Microstructure and Shear Strength of SiC Ceramics Diffusion Bonded with Ti Foils by Spark Plasma Sintering
Abstract
1. Introduction
2. Materials and Methods
2.1. Starting Materials
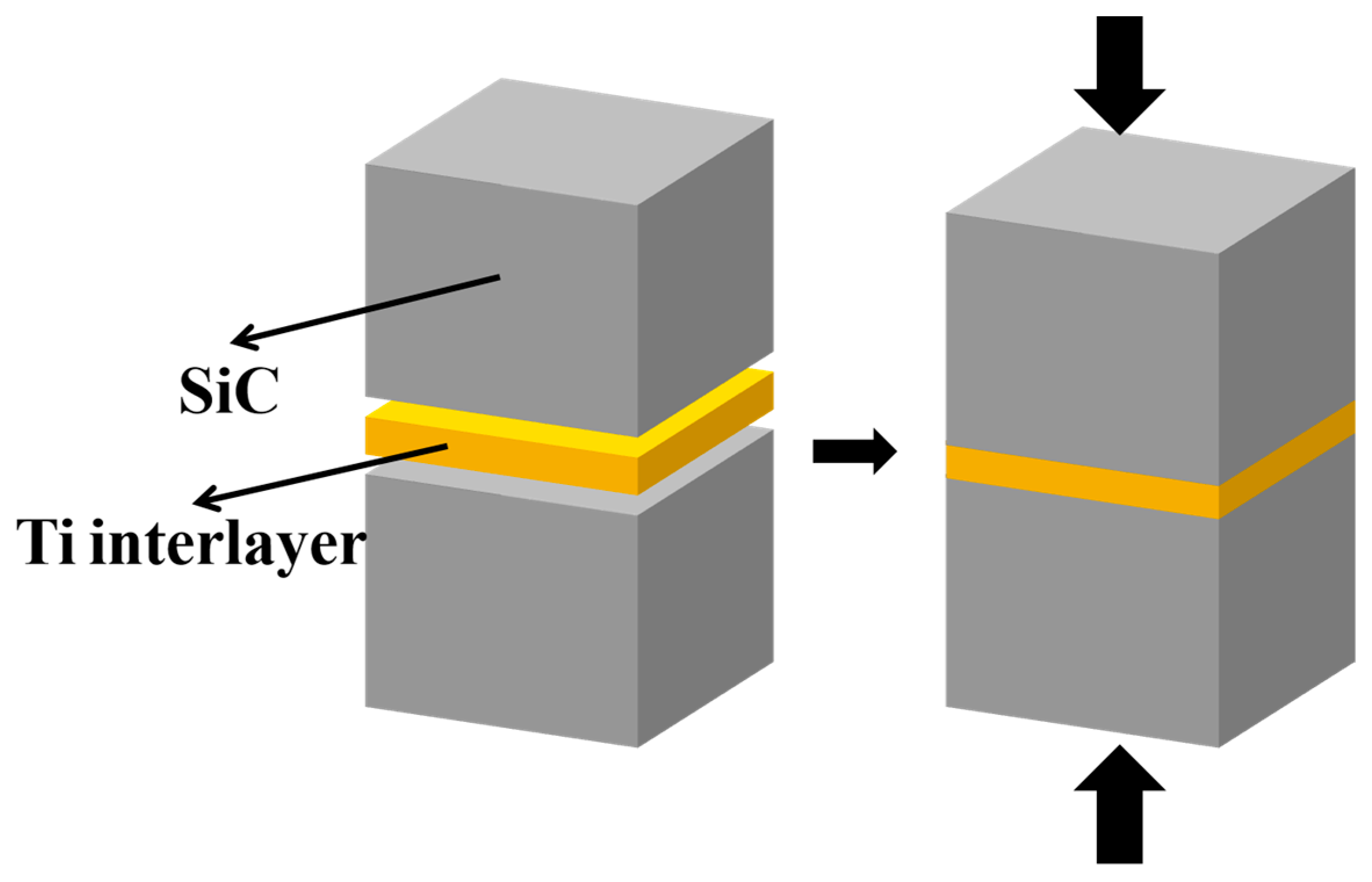
2.2. Process of Joining
2.3. Shear Strength Measurements
2.4. Microstructural Characterizations
3. Results and Discussion
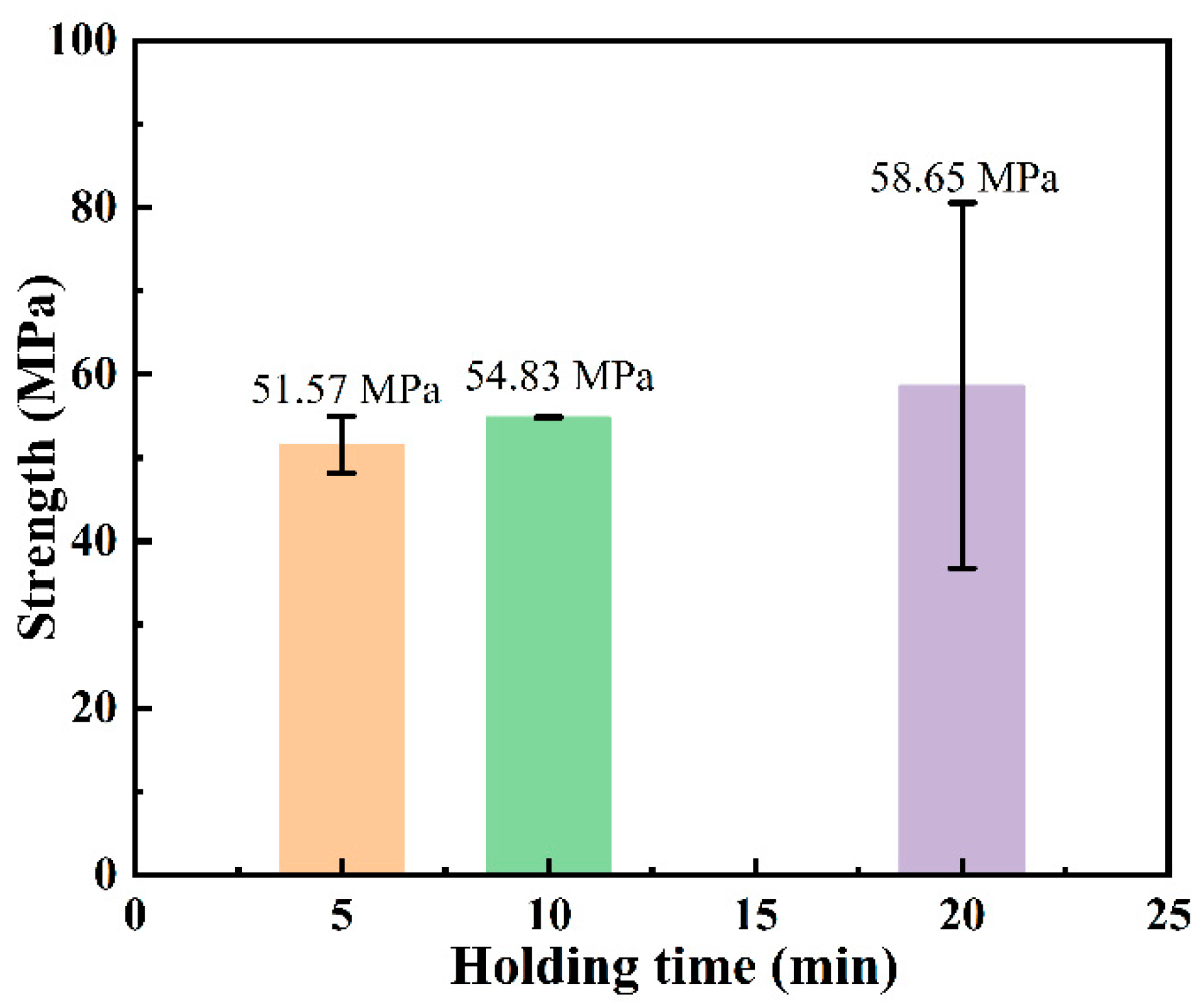
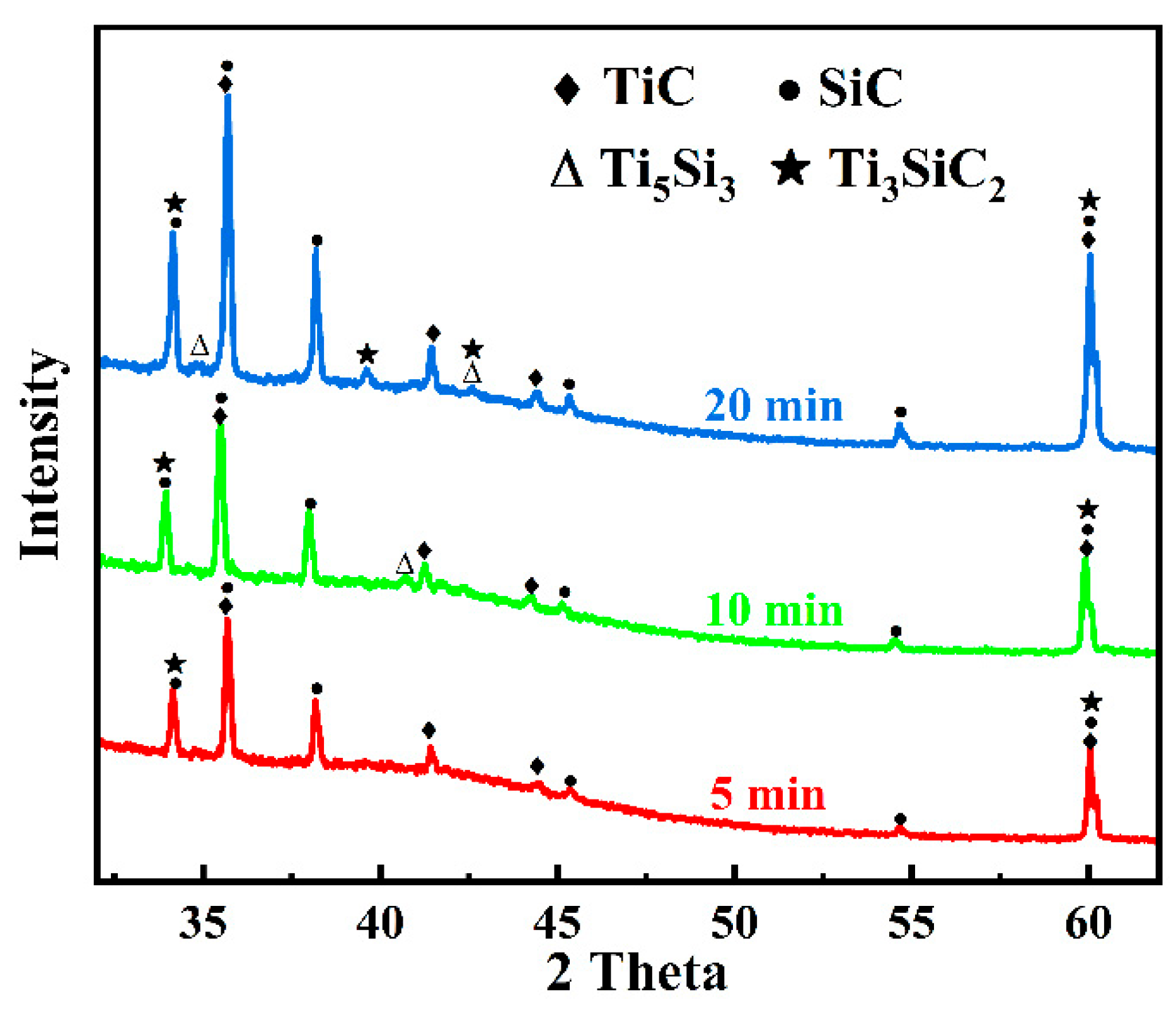
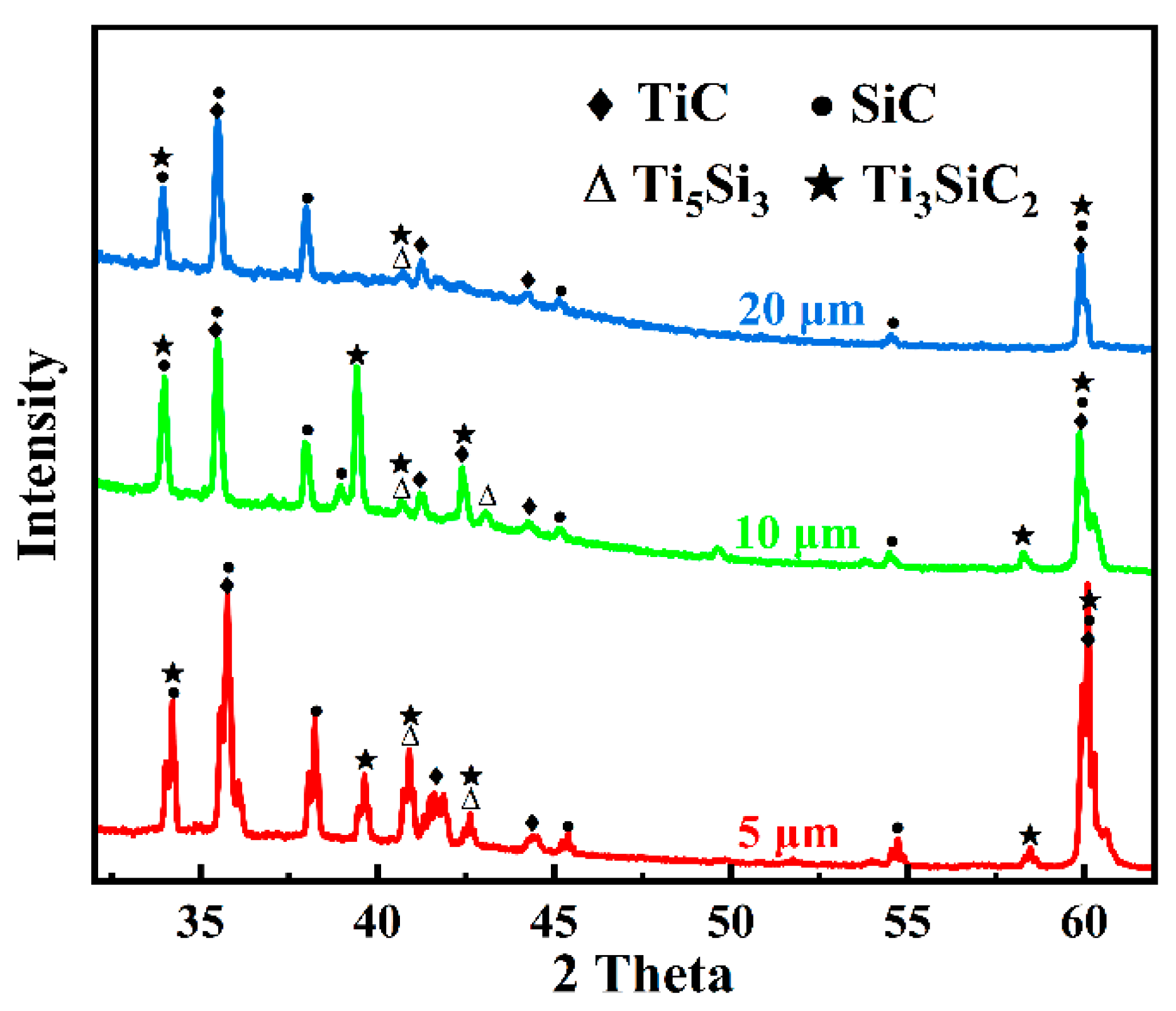
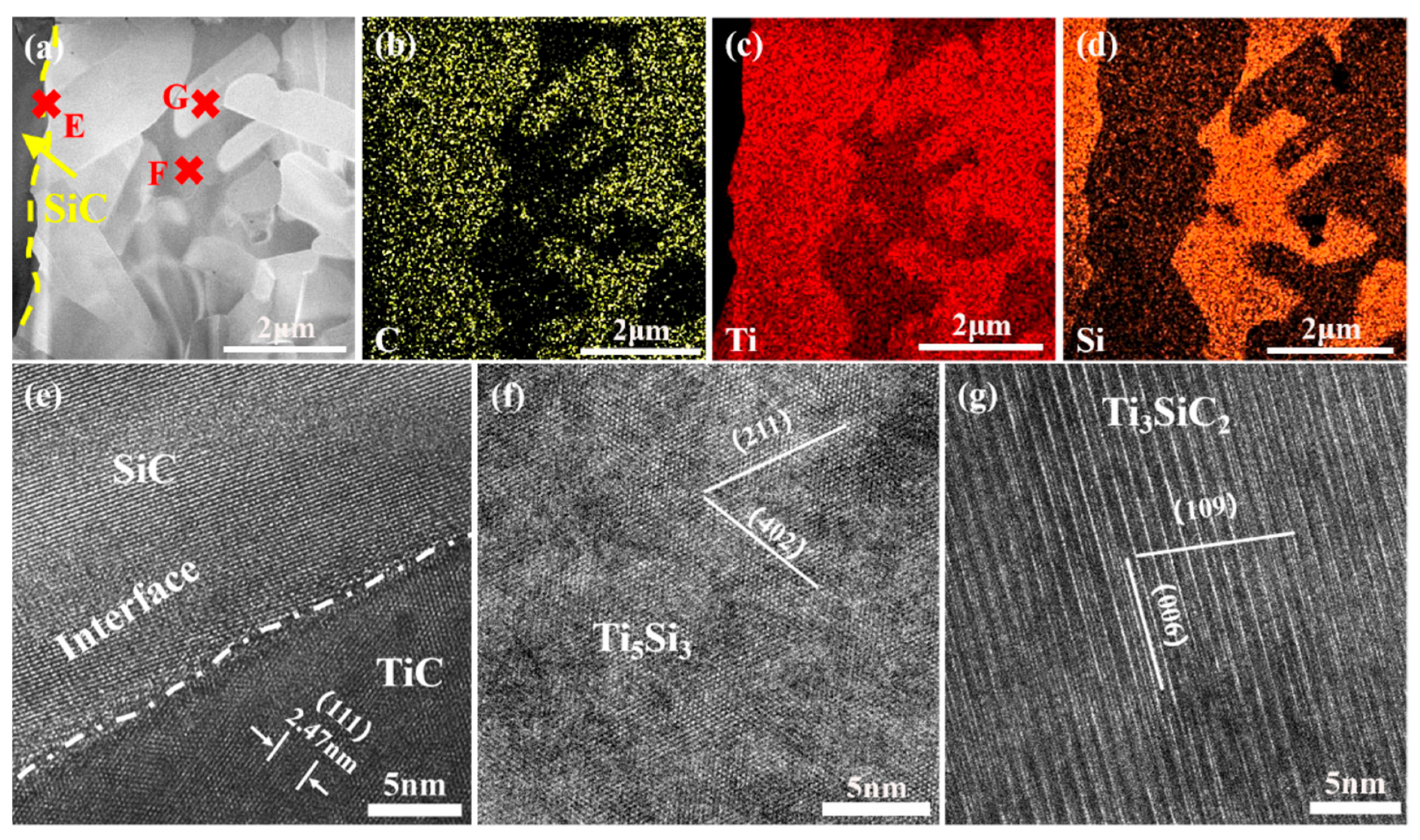
3.1. Microstructure and Phase Composition of SiC-Ti-SiC Joints Under Different Dwell Times
3.2. Microstructure and Phase Composition of SiC-Ti-SiC Joints Under Different Joining Temperatures
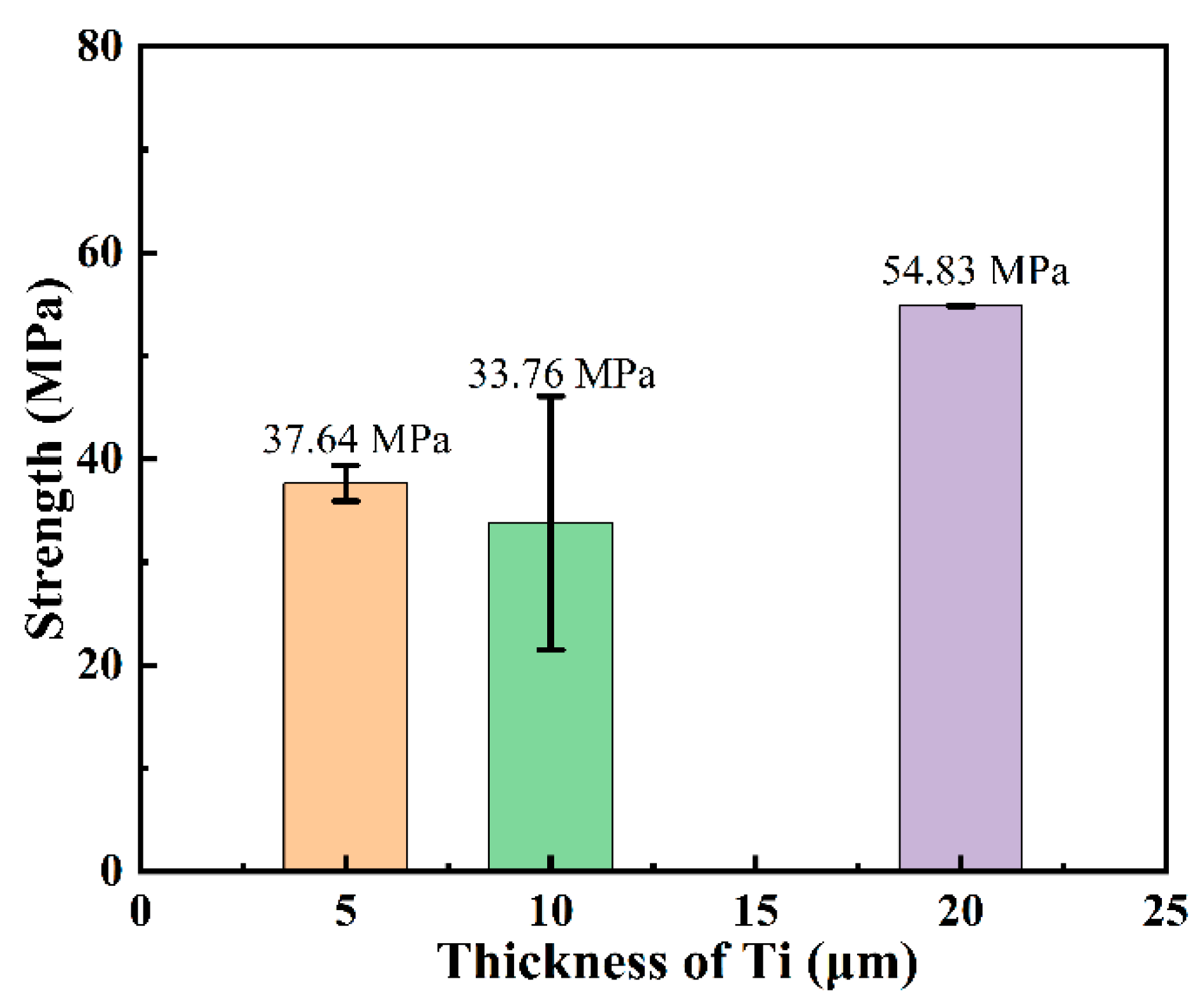
3.3. Microstructure and Phase Composition of SiC-Ti-SiC Joints Under Different Thicknesses of Ti Foils
4. Conclusions
4.1. Effect of Holding Time
4.2. Effect of Joining Temperature
4.3. Effect of Ti Foil Thickness
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koyanagi, T.; Katoh, Y.; Nozawa, T.; Snead, L.L.; Kondo, S.; Henager, C.H.; Ferraris, M.; Hinoki, T.; Huang, Q. Recent progress in the development of SiC composites for nuclear fusion applications. J. Nucl. Mater. 2018, 511, 544–555. [Google Scholar] [CrossRef]
- Katoh, Y.; Ozawa, K.; Shih, C.; Nozawa, T.; Shinavski, R.J.; Hasegawa, A.; Snead, L.L. Continuous SiC fiber, CVI SiC matrix composites for nuclear applications: Properties and irradiation effects. J. Nucl. Mater. 2014, 448, 448–476. [Google Scholar] [CrossRef]
- Zinkle, S.J.; Terrani, K.A.; Gehin, J.C.; Ott, L.J.; Snead, L.L. Accident tolerant fuels for LWRs: A perspective. J. Nucl. Mater. 2014, 448, 374–379. [Google Scholar] [CrossRef]
- Wang, Y.F.; Wei, L.F.; Yang, F.Q.; Li, H.X.; Dong, Y.; Zheng, Y.; He, Y.M.; Yang, J.G. Discharge PlasmaDiffusion Bonding of SiC Ceramic Using SiC-Al2O3-Y2O3 Interlayer: Microstructure and Strength of the Joint. Weld. Mach. 2023, 53, 1–6. [Google Scholar]
- Yu, T.; Kwon, H.; Shi, L.K.; Zhou, X.B.; Yoon, D.H.; Tatarko, P.; Xu, X.; Huang, Z.R.; Huang, Q. Joining of Ti-coated monolithic SiC using a SiCw/Ti3SiC2 filler by electric field-assisted sintering. J. Eur. Ceram. Soc. 2021, 41, 1834–1840. [Google Scholar] [CrossRef]
- Rossi, G.A.; Pelletier, P.J. Joining of SiC Parts by Polishing and Hipping. U.S. Patent 4925608, 27 September 1988. [Google Scholar]
- Herrmann, M.; Lippmann, W.; Hurtado, A. High-temperature stability of laser-joined silicon carbide components. J. Nucl. Mater. 2013, 443, 458–466. [Google Scholar] [CrossRef]
- Schaafhausen, S.; Börner, F.D.; Chand, T.; Lippmann, W.; Hurtado, A.; Müller, M.C.A. Corrosion of laser joined silicon carbide in gasification environment. Adv. Appl. Ceram. 2015, 114, 350–360. [Google Scholar] [CrossRef]
- Riccardi, B.; Nannetti, C.A.; Petrisor, T.; Woltersdorf, J.; Pippel, E.; Libera, S.; Pilloni, L. Issues of low activation brazing of SiCf/SiC composites by using alloys without free silicon. J. Nucl. Mater. 2004, 329–333, 562–566. [Google Scholar] [CrossRef]
- Prakash, P.; Mohandas, T.; Raju, P.D. Microstructural characterization of SiC ceramic and SiC-metal active metal brazed joints. Scr. Mater. 2005, 52, 1169–1173. [Google Scholar] [CrossRef]
- Luo, Z.H.; Jiang, D.L.; Zhang, J.X.; Lin, Q.L.; Chen, Z.M.; Huang, Z.R. Development of SiC–SiC joint by reaction bonding method using SiC/C tapes as the interlayer. J. Eur. Ceram. Soc. 2012, 32, 3819–3824. [Google Scholar] [CrossRef]
- Tian, W.B.; Kita, H.; Hyuga, H.; Kondo, N.; Nagaoka, T. Reaction joining of SiC ceramics using TiB2-based composites. J. Eur. Ceram. Soc. 2010, 30, 3203–3208. [Google Scholar] [CrossRef]
- Henager, C.H., Jr.; Shin, Y.; Blum, Y.; Giannuzzi, L.A.; Kempshall, B.W.; Schwarz, S.M. Coatings and joining for SiC and SiC-composites for nuclear energy systems. J. Nucl. Mater. 2007, 367–370, 1139–1143. [Google Scholar] [CrossRef]
- Ferraris, M.; Casalegno, V.; Rizzo, S.; Salvo, M.; Van Staveren, T.O.; Matejicek, J. Effects of neutron irradiation on glass ceramics as pressure-less joining materials for SiC based components for nuclear applications. J. Nucl. Mater. 2012, 429, 166–172. [Google Scholar] [CrossRef]
- Patel, M.; Singh, V.; Singh, S.; Bhanu Prasad, V.V. Micro-structural evolution during diffusion bonding of C-SiC/C-SiC composite using Ti interlayer. Mater. Charact. 2018, 135, 71–75. [Google Scholar] [CrossRef]
- Fitriani, P.; Septiadi, A.; Jeong, D.H.; Dang-Hyok, Y. Joining of SiC monoliths using a thin MAX phase tape and the elimination of joining layer by solid-state diffusion. J. Eur. Ceram. Soc. 2018, 38, 3433–3440. [Google Scholar] [CrossRef]
- Tatarko, P.; Chlup, Z.; Mahajan, A.; Casalegno, V.; Saunders, T.G.; Dlouhý, I.; Reece, M.J. High temperature properties of the monolithic CVD β-SiC materials joined with a pre-sintered MAX phase Ti3SiC2 interlayer via solid-state diffusion bonding. J. Eur. Ceram. Soc. 2017, 37, 1205–1216. [Google Scholar] [CrossRef]
- Tatarko, P.; Casalegno, V.; Hu, C.F.; Salvo, M.; Ferraris, M.; Reece, M.J. Joining of CVD-SiC coated and uncoated fibre reinforced ceramic matrix composites with pre-sintered Ti3SiC2 MAX phase using Spark Plasma Sintering. J. Eur. Ceram. Soc. 2016, 36, 3957–3967. [Google Scholar] [CrossRef]
- Jung, Y.I.; Kim, S.H.; Kim, H.G.; Park, J.Y.; Kim, W.J. Microstructures of diffusion bonded SiC ceramics using Ti and Mo interlayers. J. Nucl. Mater. 2013, 441, 510–513. [Google Scholar] [CrossRef]
- Ferraris, M.; Salvo, M.; Jung, H.C.; Casalegno, V.; Katoh, Y.; Kohyama, A.; Han, S.; Hinoki, T. Joining of SiC-based materials for nuclear energy applications. J. Nucl. Mater. 2010, 417, 379–382. [Google Scholar] [CrossRef]
- Herrmann, M.; Meisel, P.; Lippmann, W.; Hurtado, A. Joining technology—A challenge for the use of SiC components in HTRs. Nucl. Eng. Des. 2016, 306, 170–176. [Google Scholar] [CrossRef]
- Wu, L.X.; Lin, R.L.; Niu, W.B.; Zhu, L.L.; Xue, J.X.; Liao, Y.H.; Liu, T.; Guo, W.M.; Sun, S.K.; Lin, H.T. Nano-infiltration and transient eutectic (NITE) phase joining SiC ceramics at 1500 °C. Ceram. Int. 2019, 45, 24927–24931. [Google Scholar] [CrossRef]
- Xu, W.W.; Xia, F.F.; Chen, L.J.; Wu, M.; Gang, T.Q.; Huang, Y.F. High-temperature mechanical and thermodynamic properties of silicon carbide polytypes. J. Alloys Compd. 2018, 768, 722–732. [Google Scholar] [CrossRef]
- Zhou, X.B.; Han, Y.H.; Shen, X.F.; Du, S.Y.; Lee, J.; Huang, Q. Fast joining SiC ceramics with Ti3SiC2 tape film by electric field-assisted sintering technology. J. Nucl. Mater. 2015, 466, 322–327. [Google Scholar] [CrossRef]
- Wang, R.P.; Wang, G.; Ran, S.L.; Wang, W.; Yang, Y.L.; Zhao, Y.; Gui, K.X.; He, R.J.; Tan, C.W. Joining of Cf/SiC composites using AlCoCrFeNi2.1 eutectic high-entropy alloy filler by spark plasma sintering. Int. J. Appl. Ceram. Technol. 2024, 21, 3302–3310. [Google Scholar] [CrossRef]
- Pinc, W.R.; Di Prima, M.; Walker, L.S.; Wing, Z.N.; Corral, E.L.; Fahrenholtz, W. Spark Plasma Joining of ZrB2-SiC Composites Using Zirconium-Boron Reactive Filler Layers. J. Am. Ceram. Soc. 2011, 94, 3825–3832. [Google Scholar] [CrossRef]
- Zhou, W.B.; Mei, B.C.; Zhu, J.Q. Fabrication of high-purity ternary carbide Ti3AlC2 by spark plasma sintering (SPS) technique. Ceram. Int. 2007, 33, 1399–1402. [Google Scholar] [CrossRef]
- Li, H.X.; Shen, W.J.; Yang, F.Q.; Lu, C.Y.; Zheng, W.J.; Ma, Y.H.; Ren, S.D.; He, Y.M.; Yang, J.G. Microstructural, electrical, and mechanical properties of conductive SiC ceramics fabricated by spark plasma sintering. Int. J. Appl. Ceram. Technol. 2022, 19, 3376–3391. [Google Scholar] [CrossRef]
- Zhao, X.; Duan, L.Y.; Wang, Y.G. Fast interdiffusion and Kirkendall effects of SiC-coated C/SiC composites joined by a Ti-Nb-Ti interlayer via spark plasma sintering. J. Eur. Ceram. Soc. 2019, 39, 1757–1765. [Google Scholar] [CrossRef]
- Li, M.; Zhou, X.B.; Yang, H.; Du, S.Y.; Huang, Q. The critical issues of SiC materials for future nuclear systems. Scr. Mater. 2018, 143, 149–153. [Google Scholar] [CrossRef]
- Sloof, W.G.; Pei, R.Z.; McDonald, S.A. Repeated crack healing in MAX-phase ceramics revealed by 4D in situ synchrotron X-ray tomographic microscopy. Sci. Rep. 2016, 6, 23040. [Google Scholar] [CrossRef]
- Guitton, A.; Joulain, A.; Thilly, L.; Tromas, C. Evidence of dislocation cross-slip in MAX phase deformed at high temperature. Sci. Rep. 2014, 4, 6358. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, E.N.; Vinson, D.W.; Sindelar, R.L.; Tallman, D.J.; Kohse, G.; Barsoum, M.W. MAX phase carbides and nitrides: Properties for future nuclear power plant in-core applications and neutron transmutation analysis. Nucl. Eng. Des. 2012, 244, 17–24. [Google Scholar] [CrossRef]
- Gottselig, B.; Gyarmati, E.; Naoumidis, A.; Nickel, H. Joining of ceramics demonstrated by the example of SiC/Ti. J. Eur. Ceram. Soc. 1990, 6, 153–160. [Google Scholar] [CrossRef]
- Rizzo, S.; Grasso, S.; Salvo, M.; Casalegno, V.; Reece, M.J.; Ferraris, M. Joining of C/SiC composites by spark plasma sintering technique. J. Eur. Ceram. Soc. 2013, 34, 903–913. [Google Scholar] [CrossRef]
- Grasso, S.; Tatarko, P.; Rizzo, S.; Porwal, H.; Hu, C.; Katoh, Y.; Salvo, M.; Reece, M.J.; Ferraris, M. Joining of β-SiC by spark plasma sintering. J. Eur. Ceram. Soc. 2014, 34, 1681–1686. [Google Scholar] [CrossRef]
- Dong, H.Y.; Li, S.J.; Teng, Y.Y.; Ma, W. Joining of SiC ceramic-based materials with ternary carbide Ti3SiC2. Mater. Sci. Eng. B Adv. 2011, 176, 60–64. [Google Scholar] [CrossRef]
- Gan, G.Y.; Chen, J.C.; Sun, J.L.; Zhou, X.L.; Chen, X.H.; Du, Y. Chemical potential stability phase diagram of Ti-Si-C ternary system and its application. J. Kunming Univ. Sci. Technol. 2002, 1, 34–37,85. [Google Scholar]
- Zhang, Y.K.; Chen, J.C.; Zhang, J.S. Shear strength and microstructure of brazed joints between silicon carbide composites and carbon steel. Trans. China Weld. Inst. 2020, 41, 78–82. [Google Scholar]
- Naka, M.; Feng, J.C.; Schuster, J.C. Phase reaction and diffusion path of the SiC/Ti system. Metall. Mater. Trans. A 1997, 28, 1385–1390. [Google Scholar] [CrossRef]
- Yang, S.L.; Sun, Z.M.; Hashimoto, H. Reaction in Ti3SiC2 powder synthesis from a Ti/Si/TiC powder mixture. J. Alloys Compd. 2003, 368, 312–317. [Google Scholar] [CrossRef]
- Wang, J.; Li, K.Z.; Li, W.; Li, H.J.; Li, Z.Q.; Guo, L.J. The preparation and mechanical properties of carbon/carbon composite joints using Ti-Si-SiC-C filler as interlayer. Mater. Sci. Eng. A 2013, 574, 37–45. [Google Scholar] [CrossRef]
- Katoh, Y.; Snead, L.L.; Cheng, T.; Shih, C.; Lewis, W.D.; Koyanagi, T.; Hinoki, T.; Henager, C.H., Jr.; Ferraris, M. Radiation-tolerant joining technologies for silicon carbide ceramics and composites. J. Nucl. Mater. 2014, 448, 497–511. [Google Scholar] [CrossRef]
- Park, C.S.; Zheng, F.; Salamone, S.M.; Bordia, R.K. Processing of composites in the Ti-Si-C system. J. Mater. Sci. 2001, 36, 3313–3322. [Google Scholar] [CrossRef]


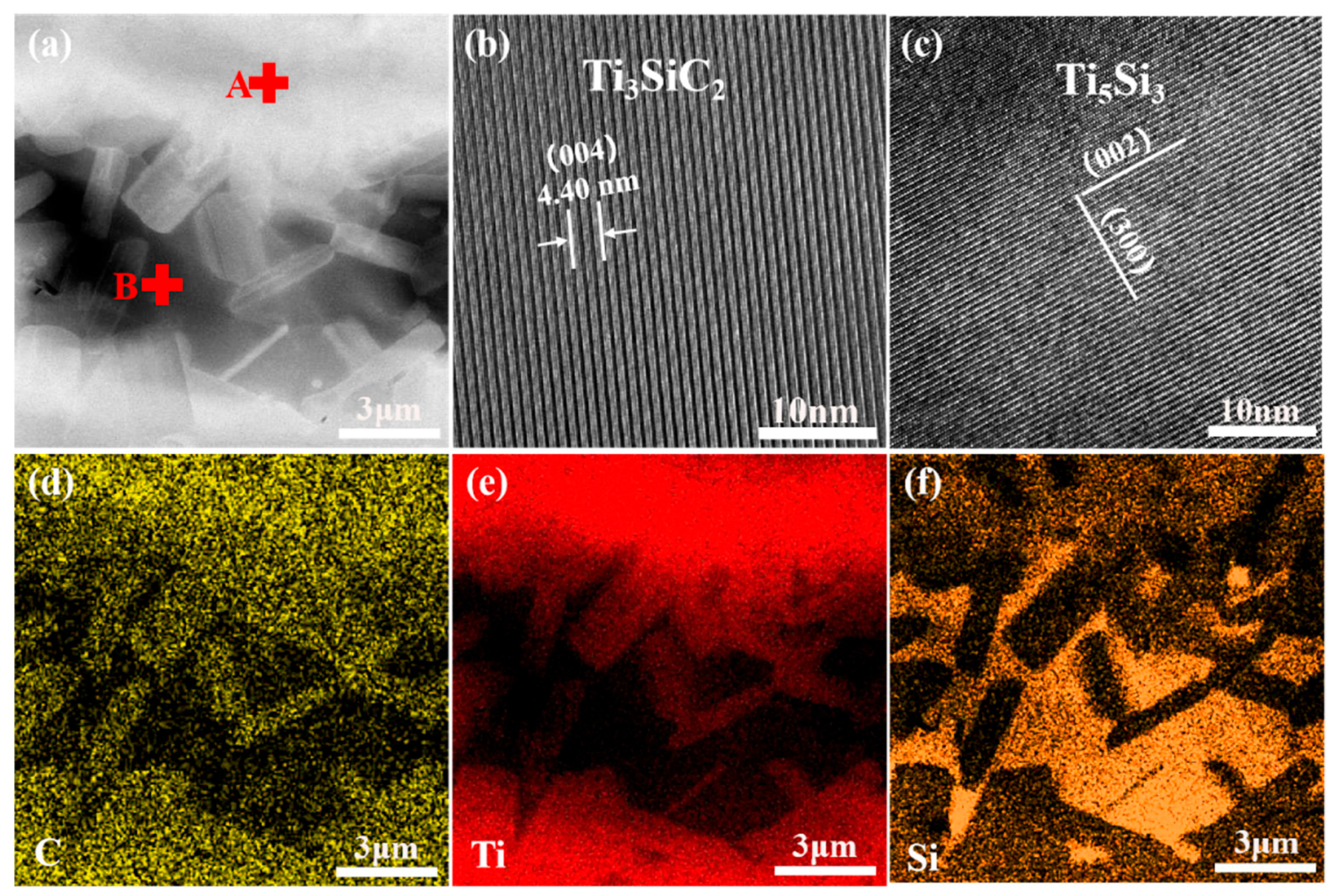
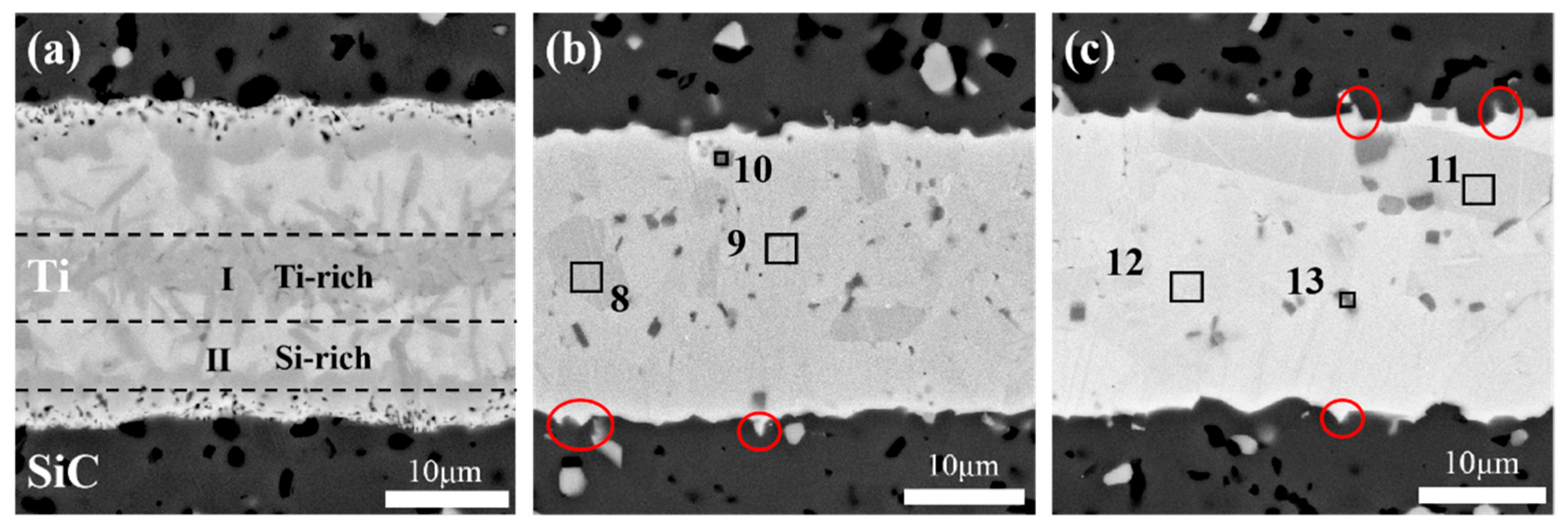



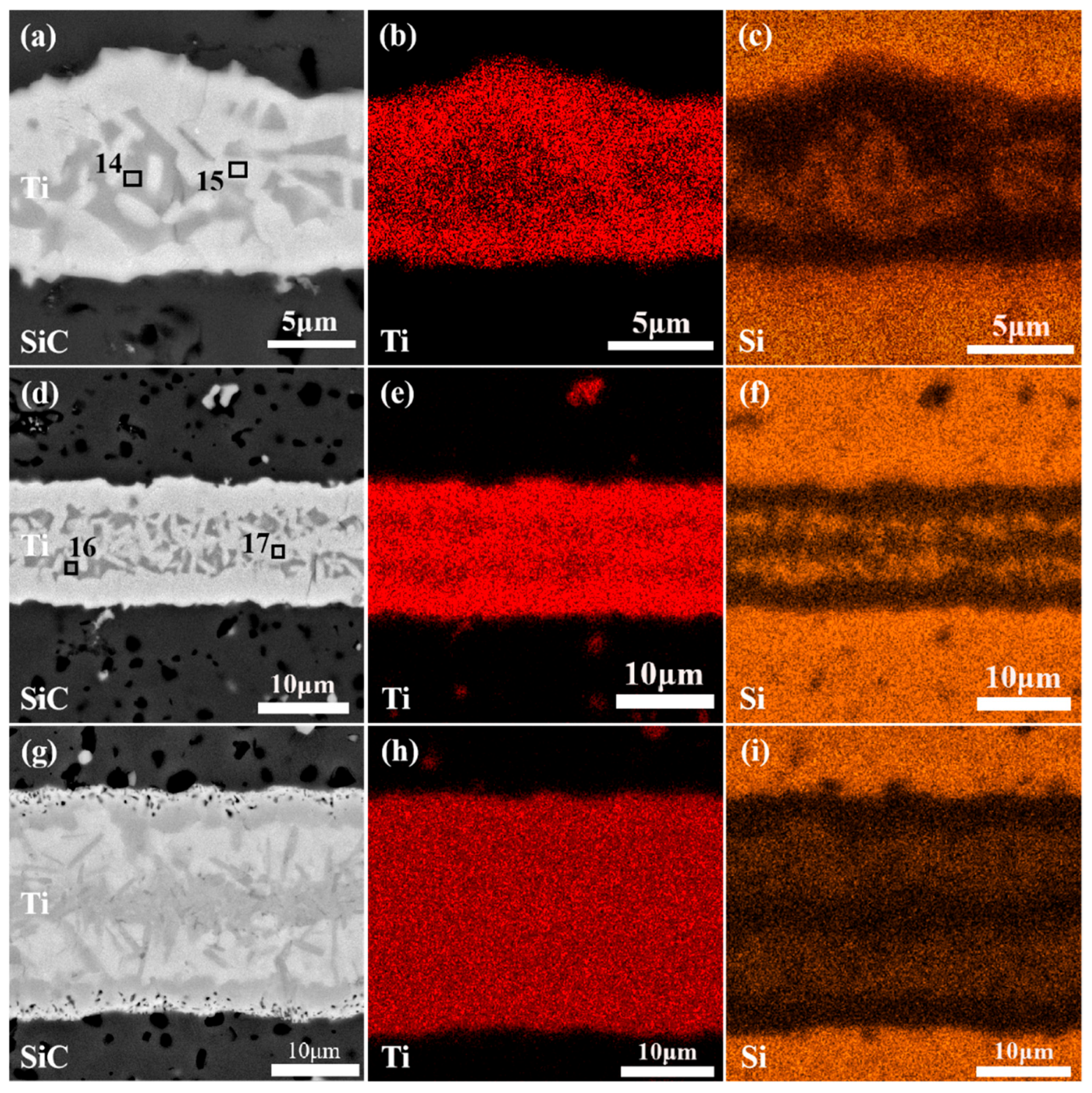
| Point | Composition in Atomic % | Probable Phase | ||
|---|---|---|---|---|
| Ti | Si | C | ||
| 1 | 74.93 | 13.08 | 11.99 | Ti3SiC2, TiC |
| 2 | 69.59 | 23.64 | 6.77 | Ti5Si3 |
| 3 | 73.75 | 12.32 | 13.92 | Ti3SiC2, TiC |
| 4 | 69.38 | 23.79 | 6.74 | Ti5Si3 |
| 5 | 59.81 | 21.00 | 19.19 | Ti3SiC2, TiC |
| 6 | 68.32 | 24.24 | 7.44 | Ti5Si3 |
| 7 | 55.72 | 36.86 | 7.42 | Ti5Si3 |
| Point | Composition in Atomic % | Probable Phase | ||
|---|---|---|---|---|
| Ti | Si | C | ||
| 8 | 71.48 | 13.29 | 15.23 | Ti3SiC2, TiC |
| 9 | 71.60 | 13.25 | 15.15 | Ti3SiC2, TiC |
| 10 | 96.22 | 3.78 | 0.00 | Ti |
| 11 | 72.27 | 12.78 | 14.95 | Ti3SiC2, TiC |
| 12 | 71.02 | 13.09 | 15.90 | Ti3SiC2, TiC |
| 13 | 94.23 | 5.05 | 0.72 | Ti |
| Point | Composition in Atomic % | Probable Phase | ||
|---|---|---|---|---|
| Ti | Si | C | ||
| 14 | 55.72 | 36.86 | 7.42 | Ti5Si3 |
| 15 | 59.80 | 21.00 | 19.19 | Ti3SiC2, TiC |
| 16 | 55.65 | 36.78 | 7.56 | Ti5Si3 |
| 17 | 66.98 | 16.02 | 17.00 | Ti3SiC2, TiC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, W.; Xie, Y.; Xu, X.; Yang, T.; Liu, H.; Tu, R.; Tan, R. Microstructure and Shear Strength of SiC Ceramics Diffusion Bonded with Ti Foils by Spark Plasma Sintering. Materials 2025, 18, 1725. https://doi.org/10.3390/ma18081725
Hu W, Xie Y, Xu X, Yang T, Liu H, Tu R, Tan R. Microstructure and Shear Strength of SiC Ceramics Diffusion Bonded with Ti Foils by Spark Plasma Sintering. Materials. 2025; 18(8):1725. https://doi.org/10.3390/ma18081725
Chicago/Turabian StyleHu, Weiqin, You Xie, Xinru Xu, Tengfei Yang, Haocheng Liu, Rui Tu, and Ruixuan Tan. 2025. "Microstructure and Shear Strength of SiC Ceramics Diffusion Bonded with Ti Foils by Spark Plasma Sintering" Materials 18, no. 8: 1725. https://doi.org/10.3390/ma18081725
APA StyleHu, W., Xie, Y., Xu, X., Yang, T., Liu, H., Tu, R., & Tan, R. (2025). Microstructure and Shear Strength of SiC Ceramics Diffusion Bonded with Ti Foils by Spark Plasma Sintering. Materials, 18(8), 1725. https://doi.org/10.3390/ma18081725






