Green Synthesis of Metallic Nanoparticles via Biological Entities
Abstract
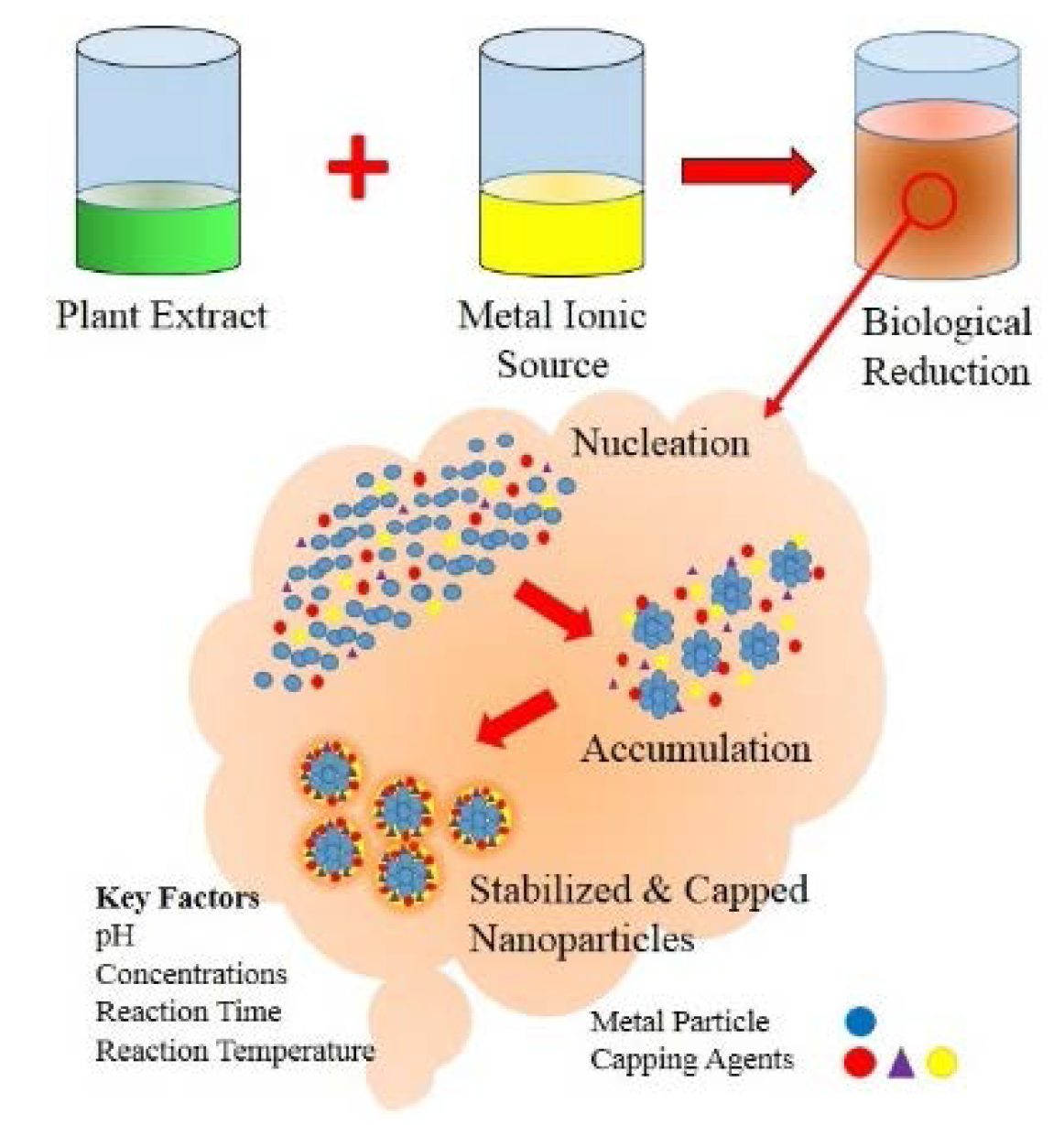
:1. Introduction
2. Characterisation Techniques
3. Biological Synthesis of Nanoparticles
| Microorganism | Nano Particle | Size (nm) | Extracellular/Intracellular | Reference |
|---|---|---|---|---|
| Actinomycetes | - | - | - | - |
| Rhodococcus sp. | Au | 5 to 15, Spherical | I | [93] |
| Thermomonospora sp. | Au | 8, Spherical | E | [47,48] |
| Algae | - | - | - | - |
| Chlorella vulgaris | Au | 40 to 60, Spheroid, polyhedral | I | [121] |
| Sargassum wightii | Au, Ag | Spheroid | E | [122] |
| Bacteria | - | - | - | - |
| Escherichia coli | CdS | 2 to 5, Spherical | I | [123] |
| Pseudomonas aeruginosa | Au | 15 to 30 Spherical | E | [53] |
| Pseudomonas stutzeri | Ag | Up to 200, various shapes | I | [109] |
| Fungus | - | - | - | - |
| Aspergillus flavus | Ag | 8 to 10 Spherical | I | [124] |
| Colletotrichum sp. | Au | 20 to 40 Spherical | E | [125] |
| Fusarium oxysporum | Au | 20 to 40, Spherical, triangular | E | [126] |
| Volvariella volvacea | Ag & Au | 20 to 150, Spherical, hexagonal | E | [127] |
| Viral | - | - | - | - |
| M13 bacteriophage | CdS, ZnS | Quantum dots, nanowires | E | [128] |
| M13 bacteriophage | HAP | Hydroxyapatite fibrils | E | [129,130] |
| Bacteriophage | Ca | Fibrils | [131,132] | |
| Tobacco mosaic virus (TMV) | Silica | Various shapes | E | [133,134] |
| Tobacco mosaic virus (TMV) | SiO2, CdS, PbS, Fe2O3 | Nanotubes on surface | E | [60,135] |
| Yeast | - | - | - | - |
| Candida glabrata | CdS | 2, Spherical | I | [62] |
| Saccharomycetes. cerevisiae | Sb2O3 | 3 to 10, Spherical | I | [77] |
| Candida glabrata (Yeast) | CdS | 3 to 100 | I | [136] |
| Yeast strain MKY3 | Ag | 2 to 5, Hexagonal | E | [63] |
| Schizosaccharomyces pombe | CdS | 1 to 2, Hexagonal | I, I | [62,110] |
| Torulopsis sp. | PbS | 2 to 5, Spherical | I | [137] |
4. Microbial Routes for Nanoparticle Synthesis
4.1. Actinomycetes
4.2. Algae
4.3. Bacteria
4.4. Fungi
4.5. Viruses
4.6. Yeasts
5. Biological Synthesis of Metal Nanoparticles via Plants
| Plant | Nanoparticle | Size (nm) | Shape | Reference |
|---|---|---|---|---|
| Aloe vera | Au & Ag | 50 to 350 | Spherical, triangular | [200] |
| Aloe vera | In2O3 | 5 to 50 | Spherical | [201] |
| Camelia sinensis | Ag, Au | 30 to 40 | Spherical, triangular, irregular | [202] |
| Citrullus colocynthis | Ag | 31 | Spherical | [203] |
| Curcuma longa | Pd | 10 to 15 | Spherical | [120] |
| Diopyros kaki | Pt | 15 to 19 | Crystalline | [204] |
| Eucalyptus macrocarpa | Au | 20 to 100 | Spherical, triangular, hexagonal | [84] |
| Ag | 10 to 100 | Spherical, cubes | [92] | |
| Mangifera indica | Ag | 20 | Spherical, triangular, hexagonal | [205] |
| Rhododendron dauricum | Ag | 25 to 40 | Spherical | [206] |
| Psidium guajava | Au | 25 to 30 | Spherical | [207] |
| Pyrus sp. (Pear fruit extract) | Au | 200 to 500 | Triangular, hexagonal | [208] |
| Terminalia catappa | Au | 10 to 35 | Spherical | [209] |
5.1. Factors Affecting Biological Synthesis of Metal Nanoparticles
5.1.1. Influence of pH
5.1.2. Influence of Reactant Concentration
5.1.3. Influence of Reaction Time
5.1.4. Influence of Reaction Temperature
5.2. Major Nanoparticles Synthesized by Plant Extracts
5.2.1. Gold and Silver Nanoparticles


5.2.2. Copper and Copper Oxide Nanoparticles
5.2.3. Palladium and Platinium Nanoparticles
5.2.4. Titanium Dioxide and Zinc Oxide Nanoparticles
5.2.5. Indium Oxide, Iron Oxide, Lead, and Selenium Nanoparticles
6. Applications of Nanoparticles & Biologically Inspired Templates
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Goodsell, D.S. Bionanomedicine in action. In Bionanotechnology: Lessons from Nature; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2004. [Google Scholar]
- Daniel, M.C.; Astruc, D. Gold nanoparticles: Assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. J. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef]
- Krolikowska, A.; Kudelski, A.; Michota, A.; Bukowska, J. SERS studies on the structure of thioglycolic acid monolayers on silver and gold. Surf. Sci. 2003, 532, 227–232. [Google Scholar] [CrossRef]
- Zharov, V.P.; Kim, J.W.; Curiel, D.T.; Everts, M. Self-assembling nanoclusters in living systems: Application for integrated photothermal nanodiagnostics and nanotherapy. Nanomed. Nanotechnol. Biol. Med. 2005, 1, 326–345. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Mandal, S.; Selvakannan, P.R.; Parischa, R.; Mandale, A.B.; Sastry, M. Investigation into the interaction between surface-bound alkylamines and gold nanoparticles. Langmuir 2003, 19, 6277–6282. [Google Scholar] [CrossRef]
- Bogunia-Kubik, K.; Sugisaka, M. From molecular biology to nanotechnology and nanomedicine. BioSystems 2002, 65, 123–138. [Google Scholar] [CrossRef]
- Perez, J.; Bax, L.; Escolano, C. Roadmap Report on Nanoparticles; Willems & Van Den Wildenberg: Barcelona, Spain, 2005. [Google Scholar]
- Sperling, R.A.; Gil, P.R.; Zhang, F.; Zanella, M.; Parak, W.J. Biological applications of gold nanoparticles. Chem. Soc. Rev. 2008, 37, 1896–1908. [Google Scholar] [CrossRef] [PubMed]
- Puvanakrishnan, P.; Park, J.; Chatterjee, D.; Krishnan, S.; Tunnel, J.W. In vivo tumor targeting of gold nanoparticles: Effect of particle type and dosing strategy. Int. J. Nanomed. 2012, 7, 1251–1258. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, R.; Murkherjee, P. Biological properties of “naked” metal nanoparticles. Adv. Drug Deliv. Rev. 2008, 60, 1284–1306. [Google Scholar] [CrossRef] [PubMed]
- Sykora, D.; Kasicka, V.; Miksik, I.; Rezanka, P.; Zaruba, K.; Matejka, P.; Kral, V. Applications of gold nanoparticles in separation sciences. J. Sep. Sci. 2010, 30, 372–387. [Google Scholar] [CrossRef] [PubMed]
- Torres-Chavolla, E.; Ranasinghe, R.J.; Alocilja, E.C. Characterization and functionalization of biogenic gold nanoparticles for biosensing enhancement. IEEE Trans. Nanobiotechnol. 2010, 9, 533–538. [Google Scholar] [CrossRef]
- Cai, W.; Gao, T.; Hong, H.; Sun, J. Applications of gold nanoparticles in cancer nanotechnology. Nanotechnol. Sci. Appl. 2008, 1, 17–32. [Google Scholar] [PubMed]
- Bhumkar, D.R.; Joshi, H.M.; Sastry, M.; Pokharkar, V.B. Chitosan reduced gold nanoparticles as novel carriers for transmucosal delivery of insulin. Pharm. Res. 2007, 24, 1415–1426. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, X.; Liao, Z.; Zhang, G.; Liu, Q.; Tang, J.; Peng, Y.; Liu, X.; Luo, Q. A randomized comparative trial between Acticoat and SD-Ag in the treatment of residual burn wounds including safety analysis. Burns 2007, 33, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Li, W.R.; Xie, X.B.; Shi, Q.S.; Duan, S.S.; Ouyang, Y.S.; Chen, Y.B. Antibacterial effect of silver nanoparticles on Staphylococcus aureus. Biometals 2011, 24, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Cox, S.G.; Cullingworth, L.; Rode, H. Treatment of paediatricburns with a nanocrystalline silver dressing compared with standard wound care in a burns unit: A cost analysis. S. Afr. Med. J. 2011, 101, 728–731. [Google Scholar] [PubMed]
- Pollini, M.; Paladini, F.; Catalno, M.; Taurino, A.; Licciulli, A.; Maffezzoli, A.; Sannio, A. Antibacterial coatings on haemodialysis catheters by photochemical deposition of silver nanoparticles. J. Mater. Sci. Mater. Med. 2011, 22, 2005–2012. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.S.; Stern, J.M.; Vanni, A.J.; Kelley, R.S.; Baumgart, E.; Field, D.; Libertino, J.A.; Summerhayes, I.C. In vitro analysis of a nanocrystalline silver-coated surgical mesh. Surg. Infect. 2007, 8, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Asha-Rani, P.V.; Mun, G.L.K.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano 2009, 3, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Hrapovic, S.; Liu, Y.; Male, K.B.; Luong, J.H.T. Electrochemical biosensing plateform using platinum nanoparticles and carbon nanotubes. Anal. Chem. 2004, 76, 1083–1088. [Google Scholar] [CrossRef] [PubMed]
- Gopidas, K.R.; Whitesell, J.K.; Fox, M.A. Synthesis, characterization and catalytic activity of a palladium nanoparticle cored dendrimier. Nano Lett. 2003, 3, 1757–1760. [Google Scholar] [CrossRef]
- Akhtar, M.S.; Panwar, J.; Yun, Y.S. Biogenic synthesis of metallic nanoparticles by plant extracts. ACS Sustain. Chem. Eng. 2013, 1, 591–602. [Google Scholar] [CrossRef]
- Mandali, P.K.; Chand, D.K. Palladium nanoparticles catalysed Suzuki cross-coupling reactions in ambient conditions. Catal. Commun. 2011, 31, 16–20. [Google Scholar] [CrossRef]
- Coccia, F.; Tonucci, L.; Bosco, D.; Bressan, M.; d’Alessandro, N. One pot synthesis of lignin-stabilized platinum and palladium nanoparticles and their catalytic behaviours in oxidation and reduction reactions. Green Chem. 2012, 14, 1073–1078. [Google Scholar] [CrossRef]
- Chen, Z.H.; Jie, J.S.; Luo, L.B.; Wang, H.; Lee, C.S.; Lee, S.T. Applications of silicon nanowires functionalized with palladium nanoparticles in hydrogen sensors. Nanotechnology 2007, 18, 1–5. [Google Scholar] [CrossRef]
- West, P.R.; Ishii, S.; Naik, G.V.; Emani, N.K.; Shalaev, V.M.; Boltasseva, A. Searching for better plasmonic materials. Laser Photonics Res. 2010, 4, 795–808. [Google Scholar] [CrossRef]
- Pankhurst, Q.A.; Connolly, J.; Jones, S.K.; Dobson, J. Applications of magnetic nanoparticles in biomedicine. J. Phys. D Appl. Phys. 2003, 36, R167–R181. [Google Scholar] [CrossRef]
- Njagi, E.C.; Huang, H.; Stafford, L.; Genuino, H.; Galindo, H.M.; Collins, J.B. Biosynthesis of iron and silver nanoparticles at room temperature using aqueous sorghum bran extracts. Langmuir 2011, 27, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Lee, G.; Jang, N.R.; Yun, J.H.; Song, J.Y.; Kim, B.S. Biological synthesis of copper nanoparticles using plants extract. Nanotechnology 2011, 1, 371–374. [Google Scholar]
- Brayner, R.; Ferrari-Iliou, R.; Brivois, N.; Djediat, S.; Benedetti, M.; Fiévet, F. Toxicological impact studies based on Escherichia coli bacteria in ultrafine ZnO nanoparticles colloidal medium. Nano Lett. 2006, 6, 866–870. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.S.; Patel, H.; Patel, T.; Patel, K.; Selvaraj, K. Biosynthesis of Se nanoparticles and its effect on UV-induced DNA damage. Colloids Surf. B Biointerfaces 2012, 103, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Mafune, F.; Kohno, J.; Takeda, Y.; Kondow, T.J. Dissociation and aggregation of gold nanoparticles under laser irradiation. J. Phys. Chem. B 2001, 105, 9050–9056. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, D.J. Fabrication of heterogeneous binary arrays of nanoparticles via colloidal lithography. J. Am. Chem. Soc. 2008, 130, 5616–5617. [Google Scholar] [CrossRef] [PubMed]
- Treguer, M.; Cointet, C.; Remita, H.; Khatouri, J.; Mostafavi, M.; Amblard, J.; Belloni, J.J. Dose rate effect on radiolytic synthesis of gold-silver bimetallic clusters in solution. J. Phys. Chem. B 1998, 102, 4310–4321. [Google Scholar] [CrossRef]
- Chen, W.; Cai, W.; Zhang, L.; Wang, G.; Zhang, L. Sonochemical processes and formation of gold nanoparticles within pores of mesoporous silica. J. Colloid Interface Sci. 2001, 238, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Eustis, S.; Hsu, H.Y.; El-Sayed, M.A. Gold nanoparticle formation from photochemical reduction of Au3+ by continuous excitation in colloidal solutions: A proposed molecular mechanism. J. Phys. Chem. B 2005, 109, 4811–4815. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Sanchez, L.; Blanco, M.C.; Lopez-Quintela, M.A. Electrochemical synthesis of silver nanoparticles. J. Phys. Chem. B 2002, 104, 9683–9688. [Google Scholar] [CrossRef]
- Starowiicz, M.; Stypula, B.; Banas, J. Electrochemical synthesis of silver nanoparticles. Electrochem. Commun. 2006, 8, 227–230. [Google Scholar] [CrossRef]
- Frattini, A.; Pellegri, N.; Nicastro, D.; de Sanctis, O. Effect of amine groups in the synthesis of Ag nanoparticles using aminosilanes. Mater. Chem. Phys. 2005, 94, 148–152. [Google Scholar] [CrossRef]
- Alexandridis, P. Gold nanoparticle synthesis, morphology control, and stabilization by functional polymers. Chem. Eng. Technol. 2011, 14, 15–38. [Google Scholar] [CrossRef]
- Wang, L.; Chen, X.; Zhan, J.; Chai, Y.; Yang, C.; Xu, L.; Zhuang, W.; Jing, B. Synthesis of gold nano and microplates in hexagonal liquid crystals. J. Phys. Chem. B 2005, 109, 3189–3194. [Google Scholar] [CrossRef] [PubMed]
- You, H.; Yang, S.; Ding, B.; Yang, H. Synthesis of colloidal metal and metal alloy nanoparticles for electrochemical energy applications. Chem. Soc. Rev. 2013, 42, 2880–2904. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, N.; Muddapur, U. Biosynthesis of metal nanoparticles: A review. J. Nanotechnol. 2014, 2014, 510246. [Google Scholar] [CrossRef]
- Ai, J.; Biazar, E.; Jafarpour, M.; Montazeri, M.; Majdi, A.; Aminifard, S.; Zafari, M.; Akbari, H.R.; Rad, H.G. Nanotoxicology and nanoparticle safety in biomedical designs. Int. J. Nanomed. 2011, 6, 1117–1127. [Google Scholar]
- Jain, N.; Bhargava, A.; Majumdar, S.; Panwar, J. Extracellular biosynthesis and characterization of silver nanoparticles using Aspergillus flavus NJP08: A mechanism prospective. Nanoscale 2011, 3, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Senapati, S.; Khan, M.I.; Kumar, R.; Sastry, M. Extracellular biosynthesis of monodisperse gold nanoparticles by a novel extremophilic actinomycete, Thermomonospora sp. Langmuir 2003, 19, 3550–3553. [Google Scholar] [CrossRef]
- Sastry, M.; Ahmad, A.; Khan, M.I.; Kumar, R. Biosynthesis of metal nanoparticles using fungi and actinomycete. Curr. Sci. 2003, 85, 162–170. [Google Scholar]
- Roh, Y.; Lauf, R.J.; McMillan, A.D.; Zhang, C.; Rawn, C.J.; Bai, J.; Phelps, T.J. Microbial synthesis and the characterization of metal-substituted magnetites. Solid State Commun. 2001, 118, 529–534. [Google Scholar] [CrossRef]
- Lengke, M.; Southam, G. Bioaccumulation of gold by sulphate-reducing bacteria cultured in the presence of gold (I)-thiosulfate complex. Acta 2006, 70, 3646–3661. [Google Scholar]
- Nair, B.; Pradeep, T. Coalescence of nanoclusters and formation of submicron crystallites assisted by Lactobacillus strains. Cryst. Growth Des. 2002, 2, 293–298. [Google Scholar] [CrossRef]
- Joerger, T.K.; Joerger, R.; Olsson, E.; Granqvist, C.G. Bacteria as workers in the living factor: Metal accumulating bacteria and their potential for materials science. Trends Biotechnol. 2001, 19, 15–20. [Google Scholar] [CrossRef]
- Husseiny, M.I.; El-Aziz, M.A.; Badr, Y.; Mahmoud, M.A. Biosynthesis of gold nanoparticles using Pseudomonas aeruginosa. Spectrochim. Acta A 2007, 67, 1003–1006. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.; Ahmad, A.; Mandal, D.; Senapati, S.; Sainkar, S.R.; Khan, M.I.; Parishcha, R.; Aiayumar, P.V.; Alam, M.; Kumar, R.; et al. Fungus-mediated synthesis of silver nanoparticles and their immobilization in the mycelia matrix: A novel biological approach to nanoparticles synthesis. Nano Lett. 2001, 1, 515–519. [Google Scholar] [CrossRef]
- Ahmad, A.; Senapati, S.; Khan, M.I.; Kumar, R.; Ramani, R.; Srinivas, V.; Sastry, M. Intracellular synthesis of gold nanoparticles by a novel alkalotolerant actinomycete Rhodococcus species. Nanotechnology 2003, 14, 824–828. [Google Scholar] [CrossRef]
- Ahmad, A.; Senapati, S.; Khan, M.I.; Kumar, R.; Sastry, M. Extra-/intracellular, biosynthesis of gold nanoparticles by an alkalotolerant fungus, Trichothecium sp. J. Biomed. Nanotechnol. 2005, 1, 47–53. [Google Scholar] [CrossRef]
- Kuber, C.; Souza, S.F. Extracellular biosynthesis of silver nanoparticles using the fungus Aspergillus fumigates. Colloids Surf. B 2006, 47, 160–164. [Google Scholar] [CrossRef]
- Philip, D. Green synthesis of gold and silver nanoparticles using Hibiscus rosa sinensis. Phys. E 2010, 42, 1417–1424. [Google Scholar] [CrossRef]
- Kumar, P.; Singh, P.; Kumari, K.; Mozumdar, S.; Chandra, R. A green approach for the synthesis of gold nanotriangles using aqueous leaf extract of Callistemon viminalis. Mater. Lett. 2011, 65, 595–597. [Google Scholar] [CrossRef]
- Lee, S.W.; Mao, C.; Flynn, C.; Belcher, A.M. Ordering of quantum dots using genetically engineered viruses. Science 2002, 296, 892–895. [Google Scholar] [CrossRef] [PubMed]
- Merzlyak, A.; Lee, S.W. Phage as template for hybrid materials and mediators for nanomaterials synthesis. Curr. Opin. Chem. Biol. 2006, 10, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Dameron, C.T.; Reeser, R.N.; Mehra, R.K.; Kortan, A.R.; Carroll, P.J.; Steigerwald, M.L.; Brus, L.E.; Winge, D.R. Biosynthesis of cadmium sulphide quantum semiconductor crystallites. Nature 1989, 338, 596–597. [Google Scholar] [CrossRef]
- Kowshik, M.; Arhtaputre, S.; Kharrazi, S.; Vogel, W.; Urban, J.; Kulkarni, S.K.; Paknikar, K.M. Extracellular synthesis of silver nanoparticles by a silver-tolerant yeast strain MKY3. Nanotechnology 2003, 14, 95–100. [Google Scholar] [CrossRef]
- Gericke, M.; Pinches, A. Biological synthesis of metal nanoparticles. Hydrometallurgy 2006, 83, 132–140. [Google Scholar] [CrossRef]
- Mohanpuria, P.; Rana, N.K.; Yadav, S.K. Biosynthesis of nanoparticles: Technological concepts and future applications. J. Nanopart. Res. 2008, 10, 507–517. [Google Scholar] [CrossRef]
- Khan, A.A.; Fox, E.K.; Gorzny, M.L.; Nikulina, E.; Brougham, D.F.; Wege, C.; Bittner, A.M. pH control of the electrostatic binding of gold and iron oxide nanoparticles to tobacco mosaic virus. Langmuir 2013, 29, 2094–2098. [Google Scholar] [PubMed]
- Courchesne, N.M.D.; Klug, M.; Chen, P.Y.; Kooi, S.E.; Yun, D.S.; Hong, N.; Fang, N.X.; Belcher, A.M. Assembly of a bacteriophage-based template for the organization of materials into nanoporous networks. Adv. Mater. 2014, 26, 3398–3404. [Google Scholar] [PubMed]
- Cao, B.; Xu, H.; Mao, C. Transmission electron microscopy as a tool to image bioinorganic nanohybrids: The case of phage-gold nanocomposites. Microsc. Res. Tech. 2011, 74, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Kale, A.; Bao, Y.; Zhou, Z.; Prevelige, P.E.; Gupta, A. Directed self-assembly of CdS quantum dots on bacteriophage P22 coat protein templates. Nanotechnology 2013, 24, 045603. [Google Scholar] [CrossRef] [PubMed]
- Ngweniform, P.; Li, D.; Mao, C. Self-assembly of drug-loaded liposomes on genetically engineered protein nanotubes: A potential anti-cancer drug delivery vector. Soft Matter 2009, 5, 954–956. [Google Scholar] [CrossRef]
- Tang, S.; Mao, C.; Liu, Y.; Kelly, D.Q.; Banerjee, S.K. Protein-mediated nanocrystal assembly for flash memory fabrication. IEEE Trans. Electron. Devices 2007, 54, 433–438. [Google Scholar] [CrossRef]
- Janardhananan, S.K.; Narayan, S.; Abbineni, G.; Hayhurst, A.; Mao, C. Architectonics of phage-liposome nanowebs as optimized photosensitizer vehicals for photodynamic cancer therapy. Mol. Cancer Ther. 2010, 9, 2524–2535. [Google Scholar] [CrossRef] [PubMed]
- Ngweniform, P.; Abbineni, G.; Cao, B.; Mao, C. Self-assembly of drug-loaded liposomes on genetically engineered target-recognizing M13 phage: A novel nanocarrier for targeted drug delivery. Small 2009, 5, 1963–1969. [Google Scholar] [CrossRef] [PubMed]
- Iravani, S. Green synthesis of metal nanoparticles using plants. Green Chem. 2011, 13, 2638–2650. [Google Scholar] [CrossRef]
- Thakkar, K.N.; Mhatre, S.S.; Parikh, R.Y. Biological synthesis of metallic nanoparticles. Nanotechnol. Biol. Med. Nanomed. 2010, 6, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Swami, A.; Selvakannan, P.R.; Pasricha, R.; Sastry, M. One-step synthesis of ordered two dimensional assemblies of silver nanoparticles by the spontaneous reduction of silver ions by pentadecylphenol Langmuir monolayers. J. Phys. Chem. B 2004, 108, 19269–19275. [Google Scholar] [CrossRef]
- Jha, A.K.; Prasad, K.; Kulkarni, A.R. Plant system: Nature’s nanofactory. Colloids Surf. B 2009, 73, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Bar, H.; Bhui, D.K.; Sahoo, G.P.; Sarkar, P.; de Sankar, P.; Misra, A. Green synthesis of silver nanoparticles using latex of Jatropha curcas. Colloids Surf. A 2009, 339, 134–139. [Google Scholar] [CrossRef]
- Shankar, S.S.; Rai, A.; Ahmad, A.; Sastry, M. Rapid synthesis of Au, Ag, and bimetallic Au core-Ag shell nanoparticles using Neem (Azadirachta indica) leaf broth. J. Colloid Interface Sci. 2004, 275, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Birnbaum, A.J.; Pique, A. Laser induced extra-planar propulsion for three dimensional microfabrication. Appl. Phys. Lett. 2011, 98, 134101–134106. [Google Scholar] [CrossRef]
- Sepeur, S. Nanotechnology: Technical Basics and Applications; Vincentz Network GmbH & Co: Hannover, Germany, 2008. [Google Scholar]
- Nielson, R.; Kaehr, B.; Shear, J.B. Micro-replication and design of biological architectures using dynamic-mask multi-photon lithography. Small 2009, 5, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Meyers, M.A.; Mishra, A.; Benson, D.J. Mechanical properties of nanocrystalline materials. Prog. Mater. Sci. 2006, 51, 427–556. [Google Scholar] [CrossRef]
- Poinern, G.E.J.; Le, X.; Chapman, P.; Fawcett, D. Green biosynthesis of gold nanometre scale plate using the leaf extracts from an indigenous Australian plant Eucalyptus macrocarpa. Gold Bull. 2013, 46, 165–173. [Google Scholar] [CrossRef]
- Naik, R.R.; Stringer, S.J.; Agarwal, G.; Jones, S.; Stone, M.O. Biomimetic synthesis and patterning of silver nanoparticles. Nat. Mater. 2002, 1, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.X.; Zheng, J.; Gao, G.; Kong, Y.F.; Zhi, X.; Wang, K.; Zhang, X.Q.; da Cui, X. Biosynthesis of gold nanoparticles using chloroplasts. Int. J. Nanomed. 2011, 6, 2899–2906. [Google Scholar] [CrossRef] [PubMed]
- Poinern, G.E.J. A Laboratory Course in Nanoscience and Nanotechnology, 1st ed.; CRC Press Taylor & Francis: Boca Raton, FL, USA, 2014. [Google Scholar]
- Eppler, A.S.; Rupprechter, G.; Anderson, E.A.; Somorjai, G.A. Thermal and chemical stability and adhesion strength of Pt nanoparticle arrays supported on silica studied by transmission electron microscopy and atomic force microscopy. J. Phys. Chem. B 2000, 104, 7286–7292. [Google Scholar] [CrossRef]
- Cao, G. Nanostructures and Nanomaterials: Synthesis, Properties and Applications; Imperial College Press: London, UK, 2004. [Google Scholar]
- Feldheim, D.L.; Foss, C.A. Metal Nanoparticles: Synthesis, Characterization, and Applications; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Devika, R.; Elumalai, S.; Manikandan, E.; Eswaramoothy, D. Biosynthesis of silver nanoparticles using fungus pleurotus ostreatus and their antimicrobial activity. Open Access Sci. Rep. 2012, 1, 1–5. [Google Scholar]
- Poinern, G.E.J.; Shah, M.; Chapman, P.; Fawcett, D. Green biosynthesis of silver nanocubes using the leaf extracts from Eucalyptus macrocarpa. Nano Bull. 2013, 2, 1–7. [Google Scholar]
- Mishra, A.; Tripathy, S.K.; Yun, S. Fungus mediated synthesis of gold nanoparticles and their conjugation with genomic DNA isolated from Escherichia coli and Staphylococcus aureus. Process Biochem. 2012, 47, 701–711. [Google Scholar] [CrossRef]
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The optical properties of metal nanoparticles: Influence of size, shape and dielectric environment. J. Phys. Chem. 2003, 107, 668–677. [Google Scholar] [CrossRef]
- Jiang, J.; Oberdörster, G.; Biswas, P. Characterization of size, surface charge, and agglomeration state of nanoparticle dispersions for toxicological studies. J. Nanopart. Res. 2009, 11, 77–89. [Google Scholar] [CrossRef]
- Klug, H.P.; Alexander, L.E. X-ray Diffraction Procedures for Poly-crystallite and Amorphous Materials, 2nd ed.; Wiley: New York, NY, USA, 1974. [Google Scholar]
- Barrett, C.S.; Cohen, J.B.; Faber, J.; Jenkins, R.; Leyden, D.E.; Russ, J.C.; Predecki, P.K. Advances in X-ray Analysis; Plenum Press: New York, NY, USA, 1986; Volume 29. [Google Scholar]
- Kneipp, K.; Wang, Y.; Kneipp, H.; Perelman, L.T.; Itzkan, I.; Dasari, R.R.; Fela, M.S. Single molecule detection using surface-enhanced Raman scattering (SERS). Phys. Rev. Lett. 1997, 78, 1667–1670. [Google Scholar] [CrossRef]
- Gowramma, B.; Keerthi, U.; Mokula, R.; Rao, D.M. Biogenic silver nanoparticles production and characterization from native stain of Corynebacterium species and its antimicrobial activity. 3 Biotech 2015, 5, 195–201. [Google Scholar] [CrossRef]
- Makarov, V.V.; Love, A.J.; Sinitsyna, O.V.; Makarova, S.S.; Yaminsky, I.V.; Taliansky, M.E.; Kalinina, N.O. Green nanotechnologies: Synthesis of metal nanoparticles using plants. Acta Naturae 2014, 6, 35–44. [Google Scholar] [PubMed]
- Baker, S.; Harini, B.P.; Rakshith, D.; Satish, S. Marine microbes: Invisible nanofactories. J. Pharm. Res. 2013, 6, 383–388. [Google Scholar] [CrossRef]
- Stephen, J.R.; Macnaughton, S.J. Developments in terrestrial bacterial remediation of metals. Curr. Opin. Biotechnol. 1999, 10, 230–233. [Google Scholar] [CrossRef]
- Bollag, J.M.; Mertz, T.; Otjen, L. Role of microorganisms in soil bioremediation. In Bioremediation through Rhizosphere Technology; American Chemical Society: Washington, DC, USA, 1994; Volume 563, pp. 2–10. [Google Scholar]
- Mann, S. Biomimetic Materials Chemistry; VCH Publishers: New York, NY, USA, 1996. [Google Scholar]
- Bhattacharya, D.; Gupta, R.K. Nanotechnology and potential of microorganisms. Crit. Rev. Biotechnol. 2005, 25, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Sastry, M.; Ahmad, A.; Khan, M.I.; Kumar, R. Microbial nanoparticle production. In Nanobiotechnology; Niemeyer, C.M., Mirkin, C.A., Eds.; Wiley-VCH: Weinheim, Germany, 2004; pp. 126–135. [Google Scholar]
- Mandal, D.; Bolander, M.E.; Mukhopadhyay, D.; Sarkar, G.; Mukherjee, P. The use of microorganisms for the formation of metal nanoparticles and their application. Appl. Microbiol. Biotechnol. 2006, 69, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Lengke, M.; Ravel, B.; Fleet, M.E.; Wanger, G.; Gordon, R.A.; Southam, G. Mechanisms of gold bioaccumulation by filamentous cyanobacteria from gold (III) chloride complex. Environ. Sci. Technol. 2006, 40, 6304–6309. [Google Scholar] [CrossRef] [PubMed]
- Belliveau, B.H.; Starodub, M.E.; Cotter, C.; Trvors, J.T. Metal resistance and accumulation in bacteria. Biotechnol. Adv. 1987, 5, 101–107. [Google Scholar] [CrossRef]
- Kowshik, M.; Deshmukh, N.; Vogel, W.; Urban, J.; Kulkarni, S.K.; Paknikar, K.M. Microbial synthesis of semiconductor CdS nanoparticles, their characterization, and their use in the fabrication of an ideal diode. Biotechnol. Bioeng. 2002, 78, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Duran, N.; Marcato, P.D.; Alves, O.L.; Souza, G.I.; Esposito, E. Mechanistic aspects of biosynthesis of silver nanoparticles by several Fusarium oxysporum strains. J. Nanobiotechnol. 2005, 3, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.K.; Chisti, Y.; Banerjee, U.C. Synthesis of metallic nanoparticles using plants. Biotechnol. Adv. 2013, 31, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Beveridge, T.J.; Murray, R.G.E. Sites of metal deposition in the cell wall of Bacillus subtilis. J. Bacteriol. 1980, 141, 876–887. [Google Scholar] [PubMed]
- Mehra, R.K.; Winge, D.R. Metal ion resistance in fungi: Molecular mechanisms and their regulated expression. J. Cell. Biochem. 1991, 45, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Southam, G.; Beveridge, T.J. The in vitro formation of placer gold by bacteria. Geochim. Cosmochim. Acta 1994, 58, 4527–4530. [Google Scholar] [CrossRef]
- Singh, R.; Gautam, N.; Mishra, A.; Gupta, R. Heavy metals and living systems: An overview. Indian J. Pharmacol. 2011, 43, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.; Ahmad, A.; Mandal, D.; Senapati, S.; Sainkar, S.R.; Khan, M.I.; Ramani, R.; Parischa, R.; Ajayakumar, P.V.; Alam, M.; et al. Bioreduction of AuCl4 ions by the fungus, Verticillium sp. and surface trapping of the gold nanoparticles formed. Angew. Chem. Int. Ed. 2001, 40, 3585–3588. [Google Scholar] [CrossRef]
- Singh, A.; Jain, D.; Upadhyay, M.K.; Khandelwal, N.; Verma, H.N. Green synthesis of silver nanoparticles using Argemone mexicana leaf extract and evaluation of their antimicrobial activity. Dig. J. Nanomater. Biostruct. 2010, 5, 483–489. [Google Scholar]
- Narayanan, K.B.; Sakthivel, N. Coriander leaf mediated biosynthesis of gold nanoparticles. Mater. Lett. 2008, 62, 4588–4590. [Google Scholar] [CrossRef]
- Sathishkumar, M.; Sneha, K.; Yun, Y.S. Palladium nanocrystals synthesis using Curcuma longa tuber extract. Int. J. Mater. Sci. 2009, 4, 11–17. [Google Scholar]
- Luangpipat, T.; Beattie, I.R.; Chisti, Y.; Haverkamp, R.G. Gold nanoparticles produced in a microalga. J. Nanopart. Res. 2011, 13, 6439–6445. [Google Scholar] [CrossRef]
- Singaravelu, G.; Arockiamary, J.S.; Kumar, V.G.; Govindaraju, K. A novel extracellular synthesis of monodisperse gold nanoparticles using marine alga, Sargassum wightii Greville. Colloids Surf. B Biointerfaces 2007, 57, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, R.Y.; Mao, C.; Gao, X.; Burt, J.L.; Belcher, A.M.; Georgiou, G.; Lverson, B.L. Bacterial biosynthesis of cadmium sulfide nanocrystals. Chem. Biol. 2004, 11, 1553–1559. [Google Scholar] [CrossRef] [PubMed]
- Vigneshwaran, N.; Ashtaputre, N.M.; Varadarajan, P.V.; Nachane, R.P.; Paralikar, K.M.; Balasubramanya, R.H. Biological synthesis of silver nanoparticles using the fungus Aspergillus flavus. Mater. Lett. 2007, 61, 1413–1418. [Google Scholar] [CrossRef]
- Shankar, S.S.; Ahmad, A.; Pasricha, R.; Sastry, M. Bioreduction of chloroaurate ions by geranium leaves and its endophytic fungus yields gold nanoparticles of different shapes. J. Mater. Chem. 2003, 13, 1822–1826. [Google Scholar] [CrossRef]
- Mukherjee, P.; Senapati, S.; Mandal, D.; Ahmad, A.; Khan, M.I.; Kumar, R.; Sastry, M. Extracellular synthesis of gold nanoparticles by the fungus Fusarium oxysporum. ChemBioChem 2002, 3, 461–463. [Google Scholar] [CrossRef]
- Philip, D. Biosynthesis of Au, Ag and Au-Ag nanoparticles using edible mushroom extract. Spectrochim. Acta A 2009, 73, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.; Flynn, C.E.; Hayhurst, A.; Sweeney, R.; Qi, J.; Georgiou, G.; Iverson, B.; Belcher, A.M. Viral assembly of oriented quantum dot nanowires. Proc. Natl. Acad. Sci. USA 2003, 100, 6946–6951. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Abbineni, G.; Cao, B.; Mao, C. Nanofibrous bio-inorganic hybrid structures formed through self-assembly and oriented mineralization of genetically engineered phage nanofibers. Small 2010, 6, 2230–2235. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Nimmo, S.L.; Cao, B.; Mao, C. Oxide formation on biological nanostructures via a structure-directing agent: Towards an understanding of precise structural transcription. Chem. Sci. 2012, 3, 2639–2645. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Cao, B.; Mao, C. Bacteriophage bundles with prealigned Ca2+ initiate the oriented nucleation and growth of hydroxylapatite. Chem. Mater. 2010, 22, 3630–3636. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Cao, B.; George, A.; Mao, C. Self-Assembly and mineralization of genetically modifiable biological nanofibers driven by β- structure formation. Biomacromolecules 2011, 12, 2193–2199. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, F.M.; Coradin, T.; Aime, C. Self-Assembly in biosilicification and biotemplated silica materials. Nanomaterials 2014, 4, 792–812. [Google Scholar] [CrossRef]
- Royston, E.S.; Brown, A.D.; Harris, M.T.; Culver, J.N. Preparation of silica stabilized tobacco mosaic virus templates for the production of metal and layered nanoparticles. J. Colloid Interface Sci. 2009, 332, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Shenton, W.; Douglas, T.; Young, M.; Stubbs, G.; Mann, S. Inorganic-organic nanotube composites from template mineralization of tobacco mosaic virus. Adv. Mater. 1999, 11, 253–256. [Google Scholar] [CrossRef]
- Gericke, M.; Pinches, A. Microbial production of gold nanoparticles. Gold Bull. 2006, 83, 132–140. [Google Scholar] [CrossRef]
- Kowshik, M.; Vogel, W.; Urban, J.; Kulkarni, S.K.; Paknikar, K.M. Microbial synthesis of semiconductor PbS nanocrystallites. Adv. Mater. 2002, 14, 815–818. [Google Scholar] [CrossRef]
- Prabhu, S.; Poulose, E.K. Silver nanoparticles: Mechanism of anti-microbial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett. 2012, 2, 1–10. [Google Scholar] [CrossRef]
- Arun, P.; Shanmugaraju, V.; Renga Ramanujam, J.; Senthil Prabhu, S.; Kumaran, E. Biosynthesis of silver nanoparticles from Corynebacterium sp. and its antimicrobial activity. Int. J. Cur. Microbiol. App. Sci. 2013, 2, 57–64. [Google Scholar]
- Selvakumar, R.; Arul Jothi, N.; Jayavignesh, V.; Karthikaiselvi, K.; Antony, G.I.; Sharmila, P.R.; Kavitha, S.; Swaminathan, K. As (V) removal using carbonized yeast cells containing silver nanoparticles. Water Res. 2011, 45, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Mie, R.; Samsudin, M.W.; Din, L.B.; Ahmad, A.; Ibrahim, N.; Adnan, S.N. Synthesis of silver nanoparticles with antibacterial activity using the lichen Parmotrema praesorediosum. Int. J. Nanomed. 2013, 9, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, K.; Selvaraj, S.; Ramachandra Murty, V. Microbial production of silver nanoparticles. Dig. J. Nanomater. Biostruct. 2010, 5, 135–140. [Google Scholar]
- Sudha, S.S.; Karthic, R.; Rengaramanujam, J. Microalgae mediated synthesis of silver nanoparticles and their antibacterial activity against pathogenic bacteria. Indian J. Exp. Biol. 2013, 51, 393–399. [Google Scholar] [PubMed]
- Abdeen, S.; Geo, S.; Sukanya, S.; Praseetha, P.K.; Dhanya, R.P. Biosynthesis of Silver nanoparticles from Actinomycetes for therapeutic applications. Int. J. Nano Dimens. 2014, 5, 155–162. [Google Scholar]
- Karthik, L.; Kumar, G.; Vishnu-Kirthi, A.; Rahuman, A.A.; Rao, V.B. Streptomyces sp. LK3 mediated synthesis of silver nanoparticles and its biomedical application. Bioprocess. Biosyst. Eng. 2014, 37, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Golinska, P.; Wypij, M.; Ingle, A.P.; Gupta, I.; Dahm, H.; Rai, M. Biogenic synthesis of metal nanoparticles from actinomycetes: Biomedical applications and cytotoxicity. Appl. Microbiol. Biotechnol. 2014, 98, 8083–8097. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, R.; Kumar, A.; Abraham, J. A biological approach to the synthesis of Silver nanoparticles with strptomyces sp JAR1 and its antimicrobial activity. Sci. Pharm. 2013, 81, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Sukanya, M.K.; Saju, K.A.; Praseetha, P.K.; Sakthivel, G. Therapeutic potential of biologically reduced silver nanoparticles from Actinomycete cultures. J. Nanosci. 2013, 2013, 940719. [Google Scholar] [CrossRef]
- Prakasham, R.S.; Buddana, S.K.; Yannam, S.K.; Guntuku, G.S. Characterization of silver nanoparticles synthesized by using marine isolate Streptomyces albidoflavus. J. Microbiol. Biotechnol. 2012, 22, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Balagurunathan, R.; Radhakrishnan, M.; Rajendran, R.B.; Velmurugan, D. Biosynthesis of gold nanoparticles by actinomycete Streptomyces viridogens strain HM10. J. Biochem. Biophys. 2011, 48, 331–335. [Google Scholar]
- Korbekandi, H.; Iravani, S.; Abbasi, S. Production of nanoparticles using organisms. Crit. Rev. Biotechnol. 2009, 29, 279–306. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Oza, G.; Pandey, S.; Sharon, M. Biogenic fabrication of gold nanoparticles using Halomonas salina. J. Microbiol. Biotechnol. Res. 2012, 2, 485–492. [Google Scholar]
- He, S.; Guo, Z.; Zhang, Y.; Gu, N. Biosynthesis of gold nanoparticles using the bacteria Rhodopseudomonas capsulata. Mater. Lett. 2007, 61, 3984–3987. [Google Scholar] [CrossRef]
- Waghmare, S.S.; Deshmukh, A.M.; Sadowski, Z. Biosynthesis, optimization, purification and characterization of gold nanoparticles. Afr. J. Microbiol. Res. 2014, 8, 138–146. [Google Scholar] [CrossRef]
- Kumar, A.; Kaur, K.; Sharma, S. Synthesis, characterization and antibacterial potential of silver nanoparticles by Morus nigra leaf extract. Indian. J. Pharm. Biol. Res. 2013, 1, 16–24. [Google Scholar]
- Xie, J.; Lee, J.Y.; Wang, D.I.C.; Ting, Y.P. Silver nanoplates: From biological to biomimetic synthesis. ACS Nano 2007, 1, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Govindaraju, K.; Kiruthiga, V.; Kumar, V.G.; Singaravelu, G. Extracellular synthesis of silver nanoparticles by a marine alga, Sargassum wightii Grevilli and their antibacterial effects. J. Nanosci. Nanotechnol. 2009, 9, 5497–501. [Google Scholar] [CrossRef] [PubMed]
- Rajasulochana, P.; Dhamotharan, R.; Murugakoothan, P.; Murugesan, S.; Krishnamoorthy, P. Biosynthesis and characterization of gold nanoparticles using the alga Kappaphycus alvarezii. Int. J. Nanosci. 2010, 9, 511–516. [Google Scholar] [CrossRef]
- Mata, Y.N.; Blázquez, M.L.; Ballester, A.; González, F.; Muñoz, J.A. Gold biosorption and bioreduction with brown alga Fucus vesiculosus. J. Hazard. Mater. 2009, 166, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Senapati, S.; Syed, A.; Moeez, S.; Kumar, A.; Ahmad, A. Intracellular synthesis of gold nanoparticles using alga Tetraselmis kochinensis. Mater. Lett. 2012, 79, 116–118. [Google Scholar] [CrossRef]
- Castro, L.; Blázquez, M.L.; Muñoz, J.A.; González, F.; Ballester, A. Biological synthesis of metallic nanoparticles using algae. IET Nanobiotechnol. 2013, 7, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, G.G.; Brar, S.K.; Kaur, S.; Verma, M. Green approach for nanoparticle biosynthesis by fungi: current trends and applications. Crit. Rev. Biotechnol. 2012, 32, 49–73. [Google Scholar] [CrossRef] [PubMed]
- Iravani, S. Bacteria in nanoparticle synthesis: Current status and future prospects. Int. Sch. Res. Not. 2014, 2014, 359316. [Google Scholar] [CrossRef]
- Sunkar, S.; Nachiyar, C.V. Biogenesis of antibacterial silver nanoparticles using the endophytic bacterium Bacillus cereus isolated from Garcinia xanthochymu. Asian Pac. J. Trop. Biomed. 2012, 12, 953–959. [Google Scholar] [CrossRef]
- Tollamadugu, N.V.K.V.; Prasad, T.; Kambala, V.S.R.; Naidu, R. A critical review on biogenic silver nanoparticles and their antimicrobial activity. Curr. Nanosci. 2011, 7, 531–544. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Fu, J.K.; Chen, P.; Yu, X.; Yang, P. Studies on biosorption of Au3+ by Bacillus megaterium. Acta Microbiol. Sin. 2000, 40, 425–429. [Google Scholar]
- Sneha, K.; Sathishkumar, M.; Mao, J.; Kwak, I.S.; Yun, Y.S. Corynebacterium glutamicum-mediated crystallization of silver ions through sorption and reduction processes. Chem. Eng. J. 2010, 162, 989–996. [Google Scholar] [CrossRef]
- Lin, Z.Y.; Fu, J.K.; Wu, J.M.; Liu, Y.Y.; Cheng, H. Preliminary study on the mechanism of non-enzymatic bioreduction of precious metal ions. Acta Phys. Chim. Sin. 2001, 17, 477–480. [Google Scholar]
- Fu, J.K.; Liu, Y.Y.; Gu, P.Y.; Liang, S.D.; Yu, L.Z.; Xin, Y.B.; Zhou, W.S. Spectroscopic characterization on the biosorption and bioreduction of Ag(I) by Lactobacillus sp A09. Acta Phys. Chim. Sin. 2000, 16, 779–782. [Google Scholar]
- Hulkoti, N.I.; Taranath, T.C. Biosynthesis of nanoparticles using microbes—A review. Colloids Surf. B Biointerfaces 2014, 121, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Yong, P.; Rowsen, N.A.; Farr, J.P.G.; Harris, I.R.; Macaskie, L.E. Bioreduction and biocrystallization of palladium by Desulfovibrio desulfuricans NCIMB 8307. Biotechnol. Bioeng. 2002, 80, 369–379. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Zhang, Y.; Guo, Z.; Gu, N. Biosynthesis of gold nanowires using extract of Rhodopseudomonas capsulata. Biotechnol. Prog. 2008, 24, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Kathiresan, K.; Manivannan, S.; Nabeel, M.A.; Dhivya, B. Studies on silver nanoparticles synthesized by a marine fungus, Penicillium fellutanum isolated from coastal mangrove sediment. Colloids Surf. B 2009, 71, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Gade, A.K.; Bonde, P.; Ingle, A.P.; Marcato, P.D.; Duran, N.; Rai, M.K. Exploitation of Aspergillus niger for synthesis of silver nanoparticles. J. Biobased Mater. Bioenergy 2008, 2, 243–247. [Google Scholar] [CrossRef]
- Narayanan, K.B.; Sakthivel, N. Biological synthesis of metal nanoparticles by microbes. Adv. Colloid Interface Sci. 2010, 156, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Volesky, B.; Holan, Z.R. Biosorption of heavy metals. Biotechnol. Prog. 1995, 11, 235–250. [Google Scholar] [CrossRef] [PubMed]
- Kumar, C.V.; McLendon, G.L. Nanoencapsulation of cytochrome c and horseradish peroxidase at the galleries of a-zirconium phosphate. Chem. Mater. 1997, 9, 863–870. [Google Scholar] [CrossRef]
- Macdonald, I.D.G.; Smith, W.E. Orientation of cytochrome c adsorbed on a citrate-reduced silver colloid surface. Langmuir 1996, 12, 706–713. [Google Scholar] [CrossRef]
- Bansal, V.; Rautaray, D.; Bharde, A.; Ahire, K.; Sanyal, A.; Ahmad, A.; Sastry, M. Fungus-mediated biosynthesis of silica and titania particles. J. Mater. Chem. 2006, 15, 2583–2589. [Google Scholar] [CrossRef]
- Kumar, S.A.; Ansary, A.A.; Abroad, A.; Khan, M.I. Extra-cellular biosynthesis of CdSe quantum dots by the fungus, Fusarium oxysporum. J. Biomed. Nanotechnol. 2007, 3, 190–194. [Google Scholar] [CrossRef]
- Bharde, A.; Rautaray, D.; Bansal, V.; Ahmad, A.; Sarkar, I.; Yusuf, S.M.; Sanyal, M.; Sastry, M. Extracellular biosynthesis of magnetite using fungi. Small 2006, 2, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Bansal, V.; Rautaray, D.; Ahmad, A.; Sastry, M. Biosynthesis of zirconia nanoparticles using the fungus Fusarium oxysporum. J. Mater. Chem. 2004, 14, 3303–3305. [Google Scholar] [CrossRef]
- Bansal, V.; Poddar, P.; Ahmad, A.; Sastry, M. Room-temperature biosynthesis of ferroelectric barium titanate nanoparticles. J. Am. Chem. Soc. 2006, 128, 11958–11963. [Google Scholar] [CrossRef] [PubMed]
- Royston, E.; Ghosh, A.; Kofinas, P.; Harris, M.T.; Culver, J.N. Self-assembly of virus-structured high surface area nanomaterials and their application as battery electrodes. Langmuir 2008, 24, 906–912. [Google Scholar] [CrossRef] [PubMed]
- Aljabali, A.A.A.; Barclay, J.E.; Lomonossoff, G.P.; Evans, D.J. Virus templated metallic nanoparticles. Nanoscale 2010, 2, 2596–2600. [Google Scholar] [CrossRef] [PubMed]
- Gorzny, M.L.; Walton, A.S.; Evans, S.D. Synthesis of high-surface-area platinum nanotubes using a viral template. Adv. Funct. Mater. 2010, 20, 1295–1300. [Google Scholar] [CrossRef]
- Kobayashi, M.; Tomita, S.; Sawada, K.; Shiba, K.; Yanagi, H.; Yamashita, I.; Uraoka, Y. Chiralmeta-molecules consisting of gold nanoparticles and genetically engineered tobacco mosaic virus. Opt. Express 2012, 20, 24856–24863. [Google Scholar] [CrossRef] [PubMed]
- Rybicki, E.P. Plant-made vaccines for humans and animals. Plant Biotechnol. J. 2010, 8, 620–637. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Wen, H.; Wen, Q.; Chen, X.; Wang, Y.; Xuan, W.; Liang, J.; Wan, S. Cucumber mosaic virus as drug delivery vehicle for doxorubicin. Biomaterials 2013, 34, 4632–4642. [Google Scholar] [CrossRef] [PubMed]
- Steinmetz, N.F.; Lin, T.; Lomonossoff, G.P.; Johnson, J.E. Structure-based engineering of anicosahedral virus for nanomedicine and nanotechnology. Viruses Nanotechnol. Curr. Top. Microbiol. Immunol. 2009, 327, 23–58. [Google Scholar]
- Love, A.J.; Makarov, V.V.; Yaminsky, I.V.; Kalinina, N.O.; Taliansky, M.E. The use of tobacco mosaic virus and cowpea mosaic virus for the production of novel metal nanomaterials. Virology 2014, 449, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Reese, R.N.; Winge, D.R. Sulfide stabilization of the cadmium-γ-glutamyl peptide complex of Schizosaccharomyces pombe. J. Biol. Chem. 1998, 263, 12832–12835. [Google Scholar]
- Agnihotri, M.; Joshi, S.; Kumar, A.R.; Zinjarde, S.S.; Kulkarni, S.K. Biosynthesis of gold nanoparticles by the tropical marine yeast Yarrowia lipolytica. Mater. Lett. 2009, 63, 1231–1234. [Google Scholar] [CrossRef]
- Pimprikar, P.S.; Joshi, S.; Kumar, A.R.; Zinjarde, S.S.; Kulkarni, S.K. Influence of biomass and gold salt concentration on nanoparticle synthesis by the tropical marine yeast Yarrowia lipolytica NCIM 3589. Colloids Surf. B 2009, 74, 30–316. [Google Scholar] [CrossRef] [PubMed]
- Marshall, A.T.; Haverkamp, R.G.; Davies, C.E.; Parsons, J.G.; Gardea-Torresdey, J.L.; van Agterveld, D. Accumulation of gold nanoparticles in Brassic juncea. Int. J. Phytoremed. 2007, 9, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Castro, L.; Blazquez, M.L.; Munoz, J.A.; Gonzalez, F.; Garcıa-Balboa, C.; Ballester, A. Biosynthesis of gold nanowires using sugar beet pulp. Process Biochem. 2011, 46, 1076–1082. [Google Scholar] [CrossRef]
- Li, X.; Xu, H.; Chen, Z.S.; Chen, G. Biosynthesis of nanoparticles by microorganisms and their applications. J. Nanomater. 2011, 2011, 270974. [Google Scholar] [CrossRef]
- Malik, P.; Shankar, R.; Malik, V.; Sharma, N.; Mukherjee, T.K. Green chemistry based benign routes for nanoparticle synthesis. J. Nanopart. 2014, 2014, 302429. [Google Scholar] [CrossRef]
- Dwivedi, A.D.; Gopal, K. Biosynthesis of silver and gold nanoparticles using Chenopodium album leaf extract. Colloids Surf. A 2010, 369, 27–33. [Google Scholar] [CrossRef]
- Chandran, S.P.; Chaudhary, M.; Pasricha, R.; Ahmad, A.; Sastry, M. Synthesis of gold nanotriangles and silver nanoparticles using Aloe vera plant extract. Biotechnol. Prog. 2006, 22, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Maensiri, S.; Laokul, P.; Klinkaewnarong, J.; Phokha, S.; Promarak, V.; Seraphin, S. Indium oxide (In2O3) nanoparticles using Aloe vera plant extract: Synthesis and optical properties. J. Optoelectron. Adv. Mater. 2008, 10, 161–165. [Google Scholar]
- Vilchis-Nestor, A.R.; Sánchez-Mendieta, V.; Camacho-López, M.A.; Gómez-Espinosa, R.M.; Arenas-Alatorre, J.A. Solventless synthesis and optical properties of Au and Ag nanoparticles using Camellia sinensis extract. Mater. Lett. 2008, 62, 3103–3105. [Google Scholar] [CrossRef]
- Satyavani, K.; Ramanathan, T.; Gurudeekan, S. Green synthesis of silver nanoparticles using stem dried callus extract of bitter apple (Citrullus colocynthis). Dig. J. Nanomater. Biostruct. 2011, 6, 1019–1024. [Google Scholar]
- Song, J.Y.; Kwon, E.Y.; Kim, B.S. Biological synthesis of platinum nanoparticles using Diopyros kaki leaf extract. Bioprocess. Biosyst. Eng. 2010, 33, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Philip, D. Mangifera indica leaf-assisted biosynthesis of well-dispersed silver nanoparticles. Spectrochim. Acta A 2011, 78, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.K.; Kaler, A.; Banerjee, U.C. Free radical scavenging and antioxidant activity of silver nanoparticles synthesized from flower extract of Rhododendron dauricum. Nano Biomed. Eng. 2012, 4, 118–124. [Google Scholar]
- Raghunandan, D.; Basavaraja, S.; Mahesh, B.; Balaji, S.; Manjunath, S.Y.; Venkataraman, A. Biosynthesis of stable polyshaped gold nanoparticles from microwave-exposed aqueous extracellular anti-amlignant Guava (Psidium guajava) leaf extract. Nanobiotechnology 2009, 5, 34–41. [Google Scholar] [CrossRef]
- Ghodake, G.; Deshpande, N.; Lee, Y.; Jin, E. Pear fruit extract-assisted room-temperature biosynthesis of gold nanoplates. Colloids Surf. B Biointerfaces 2010, 75, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Ankamwar, B. Biosynthesis of gold nanoparticles (green-gold) using leaf extract of Terminalia catappa. E J. Chem. 2010, 7, 1334–1339. [Google Scholar] [CrossRef]
- Gardea-Torresdey, J.L.; Tiemann, K.J.; Gamez, G.; Dokken, K.; Tehuacamanero, S.; Jose-Yacaman, M. Gold nanoparticles obtained by bio-precipitation from gold (III) solutions. J. Nanopart. Res. 1999, 1, 397–404. [Google Scholar] [CrossRef]
- Sathishkumar, M.; Krishnamurthy, S.; Yun, Y.S. Immobilization of silver nanoparticles synthesized using the Curcuma longa tuber powder extract on cotton cloth for bactericidal activity. Biores. Technol. 2010, 101, 7958–7965. [Google Scholar] [CrossRef] [PubMed]
- Dubey, S.P.; Lahtinen, M.; Sillanpaa, M. Tansy fruit mediated greener synthesis of silver and gold nanoparticles. Process Biochem. 2010, 45, 1065–1071. [Google Scholar] [CrossRef]
- Armendariz, V.; Herrera, I.; Peralta-Videa, J.R.; Jose-Yacaman, M.; Troiani, H.; Santiago, P.; Gardea-Torresdey, J.L. Size controlled gold nanopartilce formation by Avena sativa biomass: Use of plants in nanobiotechnology. J. Nanopart. Res. 2004, 6, 377–382. [Google Scholar] [CrossRef]
- Sathishkumar, M.; Sneha, K.; Won, S.W.; Cho, C.W.; Kim, S.; Yun, Y.S. Cinnamon zeylanicum bark extracted and powder mediated green synthesis of nano-crystalline silver particles and its bacterial activity. Colloids Surf. B Biointerfaces 2009, 73, 332–338. [Google Scholar] [CrossRef]
- Sathishkumar, M.; Sneha, K.; Kwak, I.S.; Mao, J.; Tripathy, S.J.; Yun, Y.S. Phyto-crystallization of palladium through reduction process using Cinnamon zeylanicum bark extract. J. Hazard. Mater. 2009, 171, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Li, Q.; Sun, D.; Lu, Y.; Su, Y.; Yang, X.; Wang, H.; Wang, Y.; Shao, W.; He, N.; et al. Biosynthesis of silver and gold nanoparticles by novel sundried Cinnamomum camphora leaf. Nanotechnology 2007, 18, 1–11. [Google Scholar] [CrossRef]
- Narayanan, K.B.; Sakthivel, N. Phytosynthesis of gold nanoparticles using leaf extract of Coleus amboinicus Lour. Mater. Charact. 2010, 61, 1232–1238. [Google Scholar] [CrossRef]
- Ahmad, N.; Sharma, S. Green synthesis of silver nanoparticles using extracts of Ananas comosus. Gr. Sustain. Chem. 2012, 2, 141–147. [Google Scholar] [CrossRef]
- Prathna, T.C.; Chandrasekaran, N.; Raichur, A.M.; Mukherjee, A. Kinetic evolution studies of silver nanoparticles in a bio-based green synthesis process. Colloids Surf. A 2011, 377, 212–216. [Google Scholar] [CrossRef]
- Song, J.Y.; Jang, H.K.; Kim, B.S. Biological synthesis of gold nanoparticles using Magnolia kobus and Diopyros kaki leaf extracts. Process. Biochem. 2009, 44, 1133–1138. [Google Scholar] [CrossRef]
- Kaviya, S.; Santhanalakshmi, J.; Viswanathan, B.; Muthumary, J.; Srinivasan, K. Biosynthesis of silver nanoparticles using Citrus sinensis peel extract and its antibacterial activity. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011, 79, 594–598. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.K.; Pal, T. Inter-particle coupling effect on the surface plasmon resonance of gold nanoparticles: From theory to application. Chem. Rev. 2007, 107, 4797–4862. [Google Scholar] [CrossRef] [PubMed]
- Kreibig, U.; Vollmer, M. Optical Properties of Metal Clusters; Springer Verlag: Berlin, Germany, 1995. [Google Scholar]
- Chan, W.C.W.; Nie, S.M. Quantum dot bioconjugates for ultrasensitive nonisotopic detection. Science 1998, 281, 2016–2018. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Jian, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Determination of the minimum temperature required for selective photothermal destruction of cancer cells with the use of immune-targeted gold nano-particles. Photochem. Photobiol. 2006, 82, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Paciotti, G.F.; Mayer, L.; Weinreich, D.; Goia, D.; Pavel, N.; McLaughlin, R.E.; Tamarkin, L. Colloidal gold: A novel nanoparticle vector for tumour directed drug delivery. Drug Deliv. 2006, 11, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Sondi, I.; Salopek-Sondi, B. Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interface Sci. 2004, 275, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, M.; Chen, S.; Shieh, D.; Yeh, C. One-pot synthesis of hollow Au3Cu1 spherical-like and biomineral botallackite Cu2(OH)3Cl flowerlike architectures exhibiting antimicrobial activity. J. Phys. Chem. B 2006, 110, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Das, R.K.; Gogoi, N.; Bora, U. Green synthesis of gold nanoparticles using Nyctanthes arbortristis flower extract. Bioprocess. Biosyst. Eng. 2011, 34, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.P.; Bhakat, C. Green synthesis of gold nanoparticles and silver nanoparticles from leaves and bark of Ficus carica for nanotechnological applications. Int. J. Sci. Res. Publ. 2012, 2, 1–4. [Google Scholar]
- Nellore, J.; Pauline, P.C.; Amarnath, K. Biogenic synthesis of Sphearanthus amaranthoids towards the efficient production of the biocompatible gold nanoparticles. Dig. J. Nanomater. Biostruct. 2012, 7, 123–133. [Google Scholar]
- Badole, M.R.; Dighe, V.V. Synthesis of Gold Nano particles using Putranjiva roxburghii Wall. leaves Extract. Int. J. Drug Discov. Herb. Res. 2012, 2, 275–278. [Google Scholar]
- Sotiriou, G.A.; Pratsinis, S.E. Engineering nanosilver as an antibacterial, biosensor and bioimaging material. Curr. Opin. Chem. Eng. 2011, 1, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Yadav, S.K. Plant-mediated synthesis of silver and gold nanoparticles and their applications. J. Chem. Technol. Biotechnol. 2009, 84, 151–157. [Google Scholar] [CrossRef]
- Parak, W.J.; Gerion, D.; Pellegrino, T.; Zanchet, D.; Micheel, C.; Williams, S.C.; Boudreau, R.; Le Gros, M.A.; Larabell, G.A.; Alivisatos, A.P. Biological applications of colloidal nanocrystals. Nanotechnology 2003, 14, 15–27. [Google Scholar] [CrossRef]
- Chen, X.; Schluesener, H.J. Nanosilver: A nanoproduct in medical applications. Toxicol. Lett. 2008, 176, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Simon-Deckers, A.; Loo, S.; Mayne-L’hermite, M.N.; Herlin-Boime, N.; Menguy, N.; Reynaud, C.; Gouget, B.; Carrière, M. Size composition and shape dependent toxicological impact of metal oxide nano-particles and carbon nano-tubes toward bacteria. Environ. Sci. Technol. 2009, 43, 8423–8429. [Google Scholar] [CrossRef] [PubMed]
- Edison, T.; Sethuraman, M. Instant green synthesis of silver nanoparticles using Terminalia chebula fruit extract and evaluation of their catalytic activity on reduction of Methylene Blue. Process Biochem. 2012, 47, 1351–1357. [Google Scholar] [CrossRef]
- Sotiriou, G.A.; Pratsinis, S.E. Antimicrobial activity of silver nanosilver ions and particles. Environ. Sci. Technol. 2010, 44, 5649–5654. [Google Scholar] [CrossRef] [PubMed]
- Geetha, N.; Geetha, T.S.; Manonmani, P.; Thiyagarajan, M. Green Synthesis of silver nanoparticles using Cymbopogan Citratus (Dc) Stapf. Extract and its antibacterial activity. Aust. J. Basic Appl. Sci. 2014, 8, 324–331. [Google Scholar]
- Sheny, D.S.; Mathew, T.; Philip, D. Phytosynthesis of Au, Ag and Au-Ag bimetallic nanoparticles using aqueous extract and dried leaf of Anacardium occidentale. Spectrochim. Acta A 2011, 79, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Roy, N.; Laskar, R.A.; Sk, I.; Basu, S.; Mandal, D.; Begum, N.A. Biogenic synthesis of Ag, Au and bimetallic Au/Ag alloy nanoparticles using aqueous extract of mahogany (Swietenia mahogani JACQ) leaves. Colloids Surf. B 2011, 82, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.; Mukherjee, T.; Kapoor, S. A simple approach for facile synthesis of Ag, anisotropic Au and bimetallic (Ag/Au) nanoparticles using cruciferous vegetable extracts. Mater. Sci. Eng. C 2012, 32, 1827–1834. [Google Scholar] [CrossRef]
- Subhankari, I.; Nayak, P.L. Synthesis of copper nanoparticles using Syzygium aromaticum (Cloves) aqueous extract by using green chemistry. World J. Nano Sci. Technol. 2013, 2, 14–17. [Google Scholar]
- Valodkar, M.; Jadeja, R.N.; Thounaojam, M.C.; Devkar, R.V.; Thakore, S. Biocompatible synthesis of peptide capped copper nanoparticles and their biological effect on tumor cells. Mater. Chem. Phys. 2011, 128, 83–89. [Google Scholar] [CrossRef]
- Valodkar, M.; Nagar, P.S.; Jadeja, R.N.; Thounaojam, M.C.; Devkar, R.V.; Thakore, S. Euphorbiaceaelatex induced green synthesis of non-cytotoxic metallic nanoparticle solutions: A rational approach to antimicrobial applications. Colloids Surf. A 2011, 384, 337–344. [Google Scholar] [CrossRef]
- Padil, V.V.T.; Cerník, M. Green synthesis of copper oxide nanoparticles using gum karaya as a biotemplate and their antibacterial application. Int. J. Nanomed. 2013, 8, 889–898. [Google Scholar]
- Das, D.; Nath, B.C.; Phukon, P.; Dolui, S.K. Synthesis and evaluation of antioxidant and antibacterial behavior of CuO nanoparticles. Colloids Surf. B Biointerfaces 2013, 101, 430–433. [Google Scholar] [CrossRef] [PubMed]
- Heinlaan, M.; Ivask, A.; Blinova, I.; Dubourguier, H.C.; Kakru, A. Toxicity of nanosized and bulk ZnO, CuO and TiO2 to bacteria Vibrio fischeri and crustaceans Daphnia magna and Thamnocephalus platyurus. Chemosphere 2008, 71, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Roopan, S.M.; Bharathi, A.; Kumar, R.; Khanna, V.G.; Prabhakarn, A. Acaricidal, insecticidal, and larvicidal efficacy of aqueous extract of Annona squamosa L peel as biomaterial for the reduction of palladium salts into nanoparticles. Colloids Surf. B Biointerfaces 2011, 92, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.R.; Vivekanandhan, S.; Misra, M.; Mohanty, A.; Satyanarayana, N. Soybean (Glycine max) leaf extract based green synthesis of palladium nanoparticles. J. Biomater. Nanobiotechnol. 2012, 3, 14–19. [Google Scholar] [CrossRef]
- Nadagouda, M.N.; Varma, R.S. Green synthesis of silver and palladium nanoparticles at room temperature using coffee and tea extract. Green Chem. 2008, 10, 859–862. [Google Scholar] [CrossRef]
- Jia, L.; Zhang, Q.; Li, Q.; Song, H. The biosynthesis of palladium nanoparticles by antioxidants in garden jasminoids ellis: Long life time catalyst for p-nitrotoluene hydrogenation. Nanotechnology 2009, 20, 385601. [Google Scholar] [CrossRef] [PubMed]
- Soundarrajan, C.; Sankari, A.; Dhandapani, P.; Maruthamuthu, S.; Ravichandran, S.; Sozhan, G.; Palaniswamy, N. Rapid biological synthesis of platinum nanoparticles using Ocimum sanctum for water electrolysis applications. Bioproc. Biosys. Eng. 2012, 35, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Wu, M.; Wu, B.; Kuga, S.; Endo, T.; Huang, Y. Platinum nanoparticles using wood nanomaterials: Eco-friendly synthesis, shape control and catalytic activity for p-nitrophenol reduction. Green Chem. 2011, 13, 283–287. [Google Scholar] [CrossRef]
- Roopan, S.M.; Bharathi, A.; Prabhakarn, A.; Rahuman, A.A.; Velayutham, K.; Rajakumar, G.; Padmaja, R.D.; Lekshmi, M.; Madhumitha, G. Efficient phytosynthesis and structural characterization of rutile TiO2nanoparticles using Annona squamosa peel extract. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2012, 98, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Sundrarajan, M.; Gowri, S. Green synthesis of titanium dioxide nanoparticles by nyctanthes arbor-tristis leaves extract. Chalcogenide Lett. 2011, 8, 447–451. [Google Scholar]
- Rajakumar, G.; Abdul-Rahuman, A.; Priyamvada, B.; Gopiesh-Khanna, V.; Kishore-Kumar, D.; Sujin, P.J. Eclipta prostrata leaf aqueous extract mediated synthesis of titanium dioxide nanoparticles. Mater. Lett. 2012, 68, 115–117. [Google Scholar] [CrossRef]
- Zahir, A.A.; Chauhan, I.S.; Bagavan, A.; Kamaraj, C.; Elango, G.; Shankar, J.; Arjaria, N.; Roopan, S.M.; Rahuman, A.A.; Singh, N. Green synthesis of silver and titanium dioxide nanoparticles using Euphorbia prostrate extract shows shift from apoptosis to G0/G1 arrest followed by necrotic cell death in Leishmania donovani. Antimicrob. Agents Chemother. 2015, 59, 4782–4799. [Google Scholar] [CrossRef] [PubMed]
- Velayutham, K.; Rahuman, A.A.; Rajakumar, G.; Santhoshkumar, T.; Marimuthu, S.; Jayaseelan, C.; Bagavan, A.; Kirthi, A.V.; Kamaraj, C.; Zahir, A.A.; et al. Evaluation of Catharanthus roseus leaf extract-mediated biosynthesis of titanium dioxide nanoparticles against Hippobosca maculata and Bovicola ovis. Parasitol. Res. 2011, 111, 2329–2337. [Google Scholar] [CrossRef] [PubMed]
- Santhoshkumar, T.; Rahuman, A.; Jayaseelan, C.; Rajakumar, G.; Marimuthu, S.; Kirthi, A.V.; Velayutham, K.; Thomas, J.; Venkatesan, J.; Kim, S.K. Green synthesis of titanium dioxide nanoparticles using Psidium guajava extract and its antibacterial and antioxidant properties. Asian Pac. J. Trop. Med. 2014, 7, 968–976. [Google Scholar] [CrossRef]
- Singh, R.P.; Shukla, V.K.; Yadav, R.S.; Sharma, P.K.; Singh, P.K.; Pandey, A.C. Biological approach of zinc oxide nanoparticles formation and its characterization. Adv. Mater. Lett. 2011, 2, 313–317. [Google Scholar] [CrossRef]
- Sangeetha, G.; Rajeshwari, S.; Venckatesh, R. Green synthesis of zinc oxide nanoparticles by aloe barbadensis miller leaf extract: Structure and optical properties. Mater. Res. Bull. 2011, 46, 2560–2566. [Google Scholar] [CrossRef]
- Qu, J.; Yuan, X.; Wang, X.; Shao, P. Zinc accumulation and synthesis of ZnO nanoparticles using Physalis alkekengi L. Environ. Pollut. 2011, 159, 1783–1788. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Luo, C.; Hou, J. Synthesis of ZnO nanoparticles from Zn hyper-accumulator (Sedum alfredii Hance) plants. Micro Nano Lett. 2011, 6, 174–176. [Google Scholar] [CrossRef]
- Vimala, K.; Sundarraj, S.; Paulpandi, M.; Vengatesan, S.; Kannan, S. Green synthesized doxorubicin loaded zinc oxide nanoparticles regulates the Bax and Bcl-2 expression in breast and colon carcinoma. Process Biochem. 2014, 49, 160–172. [Google Scholar] [CrossRef]
- Pattanayak, M.; Nayak, P.L. Green synthesis and characterization of zero valent iron nanoparticles from the leaf extract of Azadirachta indica (Neem). World J. Nano Sci. Technol. 2013, 2, 06–09. [Google Scholar]
- Shah, S.; Dasgupta, S.; Chakraborty, M.; Vadakkekara, R.; Hajoori, M. Green synthesis of iron nanoparticles using plant extracts. Int. J. Biol. Pharm. Res. 2014, 5, 549–552. [Google Scholar]
- Joglekar, S.; Kodam, K.; Dhaygude, M.; Hudlikar, M. Novel route for rapid biosynthesis of lead nanoparticles using aqueous extract of Jatropha curcas L. latex. Mater. Lett. 2011, 65, 3170–3172. [Google Scholar] [CrossRef]
- Sasidharan, S.; Sowmiya, R.; Balakrishnaraja, R. Biosynthesis of selenium nanoparticles using citrus reticulata peel extract. World J. Pharm. Res. 2014, 4, 1322–1330. [Google Scholar]
- Doria, G.; Conde, J.; Veigas, B.; Giestas, L.; Almeida, C.; Assunção, M.; Rosa, J.; Baptista, P.V. Nobel metal nanoparticles for biosensing applications. Sensors 2012, 12, 1657–1687. [Google Scholar] [CrossRef] [PubMed]
- Le, X.; Poinern, G.E.J.; Subramaniam, S.; Fawcett, D. Applications of nanometre scale particles as pharmaceutical delivery vehicles in medicine. Open J. Biomed. Mater. Res. 2015, 2, 11–26. [Google Scholar]
- Youns, M.; Hoheisel, J.D.; Efferth, T. Therapeutic and diagnostic applications of nanoparticles. Curr. Drug Targets 2011, 12, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Fortina, P.; Kricka, L.J.; Graves, D.J.; Park, J.; Hyslop, T.; Tam, F.; Halas, N.; Surrey, S.; Waldman, S.A. Applications of nanoparticles to diagnostics and therapeutics in colorectal cancer. Trends Biotechnol. 2007, 25, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Al-Samarrai, A.M. Nanoparticles as alternative to pesticides in management plant diseases—A Review. Int. J. Sci. Res. Publ. 2012, 2, 1–4. [Google Scholar]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Panaek, A.; Kvitek, L.; Prucek, R.; Kolar, M.; Vecerova, R.; Pizúrova, N.; Sharma, V.K.; Nevecna, T.; Zboril, R. Silver colloid nanoparticles: Synthesis, characterization and their antibacterial activity. J. Phys. Chem. B 2006, 110, 16248–16253. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.; Varghese, S.H.; Nair, B.G.; Maekawa, T.; Yoshida, Y.; Kumar, D.S. Nanoparticulate material delivery to plants. Plant Sci. 2010, 179, 154–163. [Google Scholar] [CrossRef]
- Khot, L.R.; Sankaran, S.; Maja, J.M.; Ehsani, R.; Schuster, E.W. Applications of nanomaterials in agricultural production and crop protection: A review. Crop Prot. 2012, 35, 64–70. [Google Scholar] [CrossRef]
- Vivek, M.; Kumar, P.S.; Steffi, S.; Sudha, S. Biogenic silver nanoparticles by Gelidiella acerosa extract and their antifungal effects. Avicenna J. Med. Biotechnol. 2011, 3, 143–148. [Google Scholar] [PubMed]
- Park, H.J.; Kim, S.H.; Kim, H.J.; Choi, S.H. A new composition of nanosized silica-silver for control of various plant diseases. Plant Pathol. J. 2006, 22, 295–302. [Google Scholar] [CrossRef]
- Sukirtha, R.; Priyanka, K.M.; Antony, J.J.; Kamalakkannan, S.; Thangam, R.; Gunasekaran, P.; Krishnan, M.; Achiraman, S. Cytotoxic effect of green synthesized silver nanoparticles using Melia azedarach against in vitro HeLa cell lines and lymphoma mice model. Process Biochem. 2011, 47, 273–279. [Google Scholar] [CrossRef]
- Seil, J.T.; Webster, T.J. Antimicrobial applications of nanotechnology: Methods and literature. Int. J. Nanomed. 2012, 7, 2767–2781. [Google Scholar]
- Ponarulselvam, S.; Panneerselvam, C.; Murugan, K.; Aarthi, N.; Kalimuthu, K.; Thangamani, S. Synthesis of silver nanoparticles using leaves of Catharanthus roseus Linn. G. Don and their antiplasmodial activities. Asian Pac. J. Trop. Biomed. 2012, 2, 574–580. [Google Scholar] [CrossRef]
- Subramanian, V. Green synthesis of silver nanoparticles using Coleus amboinicus lour, antioxitant activity and in vitro cytotoxicity against Ehrlich’s Ascite carcinoma. J. Pharm. Res. 2012, 5, 1268–1272. [Google Scholar]
- Hirsch, L.R.; Stafford, R.J.; Bankson, J.A.; Sershen, S.R.; Rivera, B.; Price, R.E.; Hazle, J.D.; Halas, N.J.; West, J.L. Nanoshell-mediated near-infrared thermal therapy of tumours under magnetic resonance guidance. Proc. Natl. Acad. Sci. USA 2003, 100, 13549–13554. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Sache, L. Gold nanoparticles enhance DNA damage induced by anti-cancer drugs and radiation. Radiat. Res. 2009, 172, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Samia, A.C.; Li, J.; Kenney, M.E.; Resnick, A.; Burda, C. Delivery and efficacy of a cancer drug as a function of the bond to the gold nanoparticle surface. Langmuir 2010, 26, 2248–2255. [Google Scholar] [CrossRef] [PubMed]
- Pissuwan, D.; Cortie, C.H.; Valenzuela, S.M.; Cortie, M.B. Functionalised gold nanoparticles for controlling pathogenic bacteria. Trends Biotechnol. 2009, 28, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, O.; Ghaderi, E. Cu and CuO nanoparticles immobilized by silica thin films as antibacterial materials and photocatalysts. Surf. Coat. Technol. 2012, 205, 219–223. [Google Scholar] [CrossRef]
- Hassan, M.S.; Amna, T.; Yang, O.B.; El-Newehy, M.H.; Al-Deyab, S.S.; Khil, M.S. Smart copper oxide nanocrystals: Synthesis, characterization, electrochemical and potent antibacterial activity. Colloids Surf. B Biointerfaces 2012, 97, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Stoimenov, P.K.; Klinger, R.L.; Marchin, R.L.; Klabunde, K.J. Metal oxide nanoparticles as bactericidal agents. Langmuir 2002, 18, 6679–6686. [Google Scholar] [CrossRef]
- Miller, R.J.; Bennett, S.; Keller, A.A.; Pease, S.; Lenihan, H.S. TiO2 nanoparticles are phototoxic to marine phytoplankton. PLOS Biol. 2012, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, G. Potent antibacterial activities of Ag/TiO2 nanocomposite powders synthesized by a one-Pot sol-gel method. Environ. Sci. Technol. 2009, 43, 2905–2910. [Google Scholar] [CrossRef]
- Allahverdiyev, A.M.; Abamor, E.S.; Bagirova, M.; Rafailovich, M. Antimicrobial effects of TiO2and Ag2O nanoparticles against drug-resistant bacteria and leishmania parasites. Future Microbiol. 2011, 8, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Perez-Espitia, P.J.; Ferreira-Soares, N.F.; dos Reis Coimbra, J.S.; de Andrade, N.J.; Cruz, R.S.; Medeiros, E.A.A. Zinc oxide nanoparticles: Synthesis, antimicrobial activity and food packaging applications. Food Bioprocess Technol. 2012, 5, 1447–1464. [Google Scholar] [CrossRef]
- Duran, N.; Seabra, A.B. Metallic oxide nanoparticles: State of the art in biogenic syntheses and their mechanisms. Appl. Microbiol. Biotechnol. 2012, 95, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Dujardin, E.; Peet, C.; Stubbs, G.; Culver, J.N.; Mann, S. Organization of metallic nanoparticles using tobacco mosaic virus templates. Nano Lett. 2003, 3, 413–417. [Google Scholar] [CrossRef]
- Li, D.; Qu, X.; Newton, S.M.C.; Klebba, P.E.; Mao, C. Morphology-controlled synthesis of silica nanotubes through pH and sequence-responsive morphological change bacterial flagellar biotemplates. J. Mater. Chem. 2012, 22, 15702–15709. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Li, D.; Mao, C. Genetically modified flagella as templates for silica fibers: From hybrid nanotubes to 1D periodic nanohole arrays. Adv. Funct. Mater. 2008, 18, 4007–4013. [Google Scholar] [CrossRef]
- Li, D.; Newton, S.M.C.; Klebba, P.E.; Mao, C. Flagellar display of bone- protein-derived peptides for studying peptide-mediated biomineralization. Langmuir 2012, 28, 16338–16346. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Mathew, B.; Mao, C. Biotemplated synthesis of hollow double-layered core/shell titania/silica nanotubes under ambient conditions. Small 2012, 8, 3691–3697. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.; Chhabra, R.; Cheng, A.; Brownell, J.; Liu, Y.; Yan, H. Control of self-assembly of DNA tubules through integration of gold nanoparticles. Science 2009, 323, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Xu, H.; Mao, C. Controlled self-assembly of rodlike bacterial pili particles into ordered lattices. Angew. Chem. Int. Ed. 2011, 50, 6264–6268. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, M.; Zhou, G.; Shuai, Y.; Wang, J.; Zhu, L.; Mao, C. Ca2+ induced self-assembly of Bombyx mori silk sericin into a Nanofibrous network-like protein matrix for directing controlled nucleation of hydroxyapatite nano-needles. J. Mater. Chem. B 2015, 3, 2455–2462. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Shuai, Y.; Zhang, C.; Chen, Y.; Zhu, L.; Mao, C.; OuYang, H. Biomimetic nucleation of hydroxyapatite crystals mediated by Antheraea pernyi silk sericin promotes osteogenic differentiation of human bone marrow derived mesenchymal stem cells. Biomacromolecules 2014, 15, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Zhu, Y.; Wang, L.; Mao, C. Controlled alignment of filamentous supra molecular assemblies of biomolecules into centimetre-scale highly ordered patterns by using nature-inspired magnetic guidance. Angew. Chem. Int. Ed. 2013, 52, 11750–11754. [Google Scholar] [CrossRef] [PubMed]
- Mi, C.; Wang, Y.; Zhang, J.; Huang, H.; Xu, L.; Wang, S.; Fang, X.; Fang, J.; Mao, C.; Xu, S. Biosynthesis and characterization of CdS quantum dots in genetically engineered Escherichia Coli. J. Biotechnol. 2011, 153, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.W.; Chung, W.J.; Heo, K.; Jin, H.E.; Lee, B.Y.; Wang, E.; Zueger, C.; Wong, W.; Meyer, J.; Kim, C.; et al. Biomimetic virus-based colourimetric sensors. Nat. Commun. 2014, 5, 3043. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Abbineni, G.; Mao, C. Nanocomposite films assembled from genetically engineered filamentous viruses and gold nanoparticles: Nanoarchitecture- and humidity-tunable surface plasmon resonance spectra. Adv. Mater. 2009, 21, 1001–1005. [Google Scholar] [CrossRef]
- Wang, Y.; Ju, Z.; Cao, B.; Gao, X.; Zhu, Y.; Qiu, P.; Xu, H.; Pan, P.; Bao, H.; Wang, L.; et al. Ultrasensitive rapid detection of human serum antibody biomarkers by biomarker-capturing viral nanofibers. ACS Nano 2015, 9, 4475–4483. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.S.; Rai, A.; Ankamwar, B.; Singh, A.; Ahmad, A.; Sastry, M. Biological synthesis of triangular gold nanoprisms. Naturematerials 2004, 3, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Cao, Y.; Mirkin, C.A.; Kelly, K.L.; Schatz, G.C.; Zheng, J.G. Photo-induced conversion of silver nanoparticles to nanoprisms. Science. 2001, 294, 1901–1903. [Google Scholar] [CrossRef] [PubMed]
- Malikova, N.; Pastoriza-Santos, I.; Schierhorn, M.; Kotov, N.A.; Liz-Marzan, L.M. Layer-by-layer assembled mixed spherical and planar gold nanoparticles: Control of inter-particle interaction. Langmuir. 2002, 18, 3694–3697. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, M.; Fawcett, D.; Sharma, S.; Tripathy, S.K.; Poinern, G.E.J. Green Synthesis of Metallic Nanoparticles via Biological Entities. Materials 2015, 8, 7278-7308. https://doi.org/10.3390/ma8115377
Shah M, Fawcett D, Sharma S, Tripathy SK, Poinern GEJ. Green Synthesis of Metallic Nanoparticles via Biological Entities. Materials. 2015; 8(11):7278-7308. https://doi.org/10.3390/ma8115377
Chicago/Turabian StyleShah, Monaliben, Derek Fawcett, Shashi Sharma, Suraj Kumar Tripathy, and Gérrard Eddy Jai Poinern. 2015. "Green Synthesis of Metallic Nanoparticles via Biological Entities" Materials 8, no. 11: 7278-7308. https://doi.org/10.3390/ma8115377
APA StyleShah, M., Fawcett, D., Sharma, S., Tripathy, S. K., & Poinern, G. E. J. (2015). Green Synthesis of Metallic Nanoparticles via Biological Entities. Materials, 8(11), 7278-7308. https://doi.org/10.3390/ma8115377





