Hydroxyapatite/MCM-41 and SBA-15 Nano-Composites: Preparation, Characterization and Applications
Abstract
:1. Introduction
2. Results and Discussion
2.1. XRD and BET studies
| Sample | Si/Ala | ao* (nm) | Area m2/g | Pore Vol. mL/g | Diameter ** pore (nm) | Wall thickness*** (nm) |
|---|---|---|---|---|---|---|
| MCM-41 | 25 | 2.4 | 1140 | 0.80 | 5.80 | 1.1 |
| SBA-15a | 50 | 11.3 | 1250 | 1.32 | 9.60 | 2.2 |
| SBA-15b | 32 | 11.7 | 1200 | 1.26 | 9.80 | 2.4 |
| HaP/MCM-41 | 25 | 2.4 | 960 | 0.45 | 3.25 | 1.1 |
| HaP/SBA-15a | 50 | 11.3 | 987 | 0.80 | 7.50 | 2.2 |
| HaP/SBA-15b | 32 | 11.7 | 960 | 0.85 | 7.20 | 2.4 |


2.2. FTIR studies

2.3. NMR-MAS studies

2.4. HRTEM and SEM studies


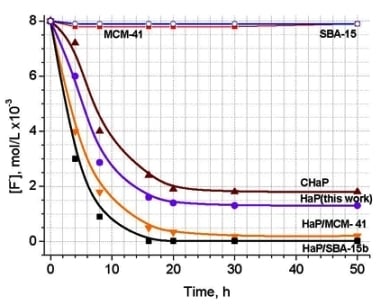
2.5. Fluoride retention

| Samples | OH- bands at 3667-3670 cm-1, Absorbance Units, %* | |||
|---|---|---|---|---|
| Time, h | ||||
| 4 | 10 | 20 | 50 | |
| CHaPa | 80 | 18 | 6 | 2 |
| HaPb | 75 | 23 | 8 | 3 |
| HaP/MCM-41 | 73 | 57 | 40 | 41 |
| HaP/SBA-15b | 71 | 60 | 45 | 43 |
3. Experimental Section
3.1. Host synthesis
3.2. Preparation of the composites
3.3. Fluoride retention essay
3.4. Characterization
4. Conclusions
Acknowledgements
References
- Elliot, J. Structure and Chemistry of the Apatite and other Calcium Orthophosphates; Elsevier: Amsterdam, The Netherlands, 1994. [Google Scholar]
- LeGros, R.Z. Calcium phosphates in oral biology and medicine. Monogr. Oral Sci. 1991, 15, 1–201. [Google Scholar] [PubMed]
- Rosalen, P.; Bowen, W.; Pearson, S. Influence of fluoride co-cystallized with sugar on caries development in desalivated rats. Arch. Oral Biol. 1997, 42, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, K.; Nakahira, A.; Okazaki, M.; Yamaguchi, S.; Kaneno, M.; Ichihara, J. Fluoride ion-promoted reaction of β-tricalcium phosphate to fluoridated hydroxyapatite. J. Fluorine Chem. 2001, 110, 75–79. [Google Scholar] [CrossRef]
- Laghzizil, A.; Elhrech, N.; Britel, O.; Bouhaouss, A.; Ferhat, M. Removal of fluoride from moroccan phosphate and synthetic fluoroapatites. J. Fluorine Chem. 2000, 101, 69–73. [Google Scholar] [CrossRef]
- Hammari, L.E.L.; Laghzizil, A.; Barboux, P.; Lahlil, K.; Saoiabi, A. Retention of fluoride ions from aqueous solution using porous hydroxyapatite: Structure and conduction properties. J. Hazard. Mater. 2004, 114, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Dalas, E.; Chrissanthopoulos, A. The overgrowth of hydroxyapatite on new functionalized polymers. J. Cryst. Growth 2003, 255, 163–169. [Google Scholar] [CrossRef]
- Rivera-Muñoz, E.; Brostow, W.; Rodriguez, J.R.; Castaño, V.M. Growth of hydroxyapatite on silica gels in the presence of organic additives: kinetics and mechanism. Mater. Res. Innov. 2001, 4, 222–230. [Google Scholar] [CrossRef]
- Lim, G.K.; Wan, J.; Ng, S.C.; Chew, C.H.; Gan, L.M. Processing of hydroxyapatite via microemulsion and emulsion routes. Biomaterials 1997, 18, 1433–1439. [Google Scholar]
- Lim, G.K.; Wang, J.; Ng, S.C.; Chew, C.H.; Gan, L.M. Nanosized hydroxyapatite powders from microemulsions and emulsions stabilized by a biodegradable surfactant. J. Mater. Chem. 1999, 9, 1635–1639. [Google Scholar] [CrossRef]
- Yao, J.; Tjandra, W.; Chen, Y.Z.; Tam, K.C.; Ma, J.; So, H.B. Hydroxyapatite nanostructure material derived using cationic surfactant as a template. J. Mater. Chem. 2003, 13, 3053–3057. [Google Scholar] [CrossRef]
- Zhao, Y.F.; Ma, J. Triblock co-polymer templating synthesis of mesostructured hydroxyapatite. Micropor. Mesopor. Mater. 2005, 87, 110–117. [Google Scholar] [CrossRef]
- Prélot, B.; Zemb, T. Calcium phosphate precipitation in catanionic templates. Mater. Sci. Eng. C 2005, 25, 553–559. [Google Scholar] [CrossRef]
- Ozin, G.A.; Varaksa, N.; Coombs, N.; Davies, J.E.; Perovic, D.D.; Ziliox, M. Bone mimetics: A composite of hydroxyapatite and calcium dodecylphosphate lamellar phase. J. Mater. Chem. 1997, 7, 1601–1607. [Google Scholar] [CrossRef]
- Soten, I.; Ozin, G.A. Porous hydroxyapatite-dodecylphosphate composite film on titania-titanium substrate. J. Mater. Chem. 1999, 9, 703–710. [Google Scholar] [CrossRef]
- Zhang, J.; Fujiwara, M.; Xu, Q.; Zhu, Y.; Iwasa, M.; Jiang, D. Synthesis of mesoporous calcium phosphate using hybrid templates. Micropor. Mesopor. Mater. 2008, 111, 411–416. [Google Scholar] [CrossRef]
- Wang, H.; Zhai, L.; Li, Y.; Shi, T. Preparation of irregular mesoporous hydroxyapatite. Mater. Res. Bull. 2008, 43, 1607–1614. [Google Scholar] [CrossRef]
- Tang, Y.J.; Tang, Y.F.; Lv, C.T.; Zhou, Z.H. Preparation of uniform porous hydroxyapatite biomaterials by a new method. Appl. Surf. Sci. 2005, 254, 5359–5362. [Google Scholar] [CrossRef]
- Anunziata, O.; Beltramone, A.; Cussa, J. Composite hydroxyapatite/Na-MCM-41 for the fluorine retention in contaminated water. In Recent Progress in Mesostructured Materials; Elsevier: Amsterdam, The Netherlands, 2007; Vol. 165, pp. 77–80. [Google Scholar]
- Anunziata, O.; Beltramone, A.; Martinez, M.L.; López Belón, L. Synthesis and characterization of SBA-3, SBA-15 and SBA-1 nanostructured catalytic materials. J. Colloid Interf. Sci. 2007, 315, 184–190. [Google Scholar] [CrossRef]
- Chen, F.; Shen, S.; Jun Xu, X.; Xu, R.; Kooli, F. Modification of micropore-containing SBA-3 by TEOS liquid phase deposition. Micropor. Mesopor. Mater. 2005, 79, 85–91. [Google Scholar] [CrossRef]
- Chen, F.; Xu, X.J.; Shen, S.; Kawi, S.; Hidajat, K. Microporosity of SBA-3 mesoporous molecular sieves. Micropor. Mesopor. Mater. 2004, 75, 231–235. [Google Scholar] [CrossRef]
- Ryoo, R.; Ko, C.H.; Kruk, M.; Antochshuk, V.; Jaroniec, M. Block-copolymer-templated ordered mesoporous silica: array of uniform mesopores or mesopore−micropore network? J. Phys. Chem. B 2000, 104, 11465–11471. [Google Scholar] [CrossRef]
- Corma, A.; Corell, C.; Fornes, V.; Kolodziejski, W.; Perez-Pariente, J. Infrared spectroscopy, thermoprogrammed desorption, and nuclear magnetic resonance study of the acidity, structure and stability of zeolite MCM-22. Zeolites 1995, 15, 576–582. [Google Scholar] [CrossRef]
- Janicke, M.; Landry, C.; Christiansen, S.; Britalan, S.; Stucky, G.; Chmelka, B. Low silica MCM-41 composites and mesoporous solids. Chem. Mater. 1999, 11, 1342–1351. [Google Scholar]
- Landau, M.V.; Varkey, S.P.; Herskowitz, M.; Regev, O.; Pevzner, S.; Sen, T.; Luz, Z. Wetting stability of Si-MCM-41 mesoporous material in neutral, acidic and basic aqueous solutions. Micropor. Mesopor. Mater. 1999, 33, 149–163. [Google Scholar] [CrossRef]
- Manjubala, I.; Sivakumar, M.; Najma, S. Synthesis and characterization of hydroxy/fluorapatite solid solutions. J. Mater. Sci. 2001, 36, 5481–5486. [Google Scholar] [CrossRef]
- Anunziata, O.; Beltramone, A.; Cussa, J. Synthesis at atmospheric pressure and characterization of highly ordered Al, V, and Ti-MCM-41 mesostructured catalysts. Catal. Today 2008, 133-135, 891–896. [Google Scholar]
- Jin, Z.W.; Wang, X.D.; Cui, X.G. Synthesis and morphological investigation of ordered SBA-15-type mesoporous silica with an amphiphilic triblock copolymer template under various conditions. Colloid. Surface. A 2008, 316, 27–36. [Google Scholar] [CrossRef]
- Dıaz, A.; Lopez, T.; Manjarrez, J.; Basaldella, E.; Martınez-Blanes, J.; Odriozola, J. Growth of hydroxyapatite in a biocompatible mesoporous ordered silica. Acta Biomaterialia 2006, 2, 173–179. [Google Scholar] [CrossRef] [PubMed]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Anunziata, O.A.; Martínez, M.L.; Beltramone, A.R. Hydroxyapatite/MCM-41 and SBA-15 Nano-Composites: Preparation, Characterization and Applications. Materials 2009, 2, 1508-1519. https://doi.org/10.3390/ma2041508
Anunziata OA, Martínez ML, Beltramone AR. Hydroxyapatite/MCM-41 and SBA-15 Nano-Composites: Preparation, Characterization and Applications. Materials. 2009; 2(4):1508-1519. https://doi.org/10.3390/ma2041508
Chicago/Turabian StyleAnunziata, Oscar A., Maria L. Martínez, and Andrea R. Beltramone. 2009. "Hydroxyapatite/MCM-41 and SBA-15 Nano-Composites: Preparation, Characterization and Applications" Materials 2, no. 4: 1508-1519. https://doi.org/10.3390/ma2041508
APA StyleAnunziata, O. A., Martínez, M. L., & Beltramone, A. R. (2009). Hydroxyapatite/MCM-41 and SBA-15 Nano-Composites: Preparation, Characterization and Applications. Materials, 2(4), 1508-1519. https://doi.org/10.3390/ma2041508