Synthesis of Green-Emitting (La,Gd)OBr:Tb3+ Phosphors
Abstract
:1. Introduction
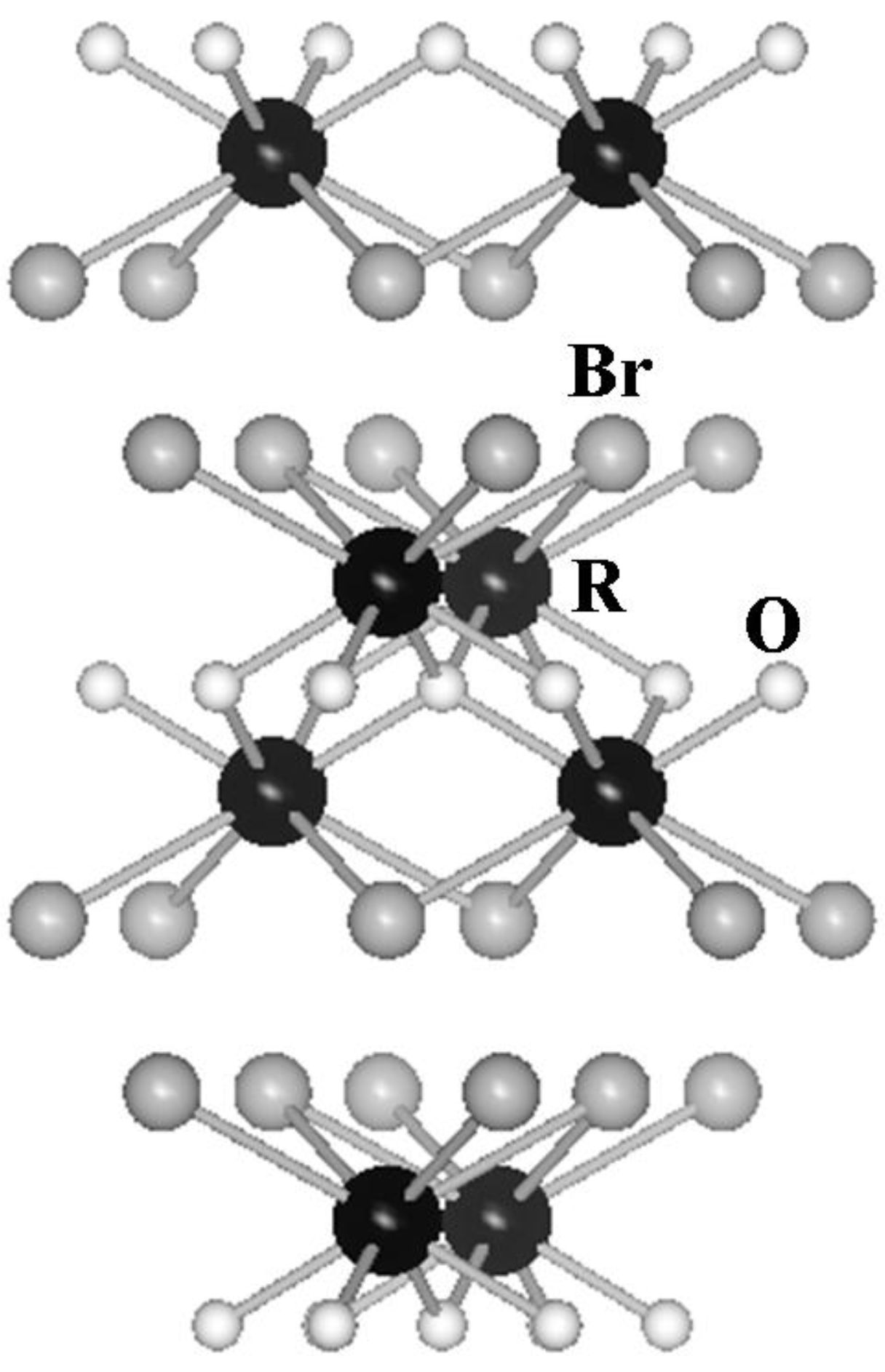
2. Results and Discussion
| Theoretical composition | Analyzed composition |
| LaOBr:5%Tb3+ | (La0.95Tb0.05)OBr |
| (La0.95Gd0.05)OBr:5%Tb3+ | (La0.9Gd0.05Tb0.05)OBr |
| (La0.9Gd0.1)OBr:5%Tb3+ | (La0.84Gd0.11Tb0.05)OBr |
| (La0.85Gd0.15)OBr:5%Tb3+ | (La0.79Gd0.16Tb0.05)OBr |
| (La0.8Gd0.2)OBr:5%Tb3+ | (La0.76Gd0.19Tb0.05)OBr |



| x = 0 | x = 0.05 | x = 0.10 | x = 0.15 | |
| 4f8-4f75d (1) (nm) | 243.9 | 243.4 | 242.8 | 242.1 |
| 4f8-4f75d (2) (nm) | 256.8 | 257.0 | 257.3 | 257.8 |
| 4f8-4f75d (3) (nm) | 282.9 | 283.6 | 284.3 | 284.8 |
| Energy separation width = (1) – (3) (cm-1) | 5652 | 5824 | 6012 | 6193 |


| x = 0 | x = 0.05 | x = 0.10 | x = 0.15 | |
| FWHM (degree) | 0.2531 | 0.2457 | 0.2457 | 0.2814 |
3. Experimental Section
4. Conclusion
Acknowledgements
References and Notes
- Kang, Y.C.; Kim, E.J.; Lee, D.Y.; Park, H.D. High Brightness LaPO4:Ce,Tb Phosphor Particles with Spherical Shape. J. Alloys Compd. 2002, 347, 166–170. [Google Scholar] [CrossRef]
- Honma, T.; Toda, K.; Ye, Z.G.; Sato, M. Concentration Quenching of the Eu3+ Activated Luminescence in Some Layered Perovskites with Two Dimensional Arrangement. J. Phys. Chem. Solids 1998, 59, 1187–1193. [Google Scholar] [CrossRef]
- Wang, Z.; Liang, H.; Wang, Q.; Luo, L.; Gong, M. Luminescent Properties of Tb3+ Activated Double Molybdates and Tungstates. Mater. Sci. Eng. B 2009, 164, 120–123. [Google Scholar] [CrossRef]
- Tamura, S.; Koyabu, K.; Masui, T.; Imanaka, N. Lithium Carbonate Flux Effect on the Luminescence Properties of Europium-doped Lanthanum Oxycarbonate Phosphor. Chem. Lett. 2004, 33, 58–59. [Google Scholar] [CrossRef]
- Masui, T.; Koyabu, K.; Tamura, S.; Imanaka, N. Synthesis of a New Green-emitting Phosphor Based on Lanthanum Oxycarbonate (La2O2CO3-II). J. Mater. Sci. 2005, 40, 1421–4123. [Google Scholar] [CrossRef]
- Masui, T.; Mayama, Y.; Koyabu, K.; Imanaka, N. Synthesis of New Green-emitting Gd2O2CO3:Tb3+ Fine Particles with High Luminescence Intensities. Chem. Lett. 2005, 34, 1236–1237. [Google Scholar] [CrossRef]
- Koyabu, K.; Masui, T.; Tamura, S.; Imanaka, N. Synthesis of a New Phosphor Based on Rare Earth Oxycarbonate. J. Alloys Compd. 2006, 408–412, 867–870. [Google Scholar] [CrossRef]
- Koyabu, K.; Mayama, Y.; Masui, T.; Imanaka, N. Synthesis of New Green Emitting Phosphor Based on Rare Earth Oxycarbonate. J. Alloys Compd. 2006, 418, 230–233. [Google Scholar] [CrossRef]
- Mayama, Y.; Koyabu, K.; Masui, T.; Tamura, S.; Imanaka, N. Synthesis of New Red Emitting Phosphor Based on Rare Earth Oxycarbonate. J. Alloys Compd. 2006, 418, 243–246. [Google Scholar] [CrossRef]
- Mayama, Y.; Masui, T.; Koyabu, K.; Imanaka, N. Enhancement of the Luminescent Intensity of the Green Emitting Gd2O2CO3:Tb Phosphor. J. Alloys Compd. 2008, 451, 132–135. [Google Scholar] [CrossRef]
- Kim, S.W.; Masui, T.; Imanaka, N. Synthesis of Red-emitting Phosphors Based on Gadolinium Oxysulfate by a Flux Method. Electrochemistry 2009, 77, 611–613. [Google Scholar] [CrossRef]
- Kim, S.W.; Masui, T.; Matsushita, H.; Imanaka, N. Synthesis of New Green-emitting Phosphor Based on Zirconium Oxyde Phosphate. Chem. Lett. 2009, 38, 1100–1101. [Google Scholar] [CrossRef]
- Haeuseler, H.; Jung, M. Single Crystal Growth and Structure of LaOBr and SmOBr. Mater. Res. Bull. 1986, 21, 1291–1294. [Google Scholar] [CrossRef]
- Hölsä, J.; Porcher, P. Crystal Field Effects in REOBr:Eu3+. J. Chem. Phys. 1982, 76, 2790–2797. [Google Scholar] [CrossRef]
- Hölsä, J.; Leskelä, M.; Niinistö, L. Thermal Stability of Rare Earth Oxybromides. Themochim. Acta 1980, 35, 79–83. [Google Scholar] [CrossRef]
- Li, Y.M.; Guillen, F.; Fouassier, C.; Hagenmuller, P. Comparative Study of Sensitization of the Luminescence of Trivalent Rare Earth Ions by Ce in LaOBr. J. Electrochem. Soc. 1985, 132, 717–721. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, W.; Yin, M. Fluorescence Spectroscopy of Er3+:LaOBr Prepared by NH4Br Solid State Reaction. Opt. Mater. 2004, 27, 605–608. [Google Scholar] [CrossRef]
- Wang, D.; Guo, Y.; Zhang, E.; Chao, X.; Yu, L.; Luo, J.; Zhang, W.; Yin, M. Synthesis and NIR-to-violet, Blue, Green, Red Upconversion Fluorescence of Er3+:LaOBr. J. Alloys Compd. 2005, 397, 1–4. [Google Scholar] [CrossRef]
- Guo, H. Two- and Three-photon Upconversion of LaOBr:Er3+. Opt. Mater. 2007, 29, 1840–1843. [Google Scholar] [CrossRef]
- Starick, D.; Golovkova, S.I.; Gurvic, A.M.; Herzog, G. Investigations on the X-ray Luminescence of LaOBr:RE3+ Phosphors. J. Lumin. 1988, 40–41, 199–200. [Google Scholar] [CrossRef]
- Wang, Q.; Bulou, A. Influence of Hydrostatic Pressure and Interatomic Distance upon the Energy Levels Scheme of Eu3+ in REOBr (RE = La, Gd or Y). Solid State Commun. 1995, 94, 309–315. [Google Scholar] [CrossRef]
- Rambabu, U.; Khanna, P.K.; Rao, I.C.; Buddhudu, S. Fluorescence Spectra of Eu3+-doped Lanthanide Oxybromide-based Powder Phosphors. Mater. Lett. 1998, 34, 269–274. [Google Scholar] [CrossRef]
- Reddy, K.R.; Aruna, V.; Balaji, T.; Annapurna, K.; Buddhudu, S. Photoluminescence Spectra of LaOBr:Eu3+ Powder Phosphors. Mater. Chem. Phys. 1998, 52, 157–160. [Google Scholar] [CrossRef]
- Hölsä, J.; Leskelä, M.; Niinistö, L. Concentration Quenching of Tb3+ Luminescence in LaOBr and Gs2O2S Phosphors. Mater. Res. Bull. 1979, 14, 1403–1409. [Google Scholar] [CrossRef]
- Hölsä, J.; Leskelä, M.; Niinistö, L. Sensitization of Tb3+ Luminescence with Ce3+ in LaOBr:Tb3+,Ce3+. J. Solid State Chem. 1981, 37, 267–270. [Google Scholar] [CrossRef]
- Zhao, F.T.; Cao, L.Y.; Xu, X.R. The Energy Transfer Between Ce3+ and Tb3+ Ions in LaOBr:Ce3+,Tb3+. J. Electrochem. Soc. 1987, 134, 3186–3190. [Google Scholar] [CrossRef]
- Starick, D.; Lange, W.; Herzog, G. Investigations on the Thermoluminescence of LaOBr:Tb3+ Phosphors. J. Thermal Anal. 1988, 33, 889–894. [Google Scholar] [CrossRef]
- Ronda, C.R.; Bechtel, H.; Kynast, U.; Welker, T. The Degradation Behavior of LaOBr:Tb Under Cathode-ray Excitation. J. Appl. Phys. 1994, 75, 4636–4641. [Google Scholar] [CrossRef]
- Yang, J.; Gong, J.; Fan, H.; Yang, L. The Structure and Luminescence Characteristics of LaOBr:Tb3+(Dy3+). J. Mater. Sci. 2005, 40, 3725–3728. [Google Scholar] [CrossRef]
- Mazurak, Z.; Garcia, A.; Fouassier, C. Luminescence Spectra and Crystal Field Analysis of LaOBr Doped with Tm3+. J. Phys.: Condens. Matter 1994, 6, 2031–2037. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta. Cryst. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Jia, P.Y.; Lin, J.; Han, X.M.; Yu, M. Pechini Sol-gel Deposition and Luminescence Properties of Y3Al5-xGdxO12:Ln3+ (Ln3+ = Eu3+, Ce3+, Tb3+; 0 ≤ x ≤ 5) Thin Films. Thin Solid Films 2005, 483, 122–129. [Google Scholar] [CrossRef]
- Blasse, G.; Bril, A. Investigations of Tb3+-Activated Phosphors. Philips Res. Rep. 1967, 22, 481–504. [Google Scholar]
- Pieterson, L.; Reid, M.F.; Burdick, G.W.; Meijerink, A. 4f➔4fn-15d1 Transitions of the Heavy Lanthanides: Experiment and Theory. Phys. Rev. B 2002, 65, 045114. [Google Scholar]
- Chong, M.K.; Pita, K.; Kam, C.H. Photoluminescence of Sol–gel-derived Y2O3:Eu3+ Thin-film Phosphors with Mg2+ and Al3+ Co-doping. Appl. Phys. A 2004, 79, 433–437. [Google Scholar]
- Sun, J.; Du, X.; Kyotani, T.; Tomita, A. Effect of Surface Treatment on Stability of Lanthanum Oxide Bromide Phosphor. React. Solids 1989, 7, 331–342. [Google Scholar]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kim, S.W.; Jyoko, K.; Masui, T.; Imanaka, N. Synthesis of Green-Emitting (La,Gd)OBr:Tb3+ Phosphors. Materials 2010, 3, 2506-2515. https://doi.org/10.3390/ma3042506
Kim SW, Jyoko K, Masui T, Imanaka N. Synthesis of Green-Emitting (La,Gd)OBr:Tb3+ Phosphors. Materials. 2010; 3(4):2506-2515. https://doi.org/10.3390/ma3042506
Chicago/Turabian StyleKim, Sun Woog, Kazuya Jyoko, Toshiyuki Masui, and Nobuhito Imanaka. 2010. "Synthesis of Green-Emitting (La,Gd)OBr:Tb3+ Phosphors" Materials 3, no. 4: 2506-2515. https://doi.org/10.3390/ma3042506
APA StyleKim, S. W., Jyoko, K., Masui, T., & Imanaka, N. (2010). Synthesis of Green-Emitting (La,Gd)OBr:Tb3+ Phosphors. Materials, 3(4), 2506-2515. https://doi.org/10.3390/ma3042506





