Thermo-Exfoliated Graphite Containing CuO/Cu2(OH)3NO3:(Co2+/Fe3+) Composites: Preparation, Characterization and Catalytic Performance in CO Conversion
Abstract
:1. Introduction
2. Results and Discussion
2.1. Composite Preparation
2.2. Powder X-Ray Diffraction

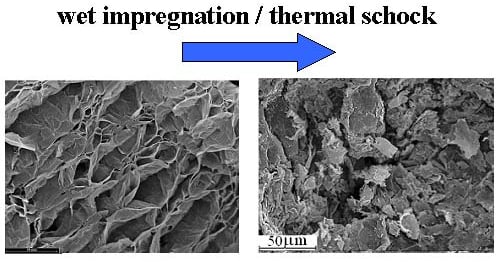
2.3. Catalytic Test, Specific Surface Area and Scanning Electron Microscopic Characterization
| Sample No | Ssp (m2·g–1) | A (mass %) | B | t100% (K) | Td (K) | Ed (kJ·mol–1) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| desorbed CO2 states | ||||||||||
| α1 | α2 | α3 | α1 | α2 | α3 | |||||
| 1 | 4 | 47 | CuO/Graphite | 528 | – | 514 | 540, 588, 678 | – | 146 | 153, 167, 193 |
| 2 | 10 | 51 | CuO/Graphite | 483 | – | 520 | 588, 683 | – | 147 | 167, 195 |
| 3 | 4 | 48 | CuO/Graphite | 488 | – | – | 539, 583, 640 | – | – | 153, 166, 183 |
| 4 | 10 | 52 | CuO/Graphite | 468 | 373 | 475 | 543 | 105 | 134 | 154 |
| 5 | 10 | 15 | CuO/Cu2(OH)3NO3 | 513 | – | 440, 476, 513 | 553, 638, 678 | – | 124, 134, 145 | 157, 182, 194 |
| 6 | 16 | 56 | CuO/Cu2(OH)3NO3 | 438 | – | 498 | 533, 588 | – | 141 | 151, 167 |
| 7 | 20 | 51 | CuO/Cu2(OH)3NO3 | 456 | – | 503 | 593, 703 | – | 142 | 169, 201 |
| 8 | 25 | 57 | CuO/Cu2(OH)3NO3 | 403 | – | 513 | 603, 693 | – | 145 | 172, 198 |

2.4. Characterization of Adsorbed Species by TPD MS after CO Conversion

3. Experimental Section
3.1. Reagents
3.1.1. Thermo-Exfoliated Graphite
3.1.2. Nitrate Mixture
3.2. Composite Preparation
- Sample 1. The TEG was wet impregnated by the nitrates mixture for 6 h, dried for 4 h at T < 373 K in air and thermal shocked at 1,073 K for 12 sec. The Cu2+/Co2+/Fe3+ nitrate mixture concentration was calculated on 47 mass % content of CuO/Cu2(OH)3NO3:(Co2+/Fe3+) in the resulted composite.
- Sample 2. The TEG was wet impregnated by the nitrates mixture for 6 h, dried for 4 h at T < 373 K in air and thermally treated at 473 K for 4 h. The Cu2+/Co2+/Fe3+ nitrate mixture concentration was calculated on 51 mass % content of CuO/Cu2(OH)3NO3:(Co2+/Fe3+) in the resulted composite.
- Sample 3. The TEG was wet impregnated by the nitrates mixture with assistance of ultrasonic agitation using an ultrasonic disperser UZDN-2T operating at 10 kHz for 6 h. The impregnated TEG was dried for 36 h at T < 373 K in air and thermally shocked at 1,073 K for 12 sec. The Cu2+/Co2+/Fe3+ nitrate mixture concentration was calculated on 48 mass % content of CuO/Cu2(OH)3NO3:(Co2+/Fe3+) in the resulted composite.
- Sample 4. The TEG was wet impregnated by the nitrates mixture with assistance of ultrasonic agitation (10 kHz) for 6 h. The impregnated TEG was for 4 h at T < 373 K in air and thermal treated at 473 K for 4 h. The Cu2+/Co2+/Fe3+ nitrate mixture concentration was calculated on 52 mass % content of CuO/Cu2(OH)3NO3:(Co2+/Fe3+) in the resulted composite.
- Sample 5. The TEG was wet impregnated by the nitrates mixture for 4 h without assistance of ultrasonic agitation (10 kHz). The Cu2+/Co2+/Fe3+ nitrate mixture concentration was calculated on 15 mass % content of CuO/Cu2(OH)3NO3:(Co2+/Fe3+) in the resulted composite. The impregnated TEG was leached with distilled water, dried at 573 K for 36 h in air and thermally treated at 453 K for 1 h.
- Sample 6. The TEG was wet impregnated by the nitrates mixture with assistance of ultrasonic agitation (10 kHz) for 4 h. The Cu2+/Co2+/Fe3+ nitrate mixture concentration was calculated on 56 mass % content of CuO/Cu2(OH)3NO3:(Co2+/Fe3+) in the resulted composite. The impregnated TEG was leached with distilled water, dried at 453 K in air and thermally treated at 453 K for 1 h.
- Sample 7. The TEG was impregnated with 63 wt % H2SO4 for 12 h. The TEG impregnated with acid was leached with distilled water up to filtrate pH = 5.5; dried at temperatures from 373 to 393 K for 8 h, and, finally, thermally shocked at 1073 K for 12 sec. The resulting TEG-H2SO4 was wet impregnated with the nitrates mixture under ultrasonic agitation (10 kHz) for 4 h, dried at 373 K and thermally treated at 453 for 1 h. The Cu2+/Co2+/Fe3+ nitrate mixture concentration was calculated based on a 51 mass % content of CuO/Cu2(OH)3NO3:(Co2+/Fe3+) in the resulting composite.
- Sample 8. The TEG was impregnated with 68 wt % HNO3 for 12 h. The TEG impregnated with acid was leached with distilled water up to filtrate pH = 5.5; dried at temperatures from 373 to 393 K for 8 h in air, and, finally, thermally shocked at 1,073 K for 12 sec. Resulted TEG-HNO3 were wet impregnated by the nitrates mixture under ultrasonic agitation (10 kHz) for 4 h, dried at 373 K and thermally treated at 453 for 1 h. The Cu2+/Co2+/Fe3+ nitrate mixture concentration was calculated on 57 mass % content of CuO/Cu2(OH)3NO3:(Co2+/Fe3+) in the resulted composite.
3.3. Composite Characterization
3.4. Catalytic CO Conversion
3.5. Characterization of Adsorbed Species over Composites Taken after CO Conversion
4. Conclusions
- The TEG impregnation with nitric or sulphuric acids with subsequent thermal shock allows achievement of complete graphite exfoliation necessary for improvement of the composites’ activity.
- The activity depends on the CuO/Cu2(OH)3NO3:(Co2+/Fe3+) component content. The high activity of (TEG)/CuO/Cu2(OH)3NO3:(Co2+/Fe3+) composites is attributed to higher dispersion over the TEG, the latter being achieved by means of the wet impregnation under ultrasonic agitation.
- Comparative studies on CO oxidation reveal that Cu2(OH)3NO3 phase, registered in the content of the composites catalysts, markedly improves their activity.
- The decrease in CO oxidation activity has been ascribed to the decomposition of Cu2(OH)3NO3, which was more active than CuO phase, and prevailing over CuO in the composite content. Catalyst with 57% of CuO/Cu2(OH)3NO3:(Co2+/Fe3+) showed the best conversion efficiency of 100% at a temperature as low as 403 K for the CO conversion to CO2. Relation between α2 and α3 states of CO2, desorbing from the surface of the composites, correlates with the catalytic activity. The high content of α2 state registered in the TPD MS spectra is a feature of the most active catalysts.
Acknowledgements
References
- Tabata, K.; Misono, M. Elimination of pollutant gases—oxidation of CO, reduction and decomposition of NO. Catal. Today 1990, 8, 249–261. [Google Scholar] [CrossRef]
- Singer, S.F. The Changing Global Environment; Springer: New York, NY, USA, 1975. [Google Scholar]
- Bailey, R.A.; Clark, H.M.; Ferris, J.P.; Krause, S.; Strong, R.L. Chemistry of the Environment; Academic Press: San Diego/London, USA/UK, 2002. [Google Scholar]
- Radwan, N.R.E.; Ei-Shall, M.S.; Hassan, H.M.A. Synthesis and characterization of nanoparticle Co3O4, CuO and NiO catalysts prepared by physical and chemical methods to minimize air pollution. Appl. Catal. A: Gen. 2007, 331, 8–18. [Google Scholar] [CrossRef]
- Wei, J. Catalysis for motor vehicle emissions. Adv. Catal. 1975, 24, 57–129. [Google Scholar]
- Dwyer, F.G. Catalysis for control of automotive emissions. Catal. Rev. 1972, 6, 261–291. [Google Scholar] [CrossRef]
- Skoglundh, M.; Fridell, E. Strategies for enhancing low-temperature activity. Topics Catal. 2004, 28, 79–87. [Google Scholar]
- Sharma, A.K. Zirconia-supported copper oxide—a catalyst for removal of vehicular exhaust gas pollutants. J. Environ. Enging. 2006, 132, 956–959. [Google Scholar] [CrossRef]
- Cheng, T.; Fang, Z.; Hu, Q.; Han, K.; Yang, X.; Zhang, Y. Low-temperature CO oxidation over CuO/Fe2O3 catalysts. Catal. Commun. 2007, 8, 1167–1171. [Google Scholar] [CrossRef]
- Cao, J.-L.; Shao, G.-S.; Wang, Y.; Liu, Y.; Yuan, Z.-Y. CuO catalysts supported on attapulgite clay for low-temperature CO oxidation. Catal. Commun. 2008, 9, 2555–2559. [Google Scholar] [CrossRef]
- Park, P.W.; Ledford, J.S. Characterization and CO oxidation activity of Cu/Cr/Al2O3 catalysts. Ind. Eng. Chem. Res. 1998, 37, 887–893. [Google Scholar] [CrossRef]
- Ribeiro, N.F.P.; Souza, M.M.V.M.; Schmal, M. Combustion synthesis of copper catalysts for selective CO oxidation. J. Power Sources 2008, 179, 329–334. [Google Scholar] [CrossRef]
- Wan, H.; Wang, Z.; Zhu, J.; Li, X.; Liu, B.; Gao, F.; Dong, L.; Chen, Y. Influence of CO pretreatment on the activities of CuO/γ-Al2O3 catalysts in CO + O2 reaction. Appl. Catal. B 2008, 79, 254–261. [Google Scholar] [CrossRef]
- Águila, G.; Gracia, F.; Araya, P. CuO and CeO2 catalysts supported on Al2O3, ZrO2, and SiO2 in the oxidation of CO at low temperature. Appl. Catal. A 2008, 343, 16–24. [Google Scholar] [CrossRef]
- Jin, L.; He, M.; Lu, J.; Luo, M.; Fang, P.; Xie, L.Y. Comparative study of CuO species on CuO/Al2O3, CuO/CeO2-Al2O3 and CuO/La2O3-Al2O3 catalysts for CO oxidation. J. Chem. Phys. 2007, 20, 582–586. [Google Scholar]
- Ishchenko, E.V.; Yatsimirskii, V.K.; Gaidai, S.V. Temperature hysteresis in oxidation of CO on complex oxide catalysts. Theor. Exper. Chem. 2005, 41, 340–345. [Google Scholar] [CrossRef]
- Chung, D.D.L. Exfoliation of graphite. J. Mater. Sci. 1987, 22, 4190–4198. [Google Scholar] [CrossRef]
- Celzard, A.; Mareche, J.F.; Furdin, G. Modelling of exfoliated graphite. Progr. Mater. Sci. 2005, 50, 93–179. [Google Scholar] [CrossRef]
- Matzui, L.; Vovchenko, L.; Zakharenko, M.; Babich, M.; Brusilovetz, A. Thermoexfoliated graphite as support for production of metal-graphite nanocomposites. J. Chem. Phys. Solids 2004, 65, 171–175. [Google Scholar] [CrossRef]
- Toyoda, M.; Inagaki, M. Heavy oil sorption by using exfoliated graphite—new application of exfoliated graphite to protect heavy oil pollution. Carbon 2000, 38, 199–210. [Google Scholar] [CrossRef]
- Vieira, F.; Cisneros, I.; Rosa, N.G.; Trindade, G.M.; Mohallem, N.D.S. Influence of the natural flake graphite particle size on the textural characteristic of exfoliated graphite used for heavy oil sorption. Carbon 2006, 44, 2587–2592. [Google Scholar] [CrossRef]
- Toyoda, M.; Aizawa, J.; Inagaki, M. Sorption and recovery of heavy oil by using exfoliated graphite. Desalination 1998, 115, 199–201. [Google Scholar] [CrossRef]
- Zheng, Y.-P.; Wang, H.-N.; Kang, F.-Y.; Wang, L.-N.; Inagaki, M. Sorption capacity of exfoliated graphite for oils sorption in and among wormlike particles. Carbon 2004, 42, 2603–2607. [Google Scholar] [CrossRef]
- Shornikova, O.N.; Sorokina, N.E.; Avdeev, V.V. Preparation and properties of exfoliated graphite doped with nickel and cobalt oxides. Inorg. Mater. 2007, 43, 938–944. [Google Scholar] [CrossRef]
- Shornikova, O.N.; Sorokina, N.E.; Avdeev, V.V. The effect of graphite nature on the properties of exfoliated graphite doped with nickel oxide. J. Phys. Chem. Solids 2008, 69, 1168–1170. [Google Scholar] [CrossRef]
- Wan, Ch.; Azumi, K.; Konno, H. Hydrated Mn(IV) oxide-exfoliated graphite composites for electrochemical capacitor. Electrochim. Acta 2007, 52, 3061–3066. [Google Scholar] [CrossRef]
- Yana, J.; Fana, Zh.; Weia, T.; Qian, W.; Zhanga, M.; Wei, F. Preparation of exfoliated graphite containing manganese oxides with high electrochemical capacitance by microwave irradiation. Carbon 2009, 47, 3365–3380. [Google Scholar] [CrossRef]
- Zhang, H.; Jin, Z.; Han, L.; Qin, C. Synthesis of nanoscale zero-valent iron supported on exfoliated graphite for removal of nitrate. Trans. Nonferrous Met. Soc. China 2006, 16, 345–349. [Google Scholar] [CrossRef]
- Yoshida, A.; Hishiyama, Y.; Inagaki, M. Exfoliation of vapor-grown graphite fibers as studied by scanning electron microscope. Carbon 1990, 28, 539–543. [Google Scholar] [CrossRef]
- Inagaki, M. New Carbons—Control of Structure and Functions; Elsevier: Amsterdam, The Netherlands, 2000. [Google Scholar]
- PDF-2 Data Base JCPDS-ICDD 1997. JCPDS—International Centre for Diffraction Data: Newtown Square, PA, USA, 1997.
- Holland, T.J.B.; Redfern, S.A.T. Unit cell refinement from powder diffraction data; the use of regression diagnostics. Mineralog. Mag. 1997, 61, 65–77. [Google Scholar] [CrossRef]
- Amenomija, Y.; Cvetanovic, R.J. Application of a temperature-programmed desorption technique to catalyst studies. Adv. Catal. 1967, 17, 103–149. [Google Scholar]
- Sample availability: Available from the authors.
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ischenko, E.V.; Matzui, L.Y.; Gayday, S.V.; Vovchenko, L.L.; Kartashova, T.V.; Lisnyak, V.V. Thermo-Exfoliated Graphite Containing CuO/Cu2(OH)3NO3:(Co2+/Fe3+) Composites: Preparation, Characterization and Catalytic Performance in CO Conversion. Materials 2010, 3, 572-584. https://doi.org/10.3390/ma3010572
Ischenko EV, Matzui LY, Gayday SV, Vovchenko LL, Kartashova TV, Lisnyak VV. Thermo-Exfoliated Graphite Containing CuO/Cu2(OH)3NO3:(Co2+/Fe3+) Composites: Preparation, Characterization and Catalytic Performance in CO Conversion. Materials. 2010; 3(1):572-584. https://doi.org/10.3390/ma3010572
Chicago/Turabian StyleIschenko, Elena V., Ludmila Yu. Matzui, Snezhanna V. Gayday, Ludmila L. Vovchenko, Tatyana V. Kartashova, and Vladyslav V. Lisnyak. 2010. "Thermo-Exfoliated Graphite Containing CuO/Cu2(OH)3NO3:(Co2+/Fe3+) Composites: Preparation, Characterization and Catalytic Performance in CO Conversion" Materials 3, no. 1: 572-584. https://doi.org/10.3390/ma3010572
APA StyleIschenko, E. V., Matzui, L. Y., Gayday, S. V., Vovchenko, L. L., Kartashova, T. V., & Lisnyak, V. V. (2010). Thermo-Exfoliated Graphite Containing CuO/Cu2(OH)3NO3:(Co2+/Fe3+) Composites: Preparation, Characterization and Catalytic Performance in CO Conversion. Materials, 3(1), 572-584. https://doi.org/10.3390/ma3010572