Metal Nanocomposites as Biosensors for Biological Fluids Analysis
Abstract
1. Introduction
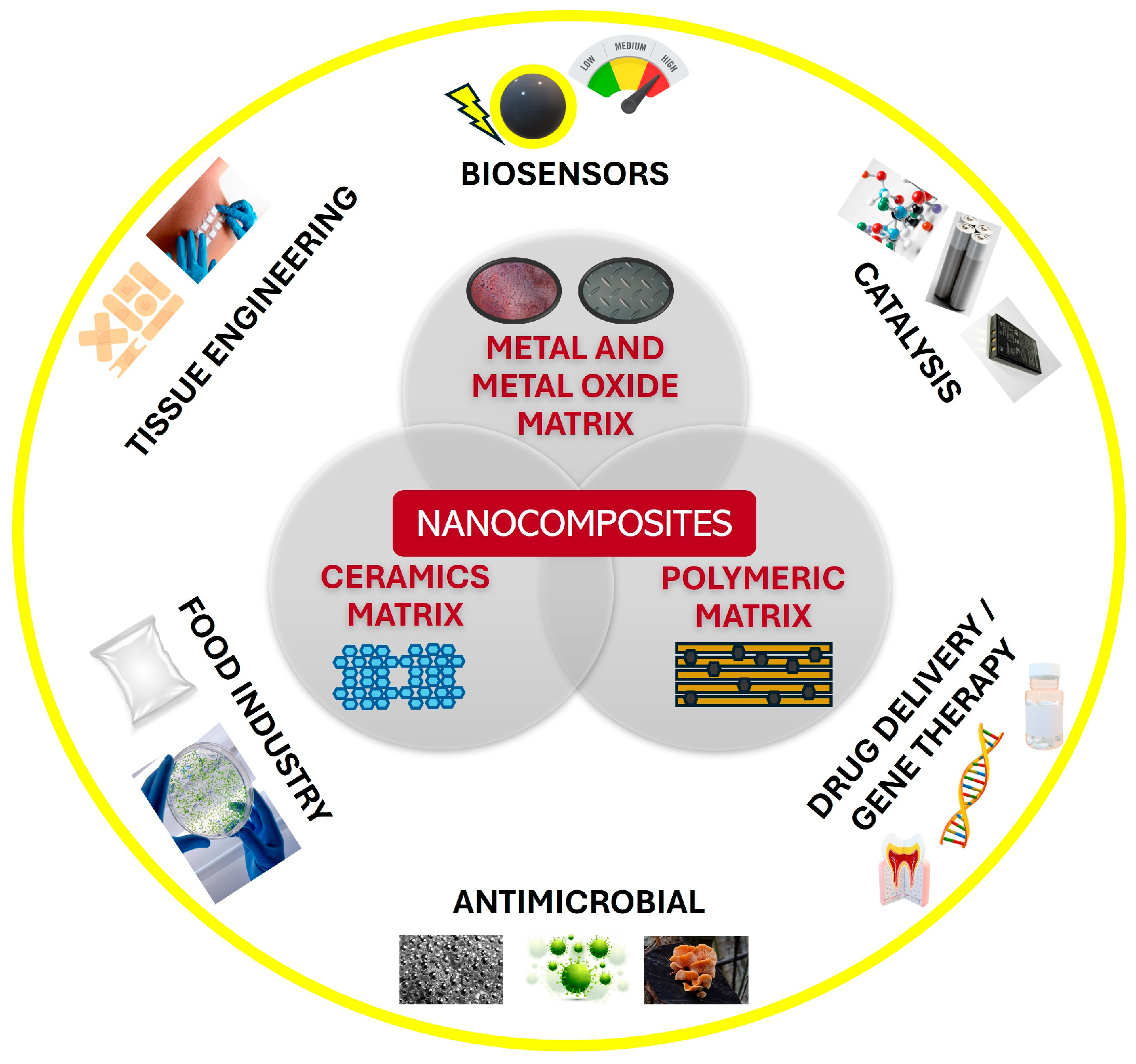
2. Fundamentals of Metal Nanocomposites
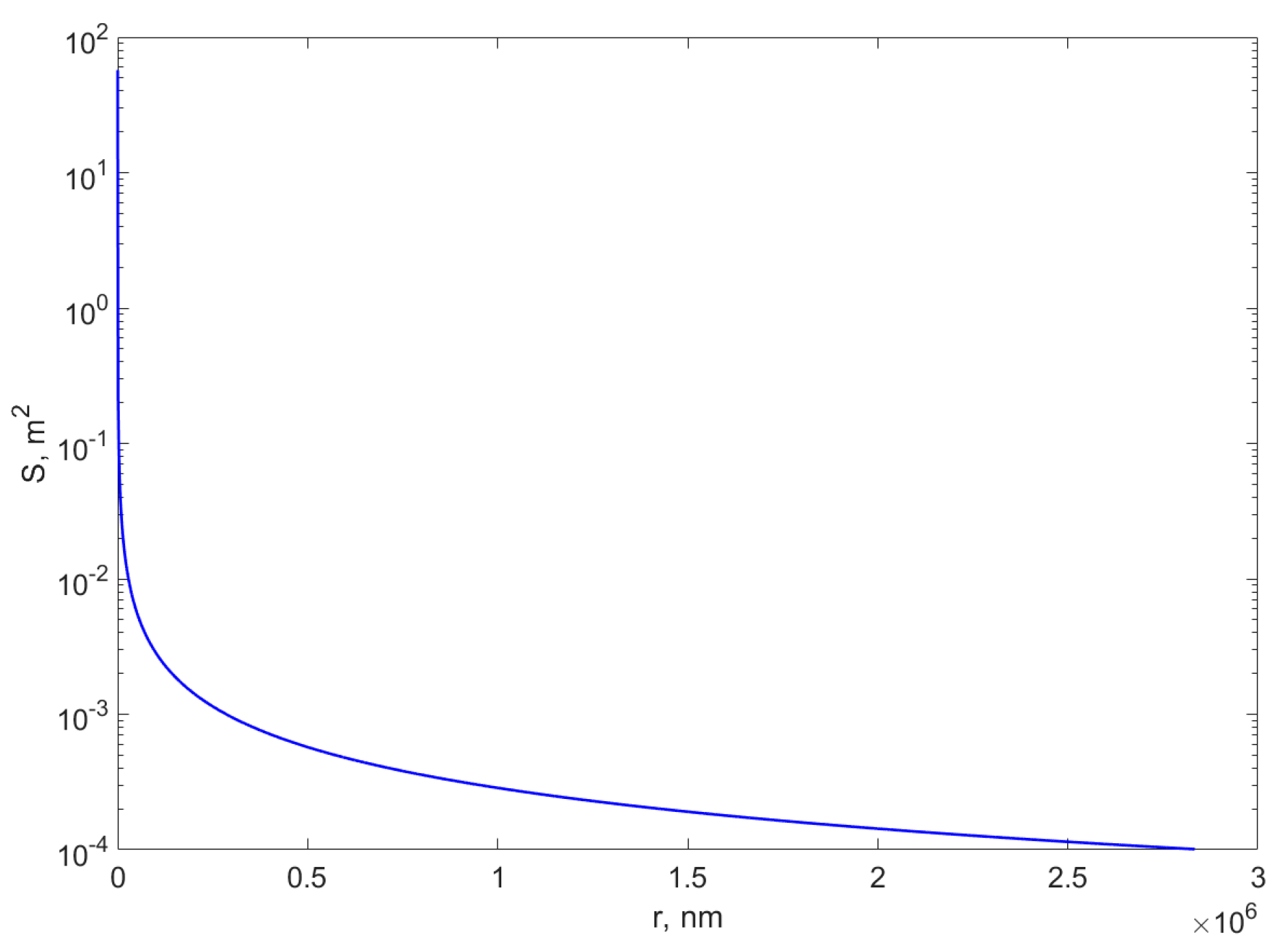
2.1. Nanocomposites Based on Metal Nanoparticles: Classification and Properties
- Challenges in the Biomedical Application of Multimetallic Nanocomposites for Biosensing
2.2. Metal Nanoparticles Syntheses
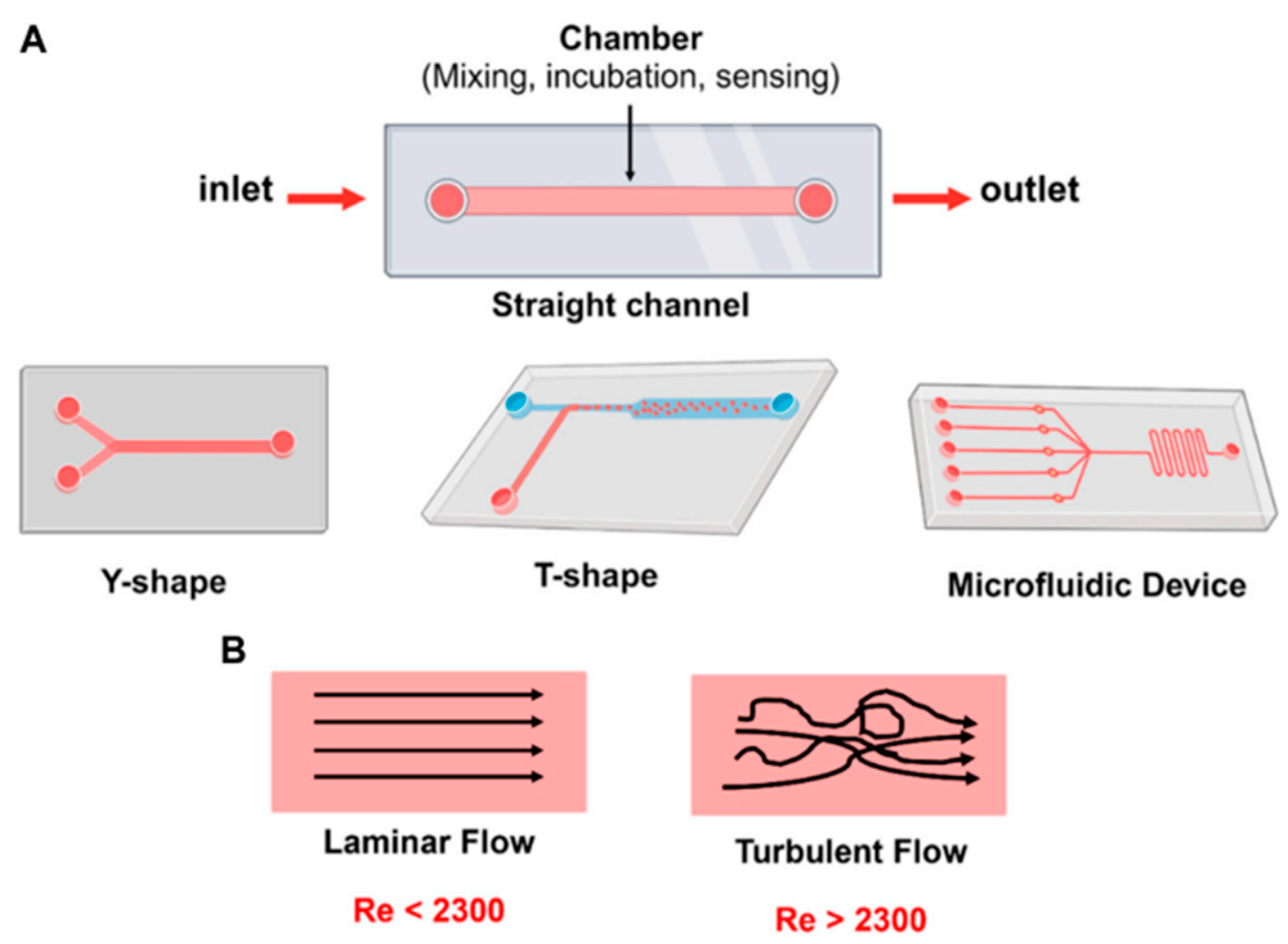
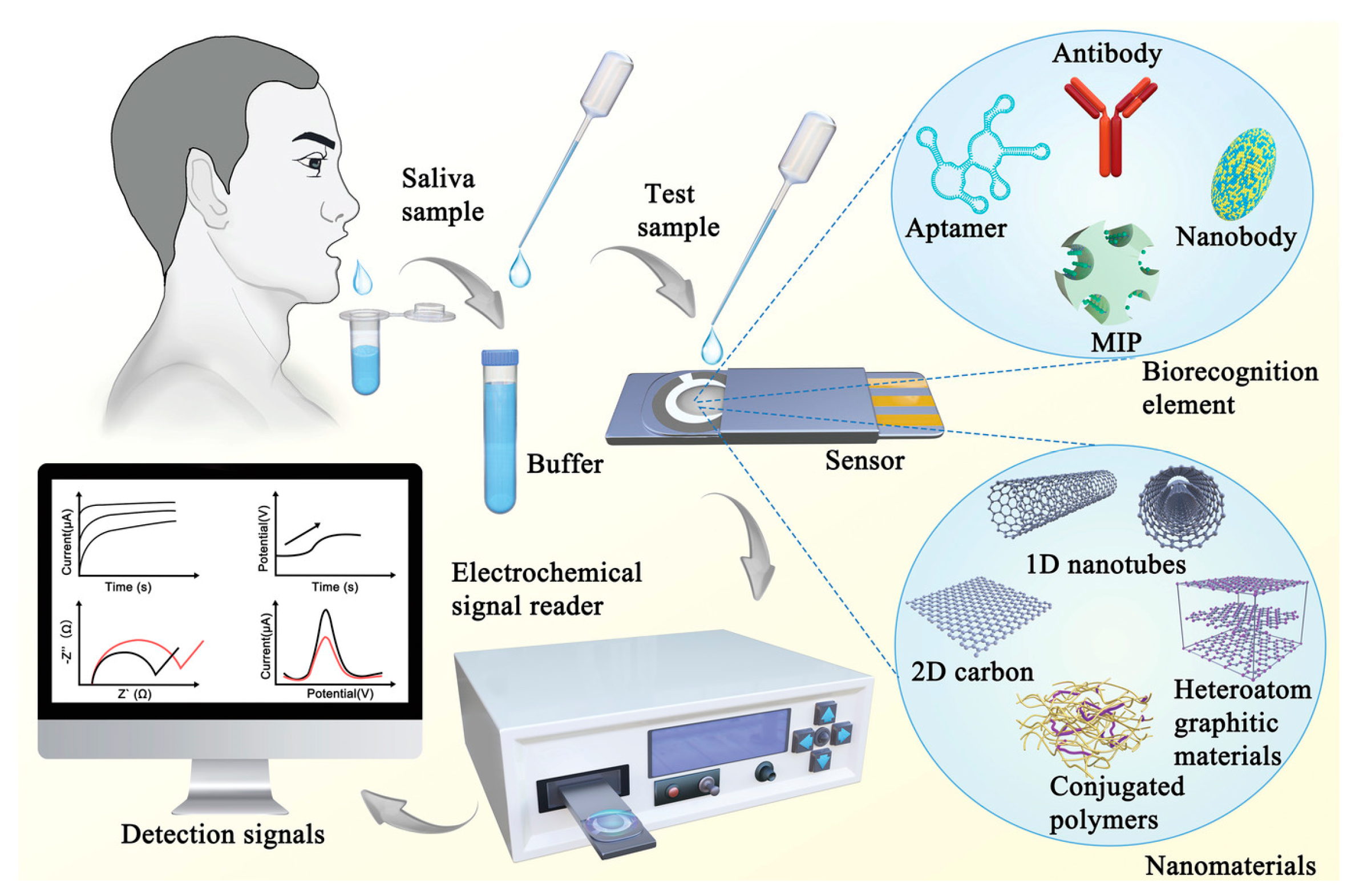
3. Microfluidic Biosensors: An Overview
3.1. The Most Used Transport Mechanisms That Govern Microfluidic Systems
3.2. The Typical Components of Microfluidic Systems Are Briefly Summarized Below
- Microchannels and Chambers
- Sample Introduction and Handling
3.3. Detection and Signal Transduction
3.4. Microfluidic Actuators and Pumps
4. Integration of Metal Nanocomposites into Microfluidic Biosensors
4.1. Types of Metal Nanocomposites Used in Microfluidic Biosensors
- Noble Metal-Based Nanocomposites
- Magnetic Nanocomposites
- Metal Oxide Nanocomposites
- Hybrid and Functionalized Metal Nanocomposites
- Fabrication Challenges in Metal Nanocomposite-Based Microfluidic Biosensors
4.2. Methods of Integrating Metal Nanocomposites into Microfluidic Devices
- Surface Immobilization Techniques
- In Situ Synthesis of Metal Nanocomposites
- Embedding Metal Nanocomposites within Polymer Matrices
- Magnetic Trapping of Metal Nanocomposites
4.3. Enhancement of Sensor Performance Through Nanocomposite Integration
- Signal Amplification
- Improved Electron Transfer
- Enhanced Biorecognition
- Increased Surface Area
- Stability and Reusability
- Practical Challenges and Solutions in Integrating Nanocomposites into Microfluidic Platforms
5. Detection of Emerging Biomarkers in Biological Fluids
5.1. Glucose
5.2. Salivary Biosensors
6. Recent Advances and Applications
6.1. Biosensors in Health Care Issues
6.2. Detection of Biological Molecules and Metals in Water
6.3. Detection of Food Pathogens Using Biosensors
6.4. Translation of Research into Commercial Products
7. Challenges and Future Perspectives
7.1. Technical and Practical Challenges in Developing Metal Nanocomposite Biosensors
- Fabrication Complexity and Scalability
- Stability and Long-Term Performance
- Biocompatibility and Toxicity
- Signal Interference and Noise
- Cost and Accessibility
- Regulatory and Standardization Challenges
- Integration with Data Analytics and Connectivity
7.2. Future Directions
7.3. Future Perspectives of Metal Nanocomposite-Based Biosensors for Biological Fluid Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Lee, J.; Kim, M.C.; Soltis, I.; Lee, S.H.; Yeo, W.H. Advances in electrochemical sensors for detecting analytes in biofluids. Adv. Sens. Res. 2023, 2, 2200088. [Google Scholar] [CrossRef]
- Corrie, S.R.; Coffey, J.W.; Islam, J.; Markey, K.A.; Kendall, M.A.F. Blood, sweat, and tears: Developing clinically relevant protein biosensors for integrated body fluid analysis. Analyst 2015, 140, 4350–4364. [Google Scholar] [CrossRef] [PubMed]
- Camargo, P.H.C.; Satyanarayana, K.G.; Wypych, F. Nanocomposites: Synthesis, structure, properties and new application opportunities. Mater. Res. 2009, 12, 1–39. [Google Scholar] [CrossRef]
- Reig, D.S.; Hummel, P.; Wang, Z.; Rosenfeldt, S.; Graczykowski, B.; Retsch, M.; Fytas, G. Well-defined metal-polymer nanocomposites: The interplay of structure, thermoplasmonics, and elastic mechanical properties. Phys. Rev. Mater. 2018, 2, 123605. [Google Scholar] [CrossRef]
- Ghazzy, A.; Naik, R.R.; Shakya, A.K. Metal–polymer nanocomposites: A promising approach to antibacterial materials. Polymers 2023, 15, 2167. [Google Scholar] [CrossRef] [PubMed]
- Jeon, I.-Y.; Baek, J.-B. Nanocomposites derived from polymers and inorganic nanoparticles. Materials 2010, 3, 3654–3674. [Google Scholar] [CrossRef]
- Lou, C.; Yang, H.; Hou, Y.; Huang, H.; Qiu, J.; Wang, C.; Sang, Y.; Liu, H.; Han, L. Microfluidic Platforms for Real-Time In Situ Monitoring of Biomarkers for Cellular Processes. Adv. Mater. 2024, 36, 2307051. [Google Scholar] [CrossRef]
- Sekhwama, M.; Mpofu, K.; Sudesh, S.; Mthunzi-Kufa, P. Integration of microfluidic chips with biosensors. Discov. Appl. Sci. 2024, 6, 458. [Google Scholar] [CrossRef]
- Hlapisi, N.; Songca, S.P.; Ajibade, P.A. Capped Plasmonic Gold and Silver Nanoparticles with Porphyrins for Potential Use as Anticancer Agents—A Review. Pharmaceutics 2024, 16, 1268. [Google Scholar] [CrossRef]
- Sanità, G.; Carrese, B.; Lamberti, A. Nanoparticle surface functionalization: How to improve biocompatibility and cellular internalization. Front. Mol. Biosci. 2020, 7, 587012. [Google Scholar] [CrossRef]
- Zhao, S.; Peng, W.; Zhou, L.; Dai, S.; Ren, W.; Xu, E.; Xiao, Y.; Zhang, M.; Huang, M.; Shen, Y.; et al. Metal-organic cage crosslinked nanocomposites with enhanced high-temperature capacitive energy storage performance. Nat. Commun. 2025, 16, 769. [Google Scholar] [CrossRef] [PubMed]
- Raza, T.; Qu, L.; Khokhar, W.A.; Andrews, B.; Ali, A.; Tian, M. Progress of Wearable and Flexible Electrochemical Biosensors With the Aid of Conductive Nanomaterials. Front. Bioeng. Biotechnol. 2021, 9, 761020. [Google Scholar] [CrossRef]
- Su, L.; Jia, W.; Hou, C.; Lei, Y. Microbial biosensors: A review. Biosens. Bioelectron. 2011, 26, 1788–1799. [Google Scholar] [CrossRef]
- Altammar, K.A. A review on nanoparticles: Characteristics, synthesis, applications, and challenges. Front. Microbiol. 2023, 14, 1155622. [Google Scholar] [CrossRef] [PubMed]
- Abbas, R.; Luo, J.; Qi, X.; Naz, A.; Khan, I.A.; Liu, H.; Yu, S.; Wei, J. Silver Nanoparticles: Synthesis, Structure, Properties and Applications. Nanomaterials 2024, 14, 1425. [Google Scholar] [CrossRef]
- Sharma, G.; Kumar, A.; Sharma, S.; Naushad, M.; Prakash Dwivedi, R.; Alothman, Z.A.; Mola, G.T. Novel development of nanoparticles to bimetallic nanoparticles and their composites: A review. J. King Saud Univ. Sci. 2019, 31, 257–269. [Google Scholar] [CrossRef]
- Rahman, M.R.; Chang Hui, J.L.; Hamdan, S.b. 1—Introduction and reinforcing potential of silica and various clay dispersed nanocomposites. In Silica and Clay Dispersed Polymer Nanocomposites; Rahman, M.R., Ed.; Woodhead Publishing: Sawston, UK, 2018; pp. 1–24. [Google Scholar]
- Greene, J.P. 12—Polymer Composites. In Automotive Plastics and Composites; Greene, J.P., Ed.; William Andrew Publishing: Nowich, NY, USA, 2021; pp. 191–222. [Google Scholar]
- Rawtani, D.; Khatri, N.; Tyagi, S.; Pandey, G. Nanotechnology-based recent approaches for sensing and remediation of pesticides. J. Environ. Manag. 2018, 206, 749–762. [Google Scholar] [CrossRef]
- Su, H.; Li, Y.; Li, X.-Y.; Wong, K.S. Optical and electrical properties of Au nanoparticles in two-dimensional networks: An effective cluster model. Opt. Express 2009, 17, 22223–22234. [Google Scholar] [CrossRef]
- Requejo-Isidro, J.; Del Coso, R.; Solis, J.; Gonzalo, J.; Afonso, C.N. Role of surface-to-volume ratio of metal nanoparticles in optical properties of Cu: Al2O3 nanocomposite films. Appl. Phys. Lett. 2005, 86, 193104. [Google Scholar] [CrossRef]
- Barbosa, A.I.; Rebelo, R.; Reis, R.L.; Bhattacharya, M.; Correlo, V.M. Current nanotechnology advances in diagnostic biosensors. Med. Devices Sens. 2021, 4, e10156. [Google Scholar] [CrossRef]
- Nicolae-Maranciuc, A.; Chicea, D.; Chicea, L.M. Ag Nanoparticles for Biomedical Applications—Synthesis and Characterization—A Review. Int. J. Mol. Sci. 2022, 23, 5778. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Hansen, M.; Lambert, C.; Hegde, S.; Jayamohan, H.; Gale, B.K.; Sant, H.J. Characterizing a silver nanoparticle-based electrochemical biosensor for Shiga toxin detection. ACS Omega 2023, 8, 40898–40903. [Google Scholar] [CrossRef] [PubMed]
- Magar, H.S.; Hassan, R.Y.; Abbas, M.N. Non-enzymatic disposable electrochemical sensors based on CuO/Co3O4@ MWCNTs nanocomposite modified screen-printed electrode for the direct determination of urea. Sci. Rep. 2023, 13, 2034. [Google Scholar] [CrossRef]
- Arif, D.; Hassan, M.; Abdullah, M.; Miran, W.; Nasir, M.A.; Batool, S.; Baig, M.A.; Liaqat, U. An electrochemical sensor based on copper oxide nanoparticles loaded on a mesoporous MCM-41 for non-enzymatic detection of glucose. Ceram. Int. 2024, 50, 12614–12620. [Google Scholar] [CrossRef]
- Foroozandeh, P.; Aziz, A.A. Insight into cellular uptake and intracellular trafficking of nanoparticles. Nanoscale Res. Lett. 2018, 13, 339. [Google Scholar] [CrossRef] [PubMed]
- Saxena, M.; Pappu, A.; Sharma, A.; Haque, R.; Wankhede, S. Composite Materials from Natural Resources: Recent Trends and Future Potentials; IntechOpen: London, UK, 2011. [Google Scholar]
- Nayak, A.K.; Mazumder, S.; Ara, T.J.; Ansari, M.T.; Hasnain, M.S. 2—Calcium fluoride-based dental nanocomposites. In Applications of Nanocomposite Materials in Dentistry; Asiri Inamuddin, A.M., Mohammad, A., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 27–45. [Google Scholar]
- Sharma, A.K.; Bhandari, R.; Sharma, C.; Dhakad, S.K.; Pinca-Bretotean, C. Polymer matrix composites: A state of art review. Mater. Today Proc. 2022, 57, 2330–2333. [Google Scholar] [CrossRef]
- Wei, Z.; Duan, H.; Weng, G.; He, J. Metals in polymers: Hybridization enables new functions. J. Mater. Chem. C 2020, 8, 15956–15980. [Google Scholar] [CrossRef]
- Al-Mutairi, N.H.; Mehdi, A.H.; Kadhim, B.J. Nanocomposites materials definitions, types and some of their applications: A review. Eur. J. Res. Dev. Sustain. 2022, 3, 102–108. [Google Scholar]
- Heness, G. 6—Metal–polymer nanocomposites. In Advances in Polymer Nanocomposites; Gao, F., Ed.; Woodhead Publishing: Sawston, UK, 2012; pp. 164–177. [Google Scholar]
- Wu, T.; Kheiri, S.; Hickman, R.J.; Tao, H.; Wu, T.C.; Yang, Z.-B.; Ge, X.; Zhang, W.; Abolhasani, M.; Liu, K. Self-driving lab for the photochemical synthesis of plasmonic nanoparticles with targeted structural and optical properties. Nat. Commun. 2025, 16, 1473. [Google Scholar] [CrossRef]
- Alam, M.M.; Rashid, M.M.O.; Foysal Hossen, F.H.; Akhter, K.N.; Nishat, S.A.; Abrar Wahab, A.W. Antibacterial activity of silver-extract nanoparticles synthesized from the combination of silver nanoparticles and M. charantia fruit extract. J. Drug Deliv. Ther. 2017, 7, 112–116. [Google Scholar] [CrossRef]
- Mohamed, M.M.; Fouad, S.A.; Elshoky, H.A.; Mohammed, G.M.; Salaheldin, T.A. Antibacterial effect of gold nanoparticles against Corynebacterium pseudotuberculosis. Int. J. Vet. Sci. Med. 2017, 5, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Pérez, R.; Guisbiers, G. Bimetallic Pt–Pd nano-catalyst: Size, shape and composition matter. Nanotechnology 2019, 30, 305702. [Google Scholar] [CrossRef]
- Yaqoob, S.B.; Adnan, R.; Rameez Khan, R.M.; Rashid, M. Gold, silver, and palladium nanoparticles: A chemical tool for biomedical applications. Front. Chem. 2020, 8, 376. [Google Scholar] [CrossRef]
- Burlec, A.F.; Corciova, A.; Boev, M.; Batir-Marin, D.; Mircea, C.; Cioanca, O.; Danila, G.; Danila, M.; Bucur, A.F.; Hancianu, M. Current overview of metal nanoparticles’ synthesis, characterization, and biomedical applications, with a focus on silver and gold nanoparticles. Pharmaceuticals 2023, 16, 1410. [Google Scholar] [CrossRef] [PubMed]
- Rahad, R.; Rakib, A.K.M.; Haque, M.A.; Sharar, S.S.; Sagor, R.H. Plasmonic refractive index sensing in the early diagnosis of diabetes, anemia, and cancer: An exploration of biological biomarkers. Results Phys. 2023, 49, 106478. [Google Scholar] [CrossRef]
- Gupta, B.D.; Sharma, A.K. Sensitivity evaluation of a multi-layered surface plasmon resonance-based fiber optic sensor: A theoretical study. Sens. Actuators B Chem. 2005, 107, 40–46. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, J.; Yang, Z.; Wilkinson, J.S.; Zhou, X. Optical biosensors based on refractometric sensing schemes: A review. Biosens. Bioelectron. 2019, 144, 111693. [Google Scholar] [CrossRef]
- Roh, S.; Chung, T.; Lee, B. Overview of the Characteristics of Micro- and Nano-Structured Surface Plasmon Resonance Sensors. Sensors 2011, 11, 1565–1588. [Google Scholar] [CrossRef]
- Chang, H.; Rho, W.-Y.; Son, B.S.; Kim, J.; Lee, S.H.; Jeong, D.H.; Jun, B.-H. Plasmonic Nanoparticles: Basics to Applications (I). In Nanotechnology for Bioapplications; Jun, B.-H., Ed.; Springer: Singapore, 2021; pp. 133–159. [Google Scholar]
- Basavegowda, N.; Baek, K.-H. Multimetallic nanoparticles as alternative antimicrobial agents: Challenges and perspectives. Molecules 2021, 26, 912. [Google Scholar] [CrossRef]
- Dikshit, P.K.; Kumar, J.; Das, A.K.; Sadhu, S.; Sharma, S.; Singh, S.; Gupta, P.K.; Kim, B.S. Green synthesis of metallic nanoparticles: Applications and limitations. Catalysts 2021, 11, 902. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Ahmad, H.; Parveen, T.; Ahmad, A.; Oves, M.; Ismail, I.M.; Qari, H.A.; Umar, K.; Mohamad Ibrahim, M.N. Recent advances in metal decorated nanomaterials and their various biological applications: A review. Front. Chem. 2020, 8, 341. [Google Scholar] [CrossRef] [PubMed]
- Karaman, D.Ş.; Manner, S.; Fallarero, A.; Rosenholm, J.M. Current approaches for exploration of nanoparticles as antibacterial agents. Antibact. Agents 2017, 61, 61–86. [Google Scholar] [CrossRef]
- García, M.C.; Torres, J.; Dan Córdoba, A.V.; Longhi, M.; Uberman, P.M. 2—Drug delivery using metal oxide nanoparticles. In Metal Oxides for Biomedical and Biosensor Applications; Mondal, K., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 35–83. [Google Scholar]
- Solanki, P.R.; Kaushik, A.; Agrawal, V.V.; Malhotra, B.D. Nanostructured metal oxide-based biosensors. NPG Asia Mater. 2011, 3, 17–24. [Google Scholar] [CrossRef]
- Sayyad, P.W.; Park, S.-J.; Ha, T.-J. Recent advances in biosensors based on metal-oxide semiconductors system-integrated into bioelectronics. Biosens. Bioelectron. 2024, 259, 116407. [Google Scholar] [CrossRef]
- Liu, B.; Liu, J. Sensors and biosensors based on metal oxide nanomaterials. TrAC Trends Anal. Chem. 2019, 121, 115690. [Google Scholar] [CrossRef]
- Yassin, M.T.; Al-Otibi, F.O.; Al-Sahli, S.A.; El-Wetidy, M.S.; Mohamed, S. Metal Oxide Nanoparticles as Efficient Nanocarriers for Targeted Cancer Therapy: Addressing Chemotherapy-Induced Disabilities. Cancers 2024, 16, 4234. [Google Scholar] [CrossRef]
- Stuparu-Cretu, M.; Braniste, G.; Necula, G.-A.; Stanciu, S.; Stoica, D.; Stoica, M. Metal oxide nanoparticles in food packaging and their influence on human health. Foods 2023, 12, 1882. [Google Scholar] [CrossRef]
- Greiner, M.T.; Lu, Z.-H. Thin-film metal oxides in organic semiconductor devices: Their electronic structures, work functions and interfaces. NPG Asia Mater. 2013, 5, e55. [Google Scholar] [CrossRef]
- Paszkiewicz, M.; Gołąbiewska, A.; Rajski, Ł.; Kowal, E.; Sajdak, A.; Zaleska-Medynska, A. Synthesis and characterization of monometallic (Ag, Cu) and bimetallic Ag-Cu particles for antibacterial and antifungal applications. J. Nanomater. 2016, 2016, 2187940. [Google Scholar] [CrossRef]
- Mahajan, R.; Suriyanarayanan, S.; Nicholls, I.A. Improved solvothermal synthesis of γ-Fe2O3 magnetic nanoparticles for SiO2 coating. Nanomaterials 2021, 11, 1889. [Google Scholar] [CrossRef]
- Nyabadza, A.; Vazquez, M.; Brabazon, D. A review of bimetallic and monometallic nanoparticle synthesis via laser ablation in liquid. Crystals 2023, 13, 253. [Google Scholar] [CrossRef]
- Nyabadza, A.; Vázquez, M.; Coyle, S.; Fitzpatrick, B.; Brabazon, D. Review of materials and fabrication methods for flexible nano and micro-scale physical and chemical property sensors. Appl. Sci. 2021, 11, 8563. [Google Scholar] [CrossRef]
- Sharma, G.; Naushad, M.; Kumar, A.; Devi, S.; Khan, M.R. Lanthanum/Cadmium/Polyaniline bimetallic nanocomposite for the photodegradation of organic pollutant. Iran. Polym. J. 2015, 24, 1003–1013. [Google Scholar] [CrossRef]
- Sharma, G.; Gupta, V.K.; Agarwal, S.; Kumar, A.; Thakur, S.; Pathania, D. Fabrication and characterization of Fe@ MoPO nanoparticles: Ion exchange behavior and photocatalytic activity against malachite green. J. Mol. Liq. 2016, 219, 1137–1143. [Google Scholar] [CrossRef]
- Godfrey, I.J.; Dent, A.J.; Parkin, I.P.; Maenosono, S.; Sankar, G. Structure of gold–silver nanoparticles. J. Phys. Chem. C 2017, 121, 1957–1963. [Google Scholar] [CrossRef]
- Arora, N.; Thangavelu, K.; Karanikolos, G.N. Bimetallic nanoparticles for antimicrobial applications. Front. Chem. 2020, 8, 412. [Google Scholar] [CrossRef] [PubMed]
- Loza, K.; Heggen, M.; Epple, M. Synthesis, structure, properties, and applications of bimetallic nanoparticles of noble metals. Adv. Funct. Mater. 2020, 30, 1909260. [Google Scholar] [CrossRef]
- Mahesh, V.; Joladarashi, S.; Kulkarni, S.M. A comprehensive review on material selection for polymer matrix composites subjected to impact load. Def. Technol. 2021, 17, 257–277. [Google Scholar] [CrossRef]
- Li, C.-H.; Li, M.-C.; Liu, S.-P.; Jamison, A.C.; Lee, D.; Lee, T.R.; Lee, T.-C. Plasmonically enhanced photocatalytic hydrogen production from water: The critical role of tunable surface plasmon resonance from gold–silver nanoshells. ACS Appl. Mater. Interfaces 2016, 8, 9152–9161. [Google Scholar] [CrossRef]
- Zaleska-Medynska, A.; Marchelek, M.; Diak, M.; Grabowska, E. Noble metal-based bimetallic nanoparticles: The effect of the structure on the optical, catalytic and photocatalytic properties. Adv. Colloid Interface Sci. 2016, 229, 80–107. [Google Scholar] [CrossRef]
- Anicetus, M.T. Synthesis, Characterisation and Applications of Bimetallic Nanoparticles; University of Birmingham: Birmingham, UK, 2010. [Google Scholar]
- Zhong, X.; Wang, X.; Cheng, L.; Tang, Y.a.; Zhan, G.; Gong, F.; Zhang, R.; Hu, J.; Liu, Z.; Yang, X. GSH-depleted PtCu3 nanocages for chemodynamic-enhanced sonodynamic cancer therapy. Adv. Funct. Mater. 2020, 30, 1907954. [Google Scholar] [CrossRef]
- Das, P.; Mudigunda, S.V.; Darabdhara, G.; Boruah, P.K.; Ghar, S.; Rengan, A.K.; Das, M.R. Biocompatible functionalized AuPd bimetallic nanoparticles decorated on reduced graphene oxide sheets for photothermal therapy of targeted cancer cells. J. Photochem. Photobiol. B Biol. 2020, 212, 112028. [Google Scholar] [CrossRef]
- Ye, X.; He, X.; Lei, Y.; Tang, J.; Yu, Y.; Shi, H.; Wang, K. One-pot synthesized Cu/Au/Pt trimetallic nanoparticles with enhanced catalytic and plasmonic properties as a universal platform for biosensing and cancer theranostics. Chem. Commun. 2019, 55, 2321–2324. [Google Scholar] [CrossRef]
- Yadav, N.; Jaiswal, A.K.; Dey, K.K.; Yadav, V.B.; Nath, G.; Srivastava, A.K.; Yadav, R.R. Trimetallic Au/Pt/Ag based nanofluid for enhanced antibacterial response. Mater. Chem. Phys. 2018, 218, 10–17. [Google Scholar] [CrossRef]
- Vaseghi, Z.; Tavakoli, O.; Nematollahzadeh, A. Rapid biosynthesis of novel Cu/Cr/Ni trimetallic oxide nanoparticles with antimicrobial activity. J. Environ. Chem. Eng. 2018, 6, 1898–1911. [Google Scholar] [CrossRef]
- Hussein, S.; Mahmoud, A.M.; Elgebaly, H.A.; Hendawy, O.M.; Hassanein, E.H.; Moustafa, S.M.; Alotaibi, N.F.; Nassar, A.M. Green synthesis of trimetallic nanocomposite (Ru/Ag/Pd)-Np and its in vitro antimicrobial and anticancer activities. J. Chem. 2022, 2022, 4593086. [Google Scholar] [CrossRef]
- Kalakonda, P.; Mandal, P.; Laxmi Mynepally, S.; Bashipangu, A.; Kethavath, A.; Khanam, S.J.; Batchu, M.; Kalakonda, P.B.; Banne, S.; Aitipamula, D. Comparison of multi-metallic nanoparticles-alternative antibacterial agent: Understanding the role of their antibacterial properties. J. Inorg. Organomet. Polym. Mater. 2024, 34, 2203–2218. [Google Scholar] [CrossRef]
- Zhang, H.; Toshima, N. Glucose oxidation using Au-containing bimetallic and trimetallic nanoparticles. Catal. Sci. Technol. 2013, 3, 268–278. [Google Scholar] [CrossRef]
- Ngan, D.T.T.; Thuy, V.T.; Van Tuan, D.; Dien, N.D.; Tam, P.D. MoS2/Ag Composite-Based Biosensor with Improved Sensitivity and Selectivity for Glucose Detection. J. Electron. Mater. 2025, 54, 3981–3993. [Google Scholar] [CrossRef]
- Atmeh, M.; Alcock-Earley, B.E. A conducting polymer/Ag nanoparticle composite as a nitrate sensor. J. Appl. Electrochem. 2011, 41, 1341–1347. [Google Scholar] [CrossRef]
- Huang, K.-J.; Wang, L.; Wang, H.-B.; Gan, T.; Wu, Y.-Y.; Li, J.; Liu, Y.-M. Electrochemical biosensor based on silver nanoparticles–polydopamine–graphene nanocomposite for sensitive determination of adenine and guanine. Talanta 2013, 114, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Wei, W.; Tao, C.; Xie, B.; Chen, X. Nano-silver/multi-walled carbon nanotube composite films for hydrogen peroxide electroanalysis. Microchim. Acta 2008, 162, 51–56. [Google Scholar] [CrossRef]
- Kumar, K.; Singh, K.R.; Rathour, R.S.; Singh, J.; Bhattacharya, S.; Pandey, S.S. Fabrication of nanobioengineered interfaces utilizing quaternary nanocomposite for highly efficient and selective electrochemical biosensing of urea. Langmuir 2024, 40, 21052–21066. [Google Scholar] [CrossRef] [PubMed]
- Ara, L.; Ali, S.L.; Daixin, Y.; Khattak, N.S. Silver nanoparticles doped polymethylmethacrylate[Ag/PMMA] nanocomposite as smart material for non-enzymatic glucose sensor. J. Dispers. Sci. Technol. 2024, 45, 1120–1128. [Google Scholar] [CrossRef]
- Liang, Z.; Zhang, X. Zn–ZnO@TiO2 nanocomposite: A direct electrode for nonenzymatic biosensors. J. Mater. Sci. 2018, 53, 7138–7149. [Google Scholar] [CrossRef]
- Yousif, N.M.; Attia, R.M.; Balboul, M.R. Adrenaline biosensors based on r Go/Ag nanocomposites functionalized textiles using advanced electron beam irradiation technique. J. Organomet. Chem. 2022, 972, 122392. [Google Scholar] [CrossRef]
- Kasturi, S.; Eom, Y.; Torati, S.R.; Kim, C. Highly sensitive electrochemical biosensor based on naturally reduced rGO/Au nanocomposite for the detection of miRNA-122 biomarker. J. Ind. Eng. Chem. 2021, 93, 186–195. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, M.; Hou, Y.; Huang, W.; Yao, C.; Wu, Q. An Au nanocomposite based biosensor for determination of cholesterol. Anal. Methods 2015, 7, 3480–3485. [Google Scholar] [CrossRef]
- Srivastava, M.; Srivastava, S.; Nirala, N.; Prakash, R. A chitosan-based polyaniline–Au nanocomposite biosensor for determination of cholesterol. Anal. Methods 2014, 6, 817–824. [Google Scholar] [CrossRef]
- Nasri, A.; Jaleh, B.; Daneshnazar, M.; Varma, R.S. Sensing Properties of g-C3N4/Au Nanocomposite for Organic Vapor Detection. Biosensors 2023, 13, 315. [Google Scholar] [CrossRef]
- Hatami, Z.; Ragheb, E.; Jalali, F.; Tabrizi, M.A.; Shamsipur, M. Zinc oxide-gold nanocomposite as a proper platform for label-free DNA biosensor. Bioelectrochemistry 2020, 133, 107458. [Google Scholar] [CrossRef] [PubMed]
- Shaikshavali, P.; Madhusudana Reddy, T.; Venu Gopal, T.; Venkataprasad, G.; Narasimha, G.; Lakshmi Narayana, A.; Hussain, O.M. Development of carbon-based nanocomposite biosensor platform for the simultaneous detection of catechol and hydroquinone in local tap water. J. Mater. Sci. Mater. Electron. 2021, 32, 5243–5258. [Google Scholar] [CrossRef]
- Nagarajan, N.; Panchatcharam, P. Biosensor nanoarchitectonics of Cu-Fe-nanoparticles/Zeolite-A/Graphene nanocomposite for enhanced electrooxidation and dopamine detection. Heliyon 2023, 9, e19741. [Google Scholar] [CrossRef] [PubMed]
- Stephanie, R.; Kim, M.W.; Kim, S.H.; Kim, J.-K.; Park, C.Y.; Park, T.J. Recent advances of bimetallic nanomaterials and its nanocomposites for biosensing applications. TrAC Trends Anal. Chem. 2021, 135, 116159. [Google Scholar] [CrossRef]
- Xuan, L.; Ju, Z.; Skonieczna, M.; Zhou, P.K.; Huang, R. Nanoparticles-induced potential toxicity on human health: Applications, toxicity mechanisms, and evaluation models. MedComm 2023, 4, e327. [Google Scholar] [CrossRef] [PubMed]
- Demishkevich, E.; Zyubin, A.; Seteikin, A.; Samusev, I.; Park, I.; Hwangbo, C.K.; Choi, E.H.; Lee, G.J. Synthesis methods and optical sensing applications of plasmonic metal nanoparticles made from rhodium, platinum, gold, or silver. Materials 2023, 16, 3342. [Google Scholar] [CrossRef]
- Coviello, V.; Forrer, D.; Amendola, V. Recent developments in plasmonic alloy nanoparticles: Synthesis, modelling, properties and applications. ChemPhysChem 2022, 23, e202200136. [Google Scholar] [CrossRef]
- Ngo, N.M.; Tran, H.-V.; Lee, T.R. Plasmonic nanostars: Systematic review of their synthesis and applications. ACS Appl. Nano Mater. 2022, 5, 14051–14091. [Google Scholar] [CrossRef]
- Kolahalam, L.A.; Viswanath, I.K.; Diwakar, B.S.; Govindh, B.; Reddy, V.; Murthy, Y. Review on nanomaterials: Synthesis and applications. Mater. Today Proc. 2019, 18, 2182–2190. [Google Scholar] [CrossRef]
- Singh, V.; Yadav, P.; Mishra, V. Chapter 3—Recent advances on classification, properties, synthesis, and characterization of nanomaterials. In Green Synthesis of Nanomaterials for Bioenergy Applications; Srivastava, N., Srivastava, M., Mishra, P.K., Gupta, V.K., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2020; pp. 83–97. [Google Scholar]
- Abid, N.; Khan, A.M.; Shujait, S.; Chaudhary, K.; Ikram, M.; Imran, M.; Haider, J.; Khan, M.; Khan, Q.; Maqbool, M. Synthesis of nanomaterials using various top-down and bottom-up approaches, influencing factors, advantages, and disadvantages: A review. Adv. Colloid Interface Sci. 2022, 300, 102597. [Google Scholar] [CrossRef]
- Lin, H.K.; Yan, T.-H.; Bashir, S.; Liu, J.L. Chapter 3—Synthesis of nanomaterials using bottom-up methods. In Advanced Nanomaterials and Their Applications in Renewable Energy, 2nd ed.; Liu, J.L., Yan, T.-H., Bashir, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 61–110. [Google Scholar]
- Ijaz, I.; Gilani, E.; Nazir, A.; Bukhari, A. Detail review on chemical, physical and green synthesis, classification, characterizations and applications of nanoparticles. Green Chem. Lett. Rev. 2020, 13, 223–245. [Google Scholar] [CrossRef]
- Ishijima, M.; Takada, T.; Cuya Huaman, J.L.; Mizutomi, T.; Sakai, O.; Shinoda, K.; Uchikoshi, M.; Mamiya, H.; Suzuki, K.; Miyamura, H. Synthesis of Electromagnetic Wave-Absorbing Co–Ni Alloys and Co–Ni Core–Shell Structured Nanoparticles. Inorg. Chem. 2022, 61, 17144–17153. [Google Scholar] [CrossRef] [PubMed]
- Besenhard, M.O.; Storozhuk, L.; LaGrow, A.P.; Panariello, L.; Maney, A.; Pal, S.; Kiefer, C.; Mertz, D.; Lees, M.R.; Thanh, N.T.K. High temperature flow synthesis of iron oxide nanoparticles: Size tuning via reactor engineering. Chem. Eng. J. 2023, 473, 144542. [Google Scholar] [CrossRef]
- Kylián, O.; Kuzminova, A.; Štefaníková, R.; Hanuš, J.; Solař, P.; Kúš, P.; Cieslar, M.; Choukourov, A.; Biederman, H. Silver/plasma polymer strawberry-like nanoparticles produced by gas-phase synthesis. Mater. Lett. 2019, 253, 238–241. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Umar, K.; Ibrahim, M.N.M. Silver nanoparticles: Various methods of synthesis, size affecting factors and their potential applications—A review. Appl. Nanosci. 2020, 10, 1369–1378. [Google Scholar] [CrossRef]
- Wender, H.; Gonçalves, R.V.; Feil, A.F.; Migowski, P.; Poletto, F.S.; Pohlmann, A.R.; Dupont, J.; Teixeira, S.r.R. Sputtering onto liquids: From thin films to nanoparticles. J. Phys. Chem. C 2011, 115, 16362–16367. [Google Scholar] [CrossRef]
- Xia, J.; Li, X.-Z.; Huang, X.; Mao, N.; Zhu, D.-D.; Wang, L.; Xu, H.; Meng, X.-M. Physical vapor deposition synthesis of two-dimensional orthorhombic SnS flakes with strong angle/temperature-dependent Raman responses. Nanoscale 2016, 8, 2063–2070. [Google Scholar] [CrossRef]
- Balachandran, A.; Sreenilayam, S.P.; Madanan, K.; Thomas, S.; Brabazon, D. Nanoparticle production via laser ablation synthesis in solution method and printed electronic application—A brief review. Results Eng. 2022, 16, 100646. [Google Scholar] [CrossRef]
- Apriliani, A.; Berliana, J.; Putri, R.; Rohilah, S.; Thifalizalfa, V.; Guniawaty, Y.; Nandiyanto, A. Synthesis of silver nanoparticles in several methods. Maghrebian J. Pure Appl. Sci. 2020, 6, 91–110. [Google Scholar] [CrossRef]
- Chicea, D.; Nicolae-Maranciuc, A.; Doroshkevich, A.S.; Chicea, L.M.; Ozkendir, O.M. Comparative synthesis of silver nanoparticles: Evaluation of chemical reduction procedures, AFM and DLS size analysis. Materials 2023, 16, 5244. [Google Scholar] [CrossRef]
- Bokov, D.; Turki Jalil, A.; Chupradit, S.; Suksatan, W.; Javed Ansari, M.; Shewael, I.H.; Valiev, G.H.; Kianfar, E. Nanomaterial by sol-gel method: Synthesis and application. Adv. Mater. Sci. Eng. 2021, 2021, 5102014. [Google Scholar] [CrossRef]
- Bharti, D.B.; Bharati, A. Synthesis of ZnO nanoparticles using a hydrothermal method and a study its optical activity. Luminescence 2017, 32, 317–320. [Google Scholar] [CrossRef]
- Richard, B.; Lemyre, J.-L.; Ritcey, A.M. Nanoparticle size control in microemulsion synthesis. Langmuir 2017, 33, 4748–4757. [Google Scholar] [CrossRef]
- Nasretdinova, G.R.; Fazleeva, R.R.; Mukhitova, R.K.; Nizameev, I.R.; Kadirov, M.K.; Ziganshina, A.Y.; Yanilkin, V.V. Electrochemical synthesis of silver nanoparticles in solution. Electrochem. Commun. 2015, 50, 69–72. [Google Scholar] [CrossRef]
- Noah, N.M.; Ndangili, P.M. Green synthesis of nanomaterials from sustainable materials for biosensors and drug delivery. Sens. Int. 2022, 3, 100166. [Google Scholar] [CrossRef]
- Coman, N.-A.; Nicolae-Maranciuc, A.; Berța, L.; Nicolescu, A.; Babotă, M.; Man, A.; Chicea, D.; Farczadi, L.; Jakab-Farkas, L.; Silva, B.; et al. Green Synthesis of Metallic Nanoparticles from Quercus Bark Extracts: Characterization and Functional Properties. Antioxidants 2024, 13, 822. [Google Scholar] [CrossRef]
- Brar, K.K.; Magdouli, S.; Othmani, A.; Ghanei, J.; Narisetty, V.; Sindhu, R.; Binod, P.; Pugazhendhi, A.; Awasthi, M.K.; Pandey, A. Green route for recycling of low-cost waste resources for the biosynthesis of nanoparticles (NPs) and nanomaterials (NMs)—A review. Environ. Res. 2022, 207, 112202. [Google Scholar] [CrossRef]
- Parveen, T.; Ahmad, I.; Aslam, M.; Fozia, F.; Khushal, A.; Mohany, M.; Al-Rejaie, S.S.; Ziaullah, Z.; Milošević, M. Green Synthesis of Pure and Zirconium-Doped Cerium Oxide Nanoparticles Using Aqueous Extract of Sanvitalia procumbens, Its Characterization, Antiplatelet and Cytotoxic Activities. Nanomater. Nanotechnol. 2025, 2025, 4276075. [Google Scholar] [CrossRef]
- Mohan, S.; Devan, M.V.; Sambathkumar, S.; Shanmugam, V.; Ravikumar, K.; Marnadu, R.; Palanivel, B.; Hegazy, H. Dual probes of Ag/Pd bimetallic NPs facilely synthesized by green process using Catharanthus leaf extract on textile dye removal and free radical capability. Appl. Nanosci. 2021, 11, 1565–1574. [Google Scholar] [CrossRef]
- Zamarchi, F.; Vieira, I.C. Determination of paracetamol using a sensor based on green synthesis of silver nanoparticles in plant extract. J. Pharm. Biomed. Anal. 2021, 196, 113912. [Google Scholar] [CrossRef]
- Usman, O.; Mohsin Baig, M.M.; Ikram, M.; Iqbal, T.; Islam, S.; Syed, W.; Al-Rawi, M.B.A.; Naseem, M. Green synthesis of metal nanoparticles and study their anti-pathogenic properties against pathogens effect on plants and animals. Sci. Rep. 2024, 14, 11354. [Google Scholar] [CrossRef]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Squires, T.M.; Quake, S.R. Microfluidics: Fluid physics at the nanoliter scale. Rev. Mod. Phys. 2005, 77, 977–1026. [Google Scholar] [CrossRef]
- AlMashrea, B.A.; Almehdi, A.M.; Damiati, S. Simple microfluidic devices for in situ detection of water contamination: A state-of-art review. Front. Bioeng. Biotechnol. 2024, 12, 1355768. [Google Scholar] [CrossRef] [PubMed]
- Erickson, D.; Li, D. Integrated microfluidic devices. Anal. Chim. Acta 2004, 507, 11–26. [Google Scholar] [CrossRef]
- Yager, P.; Edwards, T.; Fu, E.; Helton, K.; Nelson, K.; Tam, M.R.; Weigl, B.H. Microfluidic diagnostic technologies for global public health. Nature 2006, 442, 412–418. [Google Scholar] [CrossRef]
- Chin, C.D.; Linder, V.; Sia, S.K. Commercialization of microfluidic point-of-care diagnostic devices. Lab A Chip 2012, 12, 2118–2134. [Google Scholar] [CrossRef]
- Pamme, N. Magnetism and microfluidics. Lab A Chip 2006, 6, 24–38. [Google Scholar] [CrossRef]
- Friend, J.; Yeo, L.Y. Microscale acoustofluidics: Microfluidics driven via acoustics and ultrasonics. Rev. Mod. Phys. 2011, 83, 647–704. [Google Scholar] [CrossRef]
- Duffy, D.C.; McDonald, J.C.; Schueller, O.J.A.; Whitesides, G.M. Rapid Prototyping of Microfluidic Systems in Poly(dimethylsiloxane). Anal. Chem. 1998, 70, 4974–4984. [Google Scholar] [CrossRef]
- Rogers, C.I.; Qaderi, K.; Woolley, A.T.; Nordin, G.P. 3D printed microfluidic devices with integrated valves. Biomicrofluidics 2015, 9, 016501. [Google Scholar] [CrossRef] [PubMed]
- Alhalaili, B.; Popescu, I.N.; Rusanescu, C.O.; Vidu, R. Microfluidic devices and microfluidics-integrated electrochemical and optical (Bio) Sensors for pollution analysis: A review. Sustainability 2022, 14, 12844. [Google Scholar] [CrossRef]
- Pushparaj, P.N. Revisiting the micropipetting techniques in biomedical sciences: A fundamental prerequisite in good laboratory practice. Bioinformation 2020, 16, 8. [Google Scholar] [CrossRef]
- Udo, E.F.; Idowu, O.J.; Bada, A.T. Influence of successive manual pipetting of multiple samples on precision and reliability of glucose test results. Sokoto J. Med. Lab. Sci. 2024, 9, 327–332. [Google Scholar] [CrossRef]
- Siavashy, S.; Soltani, M.; Rahimi, S.; Hosseinali, M.; Guilandokht, Z.; Raahemifar, K. Recent advancements in microfluidic-based biosensors for detection of genes and proteins: Applications and techniques. Biosens. Bioelectron. X 2024, 19, 100489. [Google Scholar] [CrossRef]
- Laser, D.J.; Santiago, J.G. A review of micropumps. J. Micromech. Microeng. 2004, 14, R35. [Google Scholar] [CrossRef]
- Horiuchi, K.; Dutta, P. Electrokinetic flow control in microfluidic chips using a field-effect transistor. Lab A Chip 2006, 6, 714–723. [Google Scholar] [CrossRef] [PubMed]
- Cazorla, P.H.; Fuchs, O.; Cochet, M.; Maubert, S.; Le Rhun, G.; Robert, P.; Fouillet, Y.; Defay, E. Piezoelectric Micro-pump with PZT Thin Film for Low Consumption Microfluidic Devices. Procedia Eng. 2014, 87, 488–491. [Google Scholar] [CrossRef]
- Nishat, S.; Jafry, A.T.; Martinez, A.W.; Awan, F.R. Paper-based microfluidics: Simplified fabrication and assay methods. Sens. Actuators B Chem. 2021, 336, 129681. [Google Scholar] [CrossRef]
- Gervais, L.; Delamarche, E. Toward one-step point-of-care immunodiagnostics using capillary-driven microfluidics and PDMS substrates. Lab A Chip 2009, 9, 3330–3337. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Z.; Corstjens, P.L.A.M.; Mauk, M.G.; Bau, H.H. A disposable microfluidic cassette for DNA amplification and detection. Lab A Chip 2006, 6, 46–53. [Google Scholar] [CrossRef]
- Nguyen, N.-T.; Hejazian, M.; Ooi, C.H.; Kashaninejad, N. Recent advances and future perspectives on microfluidic liquid handling. Micromachines 2017, 8, 186. [Google Scholar] [CrossRef]
- Oh, K.W.; Ahn, C.H. A review of microvalves. J. Micromech. Microeng. 2006, 16, R13. [Google Scholar] [CrossRef]
- Unger, M.A.; Chou, H.-P.; Thorsen, T.; Scherer, A.; Quake, S.R. Monolithic microfabricated valves and pumps by multilayer soft lithography. Science 2000, 288, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Pal, R.; Yang, M.; Johnson, B.N.; Burke, D.T.; Burns, M.A. Phase Change Microvalve for Integrated Devices. Anal. Chem. 2004, 76, 3740–3748. [Google Scholar] [CrossRef]
- Rissin, D.M.; Kan, C.W.; Campbell, T.G.; Howes, S.C.; Fournier, D.R.; Song, L.; Piech, T.; Patel, P.P.; Chang, L.; Rivnak, A.J. Single-molecule enzyme-linked immunosorbent assay detects serum proteins at subfemtomolar concentrations. Nat. Biotechnol. 2010, 28, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Wiedmer, L.; Eberle, S.A.; Bedi, R.K.; Šledź, P.; Caflisch, A. A reader-based assay for m6A writers and erasers. Anal. Chem. 2019, 91, 3078–3084. [Google Scholar] [CrossRef]
- Wang, J. Electrochemical glucose biosensors. Chem. Rev. 2008, 108, 814–825. [Google Scholar] [CrossRef]
- Jokerst, J.C.; Emory, J.M.; Henry, C.S. Advances in microfluidics for environmental analysis. Analyst 2012, 137, 24–34. [Google Scholar] [CrossRef]
- Burg, T.P.; Godin, M.; Knudsen, S.M.; Shen, W.; Carlson, G.; Foster, J.S.; Babcock, K.; Manalis, S.R. Weighing of biomolecules, single cells and single nanoparticles in fluid. Nature 2007, 446, 1066–1069. [Google Scholar] [CrossRef]
- Lakshmi, S.; Sundar, S.; Menon, S.; Kesana, S.N.; Varshitha, M.G. Development of Piezoelectric Biosensors for Pathogen Detection. In Proceedings of the 24th International Conference on Circuits, Control, Communication and Computing (I4C), Bangalore, India, 21–23 December 2022. [Google Scholar]
- Nguyen, N.-T.; Wereley, S.T. Fundamentals and Applications of Microfluidics; Artech House: London, UK, 2006. [Google Scholar]
- Eijkel, J.C.; Berg, A.v.d. Nanofluidics: What is it and what can we expect from it? Microfluid. Nanofluidics 2005, 1, 249–267. [Google Scholar] [CrossRef]
- Martinez, A.W.; Phillips, S.T.; Butte, M.J.; Whitesides, G.M. Patterned paper as a platform for inexpensive, low-volume, portable bioassays. Angew. Chem. 2007, 119, 1340–1342. [Google Scholar] [CrossRef]
- Yetisen, A.K.; Akram, M.S.; Lowe, C.R. Paper-based microfluidic point-of-care diagnostic devices. Lab A Chip 2013, 13, 2210–2251. [Google Scholar] [CrossRef] [PubMed]
- Haeberle, S.; Zengerle, R. Microfluidic platforms for lab-on-a-chip applications. Lab A Chip 2007, 7, 1094–1110. [Google Scholar] [CrossRef]
- Mark, D.; Haeberle, S.; Roth, G.; Von Stetten, F.; Zengerle, R. Microfluidic lab-on-a-chip platforms: Requirements, characteristics and applications. Microfluid. Based Microsyst. Fundam. Appl. 2010, 39, 305–376. [Google Scholar] [CrossRef]
- Foudeh, A.M.; Didar, T.F.; Veres, T.; Tabrizian, M. Microfluidic designs and techniques using lab-on-a-chip devices for pathogen detection for point-of-care diagnostics. Lab A Chip 2012, 12, 3249–3266. [Google Scholar] [CrossRef]
- Ramalingam, S.; Chand, R.; Singh, C.B.; Singh, A. Phosphorene-gold nanocomposite based microfluidic aptasensor for the detection of okadaic acid. Biosens. Bioelectron. 2019, 135, 14–21. [Google Scholar] [CrossRef]
- Costa, D.; Pereira-Silva, P.; Sousa, P.; Pinto, V.; Borges, J.; Vaz, F.; Minas, G.; Sampaio, P. Critical Issues on the Surface Functionalization of Plasmonic Au-Ag/TiO2 Thin Films with Thiolated Oligonucleotide-Based Biorecognition Elements. Biosensors 2024, 14, 159. [Google Scholar] [CrossRef]
- Chowdury, M.A.; Walji, N.; Mahmud, M.A.; MacDonald, B.D. Paper-Based Microfluidic Device with a Gold Nanosensor to Detect Arsenic Contamination of Groundwater in Bangladesh. Micromachines 2017, 8, 71. [Google Scholar] [CrossRef]
- Chatterjee, S.; Lou, X.-Y.; Liang, F.; Yang, Y.-W. Surface-functionalized gold and silver nanoparticles for colorimetric and fluorescent sensing of metal ions and biomolecules. Coord. Chem. Rev. 2022, 459, 214461. [Google Scholar] [CrossRef]
- Ma, W.; Liu, L.; Zhang, X.; Liu, X.; Xu, Y.; Li, S.; Zeng, M. A microfluidic-based SERS biosensor with multifunctional nanosurface immobilized nanoparticles for sensitive detection of MicroRNA. Anal. Chim. Acta 2022, 1221, 340139. [Google Scholar] [CrossRef] [PubMed]
- Haes, A.J.; Van Duyne, R.P. A nanoscale optical biosensor: Sensitivity and selectivity of an approach based on the localized surface plasmon resonance spectroscopy of triangular silver nanoparticles. J. Am. Chem. Soc. 2002, 124, 10596–10604. [Google Scholar] [CrossRef]
- Anker, J.N.; Hall, W.P.; Lyandres, O.; Shah, N.C.; Zhao, J.; Van Duyne, R.P. Biosensing with plasmonic nanosensors. Nat. Mater. 2008, 7, 442–453. [Google Scholar] [CrossRef]
- Santos-Santos, I.d.J.; Zamora-Justo, J.A.; Vázquez-Martínez, G.R.; Cabrera-Sierra, R.; Balderas-López, J.A. Synthesis of gold nanoparticles coated with glucose oxidase using PVP as passive adsorption linkage. Front. Nanotechnol. 2024, 6, 1419239. [Google Scholar] [CrossRef]
- Li, L.; Cui, W.; He, Z.; Xue, W.; He, H. Plasmonic Sensor Based on Silver Nanoparticles for the Detection of Glucose. Plasmonics 2022, 17, 1231–1234. [Google Scholar] [CrossRef]
- Sun, L.; Li, W.; Wang, M.; Ding, W.; Ji, Y. Development of An Electrochemical Impedance Immunosensor for Myoglobin Determination. Int. J. Electrochem. Sci. 2017, 12, 6170–6179. [Google Scholar] [CrossRef]
- Park, H.; Hwang, M.P.; Lee, K.H. Immunomagnetic nanoparticle-based assays for detection of biomarkers. Int. J. Nanomed. 2013, 8, 4543–4552. [Google Scholar] [CrossRef]
- Eivazzadeh-Keihan, R.; Bahreinizad, H.; Amiri, Z.; Aliabadi, H.A.M.; Salimi-Bani, M.; Nakisa, A.; Davoodi, F.; Tahmasebi, B.; Ahmadpour, F.; Radinekiyan, F.; et al. Functionalized magnetic nanoparticles for the separation and purification of proteins and peptides. TrAC Trends Anal. Chem. 2021, 141, 116291. [Google Scholar] [CrossRef]
- Wang, J.; Xu, J.; Liu, X.; Li, X.; Xu, Z. A microfluidic chip incorporating magnetic sorting and invasive separation for isolation, culture and telomerase analysis of circulating tumor cells. Talanta 2025, 285, 127316. [Google Scholar] [CrossRef]
- Pandey, C.; Pandey, M.; Sumana, G. Langmuir–Blodgett based ordered deposition of functionalized iron oxide nanoparticles for ultrasensitive detection of Escherichia coli O157:H7. Microchem. J. 2022, 181, 107708. [Google Scholar] [CrossRef]
- Hao, L.; Xue, L.; Huang, F.; Cai, G.; Qi, W.; Zhang, M.; Han, Q.a.; Wang, Z.; Lin, J. A microfluidic biosensor based on magnetic nanoparticle separation, quantum dots labeling and mno2 nanoflower amplification for rapid and sensitive detection of Salmonella typhimurium. Micromachines 2020, 11, 281. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Wang, X.; Tian, F.; Li, M.; Xie, K.; Ma, X. Enhanced functionalization of superparamagnetic Fe3O4 nanoparticles for advanced drug enrichment and separation applications. BMC Chem. 2024, 18, 181. [Google Scholar] [CrossRef]
- Zhang, J.; Khan, M.A.; Xia, M.; Abdo, A.M.; Lei, W.; Liao, C.; Wang, F. Facile hydrothermal synthesis of magnetic adsorbent CoFe2O4/MMT to eliminate antibiotics in aqueous phase: Tetracycline and ciprofloxacin. Environ. Sci. Pollut. Res. 2019, 26, 215–226. [Google Scholar] [CrossRef]
- Chávez-Ramos, K.; del Pilar Cañizares-Macías, M. Continuous flow microfluidic system with magnetic nanoparticles for the spectrophotometric quantification of urea in urine and plasma samples. Anal. Methods 2024, 16, 7752–7762. [Google Scholar] [CrossRef]
- Marie, M.; Mandal, S.; Manasreh, O. An electrochemical glucose sensor based on zinc oxide nanorods. Sensors 2015, 15, 18714–18723. [Google Scholar] [CrossRef] [PubMed]
- Rashed, M.A.; Faisal, M.; Harraz, F.A.; Jalalah, M.; Alsaiari, M.; Alsareii, S.A. A Highly Efficient Nonenzymatic Hydrogen Peroxide Electrochemical Sensor Using Mesoporous Carbon Doped ZnO Nanocomposite. J. Electrochem. Soc. 2021, 168, 027512. [Google Scholar] [CrossRef]
- Zhang, T.; Zhu, J.; Xie, M.; Meng, K.; Yao, G.; Pan, T.; Gao, M.; Cheng, H.; Lin, Y. Highly Sensitive Wearable Sensor Based on (001)-Orientated TiO2 for Real-Time Electrochemical Detection of Dopamine, Tyrosine, and Paracetamol. Small 2024, 20, 2312238. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, R.; Aggarwal, N.; Panwar, R.S.; Kumar, N. Exploring heavy metal detection efficacy in industrial waste water using Ficus Benjamina leaf extract-mediated TiO2 nanoparticles. J. Mater. Sci. Mater. Electron. 2023, 34, 184. [Google Scholar] [CrossRef]
- Buledi, J.A.; Ameen, S.; Memon, S.A.; Fatima, A.; Solangi, A.R.; Mallah, A.; Karimi, F.; Malakmohammadi, S.; Agarwal, S.; Gupta, V.K. An improved non-enzymatic electrochemical sensor amplified with CuO nanostructures for sensitive determination of uric acid. Open Chem. 2021, 19, 481–491. [Google Scholar] [CrossRef]
- Feng, S.; Xing, X.; Hou, W. Copper oxide nanoparticles modified electrodes for high-sensitivity detection of uric acid in athletes. Alex. Eng. J. 2024, 101, 1–7. [Google Scholar] [CrossRef]
- Alam, F.A.H.; Jalal, A.H.; Forouzanfar, S.; Wang, C.; Pala, N. ZnO Nanoflakes based Enzymatic Sensor for the determination of lactic acid in sweat. In Proceedings of the 2019 IEEE Sensors, Montreal, QC, Canada, 27–30 October 2019. [Google Scholar]
- Zhang, C.; Li, L.; Ju, J.; Chen, W. Electrochemical Sensor Based on Graphene-Supported Tin Oxide Nanoclusters for Nonenzymatic Detection of Hydrogen Peroxide. Electrochim. Acta 2016, 210, 181–189. [Google Scholar] [CrossRef]
- Leau, S.-A.; Lete, C.; Lupu, S. Nanocomposite Materials based on Metal Nanoparticles for the Electrochemical Sensing of Neurotransmitters. Chemosensors 2023, 11, 179. [Google Scholar] [CrossRef]
- Liu, X.; Huang, L.; Qian, K. Nanomaterial-Based Electrochemical Sensors: Mechanism, Preparation, and Application in Biomedicine. Adv. NanoBiomed Res. 2021, 1, 2000104. [Google Scholar] [CrossRef]
- Lu, L.; Zhu, Z.; Hu, X. Multivariate nanocomposites for electrochemical sensing in the application of food. TrAC Trends Anal. Chem. 2019, 118, 759–769. [Google Scholar] [CrossRef]
- Babuji, P.; Taher, M.A.; Dar, M.H.; Rao, D.N.; Krishna, P.G.; Saikiran, V. Surface-Enhanced Raman Scattering Studies of Au-Ag Bimetallic Nanoparticles with a Tunable Surface Plasmon Resonance Wavelength Synthesized by Picosecond Laser Irradiation. Photonics 2023, 10, 1345. [Google Scholar] [CrossRef]
- Zhao, S.; Chen, L.; Luo, W.; Pi, Z.; Zhang, X.; Yang, R.; Yang, X.; Zhang, Y.; Liao, X. Electrochemical high sensitive detection of hydrogen peroxide based on platinum-palladium nano-enzyme modified microelectrode. Int. J. Electrochem. Sci. 2024, 19, 100672. [Google Scholar] [CrossRef]
- Anju, S.M.; Merin, K.A.; Varghese, S.; Shkhair, A.I.; Rajeevan, G.; Indongo, G.; George, S. Antibody-functionalized gold nanoclusters/gold nanoparticle platform for the fluorescence turn-on detection of cardiac troponin I. Microchim. Acta 2024, 191, 124. [Google Scholar] [CrossRef]
- Martino, S.; Tammaro, C.; Misso, G.; Falco, M.; Scrima, M.; Bocchetti, M.; Rea, I.; De Stefano, L.; Caraglia, M. microRNA Detection via Nanostructured Biochips for Early Cancer Diagnostics. Int. J. Mol. Sci. 2023, 24, 7762. [Google Scholar] [CrossRef]
- Jiménez-Fiérrez, F.; González-Sánchez, M.I.; Jiménez-Pérez, R.; Iniesta, J.; Valero, E. Glucose Biosensor Based on Disposable Activated Carbon Electrodes Modified with Platinum Nanoparticles Electrodeposited on Poly(Azure A). Sensors 2020, 20, 4489. [Google Scholar] [CrossRef]
- Gil, B.; Keshavarz, M.; Wales, D.; Darzi, A.; Yeatman, E. Orthogonal Surface-Enhanced Raman Scattering/Field-Effect Transistor Detection of Breast and Colorectal Cancer-Derived Exosomes using Graphene as a Tag-Free Diagnostic Template. Adv. NanoBiomed Res. 2023, 3, 2300055. [Google Scholar] [CrossRef]
- Perez Ruiz de Garibay, A.; Spinato, C.; Klippstein, R.; Bourgognon, M.; Martincic, M.; Pach, E.; Ballesteros, B.; Ménard-Moyon, C.; Al-Jamal, K.T.; Tobias, G. Evaluation of the immunological profile of antibody-functionalized metal-filled single-walled carbon nanocapsules for targeted radiotherapy. Sci. Rep. 2017, 7, 42605. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Lee, J.S.; Jang, J. Aptamer-Functionalized Three-Dimensional Carbon Nanowebs for Ultrasensitive and Free-Standing PDGF Biosensor. ACS Appl. Mater. Interfaces 2020, 12, 20882–20890. [Google Scholar] [CrossRef]
- Xiang, Y.; Wu, P.; Tan, L.H.; Lu, Y. DNazyme-functionalized gold nanoparticles for biosensing. In Biosensors Based on Aptamers and Enzymes; Advances in Biochemical Engineering/Biotechnology; Gu, M., Kim, H.S., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 93–120. [Google Scholar]
- Gimondi, S.; Ferreira, H.; Reis, R.L.; Neves, N.M. Microfluidic Devices: A Tool for Nanoparticle Synthesis and Performance Evaluation. ACS Nano 2023, 17, 14205–14228. [Google Scholar] [CrossRef] [PubMed]
- Abstiens, K.; Goepferich, A.M. Microfluidic manufacturing improves polydispersity of multicomponent polymeric nanoparticles. J. Drug Deliv. Sci. Technol. 2019, 49, 433–439. [Google Scholar] [CrossRef]
- Tejamaya, M.; Römer, I.; Merrifield, R.; Lead, J. Stability of Citrate, PVP, and PEG Coated Silver Nanoparticles in Ecotoxicology Media. Environ. Sci. Technol. 2012, 46, 7011–7017. [Google Scholar] [CrossRef]
- Yesildag, C.; Ouyang, Z.; Zhang, Z.; Lensen, M.C. Micro-patterning of PEG-based hydrogels with gold nanoparticles using a reactive micro-contact-printing approach. Front. Chem. 2019, 6, 667. [Google Scholar] [CrossRef]
- Sur, S.; Rathore, A.; Dave, V.; Reddy, K.R.; Chouhan, R.S.; Sadhu, V. Recent developments in functionalized polymer nanoparticles for efficient drug delivery system. Nano-Struct. Nano-Objects 2019, 20, 100397. [Google Scholar] [CrossRef]
- Zhang, W.J.; Hong, C.Y.; Pan, C.Y. Polymerization-induced self-assembly of functionalized block copolymer nanoparticles and their application in drug delivery. Macromol. Rapid Commun. 2019, 40, 1800279. [Google Scholar] [CrossRef]
- Thanneeru, S.; Ayers, K.M.; Anuganti, M.; Zhang, L.; Kumar, C.V.; Ung, G.; He, J. N-Heterocyclic carbene-ended polymers as surface ligands of plasmonic metal nanoparticles. J. Mater. Chem. C 2020, 8, 2280–2288. [Google Scholar] [CrossRef]
- Uson, L.; Sebastian, V.; Arruebo, M.; Santamaria, J. Continuous microfluidic synthesis and functionalization of gold nanorods. Chem. Eng. J. 2016, 285, 286–292. [Google Scholar] [CrossRef]
- Mahdy, N.K.; El-Sayed, M.; Al-Mofty, S.E.-D.; Mohamed, A.; Karaly, A.H.; El-Naggar, M.E.; Nageh, H.; Sarhan, W.A.; El-Said Azzazy, H.M. Toward scaling up the production of metal oxide nanoparticles for application on washable antimicrobial cotton fabrics. ACS Omega 2022, 7, 38942–38956. [Google Scholar] [CrossRef] [PubMed]
- Charitidis, C.A.; Georgiou, P.; Koklioti, M.A.; Trompeta, A.-F.; Markakis, V. Manufacturing nanomaterials: From research to industry. Manuf. Rev. 2014, 1, 11. [Google Scholar] [CrossRef]
- Parvin, N.; Kumar, V.; Mandal, T.K.; Joo, S.W. Advancements in Nanoporous Materials for Biomedical Imaging and Diagnostics. J. Funct. Biomater. 2024, 15, 226. [Google Scholar] [CrossRef] [PubMed]
- Radulescu, D.-M.; Surdu, V.-A.; Ficai, A.; Ficai, D.; Grumezescu, A.-M.; Andronescu, E. Green Synthesis of Metal and Metal Oxide Nanoparticles: A Review of the Principles and Biomedical Applications. Int. J. Mol. Sci. 2023, 24, 15397. [Google Scholar] [CrossRef]
- Nazir, S. Advancing point-of-care testing with nanomaterials-based screen-printing electrodes. Sens. Int. 2025, 6, 100328. [Google Scholar] [CrossRef]
- Atta, N.; Galal, A.; Ali, S. Nanobiosensors for health care. In Biosensors for Health, Environment and Biosecurity; Serra, P.A., Ed.; IntechOpen: Rijeka, Croatia, 2011. [Google Scholar]
- Gama Cavalcante, A.L.; Dari, D.N.; Izaias da Silva Aires, F.; Carlos de Castro, E.; Moreira dos Santos, K.; Sousa dos Santos, J.C. Advancements in enzyme immobilization on magnetic nanomaterials: Toward sustainable industrial applications. RSC Adv. 2024, 14, 17946–17988. [Google Scholar] [CrossRef]
- Damborska, D.; Bertok, T.; Dosekova, E.; Holazova, A.; Lorencova, L.; Kasak, P.; Tkac, J. Nanomaterial-based biosensors for detection of prostate specific antigen. Microchim. Acta 2017, 184, 3049–3067. [Google Scholar] [CrossRef]
- Geng, P.; Zhang, X.; Meng, W.; Wang, Q.; Zhang, W.; Jin, L.; Feng, Z.; Wu, Z. Self-assembled monolayers-based immunosensor for detection of Escherichia coli using electrochemical impedance spectroscopy. Electrochim. Acta 2008, 53, 4663–4668. [Google Scholar] [CrossRef]
- SadAbadi, H.; Badilescu, S.; Packirisamy, M.; Wüthrich, R. Integration of gold nanoparticles in PDMS microfluidics for lab-on-a-chip plasmonic biosensing of growth hormones. Biosens. Bioelectron. 2013, 44, 77–84. [Google Scholar] [CrossRef]
- Abou Hassan, A.; Sandre, O.; Cabuil, V.; Tabeling, P. Synthesis of iron oxide nanoparticles in a microfluidic device: Preliminary results in a coaxial flow millichannel. Chem. Commun. 2008, 15, 1783–1785. [Google Scholar] [CrossRef]
- Chicea, D.; Nicolae-Maranciuc, A. A Review of Chitosan-Based Materials for Biomedical, Food, and Water Treatment Applications. Materials 2024, 17, 5770. [Google Scholar] [CrossRef] [PubMed]
- Bîrcă, A.C.; Gherasim, O.; Niculescu, A.-G.; Grumezescu, A.M.; Neacșu, I.A.; Chircov, C.; Vasile, B.Ș.; Oprea, O.C.; Andronescu, E.; Stan, M.S.; et al. A Microfluidic Approach for Synthesis of Silver Nanoparticles as a Potential Antimicrobial Agent in Alginate–Hyaluronic Acid-Based Wound Dressings. Int. J. Mol. Sci. 2023, 24, 11466. [Google Scholar] [CrossRef]
- Jóskowiak, A.; Nogueira, C.L.; Costa, S.P.; Cunha, A.P.; Freitas, P.P.; Carvalho, C.M. A magnetic nanoparticle-based microfluidic device fabricated using a 3D-printed mould for separation of Escherichia coli from blood. Microchim. Acta 2023, 190, 356. [Google Scholar] [CrossRef]
- Unni, M.; Zhang, J.; George, T.J.; Segal, M.S.; Fan, Z.H.; Rinaldi, C. Engineering magnetic nanoparticles and their integration with microfluidics for cell isolation. J. Colloid Interface Sci. 2020, 564, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Claussen, J.C.; Kim, S.S.; Haque, A.; Artiles, M.S.; Porterfield, D.M.; Fisher, T.S. Electrochemical Glucose Biosensor of Platinum Nanospheres Connected by Carbon Nanotubes. J. Diabetes Sci. Technol. 2010, 4, 312–319. [Google Scholar] [CrossRef]
- Kim, W.-J.; Cho, H.Y.; Kim, B.K.; Huh, C.; Chung, K.H.; Ahn, C.-G.; Kim, Y.J.; Kim, A. Highly sensitive detection of cardiac troponin I in human serum using gold nanoparticle-based enhanced sandwich immunoassay. Sens. Actuators B Chem. 2015, 221, 537–543. [Google Scholar] [CrossRef]
- Ahmad, R.; Tripathy, N.; Ahn, M.-S.; Hahn, Y.-B. Solution Process Synthesis of High Aspect Ratio ZnO Nanorods on Electrode Surface for Sensitive Electrochemical Detection of Uric Acid. Sci. Rep. 2017, 7, 46475. [Google Scholar] [CrossRef]
- Almaghrabi, O.; Almulaiky, Y.Q. A biocatalytic system obtained via immobilization of urease onto magnetic metal/alginate nanocomposite: Improving reusability and enhancing stability. Biocatal. Biotransformation 2023, 41, 456–465. [Google Scholar] [CrossRef]
- Lichtenberg, J.Y.; Ling, Y.; Kim, S. Non-specific adsorption reduction methods in biosensing. Sensors 2019, 19, 2488. [Google Scholar] [CrossRef]
- Zhang, H.; Chiao, M. Anti-fouling coatings of poly (dimethylsiloxane) devices for biological and biomedical applications. J. Med. Biol. Eng. 2015, 35, 143–155. [Google Scholar] [CrossRef]
- Ladd, J.; Zhang, Z.; Chen, S.; Hower, J.C.; Jiang, S. Zwitterionic polymers exhibiting high resistance to nonspecific protein adsorption from human serum and plasma. Biomacromolecules 2008, 9, 1357–1361. [Google Scholar] [CrossRef] [PubMed]
- Van Andel, E.; Lange, S.C.; Pujari, S.P.; Tijhaar, E.J.; Smulders, M.M.; Savelkoul, H.F.; Zuilhof, H. Systematic comparison of zwitterionic and non-zwitterionic antifouling polymer brushes on a bead-based platform. Langmuir 2018, 35, 1181–1191. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Wu, L.; Gao, Y.; Ma, G. Quantification and Mitigation of Site-Preferred Nonspecific Interactions in Single-Nanoparticle Biosensors. ACS Sens. 2025, 10, 2258–2265. [Google Scholar] [CrossRef]
- Sheikh, S.; Blaszykowski, C.; Thompson, M. Sacrificial BSA to block non-specific adsorption on organosilane adlayers in ultra-high frequency acoustic wave sensing. Surf. Interface Anal. 2013, 45, 1781–1784. [Google Scholar] [CrossRef]
- Riquelme, M.V.; Zhao, H.; Srinivasaraghavan, V.; Pruden, A.; Vikesland, P.; Agah, M. Optimizing blocking of nonspecific bacterial attachment to impedimetric biosensors. Sens. Bio-Sens. Res. 2016, 8, 47–54. [Google Scholar] [CrossRef]
- Johnston, L.; Wang, G.; Hu, K.; Qian, C.; Liu, G. Advances in biosensors for continuous glucose monitoring towards wearables. Front. Bioeng. Biotechnol. 2021, 9, 733810. [Google Scholar] [CrossRef]
- Bachache, L.N.; Hasan, J.A.; Al-Neam, A.Q. A review: Non invasive sensing system for detection glucose level. Proc. J. Phys. Conf. Ser. 2021, 1963, 012125. [Google Scholar] [CrossRef]
- Antar, S.A.; Ashour, N.A.; Sharaky, M.; Khattab, M.; Ashour, N.A.; Zaid, R.T.; Roh, E.J.; Elkamhawy, A.; Al-Karmalawy, A.A. Diabetes mellitus: Classification, mediators, and complications; A gate to identify potential targets for the development of new effective treatments. Biomed. Pharmacother. 2023, 168, 115734. [Google Scholar] [CrossRef]
- Kharroubi, A.T.; Darwish, H.M. Diabetes mellitus: The epidemic of the century. World J. Diabetes 2015, 6, 850. [Google Scholar] [CrossRef]
- Bruen, D.; Delaney, C.; Florea, L.; Diamond, D. Glucose sensing for diabetes monitoring: Recent developments. Sensors 2017, 17, 1866. [Google Scholar] [CrossRef]
- Peng, Z.; Xie, X.; Tan, Q.; Kang, H.; Cui, J.; Zhang, X.; Li, W.; Feng, G. Blood glucose sensors and recent advances: A review. J. Innov. Opt. Health Sci. 2022, 15, 2230003. [Google Scholar] [CrossRef]
- Mean Fasting Blood Glucose. Available online: https://www.who.int/data/gho/indicator-metadata-registry/imr-details/2380 (accessed on 20 January 2025).
- Shang, T.; Zhang, J.Y.; Thomas, A.; Arnold, M.A.; Vetter, B.N.; Heinemann, L.; Klonoff, D.C. Products for monitoring glucose levels in the human body with noninvasive optical, noninvasive fluid sampling, or minimally invasive technologies. J. Diabetes Sci. Technol. 2022, 16, 168–214. [Google Scholar] [CrossRef] [PubMed]
- Villena Gonzales, W.; Mobashsher, A.T.; Abbosh, A. The progress of glucose monitoring—A review of invasive to minimally and non-invasive techniques, devices and sensors. Sensors 2019, 19, 800. [Google Scholar] [CrossRef]
- Tang, L.; Chang, S.J.; Chen, C.-J.; Liu, J.-T. Non-invasive blood glucose monitoring technology: A review. Sensors 2020, 20, 6925. [Google Scholar] [CrossRef] [PubMed]
- Yoo, E.-H.; Lee, S.-Y. Glucose biosensors: An overview of use in clinical practice. Sensors 2010, 10, 4558–4576. [Google Scholar] [CrossRef] [PubMed]
- Heller, A.; Feldman, B. Electrochemical glucose sensors and their applications in diabetes management. Chem. Rev. 2008, 108, 2482–2505. [Google Scholar] [CrossRef]
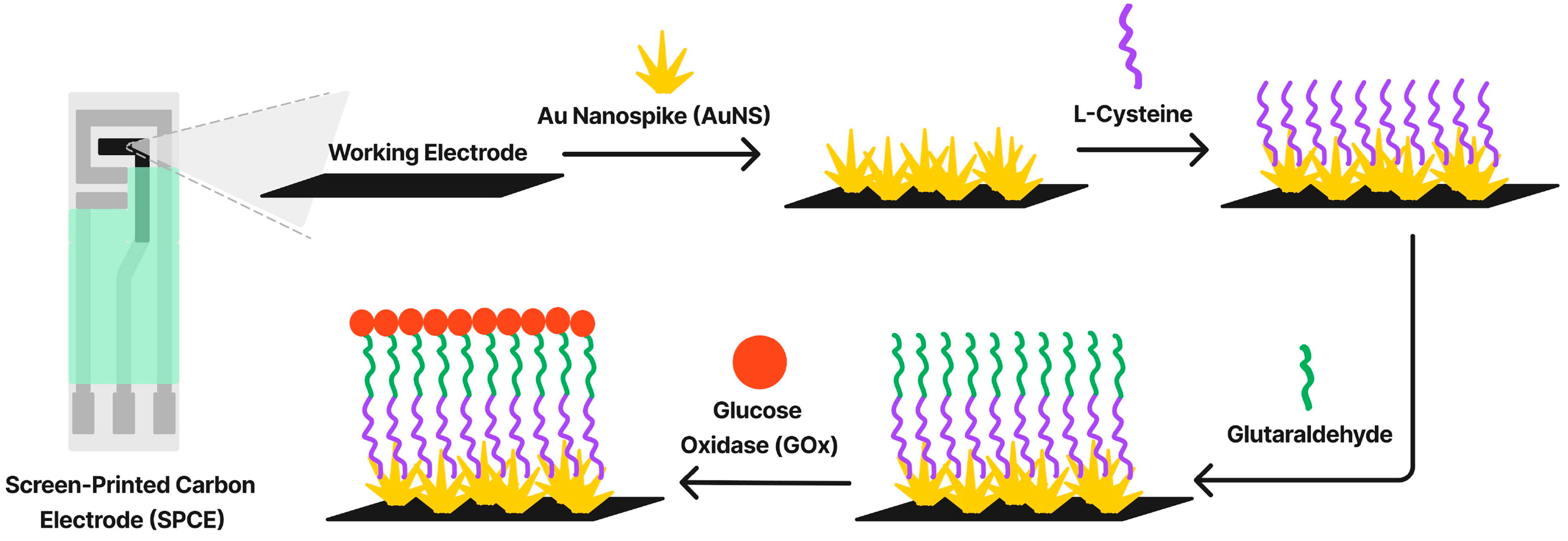
- Majidah, S.; Rizalputri, L.N.; Ariasena, E.; Raditya, A.N.; Ropii, B.; Salsabila, N.; Uperianti; Handayani, M.; Hartati, Y.W.; Anshori, I. Evaluating bioreceptor immobilization on Gold Nanospike (AuNS)–modified Screen-Printed Carbon Electrode (SPCE) as enzymatic glucose biosensor. Nanocomposites 2024, 10, 125–137. [Google Scholar] [CrossRef]
- Dong, T.; Matos Pires, N.M.; Yang, Z.; Jiang, Z. Advances in electrochemical biosensors based on nanomaterials for protein biomarker detection in saliva. Adv. Sci. 2023, 10, 2205429. [Google Scholar] [CrossRef]
- Samavati, A.; Samavati, Z.; Velashjerdi, M.; Ismail, A.F.; Othman, M.; Abdullah, M.S.; Bolurian, M. Sustainable and fast saliva-based COVID-19 virus diagnosis kit using a novel GO-decorated Au/FBG sensor. Chem. Eng. J. 2021, 420, 127655. [Google Scholar] [CrossRef]
- Wityk, P.; Terebieniec, A.; Nowak, R.; Łubiński, J.; Mroczyńska-Szeląg, M.; Wityk, T.; Kostrzewa-Nowak, D. Reusable Biosensor for Easy RNA Detection from Unfiltered Saliva. Sensors 2025, 25, 360. [Google Scholar] [CrossRef]
- Tlili, C.; Myung, N.V.; Shetty, V.; Mulchandani, A. Label-free, chemiresistor immunosensor for stress biomarker cortisol in saliva. Biosens. Bioelectron. 2011, 26, 4382–4386. [Google Scholar] [CrossRef] [PubMed]
- Sethuraman, S.; Ramalingam, K.; Ramani, P.; Kalaiyarasan, M. Nanomaterial biosensors in salivary diagnosis of oral cancer: A scoping review. Cureus 2024, 16, e59779. [Google Scholar] [CrossRef]
- Rakhshani, M.R.; Mansouri-Birjandi, M.A. A high-sensitivity sensor based on three-dimensional metal–insulator–metal racetrack resonator and application for hemoglobin detection. Photonics Nanostructures Fundam. Appl. 2018, 32, 28–34. [Google Scholar] [CrossRef]
- Chahkoutahi, A.; Emami, F.; Rafiee, E. Sensitive hemoglobin concentration sensor based on graphene-plasmonic nano-structures. Plasmonics 2022, 17, 423–431. [Google Scholar] [CrossRef]
- Sahraeian, S.; Negahdari, R.; Emami, F. Tunable terahertz absorber based on graphene-metal nanostructure as opto-fluid sensor. Optik 2021, 242, 166713. [Google Scholar] [CrossRef]
- Naseem, T.; Waseem, M. A comprehensive review on the role of some important nanocomposites for antimicrobial and wastewater applications. Int. J. Environ. Sci. Technol. 2022, 19, 2221–2246. [Google Scholar] [CrossRef]
- Brahmkhatri, V.; Pandit, P.; Rananaware, P.; D’Souza, A.; Kurkuri, M.D. Recent progress in detection of chemical and biological toxins in Water using plasmonic nanosensors. Trends Environ. Anal. Chem. 2021, 30, e00117. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Q.X.; Guo, Z.H.; Lin, J.S. Practical application of aptamer-based biosensors in detection of low molecular weight pollutants in water sources. Molecules 2018, 23, 344. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Zhang, X.; He, F. Rapid detection of Escherichia coli based on 16S rDNA nanogap network electrochemical biosensor. Biosens. Bioelectron. 2018, 118, 9–15. [Google Scholar] [CrossRef]
- Mirghani, M. Synthesis and characterizations of molybdenum-doped titanium dioxide nanoparticles for photocatalytic removal of chromium (VI) from aqueous solutions. Nanocomposites 2024, 10, 59–67. [Google Scholar] [CrossRef]
- Farhan, A.; Zulfiqar, M.; Samiah; Rashid, E.U.; Nawaz, S.; Iqbal, H.M.; Jesionowski, T.; Bilal, M.; Zdarta, J. Removal of toxic metals from water by nanocomposites through advanced remediation processes and photocatalytic oxidation. Curr. Pollut. Rep. 2023, 9, 338–358. [Google Scholar] [CrossRef]
- Velusamy, K.; Periyasamy, S.; Kumar, P.S.; Rangasamy, G.; Pauline, J.M.N.; Ramaraju, P.; Mohanasundaram, S.; Vo, D.-V.N. Biosensor for heavy metals detection in wastewater: A review. Food Chem. Toxicol. 2022, 168, 113307. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, G.; Liu, Y. An acetylcholinesterase biosensor based on platinum nanoparticles–carboxylic graphene–nafion-modified electrode for detection of pesticides. Anal. Biochem. 2013, 437, 144–149. [Google Scholar] [CrossRef]
- Chen, S.; Sun, Y.; Fan, F.; Chen, S.; Zhang, Y.; Zhang, Y.; Meng, X.; Lin, J.-M. Present status of microfluidic PCR chip in nucleic acid detection and future perspective. TrAC Trends Anal. Chem. 2022, 157, 116737. [Google Scholar] [CrossRef]
- Poltronieri, P.; Mezzolla, V.; Primiceri, E.; Maruccio, G. Biosensors for the detection of food pathogens. Foods 2014, 3, 511–526. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, D.; Bai, Y.; Cui, Y.; Wang, D.; Shi, X. Identification and characterization of two high affinity aptamers specific for Salmonella Enteritidis. Food Control 2019, 106, 106719. [Google Scholar] [CrossRef]
- Wu, W.; Li, J.; Pan, D.; Li, J.; Song, S.; Rong, M.; Li, Z.; Gao, J.; Lu, J. Gold nanoparticle-based enzyme-linked antibody-aptamer sandwich assay for detection of Salmonella Typhimurium. ACS Appl. Mater. Interfaces 2014, 6, 16974–16981. [Google Scholar] [CrossRef]
- Kalele, S.A.; Kundu, A.A.; Gosavi, S.W.; Deobagkar, D.N.; Deobagkar, D.D.; Kulkarni, S.K. Rapid detection of Escherichia coli by using antibody-conjugated silver nanoshells. Small 2006, 2, 335–338. [Google Scholar] [CrossRef]
- Soysaldı, F.; Dincyurek Ekici, D.; Soylu, M.Ç.; Mutlugun, E. Electrochemical and Optical Multi-Detection of Escherichia coli Through Magneto-Optic Nanoparticles: A Pencil-on-Paper Biosensor. Biosensors 2024, 14, 603. [Google Scholar] [CrossRef]
- Kangarshahi, B.M.; Beigi Javazm, A.; Naghib, S.M. CRISPR-integrated metal-organic frameworks for biosensing applications: Recent advances and future perspective. Sens. Bio-Sens. Res. 2025, 47, 100736. [Google Scholar] [CrossRef]
- Deveau, H.; Garneau, J.E.; Moineau, S. CRISPR/Cas system and its role in phage-bacteria interactions. Annu. Rev. Microbiol. 2010, 64, 475–493. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Singh, R.; Singh, A.P.; Wei, Y. Three-dimensional printed thermoplastic polyurethane on fabric as wearable smart sensors. Proc. Inst. Mech. Eng. Part L J. Mater. Des. Appl. 2023, 237, 1678–1692. [Google Scholar] [CrossRef]
- Suresh, R.; Vijayaraj, A.; Giribabu, K.; Manigandan, R.; Prabu, R.; Stephen, A.; Thirumal, E.; Narayanan, V. Fabrication of iron oxide nanoparticles: Magnetic and electrochemical sensing property. J. Mater. Sci. Mater. Electron. 2013, 24, 1256–1263. [Google Scholar] [CrossRef]
- Yang, Z.; Wu, S.; Gao, Y.; Kou, D.; Lu, K.; Chen, C.; Zhou, Y.; Zhou, D.; Chen, L.; Ge, J.; et al. Unveiling the Biologically Dynamic Degradation of Iron Oxide Nanoparticles via a Continuous Flow System. Small Methods 2024, 8, 2301479. [Google Scholar] [CrossRef]
- Giouroudi, I.; Keplinger, F. Microfluidic Biosensing Systems Using Magnetic Nanoparticles. Int. J. Mol. Sci. 2013, 14, 18535–18556. [Google Scholar] [CrossRef]
- Ameh, T.; Gibb, M.; Stevens, D.; Pradhan, S.H.; Braswell, E.; Sayes, C.M. Silver and copper nanoparticles induce oxidative stress in bacteria and mammalian cells. Nanomaterials 2022, 12, 2402. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, J.; Oh, J.; Bae, S.; Lee, S.; Hong, I.S.; Kim, S.H. Ion-release kinetics and ecotoxicity effects of silver nanoparticles. Environ. Toxicol. Chem. 2012, 31, 155–159. [Google Scholar] [CrossRef]
- Buff, M.; Drab, E.; Sugihara, K. Effect of the nonspecific binding in differential impedance biosensing. Biointerphases 2019, 14, 5082717. [Google Scholar] [CrossRef]
- Ciobotaru, I.C.; Oprea, D.; Ciobotaru, C.C.; Enache, T.A. Low-Cost Plant-Based Metal and Metal Oxide Nanoparticle Synthesis and Their Use in Optical and Electrochemical (Bio) Sensors. Biosensors 2023, 13, 1031. [Google Scholar] [CrossRef]
- Jaime, F.J.; Muñoz, A.; Rodríguez-Gómez, F.; Jerez-Calero, A. Strengthening privacy and data security in biomedical microelectromechanical systems by IoT communication security and protection in smart healthcare. Sensors 2023, 23, 8944. [Google Scholar] [CrossRef]
- Othman, A.; Karimi, A.; Andreescu, S. Functional nanostructures for enzyme based biosensors: Properties, fabrication and applications. J. Mater. Chem. B 2016, 4, 7178–7203. [Google Scholar] [CrossRef] [PubMed]


| Nanocomposite | Fabrication Technique | Limit of Detection | Sensitivity | Application | Ref. |
|---|---|---|---|---|---|
| MoS2 nanoflower/Ag | Chemical synthesis MoS2/Ag nanocomposite deposited on an Pt electrode | 0.06 mM and 0.0056 mM | 147.46 and 14.36 μA mM−1 cm−2 | Enzymatic glucose detection | [77] |
| PPy/Ag | Three-electrode electrochemical cell fabrication: PPy/Ag composite matrix deposited on a glassy carbon electrode | 5 μM | - | Water decontamination | [78] |
| AgNPs–Pdop@Gr | Electrochemical reaction: AgNPs integration in Graphene (Gr) coated with polydopamine (Pdop) | 4 nM for guanine and 2 nM for adenine | - | Adenine and guanine determination | [79] |
| Ag/MWCNTs/GC | AgNPs coated multi-walled carbon nanotubes (MWCNTs) dispersed on a glassy carbon electrode (GC) | 0.2 mM | - | Medical industry | [80] |
| Ag–S–Zn–O/Indium tin oxide (ITO) | Electrophoretic deposition | 0.54 mM | 12.56 μA mM−1 cm−2 | Urea detection | [81] |
| Ag/PMMA | Solution casting and sonication | 0.1 mM | 41 µA mM−1 cm−2 | Non-enzymatic glucose detection | [82] |
| TiO2-modified ZnO nanotubes | Hydrothermal method | 0.5 μM | - | Non-enzymatic glucose detection | [83] |
| rGO/Ag/cotton or polyester | Electron-beam irradiation | 9.73 nM for cotton biosensors and 3.05 nM for polyester biosensors | 0.0165 mA/cm2 for cotton biosensors and 0.0129 mA/cm2 for polyester biosensor | Adrenaline detection | [84] |
| rGO/Au | Hydrothermal reflux | 1.73 pM | - | miRNA-122 detection | [85] |
| ChOx/HRP/AuNPs/APTES/ITO | Cholesterol oxidase (ChOx) and horseradish peroxidase (HRP) immobilization on AuNPs-functionalized ITO electrode | 0.235 mg/dL | 7.5 µA mg dL−1 cm−2 | Cholesterol detection | [86] |
| ChOx/PAni–Au–Chitosan/ITO | ChOx-chitosan immobilization on AuNPs-functionalized ITO electrode | 37.89 mg/dL | 0.86 μA mg dL−1 | Cholesterol detection | [87] |
| g-C3N4/Au | Laser ablation method | 140 ppm of methanol | - | Hazardous gases detection | [88] |
| ZnO/AuNPs | Electrodeposition of ZnO/AuNPs on a glassy carbon electrode | 1.8 pM | - | DNA biosensor for Mycobacterium tuberculosis detection | [89] |
| SiSG-TYR/Fe3O4-MWCNTs/GCE | Electrochemical synthesis: Fe3O4-MWCNTs combination together with tyrosinase (TYR), and silica sol–gel (SiSG) deposited on an glassy carbon electrode (GCE) | 0.055 μM | - | Catechol and hydroquinone detection from local water | [90] |
| Cu–FeNPs/Zeolite-A/Graphene | Sol–gel spin coating | 0.058 μM | 1.97 μA μM−1 cm−2 | Dopamine detection | [91] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chicea, D.; Nicolae-Maranciuc, A. Metal Nanocomposites as Biosensors for Biological Fluids Analysis. Materials 2025, 18, 1809. https://doi.org/10.3390/ma18081809
Chicea D, Nicolae-Maranciuc A. Metal Nanocomposites as Biosensors for Biological Fluids Analysis. Materials. 2025; 18(8):1809. https://doi.org/10.3390/ma18081809
Chicago/Turabian StyleChicea, Dan, and Alexandra Nicolae-Maranciuc. 2025. "Metal Nanocomposites as Biosensors for Biological Fluids Analysis" Materials 18, no. 8: 1809. https://doi.org/10.3390/ma18081809
APA StyleChicea, D., & Nicolae-Maranciuc, A. (2025). Metal Nanocomposites as Biosensors for Biological Fluids Analysis. Materials, 18(8), 1809. https://doi.org/10.3390/ma18081809







