Development of Chitosan/Bacterial Cellulose Composite Films Containing Nanodiamonds as a Potential Flexible Platform for Wound Dressing
Abstract
:1. Introduction
2. Results and Discussion
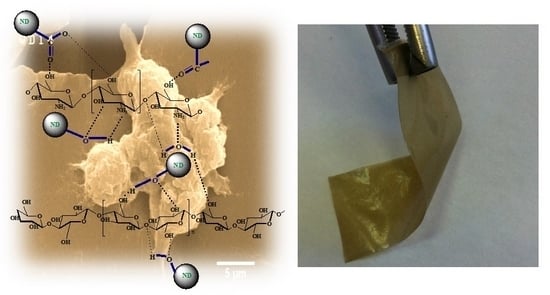
2.1. Microstructure and Chemical Interactions


2.2. Transparency and Colorimetric Analysis
| ND% | Thickness (μm) | L* | a* | b* | C* | h° | ∆E |
|---|---|---|---|---|---|---|---|
| 0 | 3 ± 26 | 86.00 | –0.22 | 24.04 | 24.04 | 90.52 | 23.22 |
| 1 | 4 ± 28 | 77.20 | 3.00 | 18.45 | 18.70 | 80.78 | 24.21 |
| 2 | 8 ± 30 | 60.77 | 6.39 | 24.4 | 25.22 | 75.31 | 41.33 |
| 3 | 5 ± 23 | 56.76 | 6.86 | 22.56 | 23.58 | 73.04 | 43.96 |
| 4 | 9 ± 27 | 51.01 | 7.69 | 21.82 | 23.14 | 70.60 | 48.92 |

2.3. Thermal Analysis and X-ray Diffraction
| ND (wt %) | Tvap (°C) | Enthalpy (J·g−1) | T (°C) | Td (°C) | Enthalpy (J·g−1) |
|---|---|---|---|---|---|
| 0 | 112.15 ± 0.42 | –334.57 ± 1.20 | 201.72 ±0 .50 | 286.85 ± 0.76 | 29.88 ± 4.12 |
| 1 | 114.39 ± 0.25 | –213.57 ± 3.02 | 205.64 ± 0.36 | 296.34 ± 0.43 | 100.17 ± 6.71 |
| 2 | 102.86 ± 0.31 | –128.48 ± 5.11 | 191.86 ± 0.87 | 296.87 ± 0.61 | 29.98 ± 4.56 |
| 3 | 99.55 ± 0.12 | –296.82 ± 2.14 | 192.87 ± 0.45 | 292.92± 0 .84 | 74.44 ± 2.69 |
| 4 | 106.15 ± 0.15 | –210.27 ± 1.15 | 203.10 ± 0.76 | 288.29 ± 0.26 | 40.44 ± 3.12 |

2.4. Mechanical Properties
| ND (%) | E (MPa) | (MPa) | (MPa) | σmax (MPa) | εmax (%) | K (kJ/m3) × 10 |
|---|---|---|---|---|---|---|
| 0 | 782 ± 20 | - | - | 60.97 ± 0.01 | 12.53 ± 0.02 | 0.674 ± 0.000 |
| 2 | 2825 ± 15 | 961 | 792000 | 46.40 ± 1.59 | 9.77 ± 1.12 | 0.399 ± 0.005 |
| 4 | 3053 ± 18 | 783 | 870000 | 38.98 ± 2.92 | 8.27 ± 1.97 | 0.348 ± 0.004 |

2.5. Cell Viability Assessment



3. Experimental Section
3.1. Materials
3.2. Sample Preparation
3.3. Materials Characterization
3.3.1. Thickness Measurement
3.3.2. Microscopic Studies
3.3.3. Optical Properties
3.3.4. Attenuated Total Reflectance FTIR
3.3.5. Thermal Stability and X-Ray Studies
3.3.6. Mechanical Measurements
3.3.7. In Vitro Assessment
3.3.8. Antibacterial Evaluation
4. Conclusions
- A fibrillar-network structure of BC on the surface of the CS films was observed. The distribution of NDs throughout the polymer matrix was uniform at concentrations ≤2%.
- The formation of hydrogen bonds between NDs and the polymer matrix was detected.
- Lower whiteness, higher redness and reduced transparency were obtained when NDs were incorporated into the polymer matrix. Nevertheless, the transparency remained at favorable level due to minimal Rayleigh scattering from the film surface and reasonable ND dispersion.
- A remarkable enhancement in the elastic modulus was obtained by dispersion of NDs in the polymer matrix.
- The addition of NDs reduced the polymer crystallinity, which led to a lower tensile strength.
- Cytotoxic evaluation via culturing of fibroblast L929 cells revealed reasonable cytocompatibility of the composite films containing NDs.
- Examinations of the cell adhesion and interactions revealed the potential of nanocomposite films to support cellular behavior in vitro.
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lam, R.; Chen, M.; Pierstorff, E.; Huang, H.; Osawa, E.; Ho, D. Nanodiamond-embedded microfilm devices for localized chemotherapeutic elution. ACS nano 2008, 2, 2095–2102. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Pierstorff, E.D.; Lam, R.; Li, S.Y.; Huang, H.; Osawa, E.; Ho, D. Nanodiamond-mediated delivery of water-insoluble therapeutics. ACS Nano 2009, 3, 2016–2022. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Q.; Chen, M.; Lam, R.; Xu, X.; Osawa, E.; Ho, D. Polymer-functionalized nanodiamond platforms as vehicles for gene delivery. ACS Nano 2009, 3, 2609–2616. [Google Scholar] [CrossRef] [PubMed]
- Wehling, J.; Dringen, R.; Zare, R.N.; Maas, M.; Rezwan, K. Bactericidal activity of partially oxidized nanodiamonds. ACS Nano 2014, 8, 6475–6483. [Google Scholar] [CrossRef] [PubMed]
- Villalba, P.; Ram, M.K.; Gomez, H.; Kumar, A.; Bhethanabotla, V.; Kumar, A. Gox-functionalized nanodiamond films for electrochemical biosensor. Mater. Sci. Eng. C 2011, 31, 1115–1120. [Google Scholar] [CrossRef]
- Mohan, N.; Chen, C.S.; Hsieh, H.H.; Wu, Y.C.; Chang, H.C. In vivo imaging and toxicity assessments of fluorescent nanodiamonds in caenorhabditis elegans. Nano Lett. 2010, 10, 3692–3699. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Mochalin, V.N.; Neitzel, I.; Knoke, I.Y.; Han, J.; Klug, C.A.; Zhou, J.G.; Lelkes, P.I.; Gogotsi, Y. Fluorescent plla-nanodiamond composites for bone tissue engineering. Biomaterials 2011, 32, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Williams, O.A.; Hees, J.; Dieker, C.; Jäger, W.; Kirste, L.; Nebel, C.E. Size-dependent reactivity of diamond nanoparticles. ACS nano 2010, 4, 4824–4830. [Google Scholar] [CrossRef] [PubMed]
- Schrand, A.M.; Dai, L.; Schlager, J.J.; Hussain, S.M.; Osawa, E. Differential biocompatibility of carbon nanotubes and nanodiamonds. Diamond Relat. Mater. 2007, 16, 2118–2123. [Google Scholar] [CrossRef]
- Andersson, Ö.H.; Kangasniemi, I. Calcium phosphate formation at the surface of bioactive glass in vitro. J. Biomed. Mater. Res. 1991, 25, 1019–1030. [Google Scholar]
- Rodrigues, A.A.; Baranauskas, V.; Ceragioli, H.J.; Peterlevitz, A.C.; Santos Junior, A.R.D.; Belangero, W.D. Preliminary viability studies of fibroblastic cells cultured on microcrystalline and nanocrystalline diamonds produced by chemical vapour deposition method. Mater. Res. 2013, 16, 252–258. [Google Scholar] [CrossRef]
- Khalil, H.A.; Davoudpour, Y.; Islam, M.N.; Mustapha, A.; Sudesh, K.; Dungani, R.; Jawaid, M. Production and modification of nanofibrillated cellulose using various mechanical processes: A review. Carbohydr. Polym. 2014, 99, 649–665. [Google Scholar] [CrossRef] [PubMed]
- Zhijiang, C.; Chengwei, H.; Guang, Y. Poly(3-hydroxubutyrate-co-4-hydroxubutyrate)/bacterial cellulose composite porous scaffold: Preparation, characterization and biocompatibility evaluation. Carbohydr. Polym. 2012, 87, 1073–1080. [Google Scholar] [CrossRef]
- Kukharenko, O.; Bardeau, J.F.; Zaets, I.; Ovcharenko, L.; Tarasyuk, O.; Porhyn, S.; Mischenko, I.; Vovk, A.; Rogalsky, S.; Kozyrovska, N. Promising low cost antimicrobial composite material based on bacterial cellulose and polyhexamethylene guanidine hydrochloride. Eur. Polym. J. 2014, 60, 247–254. [Google Scholar] [CrossRef]
- Fu, L.; Zhang, J.; Yang, G. Present status and applications of bacterial cellulose-based materials for skin tissue repair. Carbohydr. Polym. 2013, 92, 1432–1442. [Google Scholar] [CrossRef] [PubMed]
- Petersen, N.; Gatenholm, P. Bacterial cellulose-based materials and medical devices: Current state and perspectives. Appl. Microbiol. Biotechnol. 2011, 91, 1277–1286. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Dufresne, A. Nanocellulose in biomedicine: Current status and future prospect. Eur. Polym. J. 2014, 59, 302–325. [Google Scholar] [CrossRef]
- Klemm, D.; Schumann, D.; Udhardt, U.; Marsch, S. Bacterial synthesized cellulose—Artificial blood vessels for microsurgery. Prog. Polym. Sci. 2001, 26, 1561–1603. [Google Scholar] [CrossRef]
- Zahedmanesh, H.; Mackle, J.; Sellborn, A.; Drotz, K.; Bodin, A.; Gatenholm, P.; Lally, C. Bacterial cellulose as a potential vascular graft: Mechanical characterization and constitutive model development. J. Biomed. Mater. Res. Part B 2011, 97, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.H.; Rodrigues, A.F.; Almeida, I.F.; Costa, P.C.; Rosado, C.; Neto, C.P.; Silvestre, A.J.; Freire, C.S. Bacterial cellulose membranes as transdermal delivery systems for diclofenac: in vitro dissolution and permeation studies. Carbohydr. Polym. 2014, 106, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Müller, A.; Ni, Z.; Hessler, N.; Wesarg, F.; Müller, F.A.; Kralisch, D.; Fischer, D. The biopolymer bacterial nanocellulose as drug delivery system: Investigation of drug loading and release using the model protein albumin. J. Pharm. Sci. 2013, 102, 579–592. [Google Scholar] [CrossRef] [PubMed]
- Rouabhia, M.; Asselin, J.; Tazi, N.; Messaddeq, Y.; Levinson, D.; Zhang, Z. Production of biocompatible and antimicrobial bacterial cellulose polymers functionalized by RGDC grafting groups and gentamicin. ACS Appl. Mater. Interfaces 2014, 6, 1439–1446. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zheng, Y.; Wen, X.; Lin, Q.; Chen, X.; Wu, Z. Silver nanoparticle/bacterial cellulose gel membranes for antibacterial wound dressing: Investigation in vitro and in vivo. Biomed. Mater. 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Maneerung, T.; Tokura, S.; Rujiravanit, R. Impregnation of silver nanoparticles into bacterial cellulose for antimicrobial wound dressing. Carbohydr. Polym. 2008, 72, 43–51. [Google Scholar] [CrossRef]
- Croisier, F.; Jérôme, C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef]
- Yang, J.; Tian, F.; Wang, Z.; Wang, Q.; Zeng, Y.J.; Chen, S.Q. Effect of chitosan molecular weight and deacetylation degree on hemostasis. J. Biomed. Mater. Res. Part B 2008, 84, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.T.; Liu, J.H. Fabrication and characterization of poly(vinyl alcohol)/chitosan hydrogel thin films via uv irradiation. Eur. Polym. J. 2013, 49, 4201–4211. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Sudheesh Kumar, P.; Nair, S.; Tamura, H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, S.C.M.; Oliveira, L.; Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P.; Gandini, A.; Desbrieres, J. Novel transparent nanocomposite films based on chitosan and bacterial cellulose. Green Chem. 2009, 11, 2023–2029. [Google Scholar] [CrossRef]
- Phisalaphong, M.; Jatupaiboon, N. Biosynthesis and characterization of bacteria cellulose-chitosan film. Carbohydr. Polym. 2008, 74, 482–488. [Google Scholar] [CrossRef]
- Lin, W.C.; Lien, C.C.; Yeh, H.J.; Yu, C.M.; Hsu, S.H. Bacterial cellulose and bacterial cellulose-chitosan membranes for wound dressing applications. Carbohydr. Polym. 2013, 94, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, H.; Faezipour, M.; Hedjazi, S.; Mousavi, M.M.; Azusa, Y.; Heidari, A.H. Comparative study of paper and nanopaper properties prepared from bacterial cellulose nanofibers and fibers/ground cellulose nanofibers of canola straw. Ind. Crops Prod. 2013, 43, 732–737. [Google Scholar] [CrossRef]
- Yano, S.; Maeda, H.; Nakajima, M.; Hagiwara, T.; Sawaguchi, T. Preparation and mechanical properties of bacterial cellulose nanocomposites loaded with silica nanoparticles. Cellulose 2008, 15, 111–120. [Google Scholar] [CrossRef]
- Rakha, S.A.; Raza, R.; Munir, A. Reinforcement effect of nanodiamond on properties of epoxy matrix. Polym. Compos. 2013, 34, 811–818. [Google Scholar] [CrossRef]
- Ul-Islam, M.; Shah, N.; Ha, J.H.; Park, J.K. Effect of chitosan penetration on physico-chemical and mechanical properties of bacterial cellulose. Korean J. Chem. Eng. 2011, 28, 1736–1743. [Google Scholar] [CrossRef]
- Kim, J.; Cai, Z.; Lee, H.; Choi, G.; Lee, D.; Jo, C. Preparation and characterization of a bacterial cellulose/chitosan composite for potential biomedical application. J. Polym. Res. 2011, 18, 739–744. [Google Scholar] [CrossRef]
- Pan, H.; Xu, D.; Liu, Q.; Ren, H.Q.; Zhou, M. Preparation and characterization of corn starch-nanodiamond composite films. Appl. Mech. Mater. 2014, 469, 156–161. [Google Scholar] [CrossRef]
- Villalba, P.; Ram, M.K.; Gomez, H.; Bhethanabotla, V.; Helms, M.N.; Kumar, A.; Kumar, A. Cellular and in vitro toxicity of nanodiamond-polyaniline composites in mammalian and bacterial cell. Mater. Sci. Eng. C 2012, 32, 594–598. [Google Scholar] [CrossRef]
- Morimune, S.; Kotera, M.; Nishino, T.; Goto, K.; Hata, K. Poly(vinyl alcohol) nanocomposites with nanodiamond. Macromolecules 2011, 44, 4415–4421. [Google Scholar] [CrossRef]
- Mochalin, V.; Osswald, S.; Gogotsi, Y. Contribution of functional groups to the raman spectrum of nanodiamond powders. Chem. Mater. 2008, 21, 273–279. [Google Scholar] [CrossRef]
- Paci, J.T.; Man, H.B.; Saha, B.; Ho, D.; Schatz, G.C. Understanding the surfaces of nanodiamonds. J. Phys. Chem. C 2013, 117, 17256–17267. [Google Scholar] [CrossRef]
- Konwar, A.; Gogoi, N.; Majumdar, G.; Chowdhury, D. Green chitosan-carbon dots nanocomposite hydrogel film with superior properties. Carbohydr. Polym. 2015, 115, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Ajayan, P.M.; Schadler, L.S.; Braun, P.V. Nanocomposite Science and Technology; John Wiley & Sons: New York, NY, USA, 2006. [Google Scholar]
- Kittur, F.; Harish Prashanth, K.; Udaya Sankar, K.; Tharanathan, R. Characterization of chitin, chitosan and their carboxymethyl derivatives by differential scanning calorimetry. Carbohydr. Polym. 2002, 49, 185–193. [Google Scholar] [CrossRef]
- Hong, L.; Wang, Y.L.; Jia, S.R.; Huang, Y.; Gao, C.; Wan, Y.Z. Hydroxyapatite/bacterial cellulose composites synthesized via a biomimetic route. Mater. Lett. 2006, 60, 1710–1713. [Google Scholar] [CrossRef]
- Stefanescu, C.; Daly, W.H.; Negulescu, I.I. Biocomposite films prepared from ionic liquid solutions of chitosan and cellulose. Carbohydr. Polym. 2012, 87, 435–443. [Google Scholar] [CrossRef]
- Mohanasrinivasan, V.; Mishra, M.; Paliwal, J.; Singh, S.; Selvarajan, E.; Suganthi, V.; Subathra Devi, C. Studies on heavy metal removal efficiency and antibacterial activity of chitosan prepared from shrimp shell waste. 3 Biotech 2014, 4, 167–175. [Google Scholar] [CrossRef]
- Rhim, J.W.; Hong, S.I.; Park, H.M.; Ng, P.K.W. Preparation and characterization of chitosan-based nanocomposite films with antimicrobial activity. J. Agric. Food Chem. 2006, 54, 5814–5822. [Google Scholar] [CrossRef]
- Leung, V.; Hartwell, R.; Yang, H.; Ghahary, A.; Ko, F. Bioactive nanofibres for wound healing applications. In Proceedings of the Textile Bioengineering and Informatics Symposium, Melbourne, Australia, 12–15 July 2016.
- Kingkaew, J.; Kirdponpattara, S.; Sanchavanakit, N.; Pavasant, P.; Phisalaphong, M. Effect of molecular weight of chitosan on antimicrobial properties and tissue compatibility of chitosan-impregnated bacterial cellulose films. Biotechnol. Bioprocess Eng. 2014, 19, 534–544. [Google Scholar] [CrossRef]
- Ayatollahi, M.; Alishahi, E.; Doagou-R, S.; Shadlou, S. Tribological and mechanical properties of low content nanodiamond/epoxy nanocomposites. Compos. Part B 2012, 43, 3425–3430. [Google Scholar] [CrossRef]
- Wang, Z.; Cai, N.; Zhao, D.; Xu, J.; Dai, Q.; Xue, Y.; Luo, X.; Yang, Y.; Yu, F. Mechanical reinforcement of electrospun water-soluble polymer nanofibers using nanodiamonds. Polym. Compos. 2013, 34, 1735–1744. [Google Scholar] [CrossRef]
- Landel, R.F.; Nielsen, L.E. Mechanical Properties of Polymers and Composites; CRC Press: Boca Raton, FL, USA, 1993. [Google Scholar]
- Jee, A.Y.; Lee, M. Mechanical properties of polycarbonate and poly (methyl methacrylate) films reinforced with surface-functionalized nanodiamonds. J. Nanosci. Nanotechnol. 2011, 11, 533–536. [Google Scholar] [CrossRef] [PubMed]
- Hashin, Z.; Shtrikman, S. A variational approach to the theory of the elastic behaviour of multiphase materials. J. Mech. Phys. Solids 1963, 11, 127–140. [Google Scholar] [CrossRef]
- Matthews, F.L.; Rawlings, R.D. Composite Materials: Engineering and Science; Elsevier: Amsterdam, The Netherland, 1999. [Google Scholar]
- Zhu, Y.; Li, J.; Li, W.; Zhang, Y.; Yang, X.; Chen, N.; Sun, Y.; Zhao, Y.; Fan, C.; Huang, Q. The biocompatibility of nanodiamonds and their application in drug delivery systems. Theranostics 2012, 2, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Tai, H.; Mather, M.L.; Howard, D.; Wang, W.; White, L.J.; Crowe, J.A.; Morgan, S.P.; Chandra, A.; Williams, D.J.; Howdle, S.M. Control of pore size and structure of tissue engineering scaffolds produced by supercritical fluid processing. Eur. Cell Mater. 2007, 14, 64–77. [Google Scholar] [PubMed]
- Zhang, X.; Yin, J.; Kang, C.; Li, J.; Zhu, Y.; Li, W.; Huang, Q.; Zhu, Z. Biodistribution and toxicity of nanodiamonds in mice after intratracheal instillation. Toxicol. Lett. 2010, 198, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Zivanovic, S.; Li, J.; Davidson, P.M.; Kit, K. Physical, mechanical, and antibacterial properties of chitosan/peo blend films. Biomacromolecules 2007, 8, 1505–1510. [Google Scholar] [CrossRef] [PubMed]
- Mazaheri, M.; Akhavan, O.; Simchi, A. Flexible bactericidal graphene oxide-chitosan layers for stem cell proliferation. Appl. Surf. Sci. 2014, 301, 456–462. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ostadhossein, F.; Mahmoudi, N.; Morales-Cid, G.; Tamjid, E.; Navas-Martos, F.J.; Soriano-Cuadrado, B.; Paniza, J.M.L.; Simchi, A. Development of Chitosan/Bacterial Cellulose Composite Films Containing Nanodiamonds as a Potential Flexible Platform for Wound Dressing. Materials 2015, 8, 6401-6418. https://doi.org/10.3390/ma8095309
Ostadhossein F, Mahmoudi N, Morales-Cid G, Tamjid E, Navas-Martos FJ, Soriano-Cuadrado B, Paniza JML, Simchi A. Development of Chitosan/Bacterial Cellulose Composite Films Containing Nanodiamonds as a Potential Flexible Platform for Wound Dressing. Materials. 2015; 8(9):6401-6418. https://doi.org/10.3390/ma8095309
Chicago/Turabian StyleOstadhossein, Fatemeh, Nafiseh Mahmoudi, Gabriel Morales-Cid, Elnaz Tamjid, Francisco Javier Navas-Martos, Belén Soriano-Cuadrado, José Manuel López Paniza, and Abdolreza Simchi. 2015. "Development of Chitosan/Bacterial Cellulose Composite Films Containing Nanodiamonds as a Potential Flexible Platform for Wound Dressing" Materials 8, no. 9: 6401-6418. https://doi.org/10.3390/ma8095309
APA StyleOstadhossein, F., Mahmoudi, N., Morales-Cid, G., Tamjid, E., Navas-Martos, F. J., Soriano-Cuadrado, B., Paniza, J. M. L., & Simchi, A. (2015). Development of Chitosan/Bacterial Cellulose Composite Films Containing Nanodiamonds as a Potential Flexible Platform for Wound Dressing. Materials, 8(9), 6401-6418. https://doi.org/10.3390/ma8095309