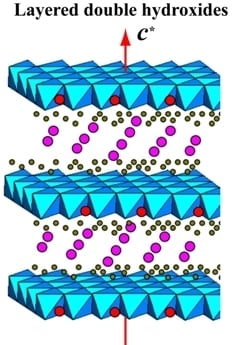
Morphologies, Preparations and Applications of Layered Double Hydroxide Micro-/Nanostructures
Abstract
:1. Introduction
2. LDH Morphology
2.1. Powdery LDHs


2.2. Spherical LDHs

2.3. 1-D LDHs Nanostructures


2.4. 2-D LDH Films
2.4.1. Parallel-Oriented LDH Films


2.4.2. Perpendicular-Oriented LDH Films

| Dimension | Morphology | Method | References |
|---|---|---|---|
| 0-D | powder | SNAS, coprecipitation, hydrothermal | 28-34 |
| sphere | LbL, coprecipitation | 15-18 | |
| 1-D | high aspect ratio | hydrothermal | 41 |
| nanowire | calcination and rehydrolysis, hydrothermal | 42, 43 | |
| nanobelt | coprecipitation | 19 | |
| nanotube | coprecipitation | 20 | |
| 2-D | parallel-oriented | solvent evaporation, electrochemical deposition spin-coating, LB, LbL, in-situ growth | 44-59 |
| perpendicular-oriented | in-situ growth | 21-26, 60-62 | |
| 3-D | 3DOM | coprecipitation | 39-40 |
3. Application of LDHs
3.1. Catalysts
3.2. Water Treatment
3.3. Additives in Concrete and Flame Retardants
3.4. LDHs in Biology and Medicine
4. Conclusions
Acknowledgements
References and Notes
- Cavani, F.; Trifiròa, F.; Vaccaria, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Rives, V. Layered Double Hydroxides: Present and Future; Nova Science Publishers: New York, NY, USA, 2001. [Google Scholar]
- Khan, A.I.; O’Hare, D. Intercalation chemistry of layered double hydroxides: Recent developments and applications. J. Mater. Chem. 2002, 12, 3191–3198. [Google Scholar] [CrossRef]
- Sideris, P.J.; Nielsen, U.G.; Gan, Z.; Grey, C.P. Mg/Al ordering in layered double hydroxides revealed by multinuclear NMR spectroscopy. Science 2008, 321, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-J.; Oh, J.-M.; Choy, J.-H. Human-related application and nanotoxicology of inorganic particles: Complementary aspects. J. Mater. Chem. 2008, 18, 615–620. [Google Scholar] [CrossRef]
- Oh, J.-M.; Biswick, T.T.; Choy, J.-H. Layered nanomaterials for green materials. J. Mater. Chem. 2009, 19, 2553–2563. [Google Scholar] [CrossRef]
- Williams, G.R.; O’Hare, D. Towards understanding, control and application of layered double hydroxide chemistry. J. Mater. Chem. 2006, 16, 3065–3074. [Google Scholar] [CrossRef]
- Xu, Z.P.; Qing, H.Z.; Gao, Q.L.; Ai, B.Y. Inorganic nanoparticles as carriers for efficient cellular delivery. Chem. Eng. Sci. 2006, 61, 1027–1040. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, F.; Evans, D.G.; Duan, X. Layered double hydroxide films: Synthesis, properties and applications. Chem. Commun. 2010, 46, 5197–5210. [Google Scholar] [CrossRef]
- Roeffaers, M.B.J.; Sels, B.F.; Uji-i, H.; De Schryver, F.C.; Jacobs, P.A.; De Vos Johan Hofkens, D.E. Spatially resolved observation of crystal-face-dependent catalysis by single turnover counting. Nature 2006, 439, 572–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Z.P.; Stevenson, G.S.; Lu, C.Q.; Lu, G.Q.; Bartlett, P.F.; Gray, P.P. Stable suspension of layered double hydroxide nanoparticles in aqueous solution. J. Am. Chem. Soc. 2006, 128, 36–37. [Google Scholar] [CrossRef] [PubMed]
- Gursky, J.A.; Blough, S.D.; Luna, C.; Gomez, C.; Luevano, A.N.; Gardner, E.A. Particle-particle interactions between layered double hydroxide nanoparticles. J. Am. Chem. Soc. 2006, 128, 8376–8377. [Google Scholar] [CrossRef] [PubMed]
- Gunawan, P.; Xu, R. Synthesis of unusual coral-like layered double hydroxide microspheres in a nonaqueous polar solvent/surfactant system. J. Mater. Chem. 2008, 18, 2112–2120. [Google Scholar] [CrossRef]
- Du, Y.; Hu, G.; O’Hare, D. Nucleation and growth of oriented layered double hydroxides on polymer resin beads. J. Mater. Chem. 2009, 19, 1160–1165. [Google Scholar] [CrossRef]
- Gunawan, P.; Xu, R. Direct assembly of snisotropic Layered Double Hydroxide (LDH) nanocrystals on spherical template for fabrication of drug-LDH hollow nanospheres. Chem. Mater. 2009, 21, 781–783. [Google Scholar] [CrossRef]
- Li, B.; He, J. Multiple Effects of Dodecanesulfonate in the Crystal Growth Control and Morphosynthesis of Layered Double hHdroxides. J. Phys. Chem. C 2008, 112, 10909–10917. [Google Scholar] [CrossRef]
- Li, L.; Ma, R.; Iyi, N.; Ebina, Y.; Takada, K.; Sasaki, T. Hollow nanoshell of layered double hyrdoxide. Chem. Commun. 2006, 42, 3125–3127. [Google Scholar] [CrossRef]
- Li, L.; Feng, Y.; Li, Y.; Zhao, W.; Shi, J. Fe3O4 core/layered double hydroxide dhell nanocomposite: Versatile magnetic matrix for anionic functional materials. Angew. Chem. Int. Ed. 2009, 48, 5888–5892. [Google Scholar] [CrossRef]
- Hu, G.; O’Hare, D. Unique layered double hydroxide morphologies using reverse microemulsion synthesis. J. Am. Chem. Soc. 2005, 127, 17808–17813. [Google Scholar] [CrossRef] [PubMed]
- De Jesús Martínez-Ortiz, M.; Lima, E.; Lara, V.; Vivar, J.M. Structural and textural evolution during folding of layers of layered double hydroxides. Langmuir 2008, 24, 8904–8911. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, F.; Fu, S.; Duan, X. In situ microstructure control of oriented layered double hydroxide monolayer films with curved hexagonal crystals as superhydrophobic materials. Adv. Mater. 2006, 18, 3089–3093. [Google Scholar] [CrossRef]
- Gao, Y.F.; Nagai, M.; Masuda, Y.; Sato, F.; Seo, W.S.; Koumoto, K. Surface precipitation of highly porous hydrotalcite-like film on al from a zinc aqueous solution. Langmuir 2006, 22, 3521–3527. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Jung, S.-H.; Jang, J.-W.; Oh, E.; Lee, K.-H. Simultaneous synthesis of Al-doped ZnO nanoneedles and zinc aluminum hydroxides through use of a seed layer. Cryst. Growth Des. 2008, 8, 4553–3558. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Huang, X.; Li, G.; Li, Z. Layered double hydroxide nano- and microstructures grown directly on metal substrates and their calcined products for application as Li-ion battery electrodes. Adv. Funct. Mater. 2008, 18, 1448–1458. [Google Scholar] [CrossRef]
- Zhang, F.; Zhao, L.; Chen, H.; Xu, S.L.; Evans, D.G.; Duan, X. Corrosion resistance of superhydrophobic layered double hydroxide films on aluminum. Angew. Chem. Int. Ed. 2008, 47, 2466–2469. [Google Scholar] [CrossRef]
- Guo, X.; Xu, S.; Zhao, L.; Lu, W.; Zhang, F.; Evans, D.G.; Duan, X. One-step hydrothermal crystallization of a layered double hydroxide/alumina bilayer film on aluminum and its corrosion resistance properties. Langmuir 2009, 25, 9894–9897. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhang, F.; Xu, S.; Evans, D.G.; Duan, X. Preparation of layered double hydroxide films with different orientations on the opposite sides of a glass substrate by in situ hydrothermal crystallization. Chem. Commun. 2009, 44, 6836–6838. [Google Scholar] [CrossRef]
- Burleigh, M.C.; Jayasundera, S.; Spector, M.S.; Thomas, C.W.; Markowitz, M.A.; Gaber, B.P. A new family of copolymers: Multifunctional periodic mesoporous organosilicas. Chem. Mater. 2004, 16, 3–5. [Google Scholar] [CrossRef]
- Hu, G.; Wang, N.; O’Hare, D.; Davis, J. One-step synthesis and AFM imaging of hydrophobic LDH monolayers. Chem. Commun. 2006, 42, 287–289. [Google Scholar] [CrossRef]
- Du, L.C.; Qu, B.J. Preparation of LLDPE/MgAl-LDH exfoliation nanocomposites with enhanced thermal properties by melt intercalation. Chin. J. Chem. 2006, 24, 1342–1345. [Google Scholar] [CrossRef]
- Hu, G.; Wang, N.; O’Hare, D.; Davis, A. Synthesis of magnesium aluminium layered double hydroxides in reverse microemulsions. J. Mater. Chem. 2007, 17, 2257–2266. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, F.; Zhang, R.; Evans, D.G.; Duan, X. Preparation of layered double-hydroxide nanomaterials with a uniform crystallite size using a new method involving separate nucleation and aging steps. Chem. Mater. 2002, 14, 4286–4291. [Google Scholar] [CrossRef]
- Gu, Z.; Xiang, X.; Fan, G.; Li, F. Facile synthesis and characterization of cobalt ferrite nanocrystals via a simple reduction-oxidation route. J. Phys. Chem. C 2008, 112, 18459–18466. [Google Scholar] [CrossRef]
- Xu, S.L.; Chen, Z.; Zhang, B.; Yu, J.; Zhang, F.Z.; Evans, D.G. Facile preparation of pure CaAl-layered double hydroxides and their application as a hardening accelerator in concrete. Chem. Eng. J. 2009, 155, 881–885. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, R.; Osada, M.; Iyi, N.; Ebina, Y.; Takada, K.; Sasaki, T. Synthesis, anion exchange, and delamination of Co-Al layered double hydroxide: Assembly of the exfoliated nanosheet/polyanion composite films and magneto-optical studies. J. Am. Chem. Soc. 2006, 128, 4872–4880. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.P.; Lu, J.; Wei, M.; Han, J.B.; Ma, J.; Li, F.; Evans, D.G.; Duan, X. Poly(p-phenylene) anionic derivative/layered double hydroxides ultra thin films with blue luminescence by layer-by-layer assembly. Angew. Chem. Int. Ed. 2009, 48, 3073–3076. [Google Scholar] [CrossRef]
- Han, J.B.; Lu, J.; Wei, M.; Wang, Z.L.; Duan, X. Heterogeneous ultrathin films fabricated by alternate assembly of exfoliated layered double hydroxides and polyanion. Chem. Commun. 2008, 44, 5188–5190. [Google Scholar] [CrossRef]
- Chen, D.; Wang, X.; Liu, T.; Wang, X.; Li, J. Electrically conductive poly(vinyl alcohol) hybrid films containing graphene and layered double hydroxide fabricated via layer-by-layer self-assembly. Appl. Mater. Interf. 2010, 2, 2005–2011. [Google Scholar] [CrossRef]
- Géraud, E.; Prévot, V.; Ghanbaja, J.; Leroux, F. Macroscopically ordered hydrotalcite-type materials using self-assembled colloidal crystal template. Chem. Mater. 2006, 18, 238–240. [Google Scholar] [CrossRef]
- Géraud, E.; Rafqah, S.; Sarakha, M.; Forano, C.; Prevot, V.; Leroux, F. Three dimensionally ordered macroporous layered double hydroxides: preparation by templated impregnation/coprecipitation and pattern stability upon calcination. Chem. Mater. 2008, 20, 1116–1125. [Google Scholar] [CrossRef]
- Tao, Q.; Zhang, Y.; Zhang, X.; Yuan, P.; He, H.P. Synthesis and characterization of layered double hydroxides with a high aspect ratio. J. Solid State Chem. 2006, 179, 708–715. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Jiao, Q.Z.; Ding, X.J.; Zhang, L.Q.; Liu, Y.Y. Hydrothermal synthesis of nanorods and nanowires of Mg/Al layered double hydroxides. Chem. Res. Chin. U. 2007, 23, 622–624. [Google Scholar] [CrossRef]
- Wu, H.; Jiao, Q.; Zhao, Y.; Huang, S.; Li, X.; Liu, H.; Zhou, M. Synthesis of Zn/Co/Fe-layered double hydroxide nanowires with controllable morphology in a water-in-oil microemulsion. Mater. Character. 2010, 61, 227–232. [Google Scholar] [CrossRef]
- Iyi, N.; Ebina, Y.; Sasaki, T. Water-swellable MgAl-LDH (Layered Double Hydroxide) hybrids: Synthesis, characterization, and film preparation. Langmuir 2008, 24, 5591–5598. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Y.; Li, C.; Liu, M.; Evans, D.G.; Duan, X. Large continuous, transparent and oriented self-supporting films of layered double hydroxides with tunable chemical composition. Chem. Commun. 2007, 43, 123–125. [Google Scholar] [CrossRef]
- Itaya, K.; Chang, H.C.; Uchida, I. Anion-exchanged hydrotalcite-like-clay modified electrodes. Inorg. Chem. 1987, 26, 624–626. [Google Scholar] [CrossRef]
- Lee, J.H.; Rhee, S.W.; Jung, D.Y. Solvothermal ion exchange of aliphatic dicarboxylates into the gallery space of layered double hydroxides immobilized on Si substrates. Chem. Mater. 2004, 16, 3774–3779. [Google Scholar] [CrossRef]
- Lee, J.H.; Rhee, S.W.; Jung, D.Y. Orientation-controlled assembly and solvothermal ion-exchange of layered double hydroxide nanocrystals. Chem. Commun. 2003, 39, 2740–2741. [Google Scholar]
- Lee, J.H.; Rhee, S.W.; Jung, D.Y. Selective layer reaction of layer-by-layer assembled layered double-hydroxide nanocrystals. J. Am. Chem. Soc. 2007, 129, 3522–3523. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Rhee, S.W.; Nam, H.J.; Jung, D.-Y. Surface Selective deposition of PMMA on layered double hydroxide nanocrystals immobilized on solid substrates. Adv. Mater. 2009, 21, 546–549. [Google Scholar] [CrossRef] [PubMed]
- Matthew, S.; Yarger, E.M.P.S.; Choi, K.-S. Electrochemical synthesis of Zn-Al layered double hydroxide (LDH) films. Inorg. Chem. 2008, 47, 5859–5865. [Google Scholar] [CrossRef] [PubMed]
- Scavetta, E.; Ballarin, B.; Gazzano, M.; Tonelli, D. Electrochemical behaviour of thin films of Co/Al layered double hydroxide prepared by electrodeposition. Electrochim. Acta 2009, 54, 1027–1033. [Google Scholar] [CrossRef]
- Khenifi, A.; Derriche, Z.; Forano, C.; Prevot, V.; Mousty, C.; Scavetta, E.; Ballarin, B.; Guadagnini, L.; Tonelli, D. Glyphosate and glufosinate detection at electrogenerated NiAl-LDH thin films. Analy. Chim. Acta 2009, 654, 97–102. [Google Scholar] [CrossRef]
- Zhang, F.; Sun, M.; Xu, S.L.; Zhao, L.; Zhang, B. Fabrication of oriented layered double hydroxide films by spin coating and their use in corrosion protection. Chem. Eng. J. 2008, 141, 362–367. [Google Scholar] [CrossRef]
- He, J.X.; Yamashita, S.; Jones, W.; Yamagishi, A. Templating effects of stearate monolayer on formation of Mg Al-Hydrotalcite. Langmuir 2002, 18, 1580–1586. [Google Scholar] [CrossRef]
- Lei, X.; Yang, L.; Zhang, F.; Evans, D.G.; Duan, X. Synthesis of oriented layered double hydroxide thin films on sulfonated polystyrene substrates. Chem. Lett. 2005, 34, 1610–1611. [Google Scholar] [CrossRef]
- Lv, Z.; Zhang, F.; Lei, X.; Yang, L.; Evans, D.G.; Duan, X. Microstructure-controlled synthesis of oriented layered double hydroxide thin films: Effect of varying the preparation conditions and a kinetic and mechanistic study of film formation. Chem. Eng. Sci. 2007, 62, 6069–6075. [Google Scholar] [CrossRef]
- Zhao, Y.; He, S.; Wei, M.; Evans, D.G.; Duan, X. Hierarchical films of layered double hydroxides by using a sol-gel process and their high adaptability in water treatment. Chem. Commun. 2010, 46, 3031–3033. [Google Scholar] [CrossRef]
- Zhang, L.H.; Li, F.; Evans, D.G.; Duan, X. Cu/Mn/Fe-layered double hydroxides and their mixed metal oxides: Physicochemical and catalytic properties in wet hydrogen peroxide oxidation of phenol. Ind. Eng. Chem. Res. 2010, 49, 5959–5968. [Google Scholar] [CrossRef]
- Lv, Z.; Zhang, F.; Lei, X.D.; Yang, L.; Xu, S.L.; Duan, X. In situ growth of layered double hydroxide films on anodic aluminum oxide/aluminum and its catalytic feature in aldol condensation of acetone. Chem. Eng. Sci. 2008, 63, 4055–4062. [Google Scholar] [CrossRef]
- Lv, L.; He, J.; Wei, M.; Duan, X. Kinetic studies on fluoride removal by calcined layered double hydroxides. Ind. Eng. Chem. Res. 2006, 45, 8623–8628. [Google Scholar] [CrossRef]
- Lv, L.; Li, L. Adsorption behavior of calcined layered double hydroxides towards removal of iodide contaminants. J. Radioanal. Nuclear Chem. 2007, 273, 221–226. [Google Scholar] [CrossRef]
- Raki, L.; Beaudoin, J.J.; Mitchell, L. Layered double hydroxide-like materials: Nanocomposites for use in concrete. Cement Concr. Res. 2004, 34, 1717–1724. [Google Scholar] [CrossRef]
- Shi, L.; Li, D.Q.; Wang, J.R.; Li, S.F.; Evans, D.G.; Duan, X. Synthesis, flame-retardant and smoke-suppressant properties of a borate-intercalated layered double hydroxide. Clays Clay Miner. 2005, 53, 294–300. [Google Scholar] [CrossRef]
- Ling, S.; Li, D.Q.; Li, S.; Wang, J.; D.G., E.; Duan, X. The structure, flame retarding and smoke suppressing properties of Zn-Mg-Al-CO32-layered double hydroxides. Chin. Sci. Bull. 2005, 50, 1101–1104. [Google Scholar]
- Wei, M.; Pu, M.; Guo, J.; Han, J.; Li, F.; He, J.; Evans, D.G.; Duan, X. Intercalation of L-dopa into layered double hydroxides: Enhancement of both chemical and stereochemical stabilities of a drug through host-guest interactions. Chem. Mater. 2008, 20, 5169–5180. [Google Scholar] [CrossRef]
- Wei, M.; Yuan, Q.; Evans, D.G.; Wang, Z.; Duan, X. Layered solids as a ‘Molecular Container’ for pharmaceutical agents: L-tyrosine-intercalated layered double hydroxides. J. Mater. Chem. 2005, 15, 1197–1203. [Google Scholar] [CrossRef]
- Ladewig, K.; Niebert, M.; Xu, Z.P.; Gray, P.P.; Lu, G.Q.M. Efficient siRNA delivery to mammalian cells using layered double hydroxide nanoparticles. Biomaterials 2010, 31, 1821–1829. [Google Scholar] [CrossRef] [PubMed]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kuang, Y.; Zhao, L.; Zhang, S.; Zhang, F.; Dong, M.; Xu, S. Morphologies, Preparations and Applications of Layered Double Hydroxide Micro-/Nanostructures. Materials 2010, 3, 5220-5235. https://doi.org/10.3390/ma3125220
Kuang Y, Zhao L, Zhang S, Zhang F, Dong M, Xu S. Morphologies, Preparations and Applications of Layered Double Hydroxide Micro-/Nanostructures. Materials. 2010; 3(12):5220-5235. https://doi.org/10.3390/ma3125220
Chicago/Turabian StyleKuang, Ye, Lina Zhao, Shuai Zhang, Fazhi Zhang, Mingdong Dong, and Sailong Xu. 2010. "Morphologies, Preparations and Applications of Layered Double Hydroxide Micro-/Nanostructures" Materials 3, no. 12: 5220-5235. https://doi.org/10.3390/ma3125220
APA StyleKuang, Y., Zhao, L., Zhang, S., Zhang, F., Dong, M., & Xu, S. (2010). Morphologies, Preparations and Applications of Layered Double Hydroxide Micro-/Nanostructures. Materials, 3(12), 5220-5235. https://doi.org/10.3390/ma3125220