An in Vitro Twist Fatigue Test of Fabric Stent-Grafts Supported by Z-Stents vs. Ringed Stents
Abstract
:1. Introduction



2. Results
2.1. Gross Observations
2.2. Observations Under Light Microscopy and SEM


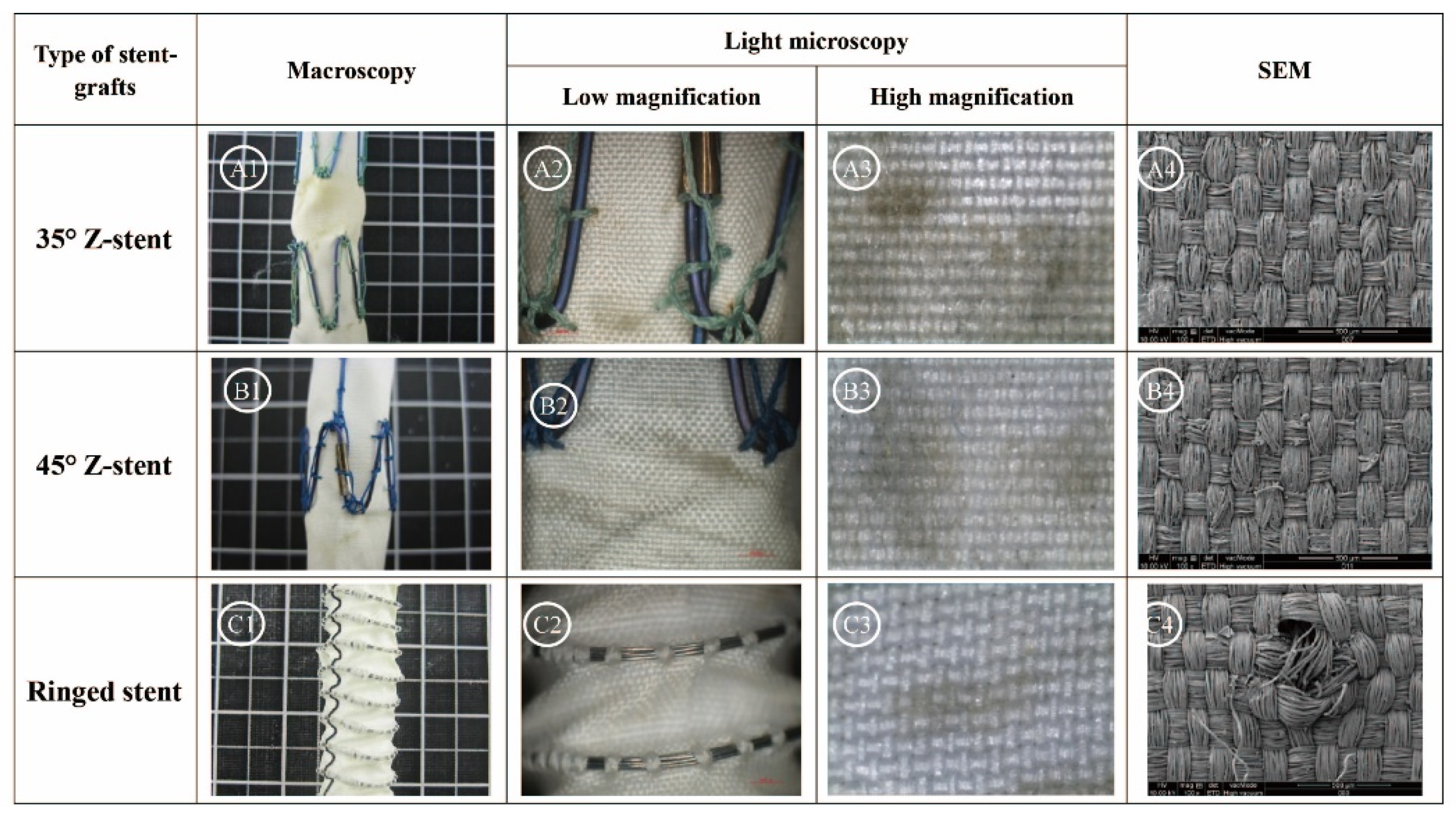

2.3. Textile Analyses of Fabrics
| Device | No. | Duration (h) | Fabric Count (mm) | Thickness (mm) | Mass (g/m2) | Porosity (%) | |
|---|---|---|---|---|---|---|---|
| Warp | Weft | ||||||
| Z-stent | N0/S0 | 0 | 7.42 ± 0.05 | 5.46 ± 0.02 | 0.110 ± 0.002 | 61.33 ± 2.31 | 59.45 |
| 35° | N1 | 24 | 7.48 ± 0.11 | 5.30 ± 0.06 | 0.113 ± 0.003 | 64.00 ± 4.00 | 58.92 |
| N2 | 7.47 ± 0.14 | 5.25 ± 0.03 | 0.114 ± 0.005 | 66.67 ± 8.33 | 57.44 | ||
| N3 | 48 | 7.45 ± 0.09 | 5.41 ± 0.02 | 0.113 ± 0.004 | 85.33 ± 4.62 | 45.13 | |
| N4 | 7.52 ± 0.09 | 5.39 ± 0.06 | 0.115 ± 0.002 | 69.33 ± 8.33 | 56.24 | ||
| N5 | 168 | 7.39 ± 0.08 | 5.42 ± 0.03 | 0.113 ± 0.003 | 73.33 ± 2.31 | 53.10 | |
| N6 | 7.39 ± 0.10 | 5.31 ± 0.06 | 0.113 ± 0.003 | 77.33 ± 8.33 | 50.36 | ||
| 45° | S1 | 24 | 7.44 ± 0.14 | 5.51 ± 0.09 | 0.110 ± 0.003 | 58.67 ± 6.11 | 61.35 |
| S2 | 7.48 ± 0.15 | 5.44 ± 0.02 | 0.109 ± 0.007 | 64.00 ± 4.00 | 57.34 | ||
| S3 | 48 | 7.48 ± 0.08 | 5.45 ± 0.02 | 0.106 ± 0.005 | 62.67 ± 4.62 | 57.20 | |
| S4 | 7.43 ± 0.16 | 5.39 ± 0.11 | 0.117 ± 0.008 | 70.67 ± 6.11 | 56.31 | ||
| S5 | 168 | 7.35 ± 0.03 | 5.30 ± 0.08 | 0.107 ± 0.003 | 65.33 ± 6.11 | 55.29 | |
| S6 | 7.37 ± 0.10 | 5.45 ± 0.01 | 0.116 ± 0.004 | 62.67 ± 4.62 | 60.68 | ||
| R-stent | R0 | 0 | 7.26 ± 0.18 | 4.92 ± 0.05 | 0.153 ± 0.009 | 81.33 ± 10.07 | 56.37 |
| R1 | 24 | 7.28 ± 0.15 | 4.89 ± 0.10 | 0.154 ± 0.007 | 84.00 ± 4.00 | 58.57 | |
| R2 | 7.22 ± 0.05 | 4.92 ± 0.01 | 0.155 ± 0.026 | 85.33 ± 8.33 | 57.04 | ||
| R3 | 48 | 7.33 ± 0.21 | 4.86 ± 0.14 | 0.155 ± 0.010 | 78.67 ± 8.33 | 64.38 | |
| R4 | 7.18 ± 0.17 | 4.85 ± 0.06 | 0.154 ± 0.016 | 85.33 ± 6.11 | 60.45 | ||
| R5 | 168 | 7.24 ± 0.14 | 4.89 ± 0.11 | 0.156 ± 0.008 | 85.33 ± 12.22 | 55.44 | |
| R6 | 7.20 ± 0.05 | 4.90 ± 0.07 | 0.160 ± 0.006 | 84.00 ± 4.00 | 63.65 | ||





3. Discussion
3.1. Potential Endotesion Issue
3.2. The Fabric Structures and Stent Shape of Stent-Grafts
3.3. The Impact of Twisting on Hemodynamics


4. Materials and Methods
4.1. Selection of Stent-Grafts

| Graft | Fabric Structure | Fabric Count (mm) | Number of Filaments | Filament Diameter | |||
|---|---|---|---|---|---|---|---|
| Warp | Weft | Warp | Weft | Warp | Weft | ||
| Experimental devices (Guangci) | Plain | 7.42 ± 0.05 | 5.46 ± 0.02 | 48 | 16 | 11.97 ± 1.21 | 15.31 ± 1.37 |
| Anaconda (Vascutek) | Plain | 7.26 ± 0.18 | 4.92 ± 0.05 | 27 | 54 | 12.87 ± 0.64 | 12.87 ± 0.64 |
| Talent (Medtronic) | 4/4 twill | 20.41 ± 0.12 | 26.30 ± 0.13 | 1 | 1 | 36.30 ± 1.05 | 35.71 ± 1.48 |
| Endurant (Medtronic) | Plain | 9.32 ± 0.14 | 5.70 ± 0.06 | 27 | 27 | 17.56 ± 0.52 | 18.14 ± 0.27 |
| Zenith TX2 (Cook) | Fancy warp-backed 1 | 5.93 ± 0.07 2 | 5.28 ± 0.05 | 54 | 54 | 15.63 ± 0.70 | 15.44 ± 1.60 |
| Graft | Thickness (mm) | Mass (g/m2) | Crystallinity (%) | Water permeability (mL/min∙cm2) |
|---|---|---|---|---|
| Experimental devices (Guangci) | 0.110 ± 0.002 | 61.33 ± 2.31 | 48.21 | 86.6 |
| Anaconda (Vascutek) | 0.153 ± 0.009 | 81.33 ± 10.07 | 44.14 | 79.1 |
| Talent(Medtronic) | 0.091 ± 0.003 | 65.91 ± 9.14 | 48.56 | 231.5 |
| Endurant (Medtronic) | 0.127 ± 0.006 | 98.67 ± 8.33 | 32.06 | 75.4 |
| Zenith TX2 (Cook) | 0.234 ± 0.010 | 117.35 ± 4.37 | 34.62 | 121.0 |

4.2. Twisting Fatigue Experiment
4.2.1. Fatigue Machine

4.2.2. Fatigue Conditions
4.3. Analyses of Stent-Grafs
4.3.1. Gross Observations

| Conditions | 0 h | 24 h | 48 h | 168 h |
|---|---|---|---|---|
| Pulsations | 0 | 8.64 × 105 | 1.73 × 106 | 6.048 × 106 |
| Twists | 0 | 8.64 × 104 | 1.73 × 105 | 6.048 × 105 |
4.3.2. Observation in Light Microscopy
4.3.3. Observation in SEM
4.4. Textile Analyses of Fabrics
4.4.1. Fabric Structure and Fabric Count
4.4.2. Thickness
4.4.3. Mass
4.4.4. Porosity
4.4.5. Fiber tensile strength
4.4.6. X-ray Diffraction (XRD)
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Miller, D.C. Through the looking glass, the first 20 years of thoracic aortic stent-grafting. J. Thorac Cardiovasc. Surg. 2013, 145, S142–S148. [Google Scholar] [CrossRef] [PubMed]
- Brunkwall, J. How to design the optimal stent graft-what have we learnt? Scand. J. Surg. 2008, 97, 191–194. [Google Scholar] [PubMed]
- Jackson, B.M.; Carpenter, J.P. Devices used for endovascular aneurysm repair, past, present and future. Sem. Interv. Radiol. 2008, 26, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.; Eaton-Evans, J.; Hillery, C.; Bakhshi, R.; You, Z.; Lu, J.; Hamilton, G.; Seifalian, A.M. AAA stent-grafts, past problems and future prospects. Ann. Biomed. Eng. 2010, 38, 1259–1275. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.L.; Morasch, M.D. Role of stents, drug-eluting stents, and stent-grafts in treatment of infrainguinal arterial disease. Semin. Vasc. Surg. 2007, 20, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Zarins, C.K.; Taylor, C.A. Endovascular device design in the future, transformation from trial and error to computational design. J. Endovasc. Ther. 2009, 16, I12–I21. [Google Scholar] [CrossRef] [PubMed]
- Scurr, J.R.H.; McWilliams, R.G. Fenestrated aortic stent grafts. Sem. Interv. Radiol. 2007, 24, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, R.K.; Clair, D.; Srivastava, S.; Bhandari, G.; Turc, A.; Hampton, J.; Popa, M.; Green, R.; Ouriel, K. Should patients with challenging anatomy be offered endovascular aneurysm repair? J. Vasc. Surg. 2003, 38, 990–996. [Google Scholar] [CrossRef]
- Chuter, T.A.M. Fenestrated and branched stent-grafts for thoracoabdominal, pararenal and juxtarenal aortic aneurysm repair. Semin. Vasc. Surg. 2007, 20, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Monahan, T.S.; Schneider, D.B. Fenestrated and branched stent grafts for repair of complex aortic aneurysms. Semin. Vasc. Surg. 2009, 22, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Ohrlander, T.; Sonesson, B.; Ivancev, K.; Resch, T.; Dias, N.; Malina, M. The chimney graft, a technique for preserving or rescuing aortic branch vessels in stent-graft sealing zones. J. Endovasc. Ther. 2008, 15, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Donas, K.P.; Torsello, G.; Bisdas, T.; Osada, N.; Schönefeld, E.; Pitoulias, G.A. Early outcomes for fenestrated and chimney endografts in the treatment of pararenal aortic pathologies are not significantly different: A systematic review with pooled data analysis. J. Endovasc. Ther. 2012, 19, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Scali, S.T.; Feezor, R.J.; Chang, C.K.; Waterman, A.L.; Berceli, S.A.; Huber, T.S.; Beck, A.W. Critical analysis of results after chimney endovascular aortic aneurysm repair raises cause for concern. J. Vasc. Surg. 2014, 60, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Tolenaar, J.L.; van Keulen, J.W.; Trimarchi, S.; Muhs, B.E.; Moll, F.L.; van Herwaarden, J.A. The chimney graft, a systematic review. Ann. Vasc. Surg. 2012, 26, 1030–1038. [Google Scholar] [CrossRef] [PubMed]
- Guidoin, R.; Marois, Y.; Douville, Y.; King, M.W.; Castonguay, M.; Traoré, A.; Formichi, M.; Staxrud, L.E.; Norgren, L.; Bergeron, P. First generation aortic endografts, analysis of explanted Stentor devices from the Eurostar registry. J. Endovasc. Ther. 2000, 7, 105–122. [Google Scholar] [CrossRef] [PubMed]
- Zarins, C.K.; Arko, F.R.; Tami, C.; Bloch, D.A.; Kenneth, O.; Allen, R.C.; White, R.A. Explant analysis of AneuRx stent-grafts, relationship between structural finding and clinical outcome. J. Vasc. Surg. 2004, 40, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Riepe, G.; Heintz, C.; Kaiser, E.; Chakfé, N.; Morlock, M.; Delling, M.; Imig, H. What can we learn from explanted endovascular devices? Eur. J. Vasc. Endovasc. Surg. 2002, 24, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Dattilo, J.B.; Brewster, D.C.; Fan, C.M.; Geller, S.C.; Cambria, R.P.; LaMuraglia, G.M.; Greenfield, A.J.; Lauterbach, S.R.; Abbott, W.M. Clinical failures of endovascular abdominal aortic aneurysm repair, incidence, causes, and management. J. Vasc. Surg. 2002, 35, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, T.; Teodorescu, V. The endovascular repair of abdominal aortic aneurysm, an update analysis of structural failure modes of endovascular stent grafts. Semin. Vasc. Surg. 2003, 16, 103–112. [Google Scholar] [CrossRef]
- Rutherford, R.B. Structural failures in abdominal aortic aneurysm stent-grafts, Threat to durability and challenge to technology. Semin. Vasc. Surg. 2004, 17, 294–297. [Google Scholar] [CrossRef] [PubMed]
- Beebe, H.G.; Cronenwett, J.L.; Katzen, B.T.; Brewster, D.C.; Green, R.M.; Investiga, V.E.T. Results of an aortic endograft trial, impact of device failure beyond 12 months. J. Vasc. Surg. 2001, 33, 55–63. [Google Scholar] [CrossRef]
- Ohki, T.; Veith, F.J.; Shaw, P.; Lipsitz, E.; Suggs, W.D.; Wain, R.A.; Bade, M.; Mehta, M.; Cayne, N.; Cynamon, J.; et al. Increasing incidence of midterm and long-term complications after endovascular graft repair of abdominal aortic aneurysms, a note of caution based on a 9-year experience. Ann. Surg. 2001, 234, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.L.; Vallabhaneni, S.R.; Desgranges, P.; Bacquemin, J.P.; van Marrewijk, C.; Laheij, R.J.F. Incidence and risk factors of late rupture, conversion and death after endovascular repair of infrarenal aortic aneurysms, the Eurostar experience. J. Vasc. Surg. 2000, 32, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Chaikof, E.L.; Blankensteijn, J.D.; Harris, P.L.; White, G.H.; Zarins, C.K.; Bernhard, V.M.; Matsumura, J.S.; May, J.; Veith, F.J.; Fillinger, M.F.; et al. Reporting standards for endovascular aortic aneurysm repair. J. Vasc. Surg. 2002, 35, 1048–1060. [Google Scholar] [CrossRef] [PubMed]
- Major, A.; Guidoin, R.; Soulez, G.; Gaboury, L.A.; Cloutier, G.; Sapoval, M.; Douville, Y.; Dionne, G.; Geelkerken, R.H.; Petrasek, P.; et al. Implant degradation and poor healing after endovascular repair of abdominal aortic aneurysms, an analysis of explanted stent-grafts. J. Endovasc. Ther. 2006, 13, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Guidoin, R.; Wang, L.; Zhang, Z.; Paynter, R.; How, T.; Nutley, M.; Wei, D.; Douville, Y.; Samis, G. Long-term resistance to fracture and/or corrosion of the Nitinol wires of the Talent stent-graft, observations from a series of explanted devices. J. Long Term Eff. Med. Implant. 2013, 23, 45–59. [Google Scholar] [CrossRef]
- Lin, J.; Guidoin, R.; Wang, L.; Zhang, Z.; Nutley, M.; Paynter, R.; Wei, D.; How, T.; Crepeay, H.; Douville, Y. Fatigue and/or failure phenomena observed in the fabric of stent-grafts explanted after adverse events. J. Long Term Eff. Med. Implant 2013, 23, 67–86. [Google Scholar] [CrossRef]
- Makaroun, M.S.; Tuchek, M.; Massop, D.; Henretta, J.; Rhee, R.; Buckley, C.; Mehta, M.; Ellozy, S.; Endurant US Pivotal Trial Investigators. One year outcomes of the United States regulatory trial of the Endurant stent graft system. J. Vasc. Surg. 2011, 54, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Mertens, R.; Bergoeing, M.; Marine, L.; Valdes, F.; Kramer, A.; Vergara, J. Ventana fenestrated stent-graft system for endovascular repair of juxtarenal aortic aneurysms. J. Endovasc. Ther. 2012, 19, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Mehta, M.; Valdes, F.E.; Nolte, T.; Mishkel, G.J.; Jordan, W.D.; Gray, B.; Eskandari, M.K.; Botti, C.; A Pivotal Clinical Study to Evaluate the Safety and Effectiveness of the Ovation Abdominal Stent Graft System Investigators. One-year outcomes from an international study of the Ovation abdominal stent graft system for endovascular aneurysm repair. J. Vasc. Surg. 2014, 59, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Malkawi, A.H.; de Bruin, J.L.; Loftus, I.M.; Thompson, M.M. Treatment of a juxtarenal aneurysm with the Nellix endovascular aneurysm sealing system and chimney stent. J. Endovasc. Ther. 2014, 21, 538–540. [Google Scholar] [CrossRef] [PubMed]
- Chaikof, E.L. Hear no evil, see no evil, speak no evil, postmarket monitoring, underreporting, and estimating the prevalence of endograft-related adverse events. J. Vasc. Surg. 2002, 35, 1299–1300. [Google Scholar] [CrossRef] [PubMed]
- Pelton, A.R.; Schroeder, V.; Mitchell, M.R.; Gong, X.Y.; Barney, M.; Robertson, S.W. Fatigue and durability of Nitinol stents. J. Mech. Behav. Biomed. Mater. 2008, 1, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Kleinstreuer, C.; Li, Z.; Basciano, C.A.; Seelecke, S.; Farber, M.A. Computational mechanics of Nitinol stent grafts. J. Biomech. 2008, 41, 2370–2378. [Google Scholar] [CrossRef] [PubMed]
- Chakfe, N.; Dieval, F.; Riepe, G.; Mathieu, D.; Zbali, I.; Thaveau, F.; Heintz, C.; Kretz, J.G.; Durand, B. Influence of the textile structure on the degradation of explanted aortic endoprostheses. Eur. J. Vasc. Endovasc. Surg. 2004, 27, 33–41. [Google Scholar] [CrossRef]
- Yao, T.; Choules, B.D.; Rust, J.P.; King, M.W. The development of an in vitro test method for predicting the abrasion resistance of textile and metal components of endovascular stent grafts. J. Biomed. Mater. Res. B 2014, 102, 488–499. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.E. Biomechanical issues in endovascular device design. J. Endovasc. Ther. 2009, 16, I1–I11. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Wang, L.; Guidoin, R.; Nutley, M.; Song, G.; Zhang, Z.; Du, J.; Douville, Y. Stent fabric fatigue of grafts supported by Z-stents versus ringed stents, An in vitro buckling test. J. Biomater. Appl. 2014, 28, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Setacci, F.; Sirignano, P.; de Donato, G.; Chisci, E.; Iacoponi, F.; Galzerano, G.; Palasciano, G.; Cappelli, A.; Setacci, C. AAA with a challenging neck, early outcomes using the Endurant stent-graft system. Eur. J. Vasc. Endovasc. Surg. 2012, 44, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Cao, P.; Ferrer, C. A new option for a wider range of anatomies: Early EU experience with the new Zenith Alpha thoracic endovascular graft. Endovasc. Today Eur. 2014, 2, 9–12. [Google Scholar]
- Chuter, T.A. Stent-graft design, the good, the bad and the ugly. Cardiovasc. Surg. 2002, 10, 7–13. [Google Scholar] [CrossRef]
- Kent, K.C. Endovascular aneurysm repair—is it durable? N. Engl. J. Med. 2010, 362, 1930–1931. [Google Scholar] [CrossRef] [PubMed]
- Dhruva, S.S.; Redberg, R.F. Medical device regulation: Time to improve performance. PLoS Med. 2012, 9, e1001277. [Google Scholar] [CrossRef] [PubMed]
- Dhruva, S.S.; Redberg, R.F. FDA regulation of cardiovascular devices and opportunities for improvement. J. Interv. Card. Electrophysiol. 2013, 36, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Gartenberg, A.J.; Peleg, A.; Dhruva, S.S.; Redberg, R.F. Presumed safe no more, lessons from the Wingspan saga on regulation of devices. Br. Med. J. 2014, 348, 390. [Google Scholar] [CrossRef] [PubMed]
- Smeds, M.R.; Jacobs, D.L.; Peterson, G.J.; Peterson, B.G. Short-term outcomes of the C3 Excluder for patients with abdominal aortic aneurysms and unfavorable proximal aortic seal zones. Ann. Vasc. Surg. 2013, 27, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Yong, T.P.T.A.; Harrison, G.J.; Duddy, M.J.; Hopkins, J.; Vohra, R.K. Endovascular repair of aortic aneurysm through bilateral common iliac stents with a repositionable stent-graft. Eur. J. Vasc. Endovasc. Surg. Extra 2012, 24, 27–28. [Google Scholar]
- Volodos, N.L. Historical perspective: The first steps in endovascular aortic repair, how it all began. J. Endovasc. Ther. 2013, 20, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Volodos, N.L.; Shekhanin, V.E.; Karpovich, I.P.; Troian, V.I.; Iua, G. A self-fixing synthetic blood vessel endoprosthesis. Vestn. Khir. Im. II Grek. 1986, 137, 123–125. [Google Scholar]
- Volodos, N.L.; Karpovich, I.P.; Troyan, V.I.; Kalashnikowa, Y.V.; Shekhanin, V.E.; Terniuk, N.E.; Neoneta, A.S.; Ustinov, N.I.; Yakovenko, L.F. Clinical experience of the use of self-fixing synthetic prostheses for remote endoprosthetics of the thoracic and the abdominal aorta and iliac arteries through the femoral artery and as intraoperative endoprosthesis for aorta reconstruction. Vasa. Suppl. 1991, 33, 93–95. [Google Scholar] [PubMed]
- Resch, T.; Ivancev, K.; Brunkwall, J.; Nyman, U.; Malina, M.; Lindblad, B. Distal migration of stent-grafts after endovascular repair of abdominal aortic aneurysms. J. Vasc. Interv. Radiol. 1999, 10, 257–264. [Google Scholar] [CrossRef]
- Zarins, C.K.; Bloch, D.A.; Crabtree, T.; Matsumoto, A.H.; White, R.A.; Fogarty, T.J. Stent graft migration after endovascular aneurysm repair, importance of proximal fixation. J. Vasc. Surg. 2003, 38, 1264–1272. [Google Scholar] [CrossRef]
- Greenberg, R.K.; Turc, A.; Haulon, S.; Srivastava, S.D.; Sarac, T.P.; O'Hara, P.J.; Lyden, S.P.; Ouriel, K. Stent-graft migration: A reappraisal of analysis methods and proposed revised definition. J. Endovasc. Ther. 2004, 11, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Malina, M.; Lindblad, B.; Ivancev, K.; Lindh, M.; Malina, J.; Brunkwall, J. Endovascular AAA exclusion: Will stents with hooks and barbs prevent stent-graft migration? J. Endovasc. Surg. 1998, 5, 310–317. [Google Scholar] [CrossRef]
- Melas, N.; Saratzis, A.; Saratzis, N.; Lazaridis, J.; Psaroulis, D.; Trygonis, K.; Kiskinis, D. Aortic and iliac fixation of seven endografts for abdominal-aortic aneurysm repair in an experimental model using human cadaveric aortas. Eur. J. Vasc. Endovasc. Surg. 2010, 40, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Bosman, W.M.P.F.; van der Steenhoven, T.J.; Suarez, D.R.; Hinnen, J.W.; Valstar, E.R.; Hamming, J.F. The proximal fixation strength of modern EVAR grafts in a short aneurysm neck. An in vitro study. Eur. J. Vasc. Endovasc. Surg. 2010, 39, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Chuter, T.A.M. The choice of stent-graft for endovascular repair of abdominal aortic aneurysm. J. Cardiovasc. Surg. 2003, 44, 519–525. [Google Scholar]
- Yin, T.; Guidoin, R.; Corriveau, M.M.; Nutley, M.; Xu, L.; Marinov, G.; Wang, L.; Merhi, Y.; McGregor, R.; Zhang, Z.; et al. Specific shortcomings of endografting design. J. Long Term Eff. Med. Implants. 2008, 18, 181–204. [Google Scholar] [CrossRef] [PubMed]
- Nutley, M.; Guidoin, R.; Yin, T.; Douville, Y.; Zhang, Z.; Marinov, G.; Wei, D.; Lin, J.; Weber, B.; Wang, L.; et al. Detailed analysis of a series of explanted Talent AAA stent-grafts, biofunctionality assessment. J. Long Term Eff. Med. Implants 2011, 21, 299–319. [Google Scholar] [CrossRef] [PubMed]
- Van Sambeck, M.; Hendriks, J.M.; Tseng, L.; Van Dijk, LC.; Van Urk, H. Sac enlargement without endoleak: When and how to convert and technical considerations. Semin. Vasc. Surg. 2004, 17, 284–287. [Google Scholar] [CrossRef]
- Heikkinen, M.A.; Arko, F.R.; Zarins, C.K. What is the significance of endoleaks and endotension. Surg. Clin. N. Am. 2001, 84, 1337–1352. [Google Scholar] [CrossRef] [PubMed]
- Hooshyav, Z.; Mehdizadeth, A. Analysis of endovascular graft features affecting endotension following endovascular aneurysm repair. World Acad. Sci. Eng. Technol. 2012, 6, 10–28. [Google Scholar]
- Molony, D.S.; Callanan, A.; Kavanagh, E.G.; Walsh, M.T.; McGloughlin, T.M. Fluid-structure interaction of a patient-specific abdominal aortic aneurysm treated with an endovascular stent-graft. Biomed. Eng. Online 2009, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Martino, E.S.D.; Bobra, A.; Scotti, C.; Finol, E.; Vorp, D.A. Wall stresses before and after endovascular repair of abdominal aortic aneurysms. In Proceeding of the IMECE04, Anaheim, CA, USA, 13–19 November 2004; Volume 35, pp. 325–326.
- Pua, U.; Tay, K.H.; Tan, B.S.; Htoo, M.M.; Sebastian, M.; Sin, K.; Chua, Y. CT appearance of complications related to thoracic endovascular aortic repair (TEVAR): A pictorial assay. Eur. Radiol. 2009, 19, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.I.; Shin, W.Y.; Choe, Y.M.; Park, J.Y.; Kim, J.Y.; Jeon, Y.S.; Cho, S.G.; Hong, K.C. Relining technique for continuous sac enlargement and module disconnection secondary to endotension after endovascular aortic aneurysm repair. Ann. Surg. Treat. Res. 2014, 86, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Gilling-Smith, G.; Brennan, J.; Harris, P.; Bakran, A.; Gould, D.; McWilliams, R. Endotension after endovascular aneurysm repair, definition, classification and strategies for surveillance and intervention. J. Endovasc. Surg. 1999, 6, 305–307. [Google Scholar] [CrossRef]
- Filippi, F.; Tirotti, C.; Stella, N.; Rizzo, L.; Taurino, M. Endotension-related aortic sac rupture treated by endograft relining. Vascular 2013, 21, 113–115. [Google Scholar] [CrossRef] [PubMed]
- Cerna, M.; Kocher, M.; Utikal, P.; Bachleda, P. Endotension after endovascular treatment of abdominal aortic aneurysm, percutaneous treatment. J. Vasc. Surg. 2009, 50, 648–651. [Google Scholar] [CrossRef] [PubMed]
- Hooshyar, Z.; Fakhrabadi, H.; Hooshyar, S.; Mehdizadeh, A. Endotension distribution in fluid-structure interaction analysis of abdominal aortic aneurysm following endovascular repair. J. Biomed. Sci. Eng. 2014, 7, 848–855. [Google Scholar] [CrossRef]
- Blum, U.; Voshage, G.; Lammer, J.; Beyersdorf, F.; Tollner, D.; Kretschmer, G.; Spillner, G.; Polterauer, P.; Nagel, G.; Holzenbein, T.; et al. Endoluminal stent-grafts for infrarenal abdominal aortic aneurysms. N. Engl. J. Med. 1997, 336, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Trocciola, S.A.; Dayal, R.; Chaer, R.A.; Lin, S.C.; DeRibertis, B.; Ryer, E.J.; Hynececk, R.L.; Pierce, M.J.; Prince, M.; Badimon, J.; et al. The development of endotension is associated with increased transmission of pressure and serous components in porous expanded polytetrafluoroethylene stent-grafts characterization using a canine model. J. Vasc. Surg. 2006, 43, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Kongrias, P.; Lin, P.H.; Dardik, A.; El Sayed, H.F.; Zhou, W. Successful treatment of endotension and aneurysm sac enlargement with endovascular stent graft reinforcement. J. Vasc. Surg. 2007, 46, 124–127. [Google Scholar]
- Schwope, R.B.; Alper, H.J.; Talenfeld, A.D.; Cohen, E.I.; Lookstein, R.A. MR angiography for patient surveillance after endovascular repair of abdominal aortic aneurysms. Am. J. Roentgenol. 2007, 188, W334–W340. [Google Scholar] [CrossRef] [PubMed]
- Estornell-Erill, J.; García-García, R.; Igual-Muñoz, B.; Gil-Alberola, O.; Talens-Ferrando, A.; Ridocci-Soriano, F. Utility of multidetector computed tomography for postprocedure evaluation of endovascular aortic stent-grafts. Rev. Esp. Cardiol. 2013, 66, 316–317. [Google Scholar] [CrossRef] [PubMed]
- Amoore, J.; Ingram, P. Quality improvement report, learning from adverse incidents involving medical devices. Br. Med. J. 2002, 325, 272–275. [Google Scholar] [CrossRef]
- Stark, N.J. A new standard for medical device adverse event classification. J. Clin. Res. Best Pract. 2009, 12, 1–8. [Google Scholar]
- O'Connor, A.B. The need for improved access to FDA reviews. Jama-J. Am. Med. Assoc. 2009, 302, 191–193. [Google Scholar] [CrossRef] [PubMed]
- Abel, D.B.; Dehdashtian, M.M.; Rodger, S.T.; Smith, A.C.; Smith, L.J.; Waninger, M.S. Evolution and future of preclinical testing for endovascular grafts. J. Endovasc. Ther. 2006, 13, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Dhruva, S.S.; Bero, L.A.; Redberg, R.F. Strength of study evidence examined by the FDA in premarket approval of cardiovascular devices. Jama-J. Am. Med. Assoc. 2009, 302, 2679–2685. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.R.; Lamm, S.D.; Han, H.C. Twist buckling behavior of arteries. Biomed. Model. Mechan. 2013, 12, 915–927. [Google Scholar] [CrossRef] [PubMed]
- Hamkins, C.P. On the mechanisms of tearing in woven fabrics. Text. Res. J. 1980, 50, 323–327. [Google Scholar] [CrossRef]
- Xu, Z.; Fan, Y.; Geelkerken, R.M.; Deng, X.; King, M.; Traoré, A.; Ingle, N.; Turgeon, S.; McGregor, R.; Dionne, G.; et al. Characterization of an endovascular prosthesis using the 3Bs rule (biocompatibility, biofunctionality and biodurability): A recommended protocol to investigate a device harvested at necropsy. J. Long Term Eff. Med. Implants 2007, 17, 237–262. [Google Scholar] [CrossRef] [PubMed]
- Veith, F.J.; Gargiulo, N.J. Endovascular aortic repair should be the gold standard for ruptured AAAs, and all vascular surgeons should be prepared to perform them. Perspect. Vasc. Surg. Endovasc. Ther. 2007, 19, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Donas, K.P.; Torsello, G. Complications and reinterventions after EVAR: Are they decreasing in incidence? J. Cardiovasc. Surg. 2011, 52, 189–192. [Google Scholar]
- Han, H.C.; Chesnutt, J.K.W.; Garcia, J.R.; Liu, Q.; Wen, Q. Artery buckling: New phenotypes, models and applications. Ann. Biomed. Eng. 2013, 41, 1399–1410. [Google Scholar] [CrossRef] [PubMed]
- Chuter, T.A.M. Durability of endovascular infrarenal aneurysm repair: When does late failure occur and why? Semi. Vasc. Surg. 2009, 22, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Badger, S.A.; O'Donnell, M.E.; Loan, W.; Hannon, R.J.; Lau, L.L.; Lee, B.; Soong, C.V. No difference in medium-term outcome between Zenith and Talent stent-grafts in endovascular aneurysm repair. Vasc. Endovasc. Surg. 2008, 41, 500–505. [Google Scholar] [CrossRef] [PubMed]
- De Bock, S.; Iannaccone, F.; De Santis, G.; De Beule, M.; Van Loo, D.; Devos, D.; Vermassen, F.; Segers, P.; Verhegghe, B. Virtual evaluation of stent graft deployment: A validated modeling and simulation study. J. Mech. Behav. Biomed. Mater. 2012, 13, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Demanget, N.; Avril, S.; Badel, P.; Orgeas, L.; Geindreau, C.; Albertini, J.N.; Favre, J.P. Computational comparison of the bending behavior of aortic stent-grafts. J. Mech. Behav. Biomed. Mater. 2012, 5, 272–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, A.; Figueiredo, L.; Bordado, J. Abrasion behaviour of polymeric textiles for endovascular stent-grafts. Tribol. Int. 2013, 63, 265–274. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, J.; Guidoin, R.; Du, J.; Wang, L.; Douglas, G.; Zhu, D.; Nutley, M.; Perron, L.; Zhang, Z.; Douville, Y. An in Vitro Twist Fatigue Test of Fabric Stent-Grafts Supported by Z-Stents vs. Ringed Stents. Materials 2016, 9, 113. https://doi.org/10.3390/ma9020113
Lin J, Guidoin R, Du J, Wang L, Douglas G, Zhu D, Nutley M, Perron L, Zhang Z, Douville Y. An in Vitro Twist Fatigue Test of Fabric Stent-Grafts Supported by Z-Stents vs. Ringed Stents. Materials. 2016; 9(2):113. https://doi.org/10.3390/ma9020113
Chicago/Turabian StyleLin, Jing, Robert Guidoin, Jia Du, Lu Wang, Graeham Douglas, Danjie Zhu, Mark Nutley, Lygia Perron, Ze Zhang, and Yvan Douville. 2016. "An in Vitro Twist Fatigue Test of Fabric Stent-Grafts Supported by Z-Stents vs. Ringed Stents" Materials 9, no. 2: 113. https://doi.org/10.3390/ma9020113
APA StyleLin, J., Guidoin, R., Du, J., Wang, L., Douglas, G., Zhu, D., Nutley, M., Perron, L., Zhang, Z., & Douville, Y. (2016). An in Vitro Twist Fatigue Test of Fabric Stent-Grafts Supported by Z-Stents vs. Ringed Stents. Materials, 9(2), 113. https://doi.org/10.3390/ma9020113






