The Synergistic Effect of Iodide and Sodium Nitrite on the Corrosion Inhibition of Mild Steel in Bicarbonate–Chloride Solution
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of Sodium Nitrite Concentration
2.2. Effect of Potassium Iodide Additives
2.3. Effect of Flow Velocity
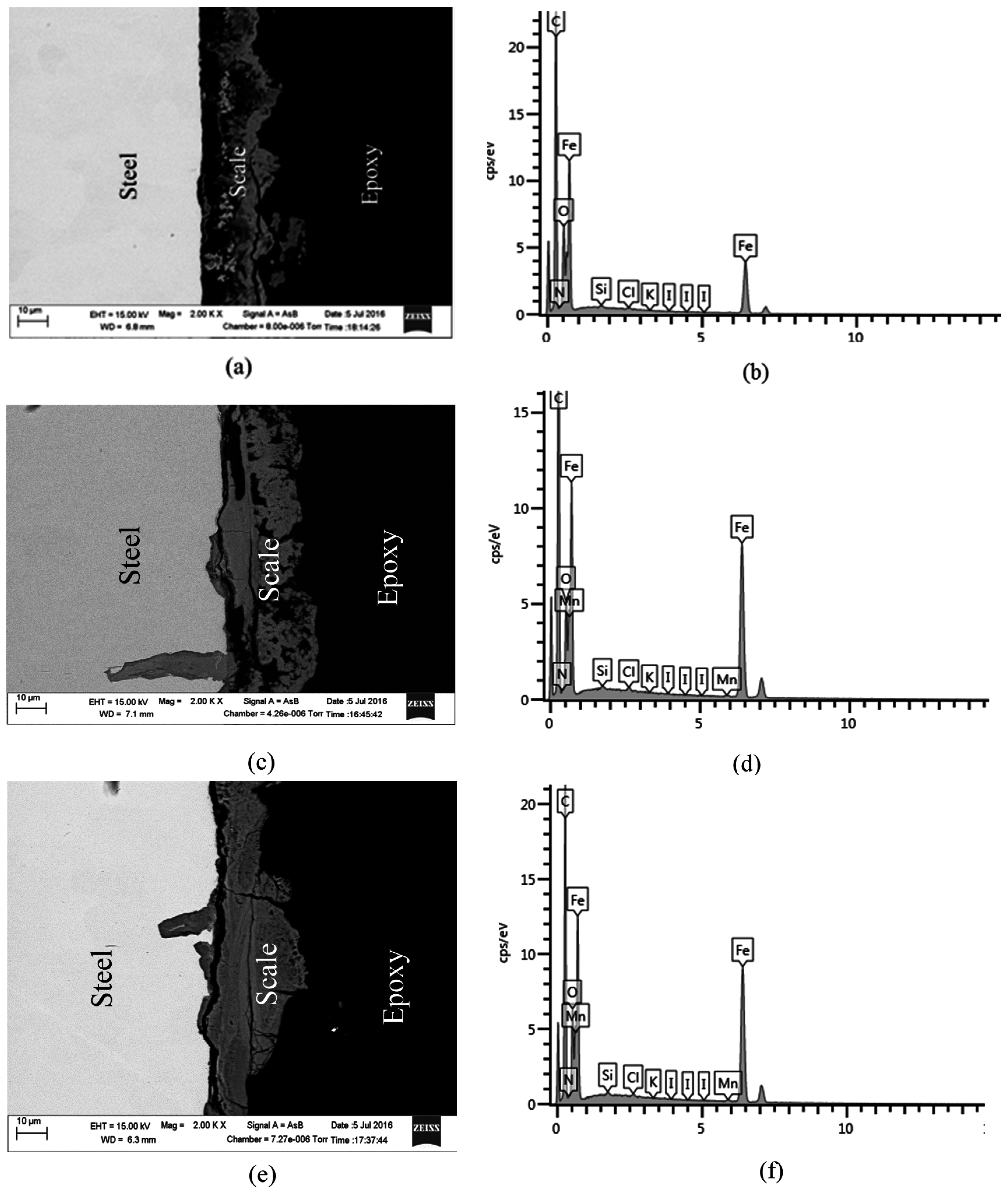
2.4. SEM and EDS Analysis
2.5. XRD Analysis
3. Experimental
3.1. Materials and Solutions
3.2. Electrochemical Test
3.3. Scanning Electron Microscopy (SEM)
3.4. X-ray Diffraction
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Han, J.; Zhang, J.; Carey, J.W. Effect of bicarbonate on corrosion of carbon steel in CO2 saturated brines. Int. J. Greenh. Gas Control 2011, 5, 1680–1683. [Google Scholar] [CrossRef]
- Alves, V.A.; Brett, C. The influence of alloying on the passive behaviour of steels in a bicarbonate medium studied by electrochemistry and XPS. Key Eng. Mater. Trans. Tech. Publ. 2002, 230, 436–439. [Google Scholar] [CrossRef]
- Castro, E.; Vilche, J.; Arvia, A. Iron dissolution and passivation in K2CO3/KHCO3 solutions. rotating ring disc electrode and XPS studies. Corros. Sci. 1991, 32, 37–50. [Google Scholar] [CrossRef]
- Davies, D.; Burstein, G. The effects of bicarbonate on the corrosion and passivation of iron. Corrosion 1980, 36, 416–422. [Google Scholar] [CrossRef]
- Linter, B.; Burstein, G. Reactions of pipeline steels in carbon dioxide solutions. Corros. Sci. 1999, 41, 117–139. [Google Scholar] [CrossRef]
- El-Etre, A.; Abdallah, M. Natural honey as corrosion inhibitor for metals and alloys. II. C-steel in high saline water. Corros. Sci. 2000, 42, 731–738. [Google Scholar] [CrossRef]
- Galliano, F.; Landolt, D. Evaluation of corrosion protection properties of additives for waterborne epoxy coatings on steel. Prog. Org. Coat. 2002, 44, 217–225. [Google Scholar] [CrossRef]
- Kim, J.-G.; Kim, Y.-W. Cathodic protection criteria of thermally insulated pipeline buried in soil. Corros. Sci. 2001, 43, 2011–2021. [Google Scholar] [CrossRef]
- Lin, B.-L.; Lu, J.-T.; Kong, G. Synergistic corrosion protection for galvanized steel by phosphating and sodium silicate post-sealing. Surf. Coat. Technol. 2008, 202, 1831–1838. [Google Scholar] [CrossRef]
- Ali, M.; Mustafa, C.; Habib, M. Effect of molybdate, nitrite and zinc ions on the corrosion inhibition of mild steel in aqueous chloride media containing cupric ions. J. Sci. Res. 2008, 1, 82–91. [Google Scholar] [CrossRef]
- Wachter, A. Sodium Nitrite as Corrosion Inhibitor for Water. Ind. Eng. Chem. 1945, 37, 749–751. [Google Scholar] [CrossRef]
- Ashassi-Sorkhabi, H.; Asghari, E. Effect of hydrodynamic conditions on the inhibition performance of l-methionine as a “green” inhibitor. Electrochim. Acta 2008, 54, 162–167. [Google Scholar] [CrossRef]
- Beecher, J.; Dinkel, C.; Corwin, S. Corrosion inhibition with sodium nitrite. J. (Am. Water Works Assoc.) 1959, 51, 1175–1180. [Google Scholar]
- Lee, D.Y.; Kim, W.C.; Kim, J.G. Effect of nitrite concentration on the corrosion behaviour of carbon steel pipelines in synthetic tap water. Corros. Sci. 2012, 64, 105–114. [Google Scholar] [CrossRef]
- Raman, R.S.; Siew, W. Role of nitrite addition in chloride stress corrosion cracking of a super duplex stainless steel. Corros. Sci. 2010, 52, 113–117. [Google Scholar] [CrossRef]
- Sanad, S.; Ismail, A.; Mahmoud, N. Inhibition effect of potassium iodide on corrosion of stainless steel in hydrochloric acid solution. J. Mater. Sci. 1992, 27, 5706–5712. [Google Scholar] [CrossRef]
- Oguzie, E.E.; Li, Y.; Wang, F.H. Corrosion inhibition and adsorption behavior of methionine on mild steel in sulfuric acid and synergistic effect of iodide ion. J. Colloid Interface Sci. 2007, 310, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Pavithra, M.K.; Venkatesha, T.V.; Vathsala, K.; Nayana, K.O. Synergistic effect of halide ions on improving corrosion inhibition behaviour of benzisothiozole-3-piperizine hydrochloride on mild steel in 0.5 M H2SO4 medium. Corros. Sci. 2010, 52, 3811–3819. [Google Scholar] [CrossRef]
- Eliyan, F.; Alfantazi, A. Sensitivity of the passive films on API-X100 steel heat-affected zones (HAZs) towards trace chloride concentrations in bicarbonate solutions at high temperature. Mater. Corros. 2014, 65, 1111–1119. [Google Scholar] [CrossRef]
- Eliyan, F.F.; Mahdi, E.-S.; Alfantazi, A. Electrochemical evaluation of the corrosion behaviour of API-X100 pipeline steel in aerated bicarbonate solutions. Corros. Sci. 2012, 58, 181–191. [Google Scholar] [CrossRef]
- Jones, D. Corrosion of central heating systems. Eng. Fail. Anal. 1997, 4, 179–194. [Google Scholar] [CrossRef]
- Bloom, M.; Goldenberg, L. γ-Fe2O3 and the passivity of iron. Corros. Sci. 1965, 5, 623–630. [Google Scholar] [CrossRef]
- Popova, A.; Sokolova, E.; Raicheva, S.; Christov, M. AC and DC study of the temperature effect on mild steel corrosion in acid media in the presence of benzimidazole derivatives. Corros. Sci. 2003, 45, 33–58. [Google Scholar] [CrossRef]
- Khaled, K.; Hackerman, N. Investigation of the inhibitive effect of ortho-substituted anilines on corrosion of iron in 1 M HCl solutions. Electrochim. Acta 2003, 48, 2715–2723. [Google Scholar] [CrossRef]
- Moretti, G.; Guidi, F.; Grion, G. Tryptamine as a green iron corrosion inhibitor in 0.5 M deaerated sulphuric acid. Corros. Sci. 2004, 46, 387–403. [Google Scholar] [CrossRef]
- Bockris, J.M.; Drazic, D.; Despic, A. The electrode kinetics of the deposition and dissolution of iron. Electrochim. Acta 1961, 4, 325–361. [Google Scholar] [CrossRef]
- Bala, H. The corrosion of iron in sulphate solutions at pH = 0–2. Electrochim. Acta 1984, 29, 119–129. [Google Scholar] [CrossRef]
- Heusler, K.; Cartledge, G. The influence of iodide ions and carbon monoxide on the anodic dissolution of active iron. J. Electrochem. Soc. 1961, 108, 732–740. [Google Scholar] [CrossRef]
- Mu, G.; Li, X. Inhibition of cold rolled steel corrosion by Tween-20 in sulfuric acid: Weight loss, electrochemical and AFM approaches. J. Colloid Interface Sci. 2005, 289, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Harek, Y.; Larabi, L. Corrosion inhibition of mild steel in 1 mol dm−3 HCl by oxalic N-phenylhydrazide-N′-phenylthiosemicarbazide. Kem. Ind. 2004, 53, 55–61. [Google Scholar]
- Kuznetsov, Y.I.; Andreev, N. Reviews on Corrosion Inhibitors Science and Technology; NACE International: Houston, TX, USA, 1996. [Google Scholar]
- Mor, E.; Scotto, V.; Wrubl, C. Hexamethylenetetramine Hydroiodide (HMTA-I) as a Corrosion Inhibitor for Steel in HCI Pickling Solutions. Br. Corros. J. 1972, 7, 276–280. [Google Scholar] [CrossRef]
- Hoinkis, E. Review of the Adsorption of Iodine on Metal and Its Behavior in Loops; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 1970. [Google Scholar]
- Attar, T.; Harek, Y. Inhibition effect of potassium iodide on the corrosion of carbon steel (XC 38) in acidic medium. Int. J. Adv. Chem. 2014, 2, 139–142. [Google Scholar] [CrossRef]
- Khadom, A.A.; Yaro, A.S. Protection of low carbon steel in phosphoric acid by potassium iodide. Prot. Met. Phys. Chem. Surf. 2011, 47, 662–669. [Google Scholar] [CrossRef]
- Cavallaro, L.; Felloni, L.; Trabanelli, G.; Pulidori, F. The anodic dissolution of iron and the behaviour of some corrosion inhibitors investigated by the potentiodynamic method. Electrochim. Acta 1964, 9, 485–494. [Google Scholar] [CrossRef]
- Abdallah, M. Corrosion behaviour of 304 stainless steel in sulphuric acid solutions and its inhibition by some substituted pyrazolones. Mater. Chem. Phys. 2003, 82, 786–792. [Google Scholar] [CrossRef]
- Bommersbach, P.; Alemany-Dumont, C.; Millet, J.-P.; Normand, B. Hydrodynamic effect on the behaviour of a corrosion inhibitor film: Characterization by electrochemical impedance spectroscopy. Electrochim. Acta 2006, 51, 4011–4018. [Google Scholar] [CrossRef]
- Eyu, G.D.; Will, G.; Dekkers, W.; MacLeod, J. Effect of hydrodynamics and surface roughness on the electrochemical behaviour of carbon steel in CSG produced water. Appl. Surf. Sci. 2015, 357, 506–515. [Google Scholar] [CrossRef]
- Branzoi, V.; Branzoi, F.; Baibarac, M. The inhibition of the corrosion of Armco iron in HCl solutions in the presence of surfactants of the type of N-alkyl quaternary ammonium salts. Mater. Chem. Phys. 2000, 65, 288–297. [Google Scholar] [CrossRef]
- Jiang, X.; Zheng, Y.; Ke, W. Effect of flow velocity and entrained sand on inhibition performances of two inhibitors for CO2 corrosion of N80 steel in 3% NaCl solution. Corros. Sci. 2005, 47, 2636–2658. [Google Scholar] [CrossRef]
- Valcarce, M.; Vázquez, M. Phosphate ions used as green inhibitor against copper corrosion in tap water. Corros. Sci. 2010, 52, 1413–1420. [Google Scholar] [CrossRef]









| Inh. Conc (g/L) | Rs (Ω·cm2) | Rct (Ω·cm2) | Qdl (F·cm−2) | n | Ra (Ω·cm2) | Qa (F·cm−2) | η (%) | Surface Coverage (θ) |
|---|---|---|---|---|---|---|---|---|
| 0 | 58.41 | 160.8 | 4.37 × 10−3 | 0.59 | 29.96 | 1.26 × 10−3 | 0 | 0 |
| 1g NO2− | 18.54 | 2048 | 1.51 × 10−3 | 0.55 | 10.68 | 5.0 × 10−1 | 92.14 | 0.921 |
| 2g NO2− | 15.33 | 4473 | 5.22 × 10−4 | 0.76 | 11.06 | 1.02 × 10−4 | 96.40 | 0.964 |
| 5g NO2− | 27.80 | 1492 | 1.59 × 10−3 | 0.78 | 303.5 | 9.4 × 10−4 | 89.22 | 0.892 |
| Inh. Conc (g/L) | Rs (Ω·cm2) | Rct (Ω·cm2) | Qdl (F·cm−2) | n | Ra (Ω·cm2) | Qa (F·cm−2) | η (%) | Surface Coverage (θ) |
|---|---|---|---|---|---|---|---|---|
| 0 | 58.41 | 160.8 | 4.37 × 10−3 | 0.59 | 29.96 | 1.26 × 10−3 | 0 | 0 |
| 1KI | 31.74 | 176.7 | 4.09 × 10−2 | 0.22 | 65.92 | 2.60 × 10−3 | 8.99 | 0.089 |
| 1NO2− | 29.57 | 1911 | 1.49 × 10−3 | 0.56 | 8.66 | 1.97 × 10−5 | 91.58 | 0.916 |
| 1NO2 + 0.5KI | 22.50 | 10,621 | 5.70 × 10−4 | 0.78 | 335.40 | 5.61 × 10−3 | 98.48 | 0.985 |
| 1NO2 + 1KI | 26.94 | 12,009 | 3.76 × 10−4 | 0.99 | 2156 | 5.31 × 10−4 | 98.66 | 0.987 |
| 1NO2 + 2KI | 93.70 | 9029 | 3.85 × 10−4 | 0.83 | 163.0 | 1.30 × 10−3 | 98.21 | 0.982 |
| Inh. Conc (g/L) | Rs (Ω·cm2) | Rct (Ω·cm2) | Qdl (F·cm−2) | n | Ra (Ω·cm2) | Qa (F·cm−2) | η (%) | Surface Coverage (θ) |
|---|---|---|---|---|---|---|---|---|
| 0 | 58.41 | 160.8 | 4.37 × 10−3 | 0.59 | 29.96 | 1.26 × 10−3 | 0 | 0 |
| 2KI | 31.81 | 46.32 | 1.47 × 10−2 | 0.59 | 33.97 | 3.30 × 10−3 | −71.19 | −0.712 |
| 2NO2− | 15.53 | 4473 | 5.22 × 10−4 | 0.76 | 11.06 | 1.02 × 10−4 | 96.40 | 0.964 oo |
| 2NO2 + 2KI | 22.30 | 13,347 | 3.78 × 10−4 | 0.75 | 78.78 | 0.9 × 10−4 | 98.79 | 0.988 |
| Rotation Speed (rpm) | Rs (Ω·cm2) | Rct (Ω·cm2) | Qdl (F·cm−2) | n | Ra (Ω·cm2) | Qa (F·cm−2) |
|---|---|---|---|---|---|---|
| 0 | 27.45 | 508.3 | 1.26 × 10−3 | 0.76 | 61.01 | 1.79 × 10−4 |
| 1000 | 25.61 | 3597 | 1.14 × 10−3 | 1 | 1536 | 5.60 × 10−4 |
| 2000 | 22.3 | 13,347 | 3.78 × 10−4 | 0.75 | 78.79 | 0.92 × 10−4 |
| 4000 | 24.87 | 3859 | 3.4 × 10−4 | 1 | 3771 | 3.92 × 10−4 |
| C | Si | Mn | Cr | Cu | Ni | Fe |
|---|---|---|---|---|---|---|
| 0.20 | 0.32 | 0.79 | 0.01 | 0.01 | 0.01 | Bal 98.7 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eyu, G.D.; Will, G.; Dekkers, W.; MacLeod, J. The Synergistic Effect of Iodide and Sodium Nitrite on the Corrosion Inhibition of Mild Steel in Bicarbonate–Chloride Solution. Materials 2016, 9, 868. https://doi.org/10.3390/ma9110868
Eyu GD, Will G, Dekkers W, MacLeod J. The Synergistic Effect of Iodide and Sodium Nitrite on the Corrosion Inhibition of Mild Steel in Bicarbonate–Chloride Solution. Materials. 2016; 9(11):868. https://doi.org/10.3390/ma9110868
Chicago/Turabian StyleEyu, Gaius Debi, Geoffrey Will, Willem Dekkers, and Jennifer MacLeod. 2016. "The Synergistic Effect of Iodide and Sodium Nitrite on the Corrosion Inhibition of Mild Steel in Bicarbonate–Chloride Solution" Materials 9, no. 11: 868. https://doi.org/10.3390/ma9110868
APA StyleEyu, G. D., Will, G., Dekkers, W., & MacLeod, J. (2016). The Synergistic Effect of Iodide and Sodium Nitrite on the Corrosion Inhibition of Mild Steel in Bicarbonate–Chloride Solution. Materials, 9(11), 868. https://doi.org/10.3390/ma9110868







