Regulation Mechanism of Different Metal Cations on the Structure and Gel Properties of Montmorillonite
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Characterization
2.2.1. XRD
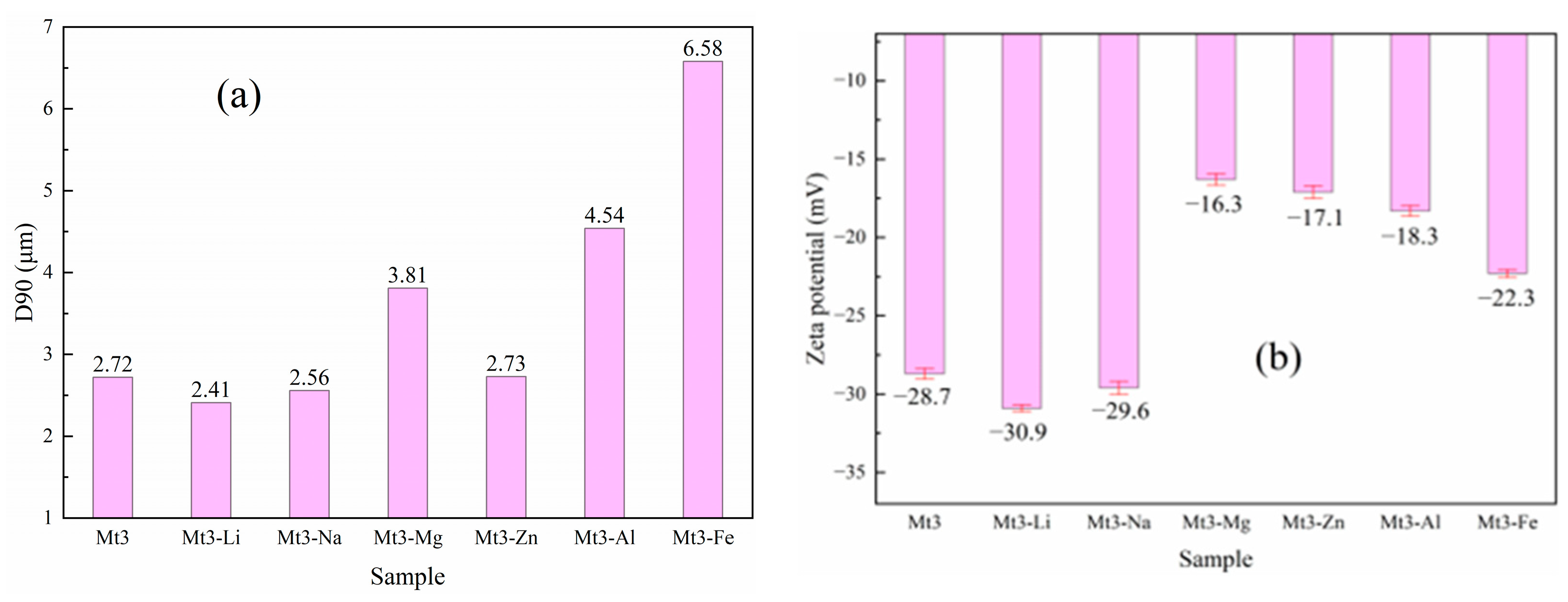
2.2.2. Particle Size Distribution (PSD)
2.2.3. Zeta Potential
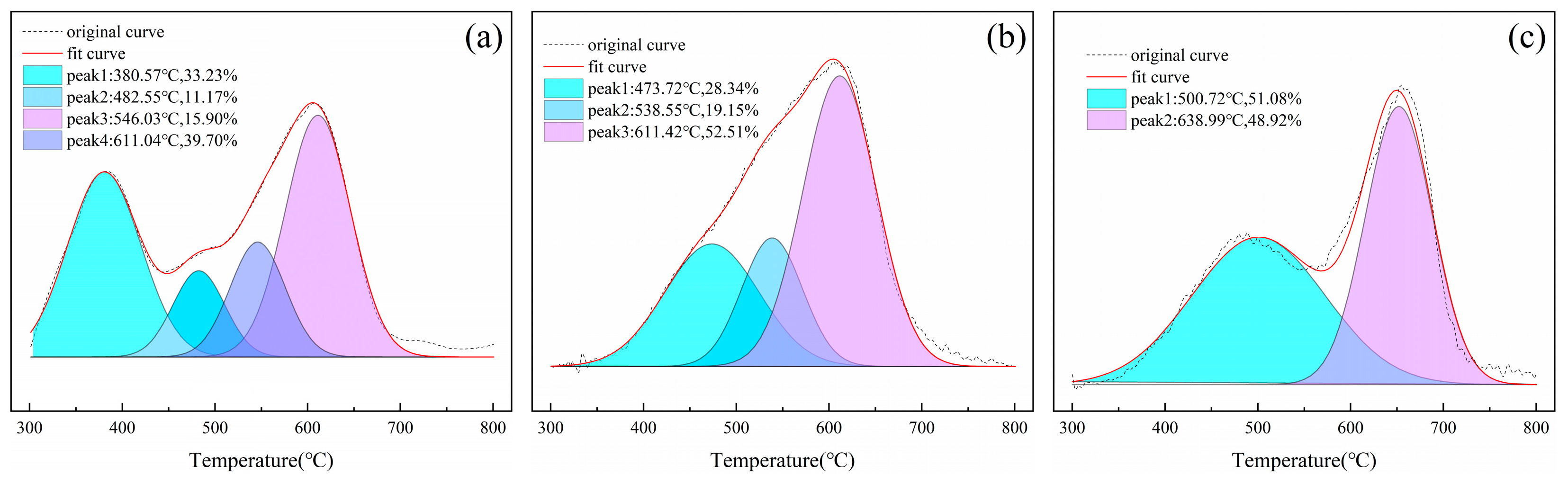
2.2.4. Thermogravimetric Analysis
2.2.5. ICP
2.2.6. 27Al Magic-Angle Spinning NMR
2.2.7. TEM
2.2.8. Viscosity
2.2.9. Swelling Capacity
3. Results
3.1. Basic Structure of Montmorillonite and Its Gel Properties
3.2. Structural Regulation of Montmorillonite by Different Metal Cations
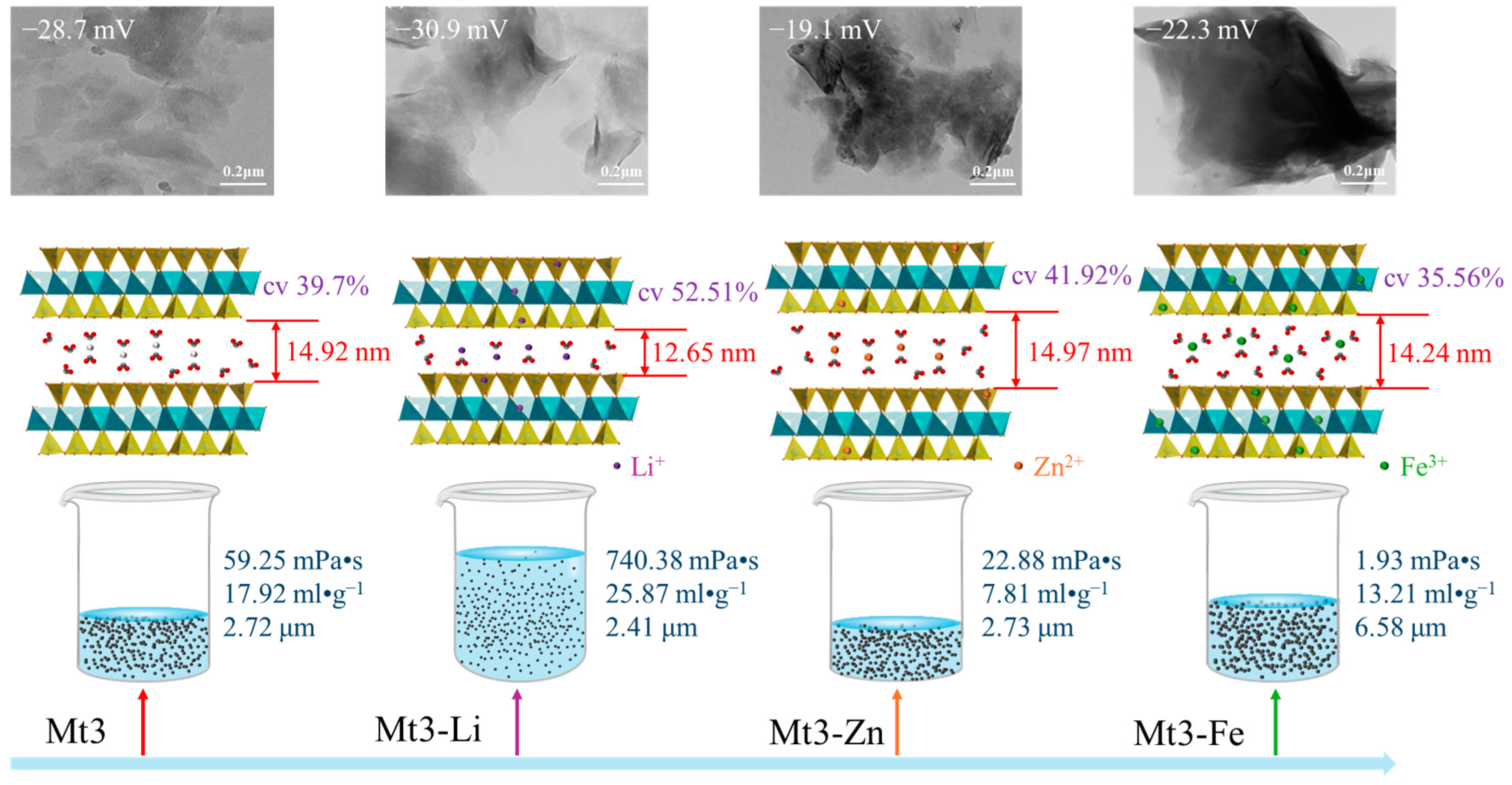
3.2.1. The Effect of Different Metal Cations Regulation on the Interlayer Structure of Montmorillonite
3.2.2. Effect of Different Metal Cations Regulation on the Central Ion of Montmorillonite Crystal Structure
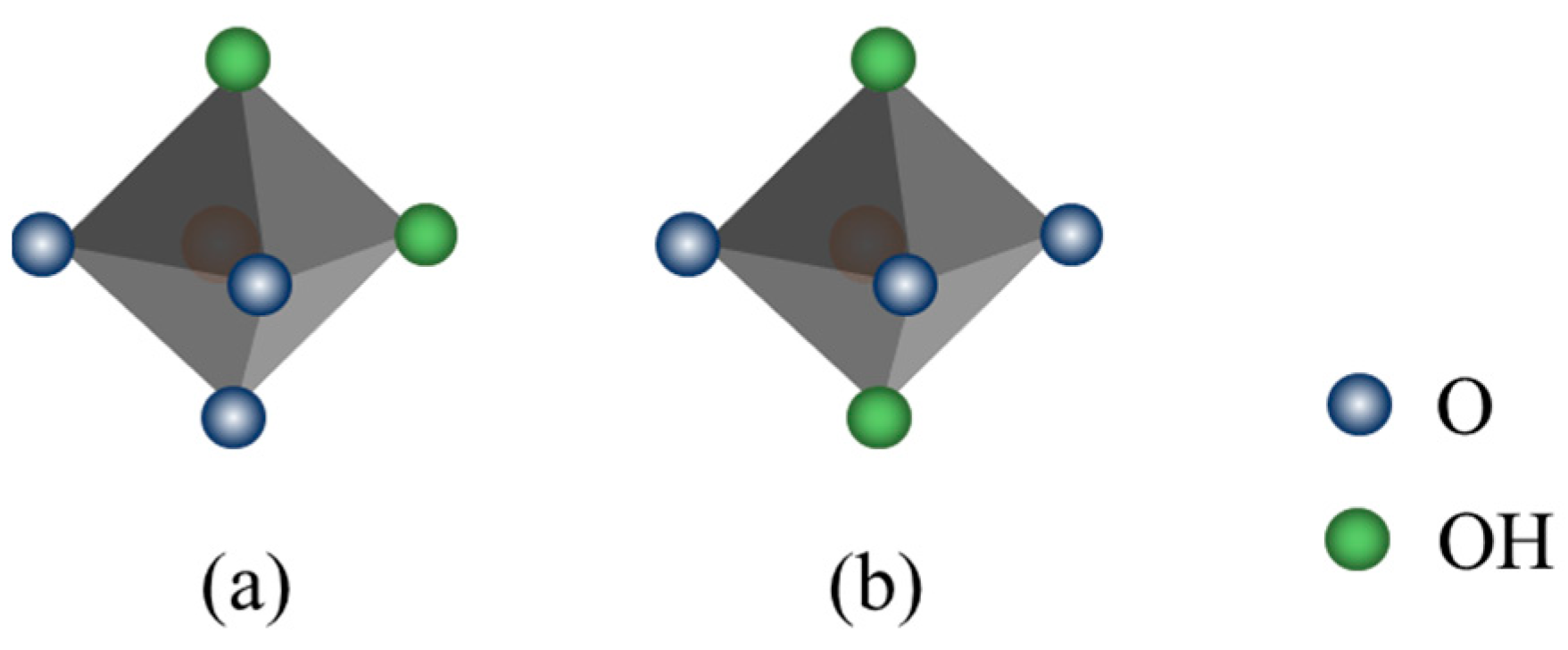
3.2.3. The Influence of Different Metal Cations Regulation on the Octahedral Configuration of Montmorillonite
3.3. Effects of Different Metal Cations Regulation on the Physical and Chemical Properties of Montmorillonite and Its Gel Properties
3.3.1. Physical and Chemical Properties of Montmorillonite with Different Metal Cations Regulation
3.3.2. Gel Properties of Montmorillonite with Different Metal Cations Regulation
3.4. Regulation Mechanism of Different Metal Cations on the Gel Properties of Montmorillonite
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yan, H.; Zhang, Z. Effect and Mechanism of Cation Species on the Gel Properties of Montmorillonite. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 611, 125824. [Google Scholar] [CrossRef]
- Faisal, H.M.N.; Katti, K.S.; Katti, D.R. Molecular Mechanics of the Swelling Clay Tactoid under Compression, Tension and Shear. Appl. Clay Sci. 2021, 200, 105908. [Google Scholar] [CrossRef]
- Harris, A.; Nosrati, A.; Addai-Mensah, J. The Influence of Pulp and Interfacial Chemistry and Mode of Electrical Power Input on Electroosmotic Dewatering of Na-Exchanged Smectite Dispersions. Appl. Clay Sci. 2018, 162, 214–222. [Google Scholar] [CrossRef]
- Luo, Q.; Liu, W.; Zhuo, Q.; Han, Y. Interaction Mechanism of Organic Carboxylate with Kaolinite and Montmorillonite: A Density Functional Theory Study. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 613, 126047. [Google Scholar] [CrossRef]
- Zheng, R.; Liu, D.; Tang, J.; Song, Q.; Yao, Q. Analysis of Montmorillonite Affecting Coke Formation during the Thermal Conversion of Heavy Oil. Fuel 2021, 288, 119687. [Google Scholar] [CrossRef]
- Morris, G.E.; Zbik, M.S. Smectite Suspension Structural Behaviour. Int. J. Miner. Process. 2009, 93, 20–25. [Google Scholar] [CrossRef]
- Qiu, J.; Li, G.; Liu, D.; Jiang, S.; Wang, G.; Chen, P.; Zhu, X.; Yao, G.; Liu, X.; Lyu, X. Effect of Layer Charge Density on Hydration Properties of Montmorillonite: Molecular Dynamics Simulation and Experimental Study. Int. J. Mol. Sci. 2019, 20, 3997. [Google Scholar] [CrossRef]
- Sun, H.; Peng, T.; Chen, Y. Influence of Interlayer Cations on Structure and Hydro-Expansive Property of Montmorillonite. Non-Met. Mines 2011, 34, 2010–2012. [Google Scholar]
- Wang, X.; Li, Y.; Wang, H. Structural Characterization of Octahedral Sheet in Dioctahedral Smectites by Thermal Analysis. Minerals 2020, 10, 347. [Google Scholar] [CrossRef]
- Delavernhe, L.; Pilavtepe, M.; Emmerich, K. Cation Exchange Capacity of Natural and Synthetic Hectorite. Appl. Clay Sci. 2018, 151, 175–180. [Google Scholar] [CrossRef]
- Garcia-Romero, E.; Lorenzo, A.; Garcia-Vicente, A.; Morales, J.; Garcia-Rivas, J.; Suarez, M. On the Structural Formula of Smectites: A Review and New Data on the Influence of Exchangeable Cations. J. Appl. Crystallogr. 2021, 54, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Jiang, S.; Wang, Y.; Chen, G.; Liu, D.; Liu, X.; Wang, G.; Wu, P.; Lyu, X. Crystal Chemistry Characteristics and Dispersion Performance of Ca-Montmorillonite with Different Layer Charge Density. Mater. Res. Express 2020, 7, aba803. [Google Scholar] [CrossRef]
- Palchik, N.A.; Razvorotneva, L.I.; Moroz, T.N.; Miroshnichenko, L.V. Crystal-Chemical Features and Sorption Properties of Natural and Synthetic Smectites. Russ. J. Inorg. Chem. 2019, 64, 308–316. [Google Scholar] [CrossRef]
- Xiao, F.; Yan, B.Q.; Zou, X.Y.; Cao, X.Q.; Dong, L.; Lyu, X.J.; Li, L.; Qiu, J.; Chen, P.; Hu, S.G.; et al. Study on Ionic Liquid Modified Montmorillonite and Molecular Dynamics Simulation. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 587, 124311. [Google Scholar] [CrossRef]
- Bailey, L.; Lekkerkerker, H.N.W.; Maitland, G.C. Rheology Modification of Montmorillonite Dispersions by Colloidal Silica. Rheol. Acta 2014, 53, 373–384. [Google Scholar] [CrossRef]
- Liu, X.; Yang, S.; Gu, P.; Liu, S.; Yang, G. Adsorption and Removal of Metal Ions by Smectites Nanoparticles: Mechanistic Aspects, and Impacts of Charge Location and Edge Structure. Appl. Clay Sci. 2021, 201, 105957. [Google Scholar] [CrossRef]
- Ohkubo, T.; Yamazaki, A.; Fukatsu, Y.; Tachi, Y. Pore Distribution of Compacted Ca-Montmorillonite Using NMR Relaxometry and Cryoporometry: Comparison with Na-Montmorillonite. Microporous Mesoporous Mater. 2021, 313, 110841. [Google Scholar] [CrossRef]
- Chen, T.; Yuan, Y.; Zhao, Y.; Rao, F.; Song, S. Effect of Layer Charges on Exfoliation of Montmorillonite in Aqueous Solutions. Colloids Surfaces A Physicochem. Eng. Asp. 2018, 548, 92–97. [Google Scholar] [CrossRef]
- Mészáros, R.; Jobbik, A.; Varga, G.; Bárány, S. Electrosurface Properties of Na-Bentonite Particles in Electrolytes and Surfactants Solution. Appl. Clay Sci. 2019, 178, 105127. [Google Scholar] [CrossRef]
- Drits, V.A.; McCarty, D.K.; Zviagina, B.B. Crystal-Chemical Factors Responsible for the Distribution of Octahedral Cations over Trans- and Cis-Sites in Dioctahedral 2:1 Layer Silicates. Clays Clay Miner. 2006, 54, 131–152. [Google Scholar] [CrossRef]
- Lavikainen, L.P.; Hirvi, J.T.; Kasa, S.; Pakkanen, T.A. Interaction of Octahedral Mg(II) and Tetrahedral Al(III) Substitutions in Aluminium-Rich Dioctahedral Smectites. Theor. Chem. Acc. 2016, 135, 2–7. [Google Scholar] [CrossRef]
- Chen, L.C.; Zhao, Y.; Chen, T.; Bai, H.; Zhang, T.; Li, H.; An, Q.; Song, S. Correlation of Aspect Ratio of Montmorillonite Nanosheets with the Colloidal Properties in Aqueous Solutions. Results Phys. 2019, 15, 102526. [Google Scholar] [CrossRef]
- Valera-Zaragoza, M.; Agüero-Valdez, D.; Lopez-Medina, M.; Dehesa-Blas, S.; Karin Navarro-Mtz, A.; Avalos-Borja, M.; Juarez-Arellano, E.A. Controlled Modification of Sodium Montmorillonite Clay by a Planetary Ball-Mill as a Versatile Tool to Tune Its Properties. Adv. Powder Technol. 2021, 32, 591–599. [Google Scholar] [CrossRef]
- Doi, A.; Ejtemaei, M.; Nguyen, A.V. Effects of Ion Specificity on the Surface Electrical Properties of Kaolinite and Montmorillonite. Miner. Eng. 2019, 143, 105929. [Google Scholar] [CrossRef]
- Whittaker, M.L.; Comolli, L.R.; Gilbert, B.; Banfield, J.F. Layer Size Polydispersity in Hydrated Montmorillonite Creates Multiscale Porosity Networks. Appl. Clay Sci. 2020, 190, 105548. [Google Scholar] [CrossRef]
- Li, Z.Y.; Xu, R.K.; Li, J.Y.; Hong, Z.N. Effect of Clay Colloids on the Zeta Potential of Fe/Al Oxide-Coated Quartz: A Streaming Potential Study. J. Soils Sediments 2016, 16, 2676–2686. [Google Scholar] [CrossRef]
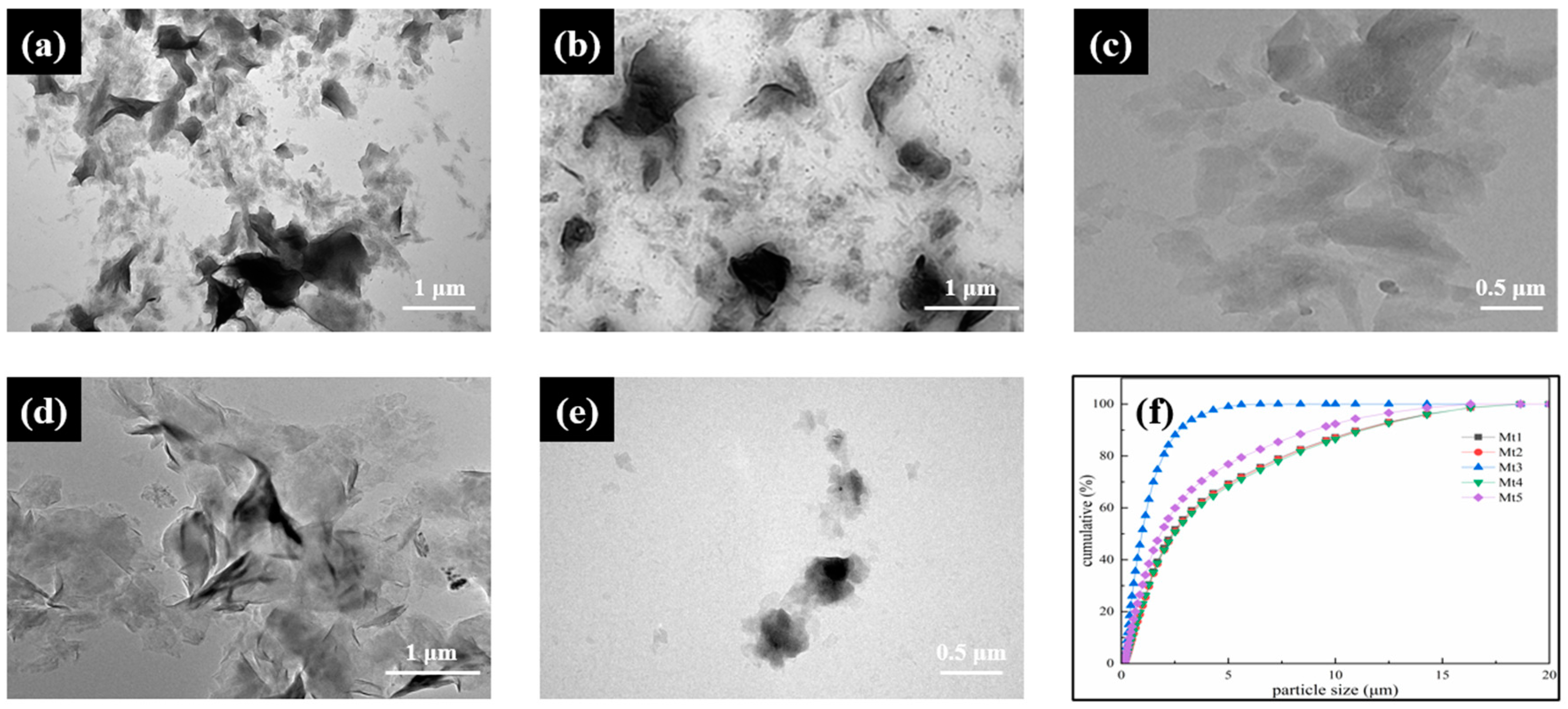
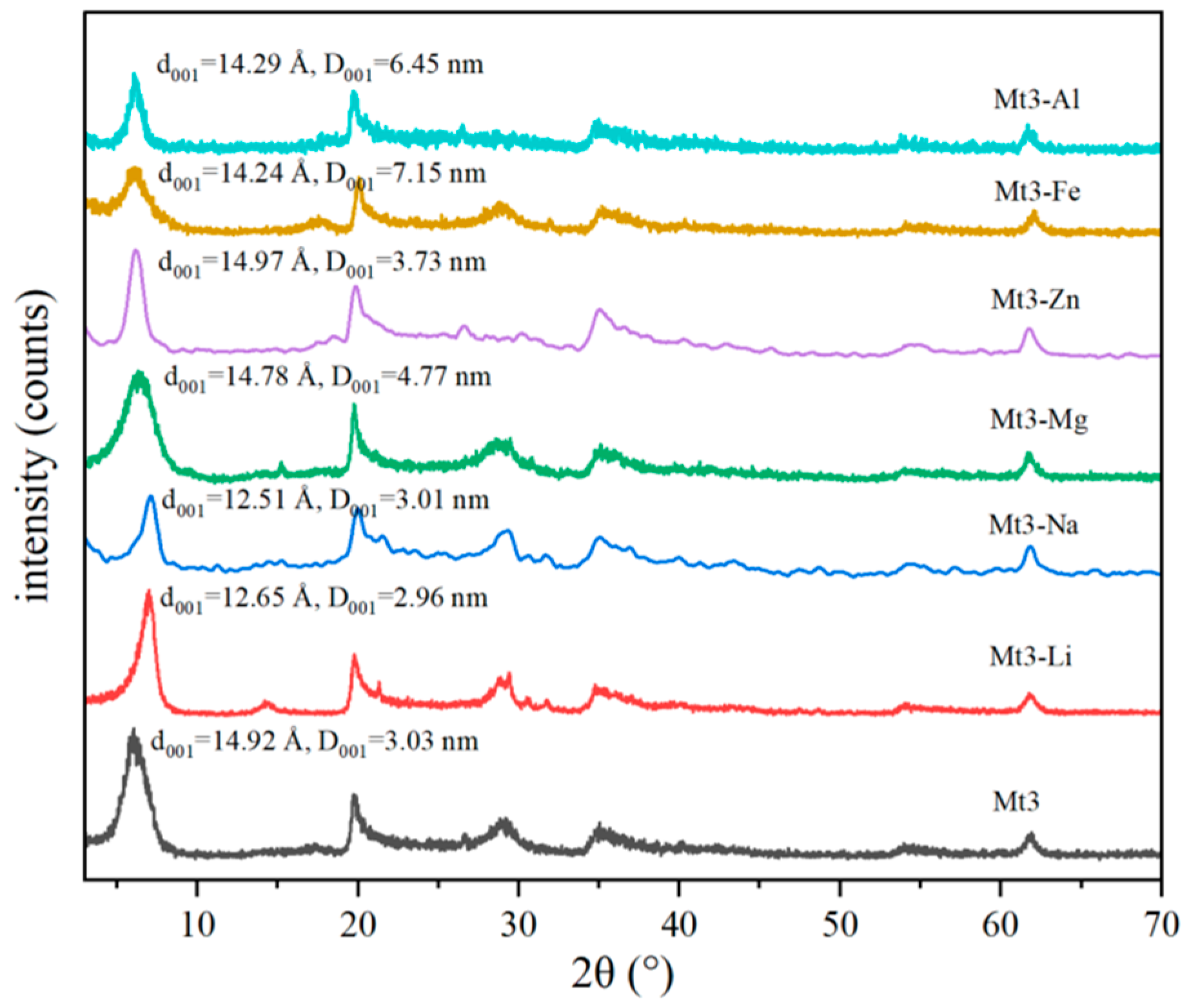
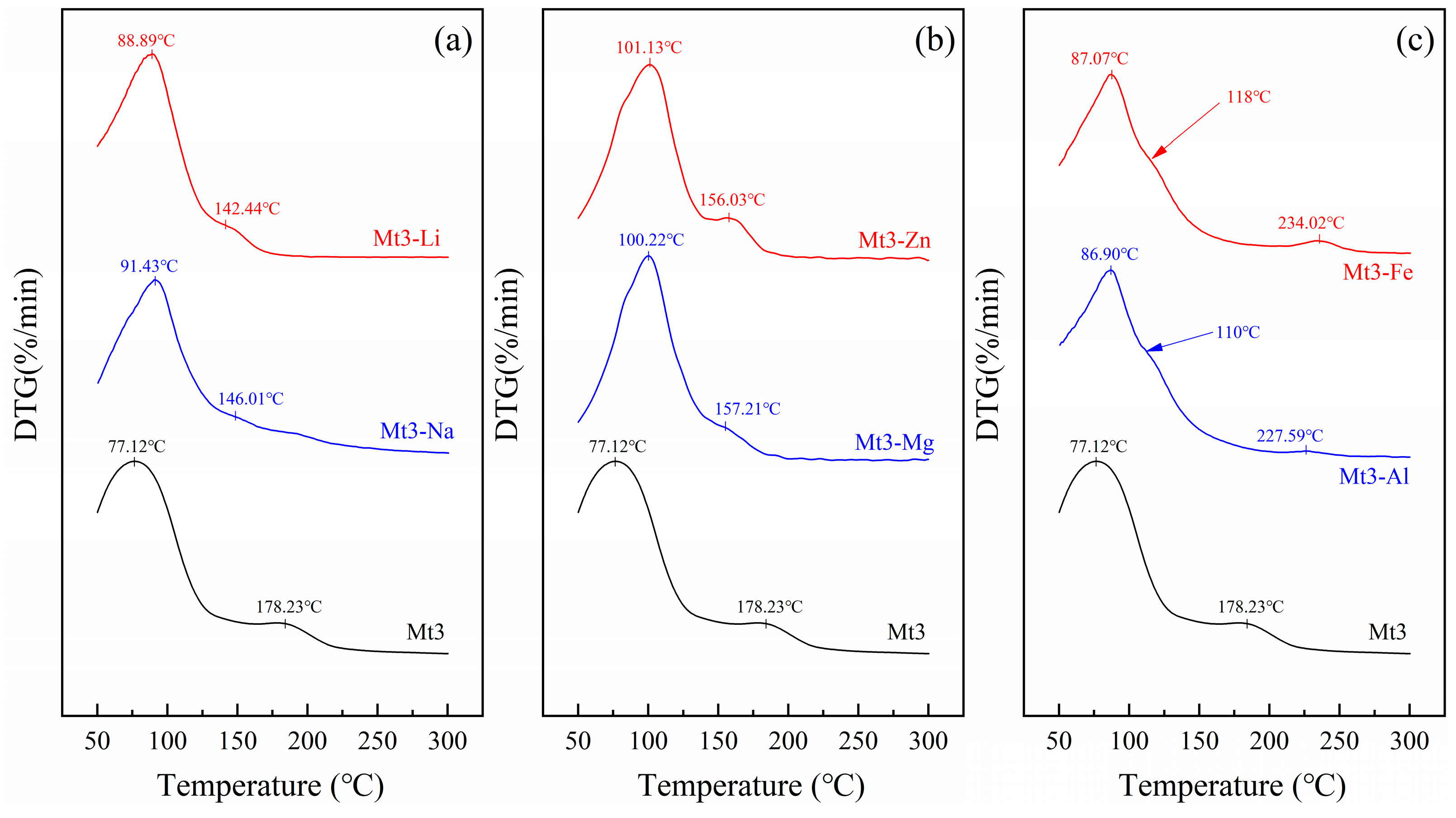
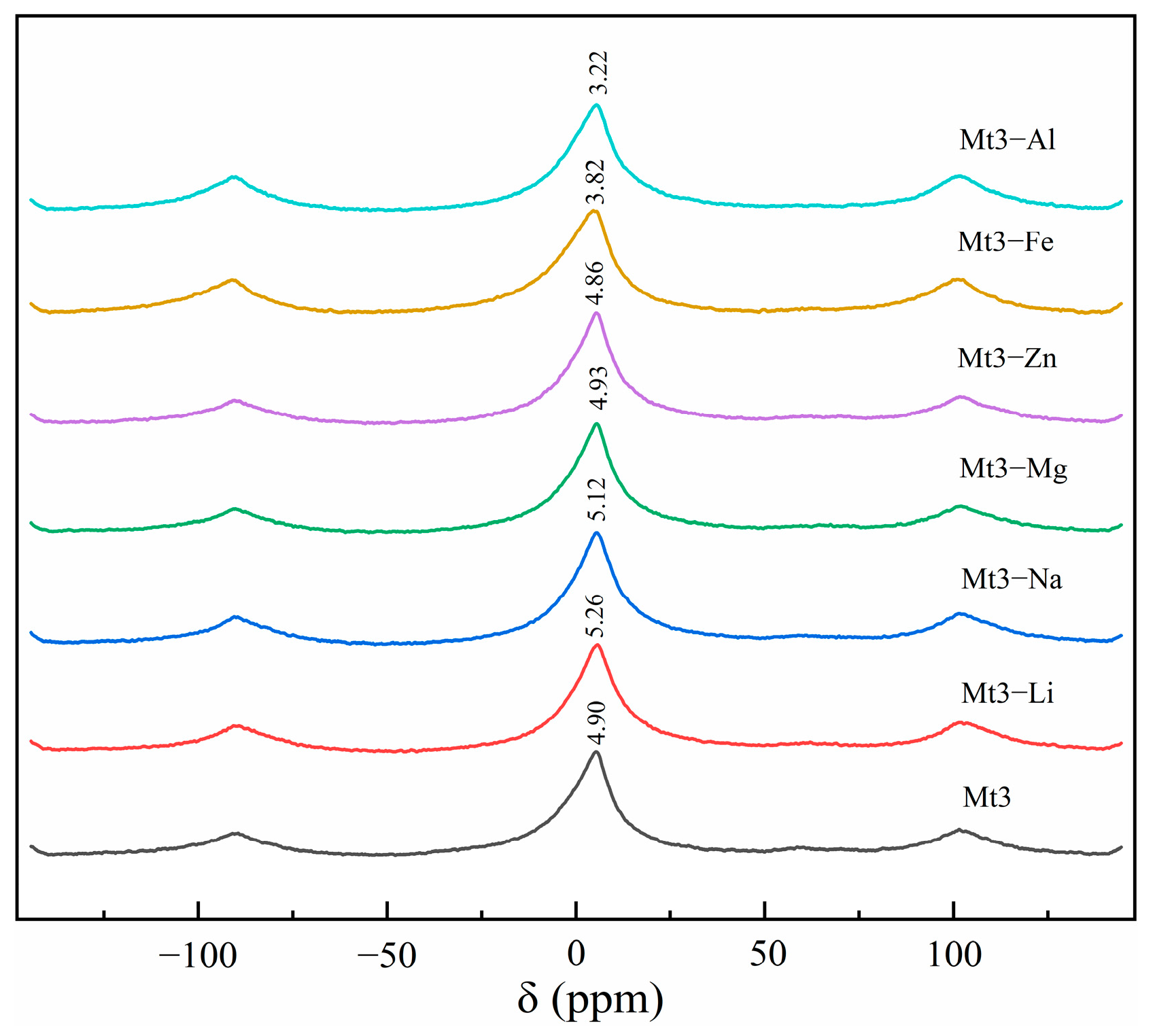

| Sample | Place of Origin | Mineral Composition/% |
|---|---|---|
| Mt1 | Qingfengshan, Liaoning | Montmorillonite 95.41, Quartz 4.59 |
| Mt2 | Shahai, Liaoning | Montmorillonite 94.59, Calcite 5.41 |
| Mt3 | Xuanhua, Hebei | Montmorillonite 99, Quartz 1 |
| Mt4 | Zhangjiakou, Hebei | Montmorillonite 91.24, Quartz 8.76 |
| Mt5 | Chifeng, Inner Mongolia | Montmorillonite 96.4, Quartz 3.6 |
| Sample | d001/Å | D001/nm | D90/μm | Zeta Potential/mV | Viscosity/mPa·s | Swelling Capacity/mL g−1 |
|---|---|---|---|---|---|---|
| Mt1 | 15.33 | 8.29 | 11.08 | −17.6 | 1.6 | 5.02 |
| Mt2 | 14.96 | 8.56 | 11.21 | −16.3 | 8.3 | 7.87 |
| Mt3 | 14.92 | 3.03 | 2.72 | −28.7 | 59.2 | 17.93 |
| Mt4 | 15.12 | 8.94 | 11.33 | −21.7 | 11.9 | 6.55 |
| Mt5 | 15.48 | 5.50 | 8.99 | −25.1 | 53.7 | 14.60 |
| Concentration | Sample | ||||||
|---|---|---|---|---|---|---|---|
| Mt3 | Mt3-Li | Mt3-Na | Mt3-Mg | Mt3-Zn | Mt3-Fe | Mt3-Al | |
| Si/ppm | 0.83 | 2.44 | 2.10 | 2.32 | 3.37 | 9.79 | 7.43 |
| Al/ppm | 0.12 | 1.32 | 0.88 | 0.12 | 0.23 | 9.64 | 233.61 |
| Sample | Mt1 | Mt1-Li | Mt1-Na | Mt1-Mg | Mt1-Zn | Mt1-Al | Mt1-Fe |
|---|---|---|---|---|---|---|---|
| Viscosity/mPa·s | 1.6 | 267.23 | 255.71 | - | 1.32 | - | 1.13 |
| Swelling capacity/mL·g−1 | 5.02 | 11.33 | 10.04 | 2.33 | 4.92 | 5.05 | 6.37 |
| Sample | Mt2 | Mt2-Li | Mt2-Na | Mt2-Mg | Mt2-Zn | Mt2-Al | Mt2-Fe |
| Viscosity/mPa·s | 8.3 | 341.7 | 301.27 | 2.6 | 6.9 | 1.41 | 1.62 |
| Swelling capacity/mL·g−1 | 7.87 | 14.52 | 12.56 | 5.58 | 4.32 | 6.01 | 7.22 |
| Sample | Mt3 | Mt3-Li | Mt3-Na | Mt3-Mg | Mt3-Zn | Mt3-Al | Mt3-Fe |
| Viscosity/mPa·s | 59.25 | 740.38 | 680.01 | 8.14 | 22.88 | 2.04 | 1.93 |
| Swelling capacity/mL·g−1 | 17.93 | 25.87 | 21.30 | 6.99 | 7.81 | 11.05 | 13.21 |
| Sample | Mt4 | Mt4-Li | Mt4-Na | Mt4-Mg | Mt4-Zn | Mt4-Al | Mt4-Fe |
| Viscosity/mPa·s | 11.9 | 372.17 | 377.33 | 4.33 | 7.2 | 2.11 | 1.81 |
| Swelling capacity/mL·g−1 | 6.55 | 15.33 | 15.46 | 3.35 | 4.22 | 5.38 | 6.09 |
| Sample | Mt5 | Mt5-Li | Mt5-Na | Mt5-Mg | Mt5-Zn | Mt5-Al | Mt5-Fe |
| Viscosity/mPa·s | 53.7 | 701.7 | 669.19 | 7.72 | 19.38 | 2.06 | 1.87 |
| Swelling capacity/mL·g−1 | 14.6 | 26.03 | 25.36 | 5.9 | 6.03 | 9.27 | 10.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Liu, D.; Zhang, T.; Yan, H.; Zhang, Z.; Geng, J. Regulation Mechanism of Different Metal Cations on the Structure and Gel Properties of Montmorillonite. Materials 2025, 18, 1878. https://doi.org/10.3390/ma18081878
Wang S, Liu D, Zhang T, Yan H, Zhang Z, Geng J. Regulation Mechanism of Different Metal Cations on the Structure and Gel Properties of Montmorillonite. Materials. 2025; 18(8):1878. https://doi.org/10.3390/ma18081878
Chicago/Turabian StyleWang, Sixiao, Dinghua Liu, Tiantian Zhang, Haowei Yan, Zepeng Zhang, and Junming Geng. 2025. "Regulation Mechanism of Different Metal Cations on the Structure and Gel Properties of Montmorillonite" Materials 18, no. 8: 1878. https://doi.org/10.3390/ma18081878
APA StyleWang, S., Liu, D., Zhang, T., Yan, H., Zhang, Z., & Geng, J. (2025). Regulation Mechanism of Different Metal Cations on the Structure and Gel Properties of Montmorillonite. Materials, 18(8), 1878. https://doi.org/10.3390/ma18081878






