Aqueous-Based Coaxial Electrospinning of Genetically Engineered Silk Elastin Core-Shell Nanofibers
Abstract
:1. Introduction
2. Experimental Section
2.1. SELP (S2E8Y) Solution Preparation
2.2. Silk Fibroin (SF) Solution Preparation
2.3. Rheology
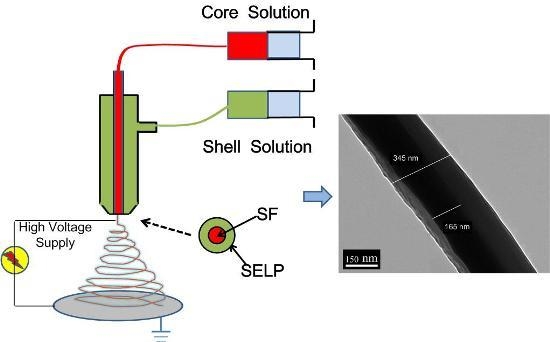
2.4. Coaxial Electrospinning
2.5. Standard Electrospinning
2.6. Morphology of the Electrospun SF-SELP Fiber Mats
2.7. Core-Shell Structure Characterization
2.8. Fourier Transform Infrared Spectroscopy (FTIR)
2.9. Methanol Treatment
2.10. Tensile Tests of Electrospun Mats
3. Results and Discussion
3.1. Protein Characterization
3.2. Rheological Behavior of Solutions
3.3. Effect of Processing on Electrospun SF-SELP Fibers
3.4. Core/Shell Structure of the Electrospun Fibers
3.5. Post-Treatment with Methanol and Structure of the Nanofiber Mats
3.6. Mechanical Testing of SF and SF-SELP Fibers Mats Before and After Methanol Treatment
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xu, Y.; Xu, H.; Chang, J. Electrospun membranes: Control of the structure and structure related applications in tissue regeneration and drug delivery. J. Mater. Chem. B 2014, 2, 5492–5510. [Google Scholar] [CrossRef]
- Alessandrino, A.; Marelli, B.; Arosio, C.; Fare, S.; Tanzi, M.C.; Freddi, G. Electrospun silk fibroin mats for tissue engineering. Eng. Life Sci. 2008, 8, 219–225. [Google Scholar] [CrossRef]
- Li, C.M.; Vepari, C.; Jin, H.J.; Kim, H.J.; Kaplan, D.L. Electrospun silk-BMP-2 scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3115–3124. [Google Scholar] [CrossRef] [PubMed]
- Vepari, C.; Kaplan, D.L. Silk as a biomaterial. Prog. Polym. Sci. 2007, 32, 991–1007. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Tang-Schomer, M.D.; Huang, W.; Xia, X.-X.; Weiss, A.S.; Kaplan, D.L. Charge-tunable autoclaved silk-tropoelastin protein alloys that control neuron cell responses. Adv. Funct. Mater. 2013, 23, 3875–3884. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.J.; Fridrikh, S.V.; Rutledge, G.C.; Kaplan, D.L. Electrospinning bombyx mori silk with poly(ethylene oxide). Biomacromolecules 2002, 3, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhang, Y.; Shao, H.; Hu, X. Electrospinning and rheology of regenerated bombyx mori silk fibroin aqueous solutions: The effects of ph and concentration. Polymer 2008, 49, 2880–2885. [Google Scholar] [CrossRef]
- Huang, W.; Tarakanova, A.; Dinjaski, N.; Wang, Q.; Xia, X.; Chen, Y.; Wong, J.; Buehler, M.J.; Kaplan, D.L. Design of multi-stimuli responsive hydrogels using integrated modeling and genetically engineered silk-elastin-like-proteins. Adv. Funct. Mater. 2016. [Google Scholar] [CrossRef]
- Wang, Q.; Xia, X.; Huang, W.; Lin, Y.; Xu, Q.; Kaplan, D.L. High throughput screening of dynamic silk-elastin-like protein biomaterials. Adv. Funct. Mater. 2014, 24, 4303–4310. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.X.; Xu, Q.B.; Hu, X.; Qin, G.K.; Kaplan, D.L. Tunable self-assembly of genetically engineered silk-elastin-like protein polymers. Biomacromolecules 2011, 12, 3844–3850. [Google Scholar] [CrossRef] [PubMed]
- Nagarsekar, A.; Crissman, J.; Crissman, M.; Ferrari, F.; Cappello, J.; Ghandehari, H. Genetic engineering of stimuli-sensitive silkelastin-like protein block copolymers. Biomacromolecules 2003, 4, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Rollett, A.; Kaplan, D.L. Silk-elastin-like protein biomaterials for the controlled delivery of therapeutics. Expert Opin. Drug Deliv. 2015, 12, 779–791. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.-X.; Wang, M.; Lin, Y.; Xu, Q.; Kaplan, D.L. Hydrophobic drug-triggered self-assembly of nanoparticles from silk-elastin-like protein polymers for drug delivery. Biomacromolecules 2014, 15, 908–914. [Google Scholar] [CrossRef] [PubMed]
- Dinerman, A.A.; Cappello, J.; Ghandehari, H.; Hoag, S.W. Swelling behavior of a genetically engineered silk-elastinlike protein polymer hydrogel. Biomaterials 2002, 23, 4203–4210. [Google Scholar] [CrossRef]
- Teng, W.; Cappello, J.; Wu, X. Recombinant silk-elastinlike protein polymer displays elasticity comparable to elastin. Biomacromolecules 2009, 10, 3028–3036. [Google Scholar] [CrossRef] [PubMed]
- Golinska, M.D.; Pham, T.T.H.; Werten, M.W.T.; de Wolf, F.A.; Stuart, M.A.C.; van der Gucht, J. Fibril formation by pH and temperature responsive silk-elastin block copolymers. Biomacromolecules 2013, 14, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, R.; Drew, C.; Mello, C.M. Polymer-micelle complex as an aid to electrospinning nanofibers from aqueous solutions. J. Phys. Chem. C 2007, 111, 16105–16108. [Google Scholar] [CrossRef]
- Ner, Y.; Stuart, J.A.; Whited, G.; Sotzing, G.A. Electrospinning nanoribbons of a bioengineered silk-elastin-like protein (SELP) from water. Polymer 2009, 50, 5828–5836. [Google Scholar] [CrossRef]
- Qiu, W.; Huang, Y.; Teng, W.; Cohn, C.M.; Cappello, J.; Wu, X. Complete recombinant silk-elastinlike protein-based tissue scaffold. Biomacromolecules 2010, 11, 3219–3227. [Google Scholar] [CrossRef] [PubMed]
- Machado, R.; da Costa, A.; Sencadas, V.; Garcia-Arevalo, C.; Costa, C.M.; Padrao, J.; Gomes, A.; Lanceros-Mendez, S.; Carlos Rodriguez-Cabello, J.; Casal, M. Electrospun silk-elastin-like fibre mats for tissue engineering applications. Biomed. Mater. 2013, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xia, Y.N. Direct fabrication of composite and ceramic hollow nanofibers by electrospinning. Nano Lett. 2004, 4, 933–938. [Google Scholar] [CrossRef]
- Li, D.; Babel, A.; Jenekhe, S.A.; Xia, Y.N. Nanofibers of conjugated polymers prepared by electrospinning with a two-capillary spinneret. Adv. Mater. 2004, 16, 2062–2066. [Google Scholar] [CrossRef]
- Loscertales, I.G.; Barrero, A.; Guerrero, I.; Cortijo, R.; Marquez, M.; Ganan-Calvo, A.M. Micro/nano encapsutation via electrified coaxial liquid jets. Science 2002, 295, 1695–1698. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.C.; Zussman, E.; Yarin, A.L.; Wendorff, J.H.; Greiner, A. Compound core-shell polymer nanofibers by co-electrospinning. Adv. Mater. 2003, 15, 1929–1932. [Google Scholar] [CrossRef]
- Ojha, S.S.; Stevens, D.R.; Hoffman, T.J.; Stano, K.; Klossner, R.; Scott, M.C.; Krause, W.; Clarke, L.I.; Gorga, R.E. Fabrication and characterization of electrospun chitosan nanofibers formed via templating with polyethylene oxide. Biomacromolecules 2008, 9, 2523–2529. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yu, J.H.; Kaplan, D.L.; Rutledge, G.C. Production of submicron diameter silk fibers under benign processing conditions by two-fluid electrospinning. Macromolecules 2006, 39, 1102–1107. [Google Scholar] [CrossRef]
- Rockwood, D.N.; Preda, R.C.; Yucel, T.; Wang, X.; Lovett, M.L.; Kaplan, D.L. Materials fabrication from bombyx mori silk fibroin. Nat. Protoc. 2011, 6, 1612–1631. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, P.; Grove, T.; Edgar, K.J.; Goldstein, A.S. Surface grafting of chitosan shell, polycaprolactone core fiber meshes to confer bioactivity. J. Bioact. Compat. Polym. 2015, 30, 258–274. [Google Scholar] [CrossRef]
- Huang, Z.M.; Zhang, Y.Z.; Ramakrishna, S.; Lim, C.T. Electrospinning and mechanical characterization of gelatin nanofibers. Polymer 2004, 45, 5361–5368. [Google Scholar] [CrossRef]
- Wong, S.-C.; Baji, A.; Leng, S. Effect of fiber diameter on tensile properties of electrospun poly(epsilon-caprolactone). Polymer 2008, 49, 4713–4722. [Google Scholar] [CrossRef]
- Huang, W.W.; Edenzon, K.; Fernandez, L.; Razmpour, S.; Woodburn, J.; Cebe, P. Nanocomposites of poly(vinylidene fluoride) with multiwalled carbon nanotubes. J. Appl. Polym. Sci. 2010, 115, 3238–3248. [Google Scholar] [CrossRef]
- Wray, L.S.; Hu, X.; Gallego, J.; Georgakoudi, I.; Omenetto, F.G.; Schmidt, D.; Kaplan, D.L. Effect of processing on silk-based biomaterials: Reproducibility and biocompatibility. J. Biomed. Mater. Res. B Appl. Biomater. 2011, 99B, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yang, Y.; Chen, X.; Shao, Z. Investigation of rheological properties and conformation of silk fibroin in the solution of amimcl. Biomacromolecules 2012, 13, 1875–1881. [Google Scholar] [CrossRef] [PubMed]
- Hodgkinson, T.; Chen, Y.; Bayat, A.; Yuan, X.-F. Rheology and electrospinning of regenerated bombyx mori silk fibroin aqueous solutions. Biomacromolecules 2014, 15, 1288–1298. [Google Scholar] [CrossRef] [PubMed]
- Tam, K.C.; Tiu, C. Steady and dynamic shear properties of aqueous polymer-solutions. J. Rheol. 1989, 33, 257–280. [Google Scholar] [CrossRef]
- Huang, Z.M.; Zhang, Y.Z.; Ramakrishna, S. Double-layered composite nanofibers and their mechanical performance. J. Polym. Sci. B Polym. Phys. 2005, 43, 2852–2861. [Google Scholar] [CrossRef]
- Yu, J.H.; Fridrikh, S.V.; Rutledge, G.C. Production of submicrometer diameter fibers by two-fluid electrospinning. Adv. Mater. 2004, 16, 1562–1566. [Google Scholar] [CrossRef]
- Yu, D.-G.; Branford-White, C.J.; Chatterton, N.P.; White, K.; Zhu, L.-M.; Shen, X.-X.; Nie, W. Electrospinning of concentrated polymer solutions. Macromolecules 2010, 43, 10743–10746. [Google Scholar] [CrossRef]
- Luo, C.J.; Edirisinghe, M. Core-liquid-induced transition from coaxial electrospray to electrospinning of low-viscosity poly(lactide-co-glycolide) sheath solution. Macromolecules 2014, 47, 7930–7938. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Huang, Z.M.; Xu, X.J.; Lim, C.T.; Ramakrishna, S. Preparation of core-shell structured PCL-r-gelatin bi-component nanofibers by coaxial electrospinning. Chem. Mater. 2004, 16, 3406–3409. [Google Scholar] [CrossRef]
- Yu, D.-G.; Branford-White, C.; Bligh, S.W.A.; White, K.; Chatterton, N.P.; Zhu, L.-M. Improving polymer nanofiber quality using a modified co-axial electrospinning process. Macromol. Rapid Commun. 2011, 32, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, L.; Qvortrup, K.; Chronakis, I.S. Phospholipid electrospun nanofibers: Effect of solvents and co-axial processing on morphology and fiber diameter. RSC Adv. 2015, 5, 53644–53652. [Google Scholar] [CrossRef]
- Pakravan, M.; Heuzey, M.-C.; Ajji, A. Core-shell structured peo-chitosan nanofibers by coaxial electrospinning. Biomacromolecules 2012, 13, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Kaplan, D.; Cebe, P. Determining beta-sheet crystallinity in fibrous proteins by thermal analysis and infrared spectroscopy. Macromolecules 2006, 39, 6161–6170. [Google Scholar] [CrossRef]
- Huang, W.W.; Krishnaji, S.; Hu, X.; Kaplan, D.; Cebe, P. Heat capacity of spider silk-like block copolymers. Macromolecules 2011, 44, 5299–5309. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Krishnaji, S.; Kaplan, D.; Cebe, P. Thermal analysis of spider silk inspired di-block copolymers in the glass transition region by tmdsc. J. Therm. Anal. Calorim. 2012, 109, 1193–1201. [Google Scholar] [CrossRef]









| Samples in Figure | Concentration % | Flow Rate mL/h | Applied Voltage kV | Working Distance cm | Mean Diameter nm | Standard Deviation ±nm | ||
|---|---|---|---|---|---|---|---|---|
| Core | Shell | Inner | Outer | |||||
| Figure 3a | 26 | 15 | 0.4 | 0.17 | 20 | 12 | - | - |
| Figure 3b | 29 | 15 | 0.4 | 0.17 | 20 | 12 | 301 | 108 |
| Figure 3c | 32 | 15 | 0.4 | 0.17 | 20 | 12 | - | - |
| Figure 4a | 29 | 12 | 0.4 | 0.17 | 20 | 10 | 373 | 129 |
| Figure 4b | 29 | 13.5 | 0.4 | 0.17 | 20 | 10 | 406 | 151 |
| Figure 4c | 29 | 15 | 0.4 | 0.17 | 20 | 10 | 361 | 98 |
| Samples in Figure | Concentration % | Flow Rate mL/h | Applied Voltage kV | Working Distance cm | Mean Diameter nm | Standard Deviation ±nm | ||
|---|---|---|---|---|---|---|---|---|
| Core | Shell | Inner | Outer | |||||
| Figure 5(A1) | 29 | 12 | 0.4 | 0.17 | 20 | 12 | 370 | 121 |
| Figure 5(A2) | 29 | 12 | 0.6 | 0.17 | 20 | 12 | 408 | 150 |
| Figure 5(A3) | 29 | 12 | 0.8 | 0.17 | 20 | 12 | 474 | 162 |
| Figure 5(B1) | 29 | 12 | 0.6 | 0.07 | 20 | 12 | 712 | 215 |
| Figure 5(B2) | 29 | 12 | 0.6 | 0.17 | 20 | 12 | 408 | 150 |
| Figure 5(B3) | 29 | 12 | 0.6 | 0.34 | 20 | 12 | - | - |
| Figure 5(C1) | 29 | 12 | 0.6 | 0.17 | 20 | 12 | 408 | 150 |
| Figure 5(C2) | 29 | 12 | 0.6 | 0.17 | 25 | 12 | - | - |
| Figure 5(C3) | 29 | 12 | 0.6 | 0.17 | 30 | 12 | - | - |
| Samples | Thickness (μm) | Young’s Modulus (MPa) | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|---|---|
| SF mats | 85 ± 8 | 1.02 ± 0.12 | 1.00 ± 0.22 | 1.05 ± 0.15 |
| SF-SELP mats | 80 ± 6 | 1.30 ± 0.35 | 1.72 ± 0.24 | 4.70 ± 0.31 |
| SF mats (MeOH) | 50 ± 5 | 2.16 ± 0.17 | 2.15 ± 0.32 | 1.38 ± 0.22 |
| SF-SELP mats (MeOH) | 45 ± 4 | 1.32 ± 0.52 | 4.81 ± 0.35 | 5.20 ± 0.57 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Huang, W.; Zhang, Q.; Ling, S.; Chen, Y.; Kaplan, D.L. Aqueous-Based Coaxial Electrospinning of Genetically Engineered Silk Elastin Core-Shell Nanofibers. Materials 2016, 9, 221. https://doi.org/10.3390/ma9040221
Zhu J, Huang W, Zhang Q, Ling S, Chen Y, Kaplan DL. Aqueous-Based Coaxial Electrospinning of Genetically Engineered Silk Elastin Core-Shell Nanofibers. Materials. 2016; 9(4):221. https://doi.org/10.3390/ma9040221
Chicago/Turabian StyleZhu, Jingxin, Wenwen Huang, Qiang Zhang, Shengjie Ling, Ying Chen, and David L. Kaplan. 2016. "Aqueous-Based Coaxial Electrospinning of Genetically Engineered Silk Elastin Core-Shell Nanofibers" Materials 9, no. 4: 221. https://doi.org/10.3390/ma9040221
APA StyleZhu, J., Huang, W., Zhang, Q., Ling, S., Chen, Y., & Kaplan, D. L. (2016). Aqueous-Based Coaxial Electrospinning of Genetically Engineered Silk Elastin Core-Shell Nanofibers. Materials, 9(4), 221. https://doi.org/10.3390/ma9040221