Novel Stable Co3O4-SnO2 Heterojunction Electrocatalysts with Low Oxygen Evolution Potential
Abstract
1. Introduction
2. Experimental Methods
2.1. Catalyst Preparation
2.2. Characterization of Catalysts
3. Results
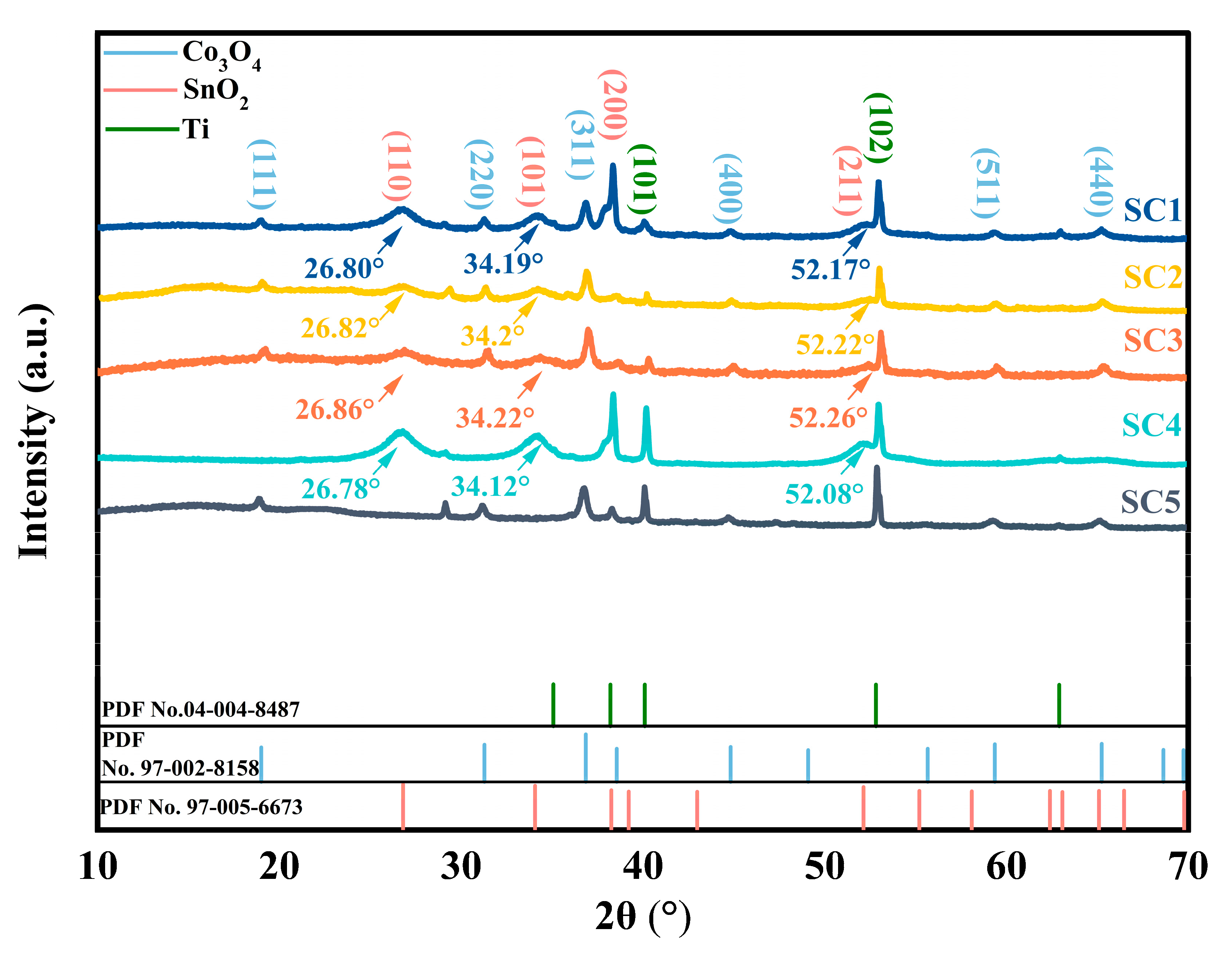
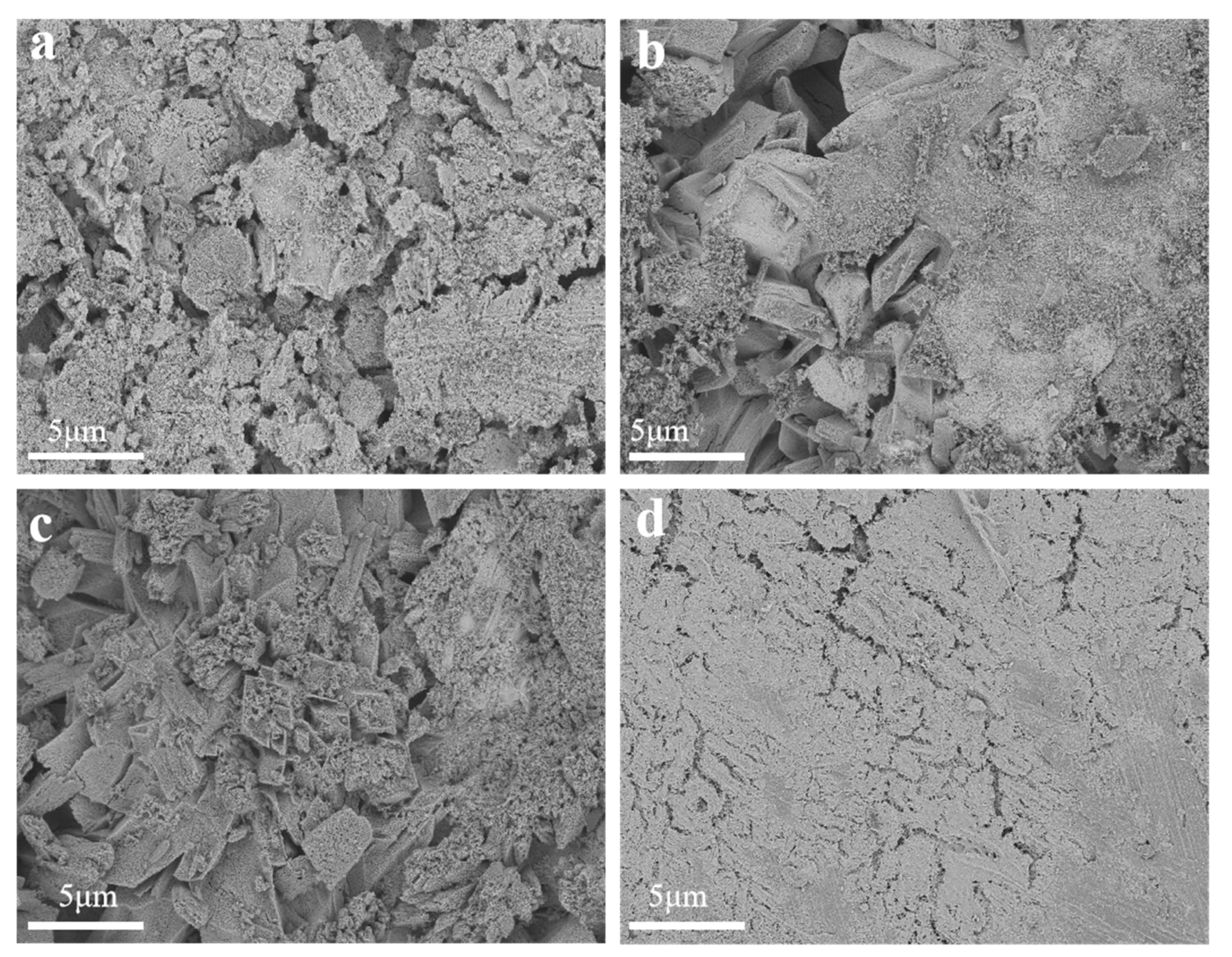
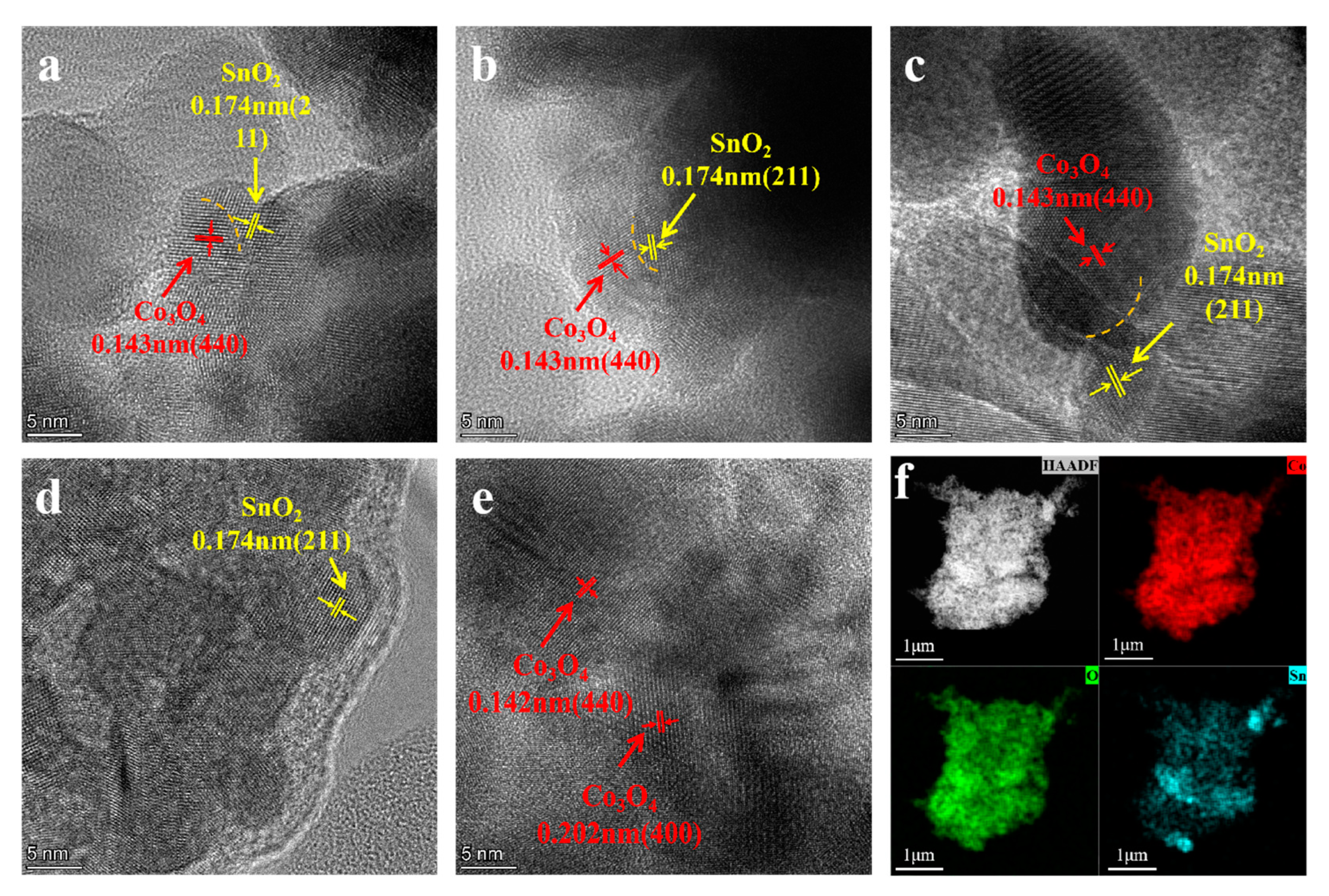
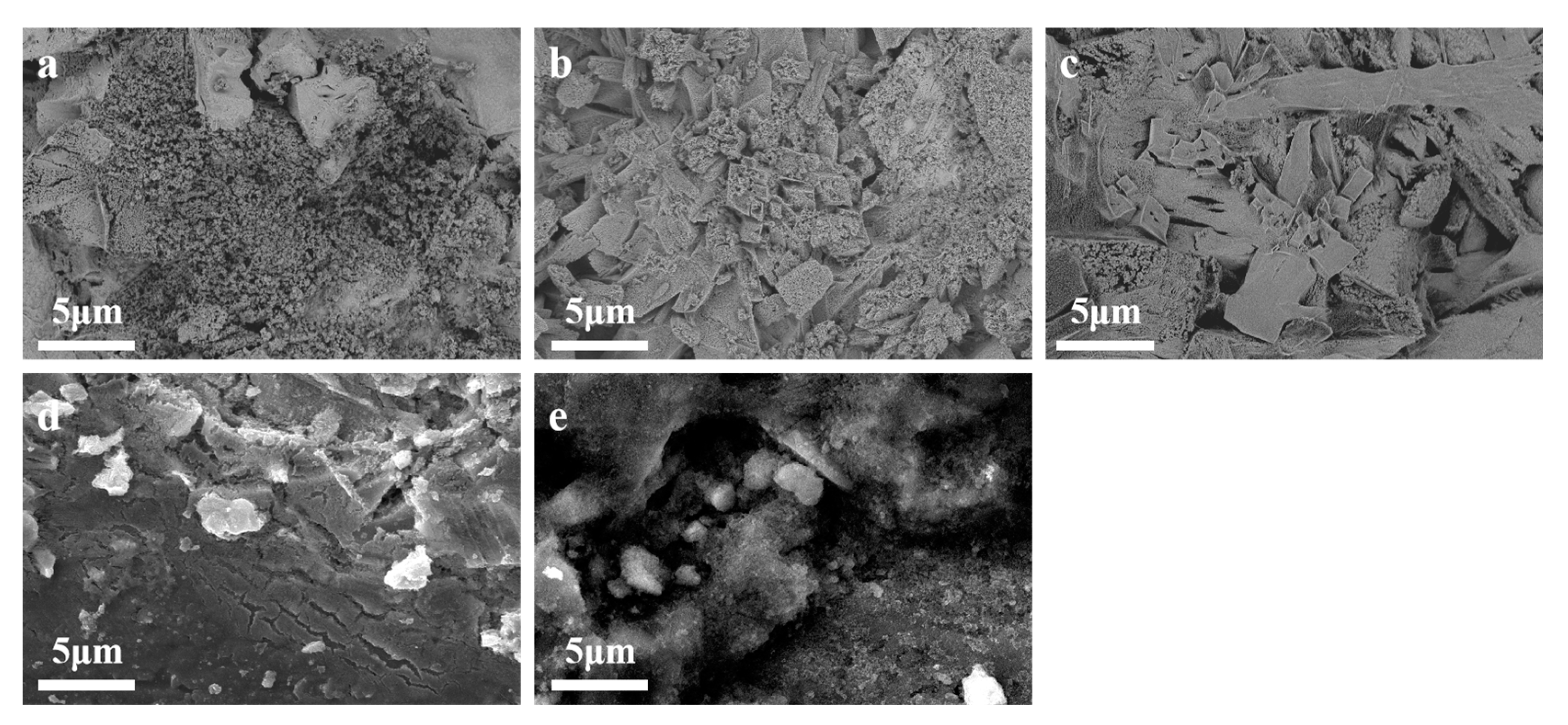
3.1. Catalyst Structural Analysis
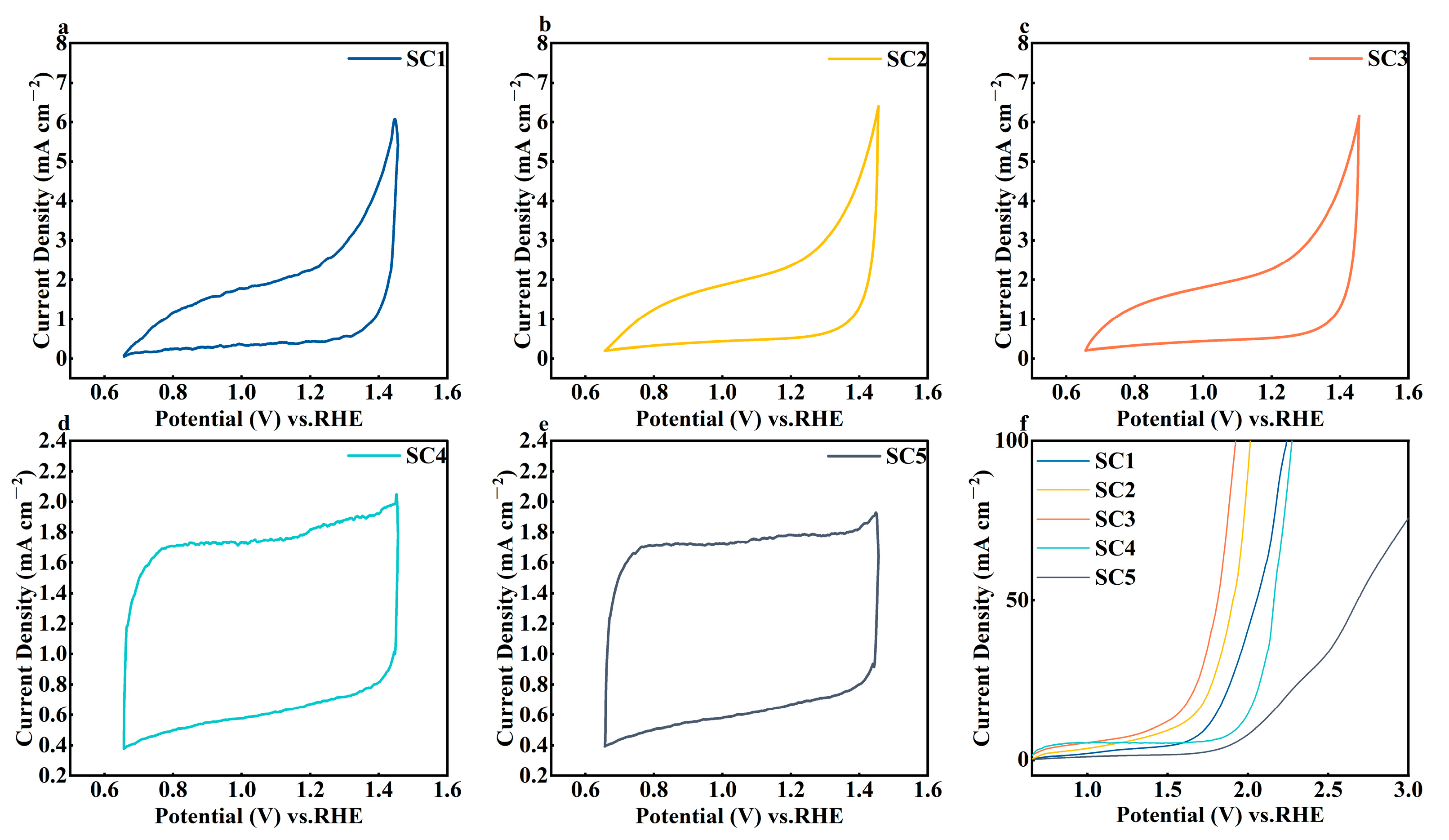
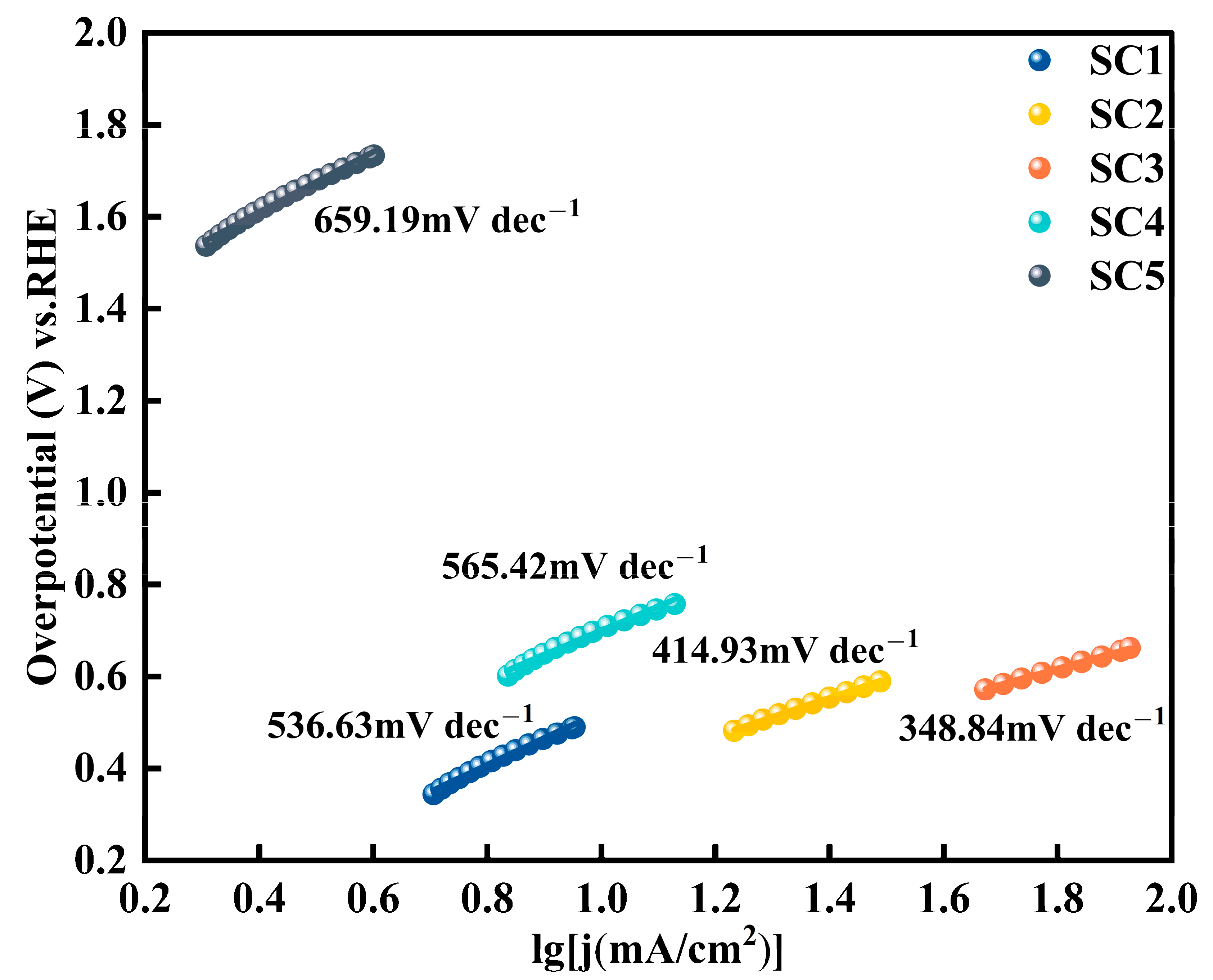
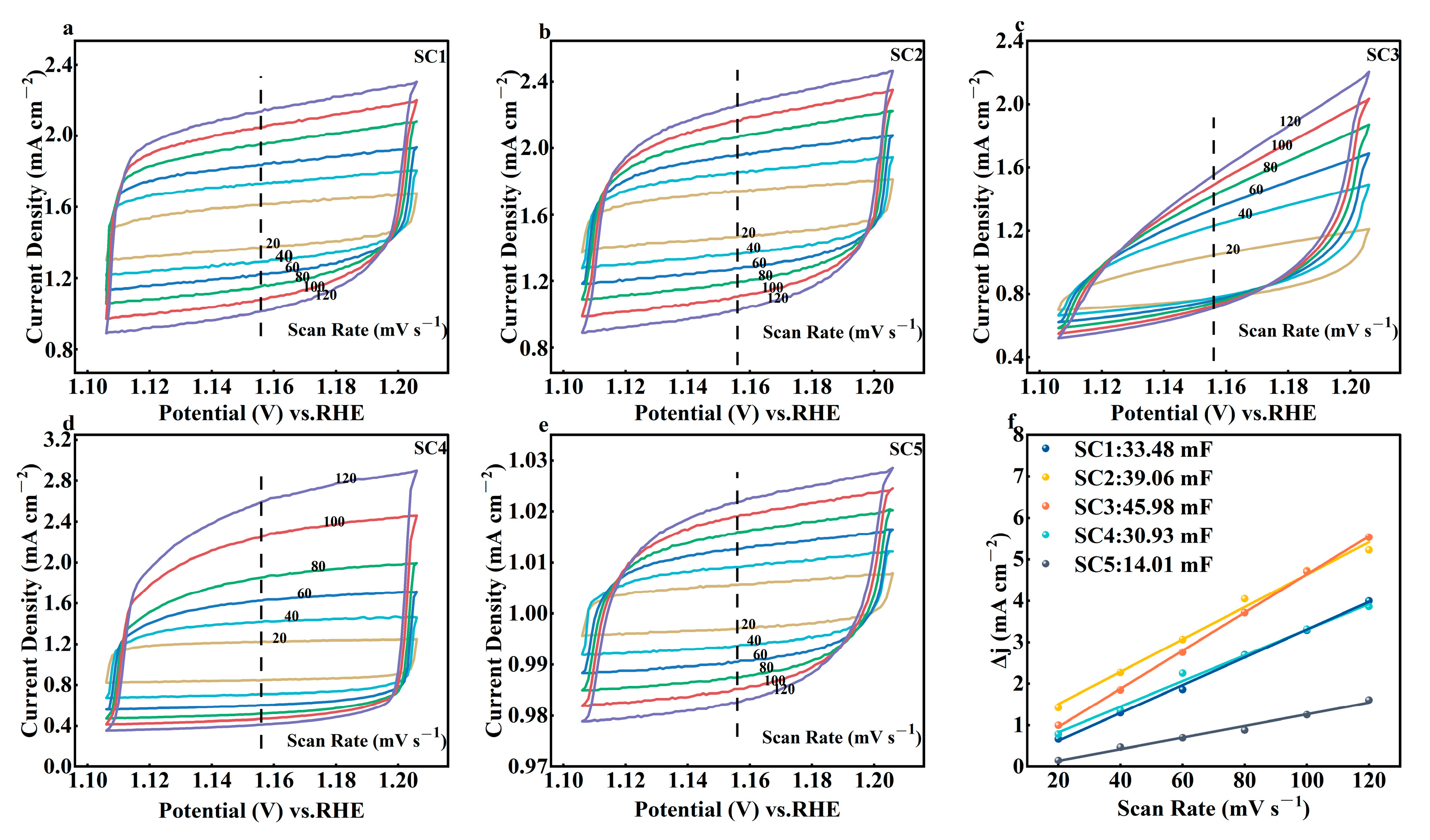
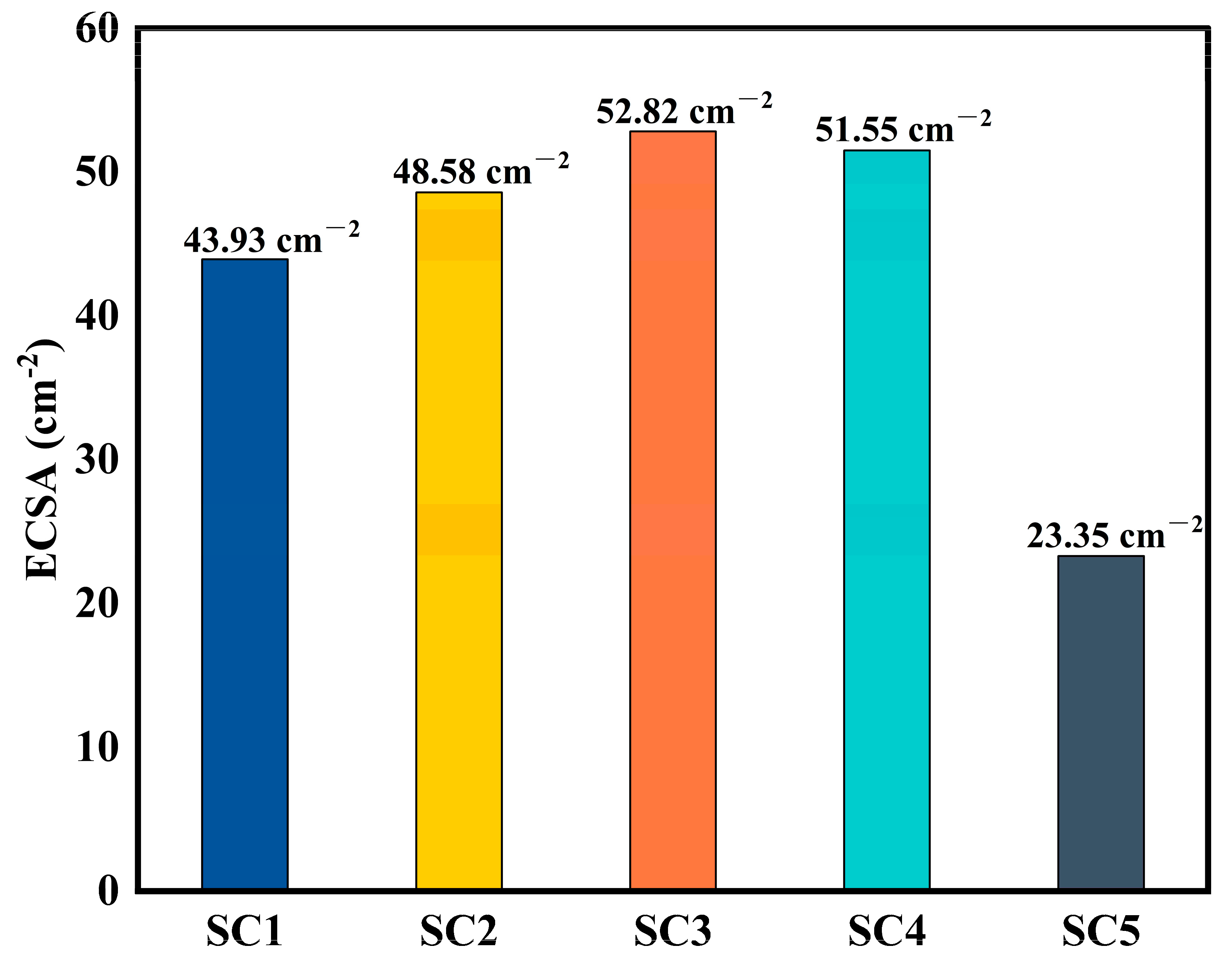
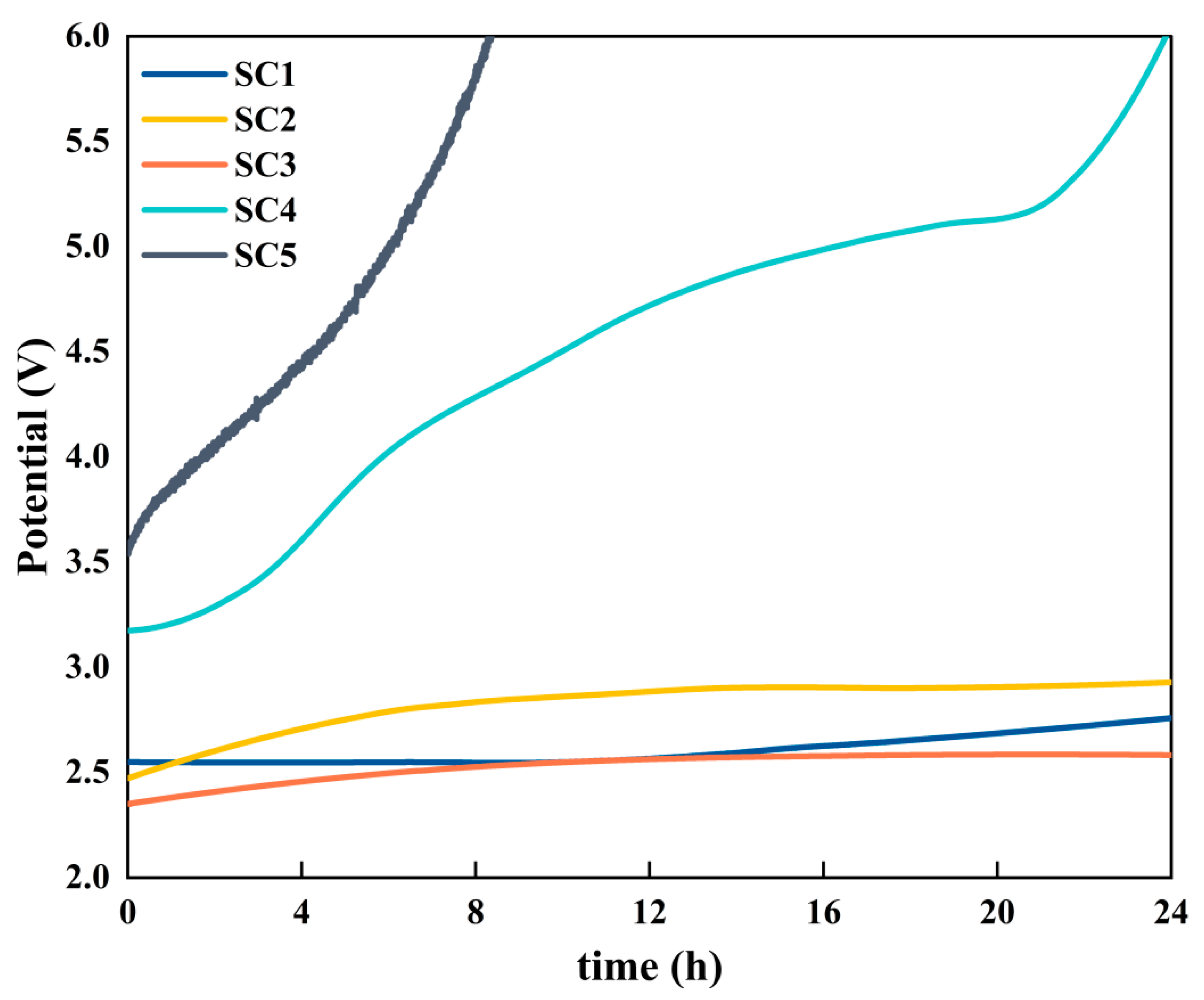
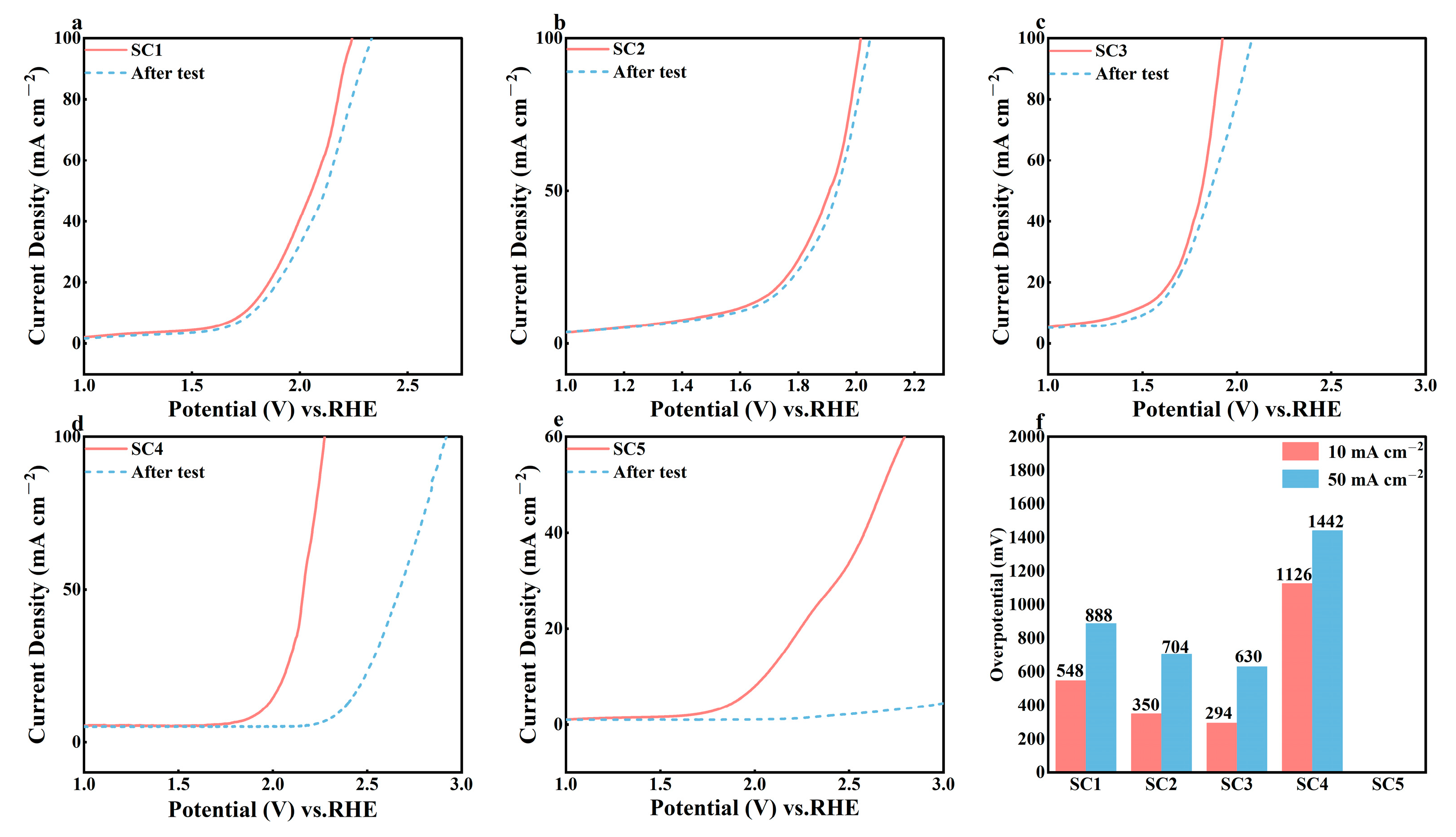
3.2. Electrochemical Performance Analysis of Catalysts
SnO(2−x)(OH) → SnO(2−x+y) + yH+ + ye−
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
List of Abbreviations
| PEM | proton exchange membrane |
| OEP | oxygen evolution potential |
| PEMWE | proton exchange membrane water electrolysis |
| OER | oxygen evolution reaction |
| XRD | X-ray diffraction |
| XPS | X-ray photoelectron spectrometer |
| FE-SEM | field emission scanning electron microscopy |
| TEM | transmission electron microscopy |
| RHE | reversible hydrogen electrode |
| LSV | linear sweep voltammetry |
| Cdl | double-layer capacitance |
| CV | cyclic voltammetry |
| HAADF | high-angle annular dark-field |
| ECSA | electrochemical surface area |
| Rct | charge transfer resistance |
| EIS | electrochemical impedance spectroscopy |
References
- Shiva Kumar, S.; Himabindu, V. Hydrogen production by PEM water electrolysis—A review. Mater. Sci. Energy Technol. 2019, 2, 442–454. [Google Scholar] [CrossRef]
- Hao, S.; Sheng, H.; Liu, M.; Huang, J.; Zheng, G.; Zhang, F.; Liu, X.; Su, Z.; Hu, J.; Qian, Y.; et al. Torsion strained iridium oxide for efficient acidic water oxidation in proton exchange membrane electrolyzers. Nat. Nanotechnol. 2021, 16, 1371–1377. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.Y.; Chen, F.Y.; Li, B.; Yu, S.-W.; Finfrock, Y.Z.; Meira, D.M.; Yan, Q.-Q.; Zhu, P.; Chen, M.-X.; Song, T.-W.; et al. Non-iridium-based electrocatalyst for durable acidic oxygen evolution reaction in proton exchange membrane water electrolysis. Nat. Mater. 2023, 22, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Huang, X.; Duan, H.; Shao, M.; Li, R.; Zhang, J.; Li, C.; Duan, X. Electrochemical synthesis in company with hydrogen production via renewable energy: Opportunities and challenges. Chin. J. Catal. 2024, 58, 1–6. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, X.; Chen, H.; Gao, R.; Shi, L.; Yang, L.; Zou, X. Iridium-containing water-oxidation catalysts in acidic electrolyte. Chin. J. Catal. 2021, 42, 1054–1077. [Google Scholar] [CrossRef]
- Chang, J.-F.; Xiao, Y.; Luo, Z.-Y.; Ge, J.-J.; Liu, C.-P.; Xing, W. Recent Progress of Non-Noble Metal Catalysts in Water Electrolysis for Hydrogen Production. Acta Phys. Chim. Sin. 2016, 32, 1556–1592. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, S.; Du, C.; Wang, Z.; Shao, Y.; Kong, F.; Lin, Y.; Yin, G. Pt/Tin Oxide/Carbon Nanocomposites as Promising Oxygen Reduction Electrocatalyst with Improved Stability and Activity. Electrochim. Acta 2014, 117, 413–419. [Google Scholar] [CrossRef]
- Ma, J.; Wang, T.; Zhao, Y.; Chang, F. Fabrication of Ti/SnO2-Sb electrodes containing RuO2 interlayer for efficient electrocatalytic oxidation of caprolactam wastewater. Int. J. Electrochem. Sci. 2024, 19, 100460. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, T.; Hu, J.; Li, J. Controlled strategy to synthesize SnO2 decorated SnS2 nanosheets with enhanced visible light photocatalytic activity. CrystEngComm 2012, 14, 5627–5633. [Google Scholar] [CrossRef]
- Pang, H.L.; Lu, J.P.; Chen, J.H.; Liu, B.; Zhang, X. Preparation of SnO2-CNTs supported Pt catalysts and their electrocatalytic properties for ethanol oxidation. Electrochim. Acta 2009, 54, 2610–2615. [Google Scholar] [CrossRef]
- Wang, G.; Takeguchi, T.; Zhang, Y.; Muhamad, E.N.; Sadakane, M.; Ye, S.; Ueda, W. Effect of SnO2 Deposition Sequence in SnO2-Modified PtRu/C Catalyst Preparation on Catalytic Activity for Methanol Electro-Oxidation. J. Electrochem. Soc. 2009, 156, B862–B869. [Google Scholar] [CrossRef]
- Chen, A.; Bin Li, B.; Miljkovic, B.; Souza, C.; Zhu, K.; Ruda, H.E. Improving the oxidation potential of Sb-doped SnO2 electrode by Zn/Sb co-doping. Appl. Phys. Lett. 2014, 105, 021606. [Google Scholar] [CrossRef]
- Wu, W.Z.; Wang, L.D.; Chen, X.; Jin, J.; Yang, Z.; Sun, W.; Qi, H.; Yu, J.; Liu, G. Preparation of Ti/SnO2-Sb-Ir-Mn Electrodes with Low Iridium Content for a Highly Efficient and Stable Oxygen Evolution Reaction. Ind. Eng. Chem. Res. 2024, 63, 4317–4328. [Google Scholar] [CrossRef]
- Saira, Y.; Li, Z.J.; Zhu, Y.; Liu, Q.; Luo, W.; Wang, Y.; Gong, M.; Fu, G.; Tang, Y. Low-loaded Ru on hollow SnO2 for enhanced electrocatalytic hydrogen evolution. Chem. Commun. 2024, 60, 2768–2771. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, F.; Huang, J.; Luo, W.; Yu, J.; Fang, X.; Lebedeva, O.E.; Wang, X. The Influence of RuO2 Distribution and Dispersion on the Reactivity of RuO2−SnO2 Composite Oxide Catalysts Probed by CO Oxidation. ChemCatChem 2019, 11, 2473–2483. [Google Scholar] [CrossRef]
- Joshi, N.C.; Gururani, P.; Kumar, N. Electrochemical performance of SnO2 after blending with Cu. Ionics 2024, 30, 6531–6547. [Google Scholar] [CrossRef]
- Ren, S.; Guo, Y.; Ma, S.; Mao, Q.; Wu, D.; Yang, Y.; Jing, H.; Song, X.; Hao, C. Co3O4 nanoparticles assembled on polypyrrole/graphene oxide for electrochemical reduction of oxygen in alkaline media. Chin. J. Catal. 2017, 38, 1281–1290. [Google Scholar] [CrossRef]
- Song, G.; Wang, Z.; Sun, J.; Yuan, D.; Zhang, L. ZnCo2S4 nanosheet array anchored on nickel foam as electrocatalyst for electrochemical water splitting. Electrochem. Commun. 2019, 105, 106487. [Google Scholar] [CrossRef]
- Pei, Y.; He, W.; Wang, M.; Wang, J.; Sun, T.; Hu, L.; Zhu, J.; Tan, Y.; Wang, J.-C. RuCo alloy trifunctional electrocatalysts with ratio-dependent activity for Zn-air batteries and self-powered water splitting. Chem. Commun. 2021, 57, 1498–1501. [Google Scholar] [CrossRef]
- Li, Y.; Li, F.M.; Meng, X.Y.; Li, S.-N.; Zeng, J.-H.; Chen, Y. Ultrathin Co3O4 Nanomeshes for the Oxygen Evolution Reaction. Acs Catal. 2018, 8, 1913–1920. [Google Scholar] [CrossRef]
- Wu, F.Y.; Tian, F.Y.; Li, M.G.; Geng, S.; Qiu, L.; He, L.; Li, L.; Chen, Z.; Yu, Y.; Yang, W.; et al. Engineering Lattice Oxygen Regeneration of NiFe Layered Double Hydroxide Enhances Oxygen Evolution Catalysis Durability. Angew. Chem. Int. Ed. 2025, 64, e202413250. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wang, Z.; Shi, Y.; Li, Z.; Ding, F.; Ren, Y.; Li, F.; Bian, H.; Wang, C.; Yang, Y.; et al. Yurong Yang Constructing a medium-entropy spinel oxide FeNiMnO4/CeO2 heterojunction as a high-performance electrocatalyst for the oxygen evolution reaction. Inorg. Chem. Front. 2024, 11, 3786–3798. [Google Scholar] [CrossRef]
- Liu, S.; Dun, C.; Jiang, Q.K.; Xuan, Z.; Yang, F.; Guo, J.; Urban, J.J.; Swihart, M.T. Challenging thermodynamics: Combining immiscible elements in a single-phase nano-ceramic. Nat. Commun. 2024, 15, 1167. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.; Chen, D.; Zhang, Y.X.; Wang, W.; Quan, Q.; Wang, W.; Meng, Y.; Lai, Z.; Yang, Z.; Yip, S.; et al. Synergistic Active Phases of Transition Metal Oxide Heterostructures for Highly Efficient Ammonia Electrosynthesis. Adv. Funct. Mater. 2023, 33, 2303803. [Google Scholar] [CrossRef]
- Wang, H.-Z.; Shao, Y.-X.; Feng, Y.-F.; Tan, Y.-J.; Liao, Q.-Y.; Chen, X.-D.; Zhang, X.-F.; Guo, Z.-H.; Li, H. Heterostructured Co3O4–SnO2 composites containing oxygen vacancy with high activity and recyclability toward NH3BH3 dehydrogenation. Rare Met. 2023, 42, 3013–3023. [Google Scholar] [CrossRef]
- Liu, Y.; Mou, G.; Yu, S.; Luo, H.; Zhong, M.; Dongc, N.; Su, B. Investigation of the Sn4+-distribution and photocatalytic performance of Sn4+/TiO2 hollow fiber nanomaterials. New J. Chem. 2022, 46, 3565–3569. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, S. Construction of SnO2/Co3O4 n-p heterojunctions by organometallic chemistry-assisted approach. Mater. Lett. 2021, 285, 129108. [Google Scholar] [CrossRef]
- Wang, Z.H.; Long, Y.; Cao, D.; Han, D.; Gu, F. A high-performance flexible supercapacitor based on hierarchical Co3O4-SnO@SnO2 nanostructures. Electrochim. Acta 2019, 307, 341–350. [Google Scholar] [CrossRef]
- Ma, J.; Gao, X.; Li, J.; Li, H. Promoting Effect of Tin on Binder-Free CoSnx-B/Ni-foam Catalysts for Fuel Conversion Efficiency in Direct Borohydride Fuel Cell. Fuel Cells 2019, 19, 609–615. [Google Scholar] [CrossRef]
- Li, Y.L.; Wang, S.C.; Wu, J.K.; Ma, J.; Cui, L.; Lu, H.; Sheng, Z. One-step hydrothermal synthesis of hybrid core-shell Co3O4@SnO2-SnO for supercapacitor electrodes. Ceram. Int. 2020, 46, 15793–15800. [Google Scholar] [CrossRef]
- Kreider, M.E.; Maldonado Santos, A.R.; Clauser, A.L.; Sweers, M.E.; Hu, L.; Volk, E.K.; Chan, A.-L.; Sugar, J.D.; Alia, S.M. Porous Transport Layers for Anion Exchange Membrane Water Electrolysis: The Impact of Morphology and Composition; ACS Electrochemistry: Washington, DC, USA, 2025. [Google Scholar]
- Correa-Lozano, B.; Comninellis, C.; Battisti, A.D. Service life of Ti/SnO2–Sb2O5 anodes. J. Appl. Electrochem. 1997, 27, 970–974. [Google Scholar] [CrossRef]
- Naresh, B.; Sreekanth, T.V.M.; Suma, C.N.; Kumar, K.S.; Yoo, K.; Kim, J. Hydrothermally synthesized NiO-SnO2 nanocomposite as an efficient electrocatalyst for oxygen evolution reaction (OER) and urea oxidation reaction (UOR). J. Alloys Compd. 2025, 1010, 177865. [Google Scholar] [CrossRef]
- Sreekanth, T.V.M.; Prasad, K.; Yoo, J.; Kim, J.; Yoo, K. CuO-SnO2 nanocomposites: Efficient and cost-effective electrocatalysts for urea oxidation. Mater. Lett. 2023, 353, 135243. [Google Scholar] [CrossRef]
- Li, H.Y.; Xu, Y.X.; Lv, N.; Zhang, Q.; Zhang, X.; Wei, Z.; Wang, Y.; Tang, H.; Pan, H. Ti-Doped SnO2 Supports IrO2 Electrocatalysts for the Oxygen Evolution Reaction (OER) in PEM Water Electrolysis. Acs Sustain. Chem. Eng. 2023, 11, 1121–1132. [Google Scholar] [CrossRef]




| Electrode Name | Cobalt–Tin Molar Ratio | Cobalt Loading per Monolith (mg cm−2) | Tin Loading per Monolith (mg cm−2) |
|---|---|---|---|
| SC1 | 1:5 | 2.7 | 10 |
| SC2 | 1:4 | 3.4 | 10 |
| SC3 | 1:3 | 4.5 | 10 |
| SC4 | No cobalt | 0 | 10 |
| SC5 | No tin | 10 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, B.; Liu, W.; Sun, Y.; Gao, M.; Chen, A.; Zhang, J. Novel Stable Co3O4-SnO2 Heterojunction Electrocatalysts with Low Oxygen Evolution Potential. Materials 2025, 18, 1869. https://doi.org/10.3390/ma18081869
Yan B, Liu W, Sun Y, Gao M, Chen A, Zhang J. Novel Stable Co3O4-SnO2 Heterojunction Electrocatalysts with Low Oxygen Evolution Potential. Materials. 2025; 18(8):1869. https://doi.org/10.3390/ma18081869
Chicago/Turabian StyleYan, Bingfeng, Wen Liu, Youchen Sun, Meng Gao, Aqing Chen, and Jun Zhang. 2025. "Novel Stable Co3O4-SnO2 Heterojunction Electrocatalysts with Low Oxygen Evolution Potential" Materials 18, no. 8: 1869. https://doi.org/10.3390/ma18081869
APA StyleYan, B., Liu, W., Sun, Y., Gao, M., Chen, A., & Zhang, J. (2025). Novel Stable Co3O4-SnO2 Heterojunction Electrocatalysts with Low Oxygen Evolution Potential. Materials, 18(8), 1869. https://doi.org/10.3390/ma18081869





