2. Results and Discussion
In our previous work we showed that, in common with clusters of later transition ions, molecular V(III) phosphonates can be prepared from {M
3(μ
3-O)} building blocks. We found that these {V(III)
3} triangles could be formed
in situ which is simpler than preparing and isolating the air sensitive [V
3O(O
2CR)
6L
3]
+ (L = terminal ligand) basic metal carboxylates. For example, [V
III6(O)
2(O
2C
tBu)
8(HO
2C
tBu)
2(HO
3P
tBu)
2(O
3P
tBu)
2] (
1) is prepared from the one-pot reaction of pivalic acid, VCl
3 and
t-butylphosphonate in MeCN with Et
3N as base [
8]. Analogues of
1 can be prepared similarly. If [VCl
3(thf)
3] is used in place of VCl
3 in an otherwise identical reaction then [V
III6(μ
3-O)
2(
tBuPO
3)
2(
tBuPO
3H)
2(
tBuCO
2)
8(thf)
2] (
2) is formed.
2 is centrosymmetric with two oxo-centred vanadium triangles linked via four phosphonates (
Figure 1). The two fully deprotonated RPO
32- 1,3-bridge one edge of each triangle, formally replacing one carboxylate in the “parent” [V
3O(O
2C
tBu)
6L
3]
+ triangle, with the third arm providing a μ
2-bridge on the second triangle ([4.211]-binding mode in Harris notation [
10]). The two singly deprotonated (RPO
3H)
- 1,3-bridge between the triangles, with the two coordinated arms binding terminally ([2.110]-binding mode). The two thf molecules act as terminal ligands at the vanadium ions not involved in linking the two triangles – in complex
1 these are replaced by pivalic acid.
Figure 1.
Structure of 2 in the crystal.
Figure 1.
Structure of 2 in the crystal.
The bridging carboxylates can also be substituted: reaction of [VCl3(thf)3], PhCO2H and tBuPO3H2 (6:8:4) with Et3N in EtOH gives [VIII6(O)2(tBuPO3)2(tBuPO3H)2(PhCO2)8(EtOH)2] (3). Now eight benzoates span edges of the triangles and alcohol is the terminal ligand. If a higher proportion of phosphonic acid is used in the reaction then phosphonate can also act as the terminal ligand: reaction of [VCl3(thf)3], tBuCO2H and tBuPO3H2 (6:8:6) with Et3N in MeCN gives (Et3NH)2[VIII6(O)2(tBuPO3)2(tBuPO3H)2(tBuCO2)8(tBuPO3H)2] (4). Complex 4 has two singly deprotonated (RPO3H)- terminal ligands making it a dianion.
Much larger clusters result if the reactions in alcohols are performed solvothermally. We previously reported [V
III12(V
IVO)(μ
3-OH)
4(μ
2-OH)
8(μ
2-OEt)
4(EtOH)
4(PhCO
2)
4(O
3P
tBu)
8]Cl
2 (
5) from reaction of [VCl
3(thf)
3], PhCO
2H and
tBuPO
3H (13:8:4) with KOEt in EtOH at 150 °C [
8]. As with the hexametallic
1, we can prepare a number of analogues of
5, substituting the phosphonate RPO
3H
2, carboxylic acid R’CO
2H and alcohol R”OH to give [V
12(VO)(
μ3-OH)
4(
μ2-OH)
8(
μ2-OR’’)
4(R’’OH)
4(R’CO
2)
4(RPO
3)
8]X
2 where R =
tBu, R’ =
tBu, R’’ = Et, X
2 = (OH)Cl (
6); R =
tBu, R’ = Ph
2C(H), R’’ = Et, X
2 = (OH)Cl (
7); R = PhCH
2, R’ =
tBu, R’’ = Et, X = Cl (
8); R =
tBu, R’ =
tBu, R’’ = Me, X
2 = (OH)Cl (
9). The structures of
5–
9 are similar, being based on a square of{V
III3(
μ3-OH)} triangles with a central vanadyl ion (V1 in
Figure 2) bound to the center of the cage. The square of triangles is formed so that eight V
III centres are in one plane with the remaining four V
III ions forming a square above this octagon. Four phosphonates each bind a face of each triangle, each arm binding terminally ([3.111]-binding mode;
Figure 3). The other four phosphonates (P2 and P5 and symmetry equivalents in
Figure 3b) link the triangles, 1,3-bridging edges of neighbouring triangles with one arm binding to both ([4.211] binding mode,
Figure 3). The {VO}
2+ ion is bridged by four μ
2-OH to the four V
III centers within the square plane. Each of these V
III ions has a terminal R’’OH, all of which H-bond to one of the counter-ions. The bridging in the octagonal plane alternates between a [3.111]-phosphonate with an alkoxide and a μ
3-OH, and a [4.211]-phosphonate with a μ
2-carboxylate and a μ
2-OH. The four hydroxides lie towards the centre of the cavity. The central vanadium ion binds the only terminal oxide in the structure and is in the +4 oxidation state. All other metal ions in the complexes are in the +3 oxidation state.
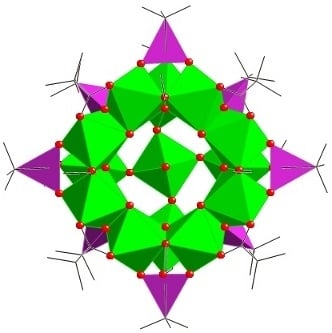
Figure 2.
(left) Structure of the cation of 6 in the crystal. (right) “Side-on” view highlighting layered structure. R, R’ and R’’ groups removed for clarity.
Figure 2.
(left) Structure of the cation of 6 in the crystal. (right) “Side-on” view highlighting layered structure. R, R’ and R’’ groups removed for clarity.
Figure 3.
Fragment of structure of 6 highlighting the phosphonate binding modes. The top three vanadium ions are in the “upper” V8 ring.
Figure 3.
Fragment of structure of 6 highlighting the phosphonate binding modes. The top three vanadium ions are in the “upper” V8 ring.
We have undertaken magnetic studies on two representative examples of the hexa- and trideca-metallic families,
3 and
5. The room temperature
χMT value of
3 is 6.5 cm
3 K mol
−1; assuming we can treat the V
III, d
2 ion as
s = 1 we would expect 6.0 cm
3 K mol
−1 for six non-interacting ions with
g = 2.0.
χMT decreases slightly on cooling and then increases slowly to a maximum at ca. 25 K before collapsing to 2.1 cm
3 K mol
−1 at 2 K (
Figure 4). We have attempted to model this behaviour. The simplest meaningful model has three unique exchange interactions [
Figure 5, Hamiltonian (1)]: (i) the carboxylate-bridged edges within each {V
3(μ
3-O)} unit,
J1 (ii) the unique, phosphonate-bridged edge with each triangle,
J2; (iii) between vanadium ions in different triangles, bridged by 1,3-phophonates,
J3.
Figure 4.
Magnetic data for 3 (triangles) and 5 (squares) and calculated curve for 3 (solid line) using the values in the text.
Figure 4.
Magnetic data for 3 (triangles) and 5 (squares) and calculated curve for 3 (solid line) using the values in the text.
Figure 5.
Model for exchange coupling in complex 3.
Figure 5.
Model for exchange coupling in complex 3.
A reasonable, but not good, fit is found with
J1 = +7.2,
J2 = −4.5 and
J3 = −0.95 cm
−1 with
g = 1.95. The weak exchange interactions are not surprising given precedent in the literature from dimers and trimers with related bridging motifs [
9]. These
J-values can be interpreted as giving an
S = 3 ground state for each triangle which are then antiferromagnetically coupled to each other. This gives an
S = 0 ground state but with many low-lying excited states; this is consistent with low temperature magnetization (
M)
vs. applied magnetic field (
H) which fail to saturate up to 7 T and 1.8 K.
Complex
5 has a room temperature
χMT value of 9.94 cm
3 K mol
−1 and is already decreasing rapidly with decreasing temperature (
Figure 4).
χMT plateaus in the 20–30 K region at ca. 3.7 cm
3 K mol
−1. We have not attempted to model this behaviour, but we can show that it is consistent with the odd-integer electron count arising from {V
III12(V
IVO)}: a low temperature W-band (94 GHz) EPR spectrum of
5 is characteristic of an
S = 5/2 ground state for the cluster (
Figure 6). This would be expected to give rise to a low temperature limiting
χMT value of 4.2 cm
3 K mol
−1 for
g = 1.95.
Figure 6.
W-band EPR spectrum of a polycrystalline sample of 5 (black) and simulation (red) with S = 5/2, D = −0.14 cm-1 and g = 1.96.
Figure 6.
W-band EPR spectrum of a polycrystalline sample of 5 (black) and simulation (red) with S = 5/2, D = −0.14 cm-1 and g = 1.96.
3. Experimental Section
All manipulations were conducted under anaerobic conditions (dinitrogen purged glove box and Schlenk line). Solvents were dried before using. All the vanadium compounds are very sensitive to moisture and air.
[VIII6(μ3-O)2(tBuPO3)2(tBuPO3H)2(tBuCO2)8(thf)2] (2): [VCl3(thf)3] (0.37 g, 1 mmol) was added to a solution of tBuPO3H2 (0.09 g, 0.66 mmol) in MeCN (10 mL). tBuCO2Na (0.16 g, 1.32 mmol) was added to the resultant suspension and the mixture stirred for 24 h and filtered. Green crystals suitable for X-ray analysis were obtained in three weeks (11%). Elemental analysis calcd (%) for C64H126O32P4V6: C 41.83, H 6.91, P 6.74, V 16.64; found: C 40.94, H 6.7, P 6.38, V 15.4.
[VIII6(O)2(tBuPO3)2(tBuPO3H)2(PhCO2)8(EtOH)2] (3): [VCl3(thf)3] (0.37 g, 1 mmol) was added to a solution of tBuPO3H2 (0.09 g, 0.66 mmol) in EtOH (10 mL). PhCO2H (0.16 g, 1.32 mmol) and Et3N (0.3 mL, 2.3 mmol) were added to the resultant suspension, and the mixture stirred for 24 h and filtered. Green crystals were obtained in two-to-three weeks (16%). Elemental analysis calcd (%) for C76H90O32P4V6: C 46.93, H 4.66, P 6.37, V 15.71; found: C 46.58, H 4.55, P 6.08, V 14.82.
(Et3NH)2[VIII6(O)2(tBuPO3)2(tBuPO3H)2(tBuCO2)8(tBuPO3H)2] (4): [VCl3(thf)3] (0.185 g, 0.5 mmol) was added to tBuPO3H2 (0.069 g, 0.5 mmol) in MeCN (10 mL). tBuCO2H (0.07 g, 0.66 mmol) and Et3N (0.17 mL, 1.33 mmol) were added and the mixture stirred for 24 h and filtered. Green crystals were obtained after two weeks (20%). Elemental analysis calcd (%) for C76H162N2O36P6V6: C 42.03, H 7.52, N 1.29, P 8.56, V 14.07; found: C 42.53, H 7.67, N 1.31, P 8.23, V 13.35.
[V12(VO)(μ3-OH)4(μ2-OH)8(μ2-OEt)4(EtOH)4(tBuCO2)4(tBuPO3)8](OH)Cl (6): [VCl3(thf)3] (0.3 g, 0.8 mmol), tBuPO3H2 (0.069 g, 0.5 mmol), tBuCO2H (0.026 g, 0.25 mmol) and KOEt (0.10 g, 1.3 mmol) in EtOH (14 mL) were heated at 150 °C in a sealed Teflon-lined autoclave for 12 h then cooled to give an insoluble solid under a green solution, which was filtered. Green crystals grew from the filtrate after two weeks (36%). Elemental analysis calcd (%) for C68H165V13O54P8Cl1·1.5EtOH: C 29.7, H 6.11, P 8.87, Cl 1.24, V 23.08; found: C 29.47, H 5.96, P 8.58, Cl 1.15, V 21.8
Compounds 7–9 were synthesized by analogous reactions. 7: Yield 30%. Elemental analysis calcd (%) for C104H173V13O54P8Cl1·2EtOH: C 39.01, H 5.68, P 7.45, Cl 1.07, V 19.91; found: C 39.71, H 5.85, P 7.14, Cl 1.1, V 18.59. 8: Yield 36%. Elemental analysis calcd (%) for C100H132V13O53P8Cl2: C 37.96, H 4.21, P 7.83, Cl 2.24, V 20.94; found: C 37.71, H 4.08, P 7.64, Cl 2.14, V 19.63. 9: Yield 35%. Elemental analysis calcd (%) for C62H149V13O54P8Cl1: C 26.89, H 5.6, P 9.24, V 24.71; found: C 27.12, H 5.78, P 8.98, V 23.4.
X-ray data for compounds
2–4 and
9 were collected on an Oxford Instruments CCD diffractometer (Mo K
α, λ= 0.71073 Å), and data for
6–
8 on a on Bruker SMART CCD diffractometer using synchrotron radiation (λ=0.67090 Å and 0.69260 Å). In all cases the selected crystals were mounted on the tip of a glass pin by using Paratone-N oil and placed in the cold flow produced by an Oxford Cryo-cooling device. Complete hemispheres of data were collected using ω scans (0.3°, 30–50 s/frame). Integrated intensities were obtained with SAINT+, and they were corrected for absorption using SADABS. Structure solution and refinement was performed with the SHELX package [
11]. The structures were solved by direct methods and completed by iterative cycles of ∇F syntheses and full-matrix least-squares refinement against F
2. Crystal data and refinement parameters are given in
Table 1 and
Table 2. Cif files are in electronic supplementary information. CCDC deposition numbers 760561 – 760567.
Table 1.
Crystal data for 2–4.
Table 1.
Crystal data for 2–4.
| Compound | 2 | 3 | 4 |
|---|
| formula | C64H126V6O32P4 | C79H104V6O36P4 | C76H162V6N2O36P6 |
| M | 1837.17 | 2059.14 | 2171.54 |
| cryst syst | orthorhombic | monoclinic | monoclinic |
| space group | Pbca | P21/n | P21/n |
| a/Å | 21.2682(11) | 17.5267(14) | 14.7996(16) |
| b/Å | 19.6989(10) | 14.7323(13) | 21.810(3) |
| c/Å | 22.0589(11) | 20.2027(17) | 17.6498(17) |
| α/deg | 90 | 90 | 90 |
| β/deg | 90 | 103.887(8) | 107.009(12) |
| γ/deg | 90 | 90 | 90 |
| U/Å3 | 9241.8(8) | 5064.0(7) | 5447.9(10) |
| T/K | 100(2) | 100(2) | 100(2) |
| Z | 4 | 2 | 2 |
| μ/mm-1 | 1.320 | 1.350 | 1.324 |
| unique data | 8174 | 5138 | 4255 |
| data with Fo > 4σ (Fo) | 7011 | 2003 | 2430 |
| R1, wR2a | 0.0553, 0.1642 | 0.0711, 0.1524 | 0.0955, 0.2177 |
Table 2.
Crystal data for 6–9.
Table 2.
Crystal data for 6–9.
| Compound | 6 | 7 | 8 | 9 |
|---|
| formula | C68.5H168.75V13Cl1O56P8 | C108H184V13Cl1O56P8 | C100H134V13Cl2O55P8 | C62.75H162V13Cl1O57.75P8 |
| M | 2834.21 | 3323.98 | 3196.96 | 2786.35 |
| cryst syst | orthorhombic | tetragonal | monoclinic | monoclinic |
| space group | Aba2 | P4/n | C2/c | C2/c |
| a/Å | 50.480(10) | 21.229(5) | 28.2520(13) | 55.477(2) |
| b/Å | 34.466(9) | 21.229(5) | 26.0681(13) | 16.4228(3) |
| c/Å | 34.340(9) | 18.408(5) | 20.1169(10) | 29.2038(8) |
| α/deg | 90 | 90 | 90 | 90 |
| β/deg | 90 | 90 | 93.5800(10) | 93.861(3) |
| γ/deg | 90 | 90 | 90 | 90 |
| U/Å3 | 59746(25) | 8296(3) | 14786.7(12) | 26546.9(14) |
| T/K | 150(2) | 150(2) | 150(2) | 100(2) |
| Z | 16 | 2 | 8 | 8 |
| μ/mm-1 | 1.260 | 1.331 | 1.436 | 1.394 |
| unique data | 53420 | 7338 | 16284 | 20725 |
| data with Fo > 4σ (Fo) | 42216 | 4641 | 12222 | 11195 |
| R1, wR2a | 0.0671, 0.1742 | 0.0879, 0.2685 | 0.0626, 0.1859 | 0.0802, 0.2156 |