Order-Induced Selectivity Increase of Cu60Pd40 in the Semi-Hydrogenation of Acetylene
Abstract
:1. Introduction
2. Results and Discussion
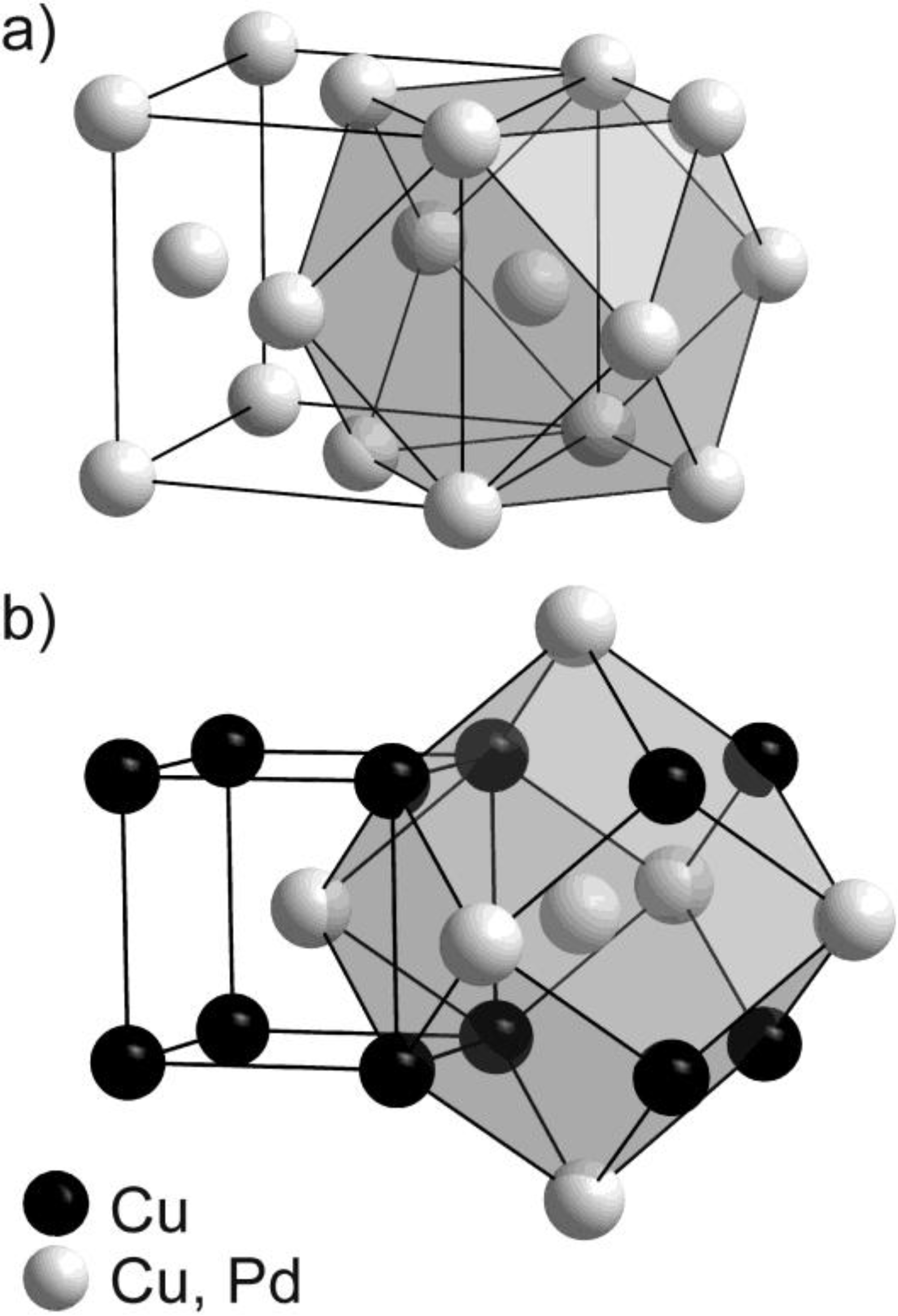
2.1. Synthesis and Phase Transformation


2.2. Electronic Structure Calculations



2.3. Catalysis


2.4. Ex-situ Characterization

2.5. In-situ Characterization
2.5.1. PGAA
2.5.2. XPS

3. Experimental Section
4. Conclusions
Acknowledgments
References
- Bond, G.C.; Dowden, D.A.; Mackenzie, N. The selective hydrogenation of acetylene. Trans. Farad. Soc. 1958, 54, 1537–1546. [Google Scholar] [CrossRef]
- Borodzinski, A.; Bond, G.C. Selective hydrogenation of ethyne in ethene-rich streams on palladium catalysts. Part 1. Effect of changes to the catalyst during reaction. Catal. Rev. 2006, 48, 91–144. [Google Scholar] [CrossRef]
- Lamberov, A.A.; Egorova, S.R.; Il’yasov, I.R.; Gil’manov, K.K.; Trifonov, S.V.; Shatilov, V.M.; Ziyatdinov, A.S. Changes in the course of reaction and regeneration of a Pd–Ag/Al2O3 catalyst for the selective hydrogenation of acetylene. Kinet. Catal. 2007, 48, 136–142. [Google Scholar] [CrossRef]
- Sachtler, W.M.H. Chemisorption complexes on alloy surfaces. Catal. Rev. Sci. Eng. 1976, 14, 193–210. [Google Scholar] [CrossRef]
- Zea, H.; Lester, K.; Datye, A.K.; Rightor, E.; Gulotty, R.; Waterman, W.; Smith, M. The influence of Pd–Ag catalyst restructuring on the activation energy for ethylene hydrogenation in ethylene-acetylene mixtures. Appl. Catal. A Gen. 2005, 282, 237–245. [Google Scholar] [CrossRef]
- Armbrüster, M.; Kovnir, K.; Behrens, M.; Teschner, D.; Grin, Y.; Schlögl, R. Pd−Ga intermetallic compounds as highly selective semihydrogenation catalysts. J. Amer. Chem. Soc. 2010, 132, 14745–14747. [Google Scholar] [CrossRef]
- Kovnir, K.; Armbrüster, M.; Teschner, D.; Venkov, T.V.; Jentoft, F.C.; Knop-Gericke, A.; Grin, Yu.; Schlögl, R. A new approach to well-defined, stable and site-isolated catalysts. Sci. Technol. Adv. Mater. 2007, 8, 420–427. [Google Scholar] [CrossRef]
- Massalski, T.B.; Okamoto, H.; Subramanian, P.R.; Kacprzak, L. Binary Alloy Phase Diagrams, 2nd ed.; ASM International: Geauga County, OH, USA, 1990. [Google Scholar]
- Friedrich, M.; Ormeci, A.; Grin, Y.; Armbrüster, M. PdZn or ZnPd: Charge transfer and Pd–Pd bonding as the driving force for the tetragonal distortion of the cubic crystal structure. Z. Anorg. Allg. Chem. 2010, 636, 1735–1739. [Google Scholar] [CrossRef]
- Borelius, G.; Johansson, C.H.; Linde, J.O. Die Gitterstrukturumwandlungen in Metallischen Mischkristallen (in German). Ann. Phys. 1928, 391, 299–318. [Google Scholar] [CrossRef]
- Gmelin—Handbuch der Anorg. Chemie(in German), 8th ed.; Springer-Verlag: Weinheim, Germany, 1949.
- Rienäcker, G.; Wessing, G.; Trautmann, G. Der Einfluß der Atomanordnung der Kupfer-Palladium-Mischkristalle auf ihre Eigenschaften als Katalysatoren des Ameisensäuredampf-Zerfalles. (in German). Z. Anorg. Allg. Chem. 1938, 236, 252–262. [Google Scholar] [CrossRef]
- Rienäcker, G.; Vormum, G. Die Parawasserstoff-Umwandlung an Kupfer-Nickel- und Kupfer-Palladium-Legierungen. (in German). Z. Anorg. Chem. 1956, 283, 287–298. [Google Scholar] [CrossRef]
- Rienäcker, G.; Müller, E.; Burmann, R. Die Eigenschaften von Kupfer-Palladium- und Kupfer-Platin-Legierungen als Katalysatoren der Äthylenhydrierung. (in German). Z. Anorg. Chem. 1943, 251, 55–70. [Google Scholar] [CrossRef]
- Guczi, L.; Schay, Z.; Weiss, A.H.; Nair, V.; Leviness, S. Acetylene hydrogenation selectivity control using Pd/Cu catalysts. React. Kinet. Catal. Lett. 1985, 27, 147–151. [Google Scholar] [CrossRef]
- Leviness, S.; Nair, V.; Weiss, A.H.; Schay, Z.; Guczi, L. Acetylene hydrogenation selectivity control on PdCu/Al2O3 catalysts. J. Mol. Catal. 1984, 25, 131–140. [Google Scholar] [CrossRef]
- Kim, S.K.; Lee, J.H.; Ahn, I.Y.; Kim, W.-J.; Moon, S.H. Performance of Cu-promoted Pd catalysts prepared by adding Cu using a surface redox method in acetylene hydrogenation. Appl. Catal. A 2011, 401, 12–19. [Google Scholar] [CrossRef]
- Ledesma, C.; Llorca, J. Dimethyl ether steam reforming over Cu–Zn–Pd/CeO2–ZrO2 catalytic monoliths. The role of Cu species on catalyst stability. J. Phys. Chem. C 2011, 115, 11624–11632. [Google Scholar] [CrossRef]
- Kugai, J.; Miller, J.T.; Guo, N.; Song, C. Oxygen-enhanced water gas shift on ceria-supported Pd–Cu and Pt–Cu bimetallic catalysts. J. Catal. 2011, 277, 46–53. [Google Scholar] [CrossRef]
- Yin, Z.; Zhou, W.; Gao, Y.; Ma, D.; Kiely, C.J.; Bao, X. Supported Pd–Cu bimetallic nanoparticles that have high activity for the electrochemical oxidation of methanol. Chem. Eur. J. 2012, 18, 4887–4893. [Google Scholar] [CrossRef] [PubMed]
- Efremenko, I.; Matatov-Meytal, U.; Sheintuch, M. Hydrodentrification with Pd–Cu catalysts: Catalyst optimization by experimental and quantum chemical approaches. Isr. J. Chem. 2006, 46, 1–15. [Google Scholar] [CrossRef]
- Sa, J.; Gross, S.; Vinek, H. Effect of the reducing step on the properties of Pd–Cu bimetallic catalysts used for denitration. Appl. Catal. A Gen. 2005, 294, 226–234. [Google Scholar] [CrossRef]
- Toshima, N.; Wang, Y. Novel preparation, characterization and catalytic properties of polymer-protected Cu/Pd bimetallic colloid. Chem. Lett. 1993, 9, 1611–1614. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.; Toshima, N. Nanoscopic naked Cu/Pd powder as air-resistant active catalyst for selective hydration of acrylonitrile to acrylamide. J. Phys. Chem. 1996, 100, 19533–19537. [Google Scholar] [CrossRef]
- Brayner, R.; Viau, G.; Bozon-Verduraz, F. Liquid-phase hydrogenation of hexadienes on metallic colloidal nanoparticles immobilized on supports via coordination capture by bifunctional organic molecules. J. Mol. Catal. A 2002, 227–238. [Google Scholar]
- Hähnlein, M.; Prüsse, U.; Hörold, S.; Vorlop, K.-D. Selektivitätsverbesserung des katalytischen Nitratabbaus durch Verwendung von Katalysatorgemischen aus nitratreduzierenden PdCu/Al2O3- und nitritreduzierenden Pd/Al2O3-Katalysatoren. (in German). Chem. Ing. Tech. 1996, 69, 90–93. [Google Scholar] [CrossRef]
- Green, D.J.; Lange, F.F.; James, M.R. Factors influencing residual surface stress due to a stress-induced phase transformation. J. Amer. Ceram. Soc. 1983, 66, 623–629. [Google Scholar] [CrossRef]
- Gogotsi, Y.; Baek, C.; Kirscht, F. Raman microscopy study of processing-induced phase transformations and residual stress in silicon. Semicond. Sci. Technol. 1999, 14, 936–944. [Google Scholar] [CrossRef]
- Friedrich, M.; Armbrüster, M. Crystallite size controls the crystal structure of Cu60Pd40 nanoparticles. Chem. Mater. 2009, 21, 5886–5891. [Google Scholar] [CrossRef]
- Teschner, D.; Borsodi, J.; Wootsch, A.; Revay, Z.; Hävecker, M.; Knop-Gericke, A.; Jackson, S.D.; Schlögl, R. The roles of subsurface carbon and hydrogen in palladium-catalyzed alkyne hydrogenation. Science 2008, 320, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Teschner, D.; Vass, E.; Hävecker, M.; Zafeiratos, S.; Schnörch, P.; Sauer, H.; Knop-Gericke, A.; Schlögl, R.; Chamam, M.; Wootsch, A.; et al. Alkyne hydrogenation over Pd catalysts: A new paradigm. J. Catal. 2006, 242, 26–37. [Google Scholar] [CrossRef]
- Galipaud, J.; Martin, M.H.; Roué, L.; Guay, D. Measurement of hydrogen solubility in PdxCu100−x thin films prepared by pulsed laser deposition: An electrochemical in situ X-ray diffraction analysis. J. Phys. Chem. C 2013, 117, 2688–2698. [Google Scholar] [CrossRef]
- Hedman, J.; Klasson, M.; Nilsson, R.; Nordling, C.; Sorokina, M.F.; Kljushnikov, O.I.; Nemnonov, S.A.; Trapeznikov, V.A.; Zyryanov, V.G. The electronic structure of some palladium alloys studied by ESCA and X-ray spectroscopy. Phys. Scr. 1971, 4, 195–201. [Google Scholar] [CrossRef]
- Studt, F.; Abild-Pedersen, F.; Bligaard, T.; Sørensen, R.Z.; Christensen, C.H.; Nørskov, J.K. Identification of non-precious metal alloy catalysts for selective hydrogenation of Acetylene. Science 2008, 320, 1320–1322. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.A.; Fernández-García, M.; Haller, G.L. Surface and bulk characterisation of metallic phases present during CO hydrogenation over Pd–Cu/KL zeolite catalysts. J. Catal. 1996, 164, 477–483. [Google Scholar]
- Zafeiratos, S.; Piccinin, S.; Teschner, D. Alloys in catalysis: Phase separation and surface segregation phenomena in response to the reactive environment. Catal. Sci. Technol. 2012, 2, 1787–1801. [Google Scholar] [CrossRef]
- Friedrich, M.; Teschner, D.; Knop-Gericke, A.; Armbrüster, M. Influence of bulk composition of the intermetallic compound ZnPd on surface composition and methanol steam reforming properties. J. Catal. 2012, 285, 41–47. [Google Scholar] [CrossRef]
- Kyriakou, G.; Boucher, M.B.; Jewell, A.D.; Lewis, E.A.; Lawton, T.J.; Baber, A.E.; Tierney, H.L.; Flytzani-Stephanopoulos, M.; Sykes, E.C.H. Isolated metal atom geometries as a strategy for selective heterogeneous hydrogenations. Science 2012, 335, 1209–1212. [Google Scholar] [CrossRef] [PubMed]
- Powder Cell for Windows, version 2.4; BAM: Berlin, Germany, 2000.
- Knop-Gericke, A.; Kleimenov, E.; Hävecker, M.; Blume, R.; Teschner, D.; Zafeiratos, S.; Schlögl, R.; Bukhtiyarov, V.I.; Kaichev, V.V.; Prosvirin, I.P.; et al. X-Ray photoelectron spectroscopy for investigation of heterogeneous catalytic processes. Adv. Catal. 2009, 52, 213–272. [Google Scholar]
- Casa XPS, version 2.3.16; Casa Software Ltd.: Teignmouth, UK, 2010.
- Yeh, J.J.; Lindau, I. Atomic subshell photoionization cross sections and asymmetry parameters: 1 ≤ Z ≤ 103. At. Data Nucl. Data Tables 1985, 32, 1–155. [Google Scholar] [CrossRef]
- NIST Electron Inelastic-Mean-Free-Path Database, version 1.2; NIST Data Gateway: Gaithersburg, MD, USA, 2010.
- Tanuma, S.; Powell, C.J.; Penn, D.R. Calculations of electron inelastic mean free paths. Surf. Interface Anal. 1991, 17, 911–926. [Google Scholar] [CrossRef]
- Vickerman, J.S.; Gilmore, I.S. Surface Analysis—The Principal Techniques; Wiley: Chichester, UK, 2009. [Google Scholar]
- Szentmiklósi, L.; Belgya, T.; Révay, Z.; Kis, Z. Upgrade of the prompt-gamma activation analysis (PGAA) and the neutron induced prompt-gamma spectroscopy (NIPS) facilities at the budapest research reactor. J. Radioanal. Nucl. Chem. 2010, 286, 501–505. [Google Scholar] [CrossRef]
- Molnár, G.L. Handbook of Prompt Gamma Activation Analysis with Neutron Beams; Kluwer Academic Publishers: Dordrecht, the Netherlands, 2004. [Google Scholar]
- Koepernik, K.; Eschrig, H. Full-potential nonorthogonal local-orbital minimum-basis band-structure scheme. Phys. Rev. B 1999, 59, 1743–1757. [Google Scholar] [CrossRef]
- Perdew, J.P.; Wang, Y. Accurate and simple representation of the electron-gas correlation energy. Phys. Rev. B. 1992, 45, 13244–13249. [Google Scholar] [CrossRef]
- Rao, C.N.; Rao, K.K. Effect of temperature on lattice parameters of some silver-palladium alloys. Can. J. Phys. 1964, 42, 1336–1342. [Google Scholar]
- Koepernik, K.; Velicky, B.; Hayn, R.; Eschrig, H. Self-consistent LCAO-CPA method for disordered alloys. Phys. Rev. B 1997, 55, 5717–5729. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Friedrich, M.; Villaseca, S.A.; Szentmiklósi, L.; Teschner, D.; Armbrüster, M. Order-Induced Selectivity Increase of Cu60Pd40 in the Semi-Hydrogenation of Acetylene. Materials 2013, 6, 2958-2977. https://doi.org/10.3390/ma6072958
Friedrich M, Villaseca SA, Szentmiklósi L, Teschner D, Armbrüster M. Order-Induced Selectivity Increase of Cu60Pd40 in the Semi-Hydrogenation of Acetylene. Materials. 2013; 6(7):2958-2977. https://doi.org/10.3390/ma6072958
Chicago/Turabian StyleFriedrich, Matthias, Sebastián Alarcón Villaseca, László Szentmiklósi, Detre Teschner, and Marc Armbrüster. 2013. "Order-Induced Selectivity Increase of Cu60Pd40 in the Semi-Hydrogenation of Acetylene" Materials 6, no. 7: 2958-2977. https://doi.org/10.3390/ma6072958
APA StyleFriedrich, M., Villaseca, S. A., Szentmiklósi, L., Teschner, D., & Armbrüster, M. (2013). Order-Induced Selectivity Increase of Cu60Pd40 in the Semi-Hydrogenation of Acetylene. Materials, 6(7), 2958-2977. https://doi.org/10.3390/ma6072958




