Floating Hydrogel with Self-Generating Micro-Bubbles for Intravesical Instillation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
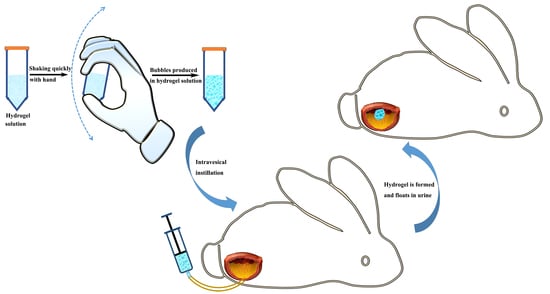
2.2. Preparation and Optimization of Floating Hydrogel
2.3. Characterizations of Floating Hydrogel
2.4. Incorporation of Drug in Floating Hydrogel
2.5. Release Study In Vitro
2.6. Verification of Hydrogel Floating In Vivo
2.7. Release Study In Vivo
2.8. The Efficacy of Floating Hydrogel in Acute Bladder Injury Model
3. Results
3.1. Preparation and Optimization of Floating Hydrogel
3.2. Characterization of Floating Hydrogel
3.3. Release Study In Vitro
3.4. Release Study In Vivo
3.5. The Efficacy of Floating Hydrogel in Acute Bladder Injury Model
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- GuhaSarkar, S.; Banerjee, R. Intravesical drug delivery: Challenges, current status, opportunities and novel strategies. J. Controll. Release: Off. J. Controll. Release Soc. 2010, 148, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Gasion, J.P.; Cruz, J.F. Improving efficacy of intravesical chemotherapy. Eur. Urol. 2006, 50, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, P.; Li, Z.; Chancellor, M.; De Groat, W.C.; Yoshimura, N.; Huang, L. Sustained intravesical drug delivery using thermosensitive hydrogel. Pharm. Res. 2004, 21, 832–837. [Google Scholar] [CrossRef] [PubMed]
- Men, K.; Liu, W.; Li, L.; Duan, X.; Wang, P.; Gou, M.; Wei, X.; Gao, X.; Wang, B.; Du, Y.; Huang, M. Delivering instilled hydrophobic drug to the bladder by a cationic nanoparticle and thermo-sensitive hydrogel composite system. Nanoscale 2012, 4, 6425–6433. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Sun, P.; Li, P.; Xue, A.; Zhang, X.; Zhang, H.; Jin, X. A magnetic chitosan hydrogel for sustained and prolonged delivery of Bacillus Calmette-Guerin in the treatment of bladder cancer. Biomaterials 2013, 34, 10258–10266. [Google Scholar] [CrossRef] [PubMed]
- Collado, A.; Chechile, G.E.; Salvador, J.; Vicente, J. Early complications of endoscopic treatment for superficial bladder tumors. J. Urol. 2000, 164, 1529–1532. [Google Scholar] [CrossRef]
- Lin, T.; Wu, J.; Zhao, X.; Lian, H.; Yuan, A.; Tang, X.; Zhao, S.; Guo, H.; Hu, Y. In situ floating hydrogel for intravesical delivery of adriamycin without blocking urinary tract. J. Pharm. Sci. 2014, 103, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Zhang, Y.; Wu, J.; Zhao, X.; Lian, H.; Wang, W.; Guo, H.; Hu, Y. A Floating Hydrogel System Capable of Generating CO Bubbles to Diminish Urinary Obstruction after Intravesical Instillation. Pharm. Res. 2014, 31, 2655–2663. [Google Scholar] [CrossRef] [PubMed]
- Buckton, G.; Machiste, E.O. Differences between dynamic and equilibrium surface tension of poly(oxyethylene)-poly(oxypropylene)-poly(oxyethylene) block copolymer surfactants (poloxamers P407, P237, and P338) in aqueous solution. J. Pharm. Sci. 1997, 86, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Golemanov, K.; Denkov, N.D.; Tcholakova, S.; Vethamuthu, M.; Lips, A. Surfactant mixtures for control of bubble surface mobility in foam studies. Langmuir ACS J. Surf. Colloids 2008, 24, 9956–9961. [Google Scholar] [CrossRef] [PubMed]
- Shelke, S.; Shahi, S.; Jadhav, K.; Dhamecha, D.; Tiwari, R.; Patil, H. Thermoreversible nanoethosomal gel for the intranasal delivery of Eletriptan hydrobromide. J. Mater. Sci. Mater. Med. 2016, 27, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.; Sousa Lobo, J.M. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Parsons, C.L.; Mulholland, S.G. Successful Therapy of Interstitial Cystitis with Pentosanpolysulfate. J. Urol. 1987, 138, 513–516. [Google Scholar] [PubMed]
- Dumortier, G.; Grossiord, J.L.; Agnely, F.; Chaumeil, J.C. A review of poloxamer 407 pharmaceutical and pharmacological characteristics. Pharm. Res. 2006, 23, 2709–2728. [Google Scholar] [CrossRef] [PubMed]
- Ricci, E.J.; Lunardi, L.O.; Nanclares, D.M.; Marchetti, J.M. Sustained release of lidocaine from Poloxamer 407 gels. Int. J. Pharm. 2005, 288, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Shelke, S.; Shahi, S.; Jalalpure, S.; Dhamecha, D. Poloxamer 407-based intranasal thermoreversible gel of zolmitriptan-loaded nanoethosomes: Formulation, optimization, evaluation and permeation studies. J. Liposome Res. 2016, 26, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Fdhila, R.B.; Duineveld, P.C. The effect of surfactant on the rise of a spherical bubble at high Reynolds and Peclet numbers. Phys. Fluids 1996, 8, 310–321. [Google Scholar] [CrossRef]
- Hetsroni, G.; Mosyak, A.; Pogrebnyak, E.; Sher, I.; Segal, Z. Bubble growth in saturated pool boiling in water and surfactant solution. Int. J. Multiphas Flow 2006, 32, 159–182. [Google Scholar] [CrossRef]
- Giribabu, K.; Ghosh, P. Binary coalescence of air bubbles in viscous liquids in presence of non-ionic surfactant. Can. J. Chem. Eng. 2008, 86, 643–650. [Google Scholar] [CrossRef]
- Zhang, L.; Parsons, D.L.; Navarre, C.; Kompella, U.B. Development and in vitro evaluation of sustained release poloxamer 407 (P407) gel formulations of ceftiofur. J. Controll. Release: Off. J. Controll. Release Soc. 2002, 85, 73–81. [Google Scholar] [CrossRef]
- Bruskewitz, R.C.; Iversen, P.; Madsen, P.O. Value of postvoid residual urine determination in evaluation of prostatism. Urology 1982, 20, 602–604. [Google Scholar] [CrossRef]
- Eckford, S.D.; Persad, R.A.; Brewster, S.F.; Gingell, J.C. Intravesical foreign bodies: Five-year review. Br. J. Urol. 1992, 69, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Chancellor, M.B.; Yoshimura, N. Treatment of interstitial cystitis. Urology 2004, 63, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Nickel, J.C.; Downey, J.; Morales, A.; Emerson, L.; Clark, J. Relative efficacy of various exogenous glycosaminoglycans in providing a bladder surface permeability barrier. J. Urol. 1998, 160, 612–614. [Google Scholar] [CrossRef]
- Parsons, C.L. The role of the urinary epithelium in the pathogenesis of interstitial cystitis/prostatitis/urethritis. Urology 2007, 69, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Parsons, C.L. Epithelial coating techniques in the treatment of interstitial cystitis. Urology 1997, 49, 100–104. [Google Scholar] [CrossRef]









© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, T.; Zhao, X.; Zhang, Y.; Lian, H.; Zhuang, J.; Zhang, Q.; Chen, W.; Wang, W.; Liu, G.; Guo, S.; et al. Floating Hydrogel with Self-Generating Micro-Bubbles for Intravesical Instillation. Materials 2016, 9, 1005. https://doi.org/10.3390/ma9121005
Lin T, Zhao X, Zhang Y, Lian H, Zhuang J, Zhang Q, Chen W, Wang W, Liu G, Guo S, et al. Floating Hydrogel with Self-Generating Micro-Bubbles for Intravesical Instillation. Materials. 2016; 9(12):1005. https://doi.org/10.3390/ma9121005
Chicago/Turabian StyleLin, Tingsheng, Xiaozhi Zhao, Yifan Zhang, Huibo Lian, Junlong Zhuang, Qing Zhang, Wei Chen, Wei Wang, Guangxiang Liu, Suhan Guo, and et al. 2016. "Floating Hydrogel with Self-Generating Micro-Bubbles for Intravesical Instillation" Materials 9, no. 12: 1005. https://doi.org/10.3390/ma9121005
APA StyleLin, T., Zhao, X., Zhang, Y., Lian, H., Zhuang, J., Zhang, Q., Chen, W., Wang, W., Liu, G., Guo, S., Wu, J., Hu, Y., & Guo, H. (2016). Floating Hydrogel with Self-Generating Micro-Bubbles for Intravesical Instillation. Materials, 9(12), 1005. https://doi.org/10.3390/ma9121005