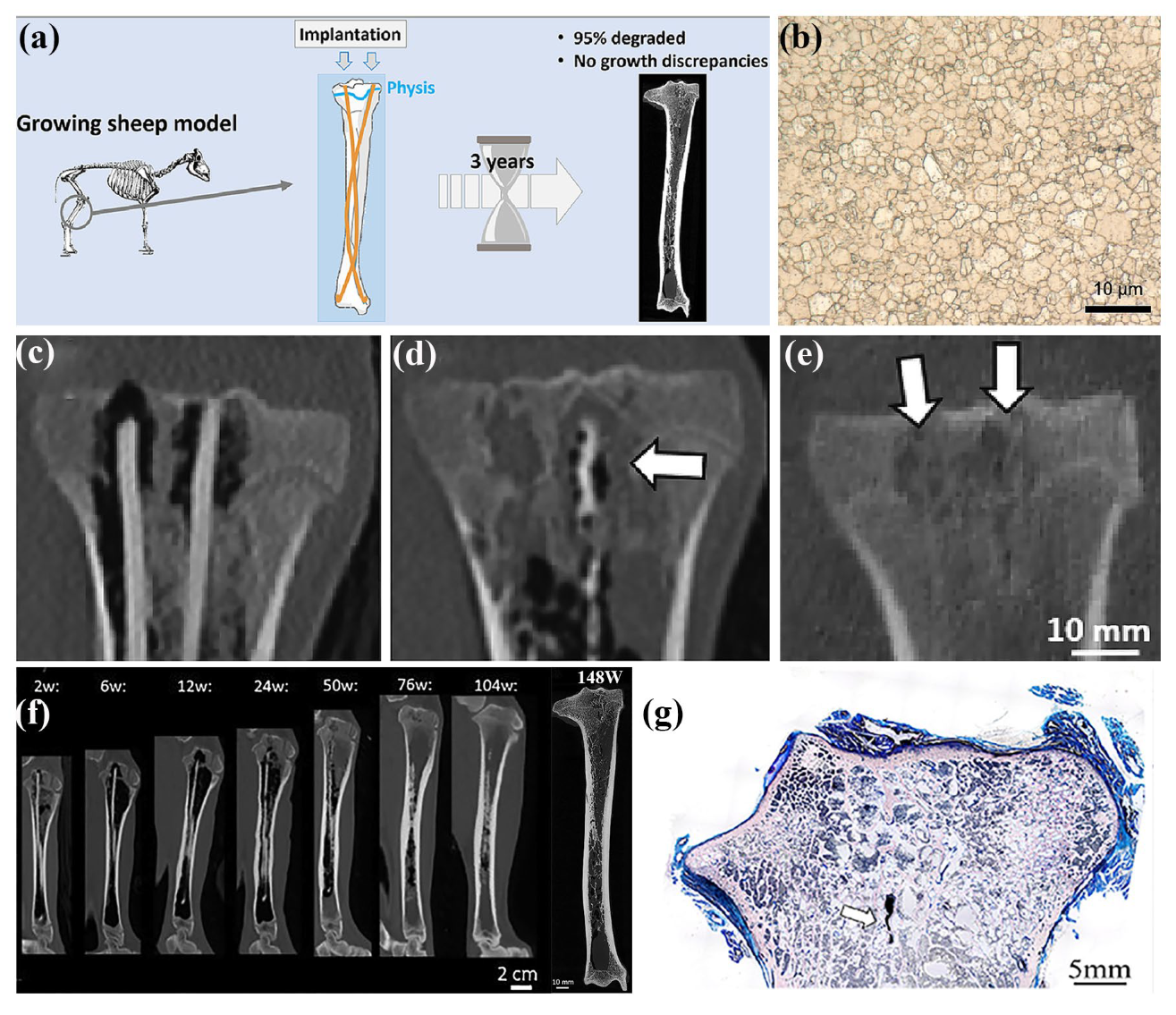
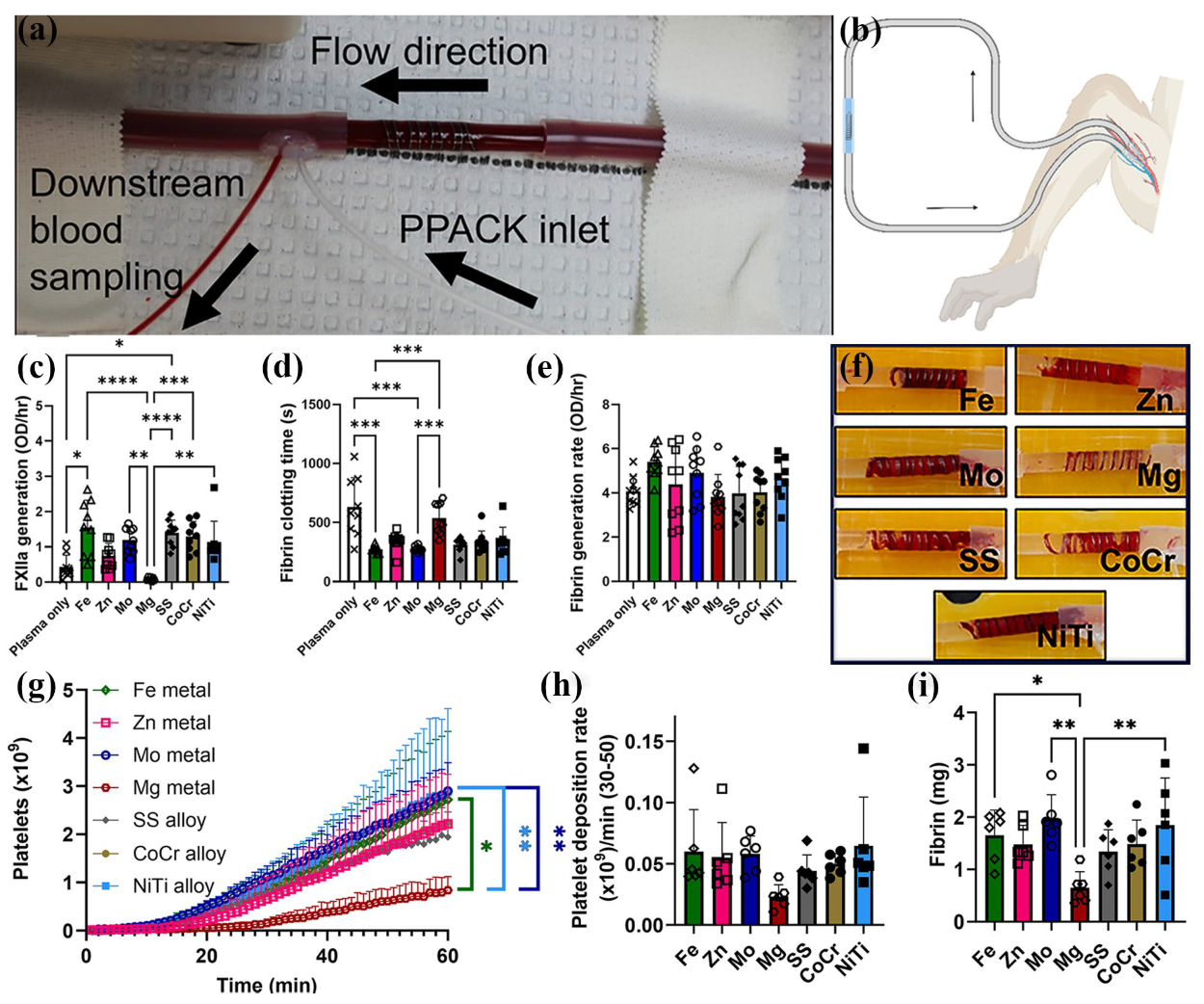
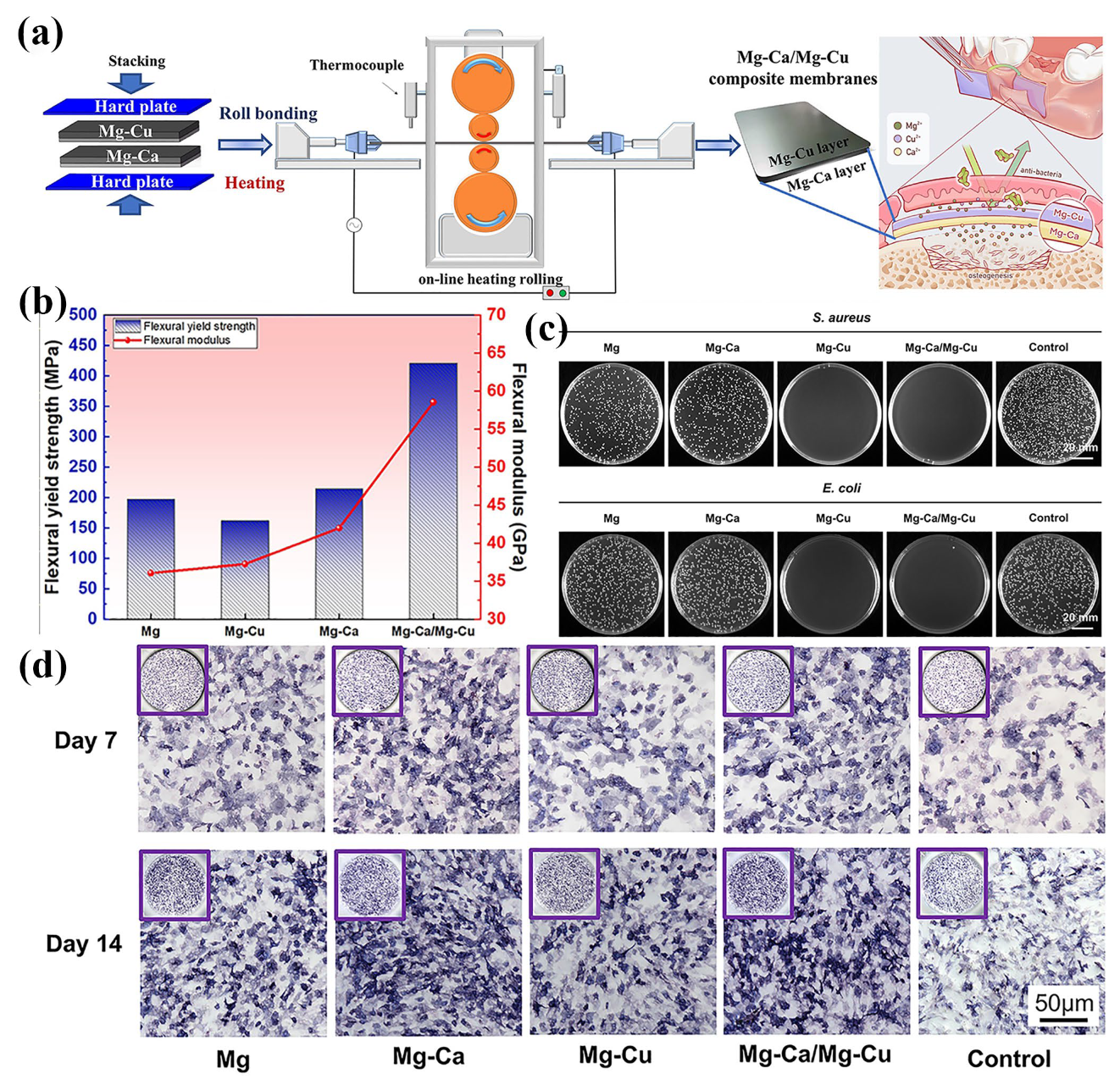
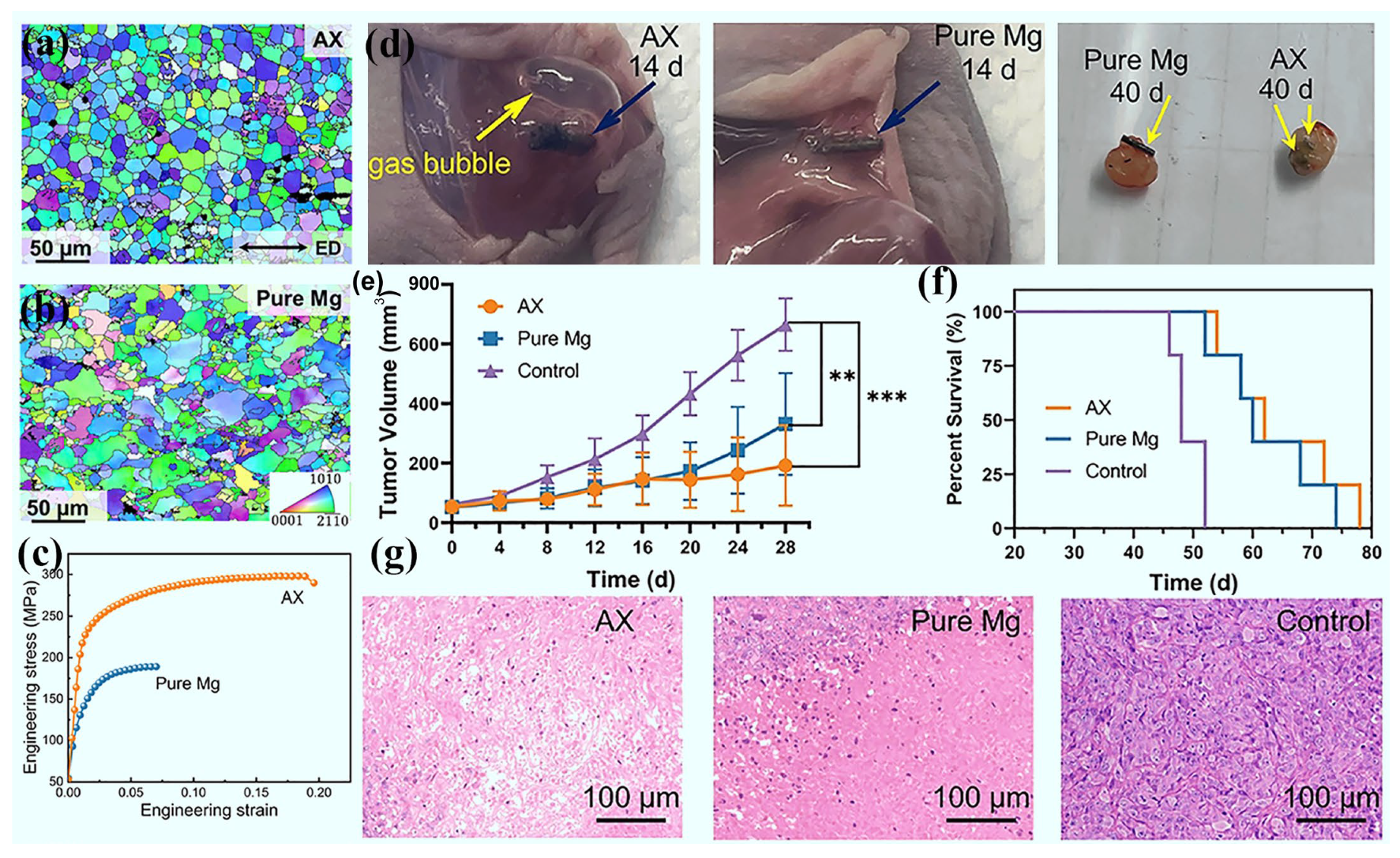
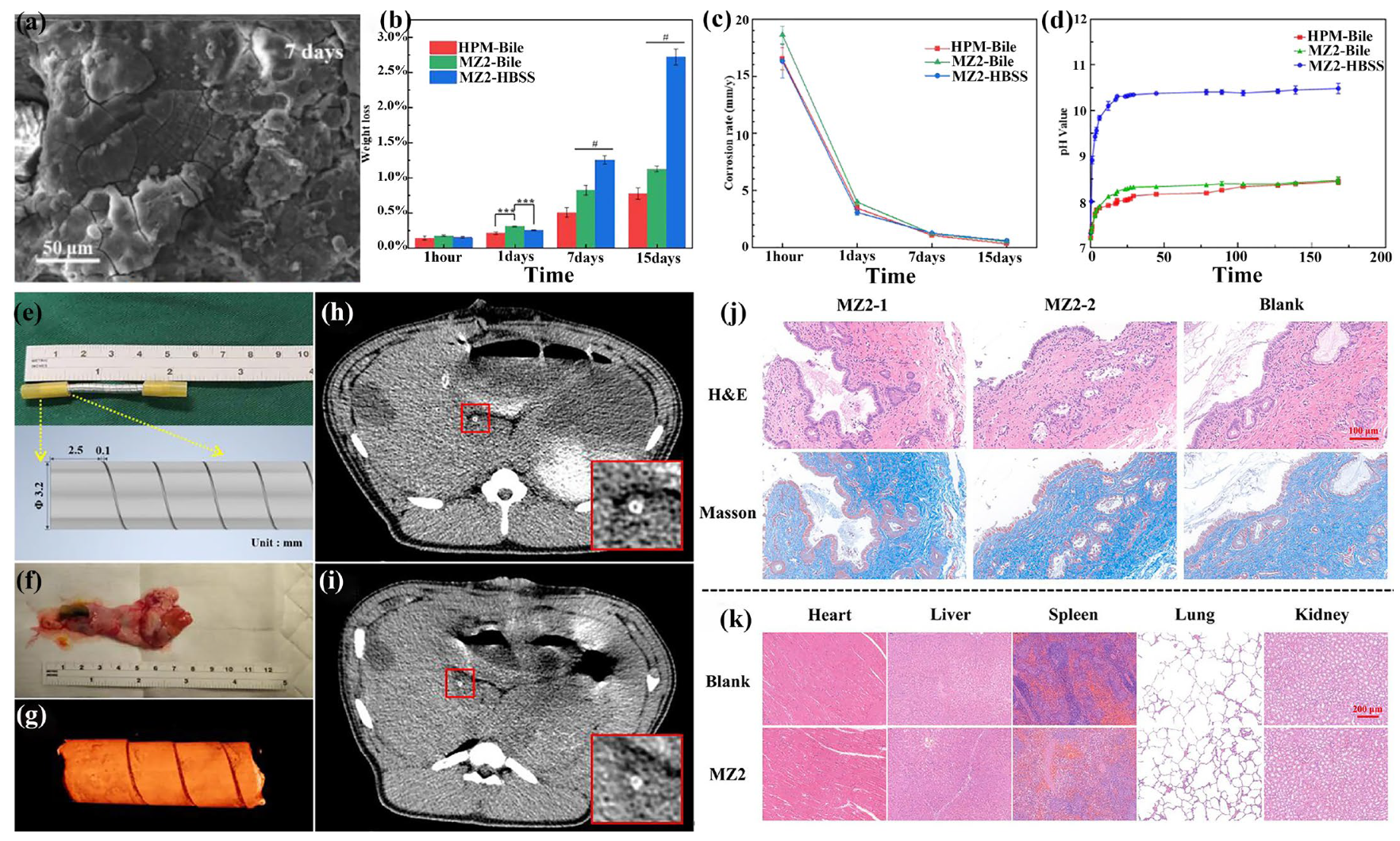
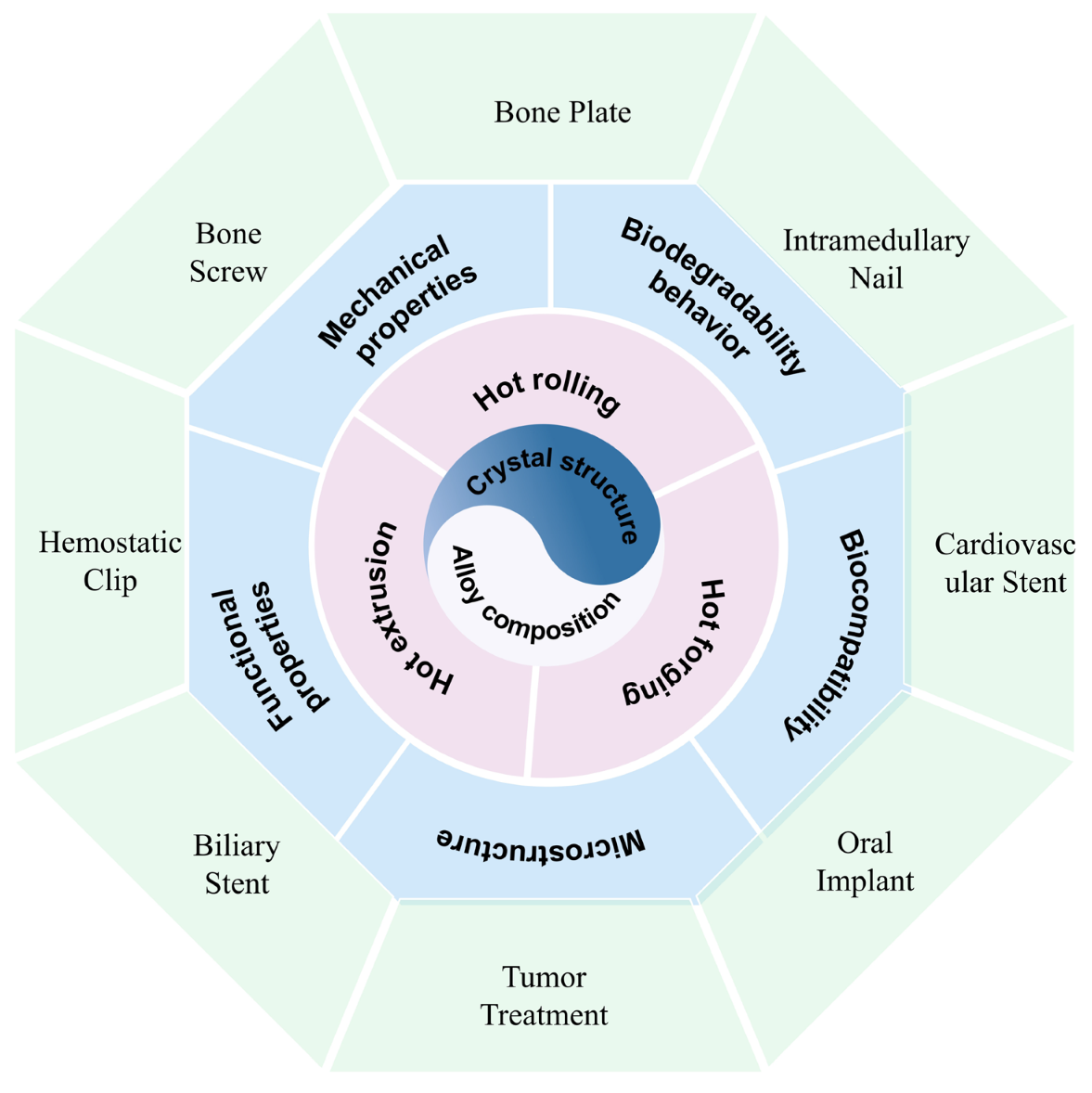
| Alloying Element | Biological Benefits | Performance Benefits | Reference |
|---|---|---|---|
| Zn | Non-cytotoxic element, good cytocompatibility | Grain refinement, increasing corrosion resistance and mechanical properties. | [65] |
| Mn | Low hemolysis rate, regulating immunity effect, and good cytocompatibility | Increasing corrosion resistance and strength. | [66] |
| Ca | Essential bone component, good cytocompatibility, and anticarcinogenic function | Decreasing corrosion resistance, increasing strength and ductility. | [67] |
| Sr | Osteogenesis promotion and good cytocompatibility | Grain refinement, increasing corrosion resistance. | [68] |
| Sn | Essential trace element of human body, inducing tissue regeneration and good cytocompatibility | Improving corrosion resistance. | [69] |
| Li | Nutrient element for central nervous system and good cytocompatibility | Increasing specific strength, and promoting the activity of slip system. | [21] |
| Y | Relative good cytocompatibility at low content | Grain refinement, promoting the activity of non-basal slip systems, improving ductility and corrosion resistance. | [70] |
| Nd | Relative good cytocompatibility at low content | Grain refinement, precipitation strengthening, improving corrosion resistance, but detrimental to ductility. | [70] |
| Ce | Relative good cytocompatibility at low content and osseointegration | Grain refinement, precipitation strengthening but detrimental to corrosion resistance. | [71] |
| Gd | Relative good cytocompatibility at low content | Grain refinement, precipitation strengthening, but detrimental to ductility. | [72] |
| Zr | Relative good cytocompatibility at low content | Grain refinement, increasing strength and ductility. | [72] |
| Dy | Relative good cytocompatibility at low content | Grain refinement, promoting the activity of non-basal slip systems, improving ductility and corrosion resistance. | [73] |
| Thermomechanical Processing | Advantages | Disadvantages | Reference |
|---|---|---|---|
| Conventional extrusion (Direct extrusion) | (1) Grain refinement; (2) Wide applicability; (3) Reduction in metallurgical defects; (4) Improvement in compactness; (5) High surface quality. | (1) Limited billet length; (2) Prone to porosity, cold separation, poor filling, cracking, and cold interlayers; (3) High sensitivity to temperature control; (4) Uneven deformation. | [88] |
| Equal-channel angular pressing | (1) Grain refinement; (2) Improved microstructure uniformity; (3) Significant improvement in mechanical properties; (4) Formation of nanocrystalline. | (1) Limited billet size; (2) Limited application in alloys; (3) Extremely high crystal defects and possible damage. | [89,90] |
| Cyclic extrusion compression | (1) Grain refinement; (2) Improvement in mechanical properties; (3) Obtaining ultra-fine grains and uniformly distributed secondary phase; (4) Continuous processing. | (1) Accurate reverse compression parameters; (2) Having critical passes and minimum grain size; (3) Cracking occurs in exceeded critical passes. | [91,92,93,94] |
| Conventional rolling | (1) Grain refinement; (2) High production efficiency; (3) Improved mechanical properties; (4) Good uniformity; (5) Wide range of applications. | (1) Uneven deformation; (2) Low product accuracy; (3) Stress concentration issues; (4) Poor deformation coordination, wrinkling, low secondary formability, and high cracking susceptibility. | [2,95,96,97] |
| Cross-rolling | (1) Homogenization of mechanical properties; (2) Reduction in internal stress; (3) Grain refinement; (4) Improvement in the surface quality; (5) Reduction in cracks and defects. | (1) Complex process control; (2) Limited scope of application; (3) Potential introduction of defects; (4) Low production efficiency. | [98] |
| Accumulative roll bonding | (1) Ultra-fine microstructure; (2) Diversified applications; (3) Capability to process large billet; (4) High stability. | (1) Interlayer bonding issues, local necking, and fragmentation; (2) Uneven thickness distribution across layers; (3) Non-uniform dislocation slip; (4) Complex process. | [99,100,101,102] |
| Conventional forging | (1) Grain refinement; (2) Industrialized mass processing; (3) Fewer processing defects. | (1) Cracks and localized excessive deformation; (2) Limited shape complexity; (3) Uneven microstructure. | [82,103] |
| Multi-directional forging | (1) Fewer processing defects; (2) Suitable for large-size and complex-shaped workpieces; (3) Improved product quality; (4) Enhanced isotropy; (5) Reduced residual stress. | (1) Complex equipment and process control; (2) Limited scope of application; (3) Relatively low production efficiency; (4) Potential for local deformation of the material. | [104,105,106] |
| Radial forging (Rotary swaging) | (1) Grain refinement; (2) High material utilization; (3) Enhanced microstructure uniformity; (4) Low residual stress; (5) High machining accuracy. | (1) Complex process control; (2) Limited shape adaptability; (3) Unstable billet flow; (4) High billet size requirements. | [82] |
| Alloys | Processing | Yield Strength (MPa) | Tensile Strength (MPa) | Elongation (%) | Reference |
|---|---|---|---|---|---|
| Mg-3Zn-0.2Ca | HE | 215.9 | 270.2 | 11.14 | [50] |
| Mg-Zn-Ca-Mn | HR | 146 | 229 | 1.6 | [60] |
| Mg-2Zn-0.7Ca-1Mn | HE | 229 | 278 | 10 | [66] |
| Mg-0.8Ca | MDF | 199 | 264 | 9.4 | [67] |
| Mg-1Zr-0.5/1Sr-0.5/1/1.5/2Dy | HE | 163.7–231.8 | 229.4–258.3 | 11.6–23.9 | [80] |
| Mg-2.22Zn-2.25Ga | HE | 128 | 261 | 22.0 | [176] |
| Mg-2.9Zn-1.1Ca-0.5Mn | HE | 352.5 | 382.3 | 7.1 | [107] |
| Mg-1.03Zn-0.66Ca (ZX 11) | RS | 210 | 276 | 18.3 | [117] |
| Mg-1Zn-0.5Sn | HE | 115 | 239 | \ | [122] |
| Mg-2Zn-0.6Zr-0.6Nd | HE | 242–269 | 274–298 | 25.6–26.1 | [121] |
| Mg-4Zn-4Ga | HE | 256 | 343 | 14.2 | [66] |
| Mg-2.0Zn-1.6Ca | HE | / | 283.47–393.96 | 10.84–18.08 | [189] |
| Mg-4/6Zn-0.6/0.8Y-0.5Nd | HE | 153–252.6 | 245.6–308.8 | 10.0–20.2 | [123] |
| Mg-2Zn-0.46Y-0.5Nd | HE | 139.4 | 249.4 | 21.1 | [186] |
| Mg-1.5Y-1.2Zn-0.44Zr | HE | 178 | 236 | 28 | [190] |
| Mg-4Zn-0.8Y-0.5Nd | HE | 252.6 | 308.8 | 10.0 | [191] |
| Mg-0.035/0.5/1/3Zr-0.2/0.5/1/3Sr | HE | 210–275 | 253–302 | 5.9–11.1 | [192] |
| Mg-2Zn-0.6Ca-1Er | HE | 128 | 225 | 17.2 | [117] |
| Mg-3Nd-0.5Zn | HE | 251–337 | 271–338 | 0.5–5.9 | [193] |
| Mg-3Zn-0.5Sr | HE | 164 | 254 | 19 | [194] |
| Mg-3Zn-0.5Sr-0.2/0.5Ca | HE | 126–185 | 257–305 | 15–29 | [194] |
| Mg-4.12Y-2.15Nd-0.43Zr-0.26La | ECAP + Ex | 225–350 | 325–398 | 9.45–12.20 | [195] |
| Mg-0.24Sn-0.04Mn | HR | 117.9 | 178.8 | 9.1 | [196] |
| Mg-0.24Sn-0.04Mn | HE | 129 | 219.6 | 7.9 | [196] |
| Mg-0.24Sn-1.16Zn-0.04 Mn | HR | 178 | 223 | 5.2 | [196] |
| Mg-0.24Sn-1.16Zn-0.04 Mn | HE | 142.3 | 269.2 | 16.5 | [196] |
| Alloys | Processing | Corrosion Medium | Corrosion Rate (mm/y) | Reference |
|---|---|---|---|---|
| Mg-1Zr-0.5Sr-1Dy | HE | SBF | 3.37 | [80] |
| Mg-4Zn-0.5Ca-0.75Mn | HE | SBF | 0.12 | [200] |
| Mg-1.03Zn-0.6Ca | RS | FBS | 0.44 | [117] |
| Mg-1Zn-0.1Ca | HE | SBF | 3.2 | [197] |
| Mg-0.45Zn-0.45Ca | HE | α-MEM medium | 1.04 | [201] |
| Mg-1Zn-0.3Ca | HE | Right tibia of sheep | 0.27 | [202] |
| Mg-2Sr-Zn | HR | HBSS | 0.85 | [68] |
| Mg-2Sr-Ca | HR | HBSS | 1.10 | [68] |
| Mg–2Sr | HR | HBSS | 1.37 | [68] |
| Mg-3Zn-0.2Ca-0.5Y | HE | SBF | 5 | [49] |
| Mg-2Zn-0.7Ca-1Mn | HE | HBSS | 0.3 | [203] |
| Mg-1.0Zn-0.3Ca | HE | SBF | 0.091 | [204] |
| Mg-1.5Zn-0.25Ca | HE | SBF | 0.123 | [204] |
| Mg-3.0Gd-2.7Zn-0.4Zr-0.1Mn | HE | HBSS | 0.46 ± 0.19 | [205] |
| Mg-2Zn-0.6Ca-1Er | HE | 0.5 wt.% NaCl solution at 25 °C | 7.83 | [206] |
| Mg-2Zn-0.6Ca-1Er | HE | PBS at 37 °C | 1.55 | [206] |
| Mg-0.5Zn-0.35Zr-0.15Mn-2Tb | HE | HBSS | 0.10 | [207] |
| Mg-0.24Sn-0.04Mn | HR | HBSS | 0.51 | [196] |
| Mg-0.24Sn-0.04Mn | HE | HBSS | 0.71 | [196] |
| Mg-0.24Sn-1.16Zn-0.04 Mn | HR | HBSS | 2.87 | [196] |
| Mg-0.24Sn-1.16Zn-0.04 Mn | HE | HBSS | 0.95 | [196] |
| Mg-6Zn | MDF | 0.1 M NaCl solution | 0.34 | [65] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, H.; Cheng, J.; Zhao, C.; Wen, M.; Wang, R.; Wu, D.; Wu, Z.; Yang, F.; Sheng, L. The Recent Developments of Thermomechanical Processing for Biomedical Mg Alloys and Their Clinical Applications. Materials 2025, 18, 1718. https://doi.org/10.3390/ma18081718
Zhao H, Cheng J, Zhao C, Wen M, Wang R, Wu D, Wu Z, Yang F, Sheng L. The Recent Developments of Thermomechanical Processing for Biomedical Mg Alloys and Their Clinical Applications. Materials. 2025; 18(8):1718. https://doi.org/10.3390/ma18081718
Chicago/Turabian StyleZhao, Hui, Jing Cheng, Chaochao Zhao, Min Wen, Rui Wang, Di Wu, Zhaoying Wu, Fang Yang, and Liyuan Sheng. 2025. "The Recent Developments of Thermomechanical Processing for Biomedical Mg Alloys and Their Clinical Applications" Materials 18, no. 8: 1718. https://doi.org/10.3390/ma18081718
APA StyleZhao, H., Cheng, J., Zhao, C., Wen, M., Wang, R., Wu, D., Wu, Z., Yang, F., & Sheng, L. (2025). The Recent Developments of Thermomechanical Processing for Biomedical Mg Alloys and Their Clinical Applications. Materials, 18(8), 1718. https://doi.org/10.3390/ma18081718








