Higher Ratios of Hyaluronic Acid Enhance Chondrogenic Differentiation of Human MSCs in a Hyaluronic Acid–Gelatin Composite Scaffold
Abstract
:1. Introduction
2. Results
2.1. Production of Hyaluronic Acid (HA)-Based Composite Scaffolds
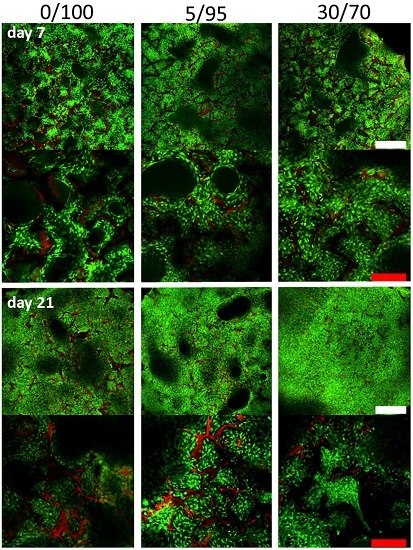
2.2. Seeding, Adhesion and Viability of MSCs
2.3. Chondrogenic Differentiation of Seeded MSC—Gene Expression Analysis and Extracellualar Matrix Production
2.4. Histomorphometric Appearance
2.5. Immunohistochemistry
2.6. TGF-Beta Concentrations in Supernatant under Cell Free Scaffold Conditions
3. Discussion
4. Materials and Methods
4.1. Isolation and Culture of MSCs
4.2. Production of Scaffolds
4.3. Seeding and Culture of MSCs in Composite Scaffolds
4.4. Cell Viability Measurement
4.5. Gene Expression Analysis
4.6. Histology
4.7. Immunohistochemistry
4.8. Confocal Microscopy
4.9. Analysis of Extracellular Matrix Production
4.10. Analysis of TGF-Beta Concentrations in Supernatant in Cell Free Scaffold Conditions
4.11. Staistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| MSC | mesenchymal stem cell |
| ELISA | enzyme-linked immunosorbent assay |
| ANOVA | analysis of variance |
| RNA | ribonucleic acid |
| DNA | desoxy-ribonucleic acid |
| HA | hyaluronic acid |
| TGF-beta | transforming growth factor beta |
Appendix
| Primer | Forward | Reversed |
|---|---|---|
| Collagen 2 | 5′ GGGCAATAGCAGGTTCACGTA 3′ | 5′ TGTTTCGTGCAGCCATCCT 3′ |
| Collagen 1 | 5′ ACGTCCTGGTGAAGTTGGTC 3′ | 5′ ACCAGGGAAGCCTCTCTCTC 3′ |
| Collagen 10 | 5′ CCCTCTTGTTAGTGCCAACC 3′ | 5′ AGATTCCAGTCCTTGGGTCA 3′ |
| Sox 9 | 5′ ACACACAGCTCACTCGACCTTG 3′ | 5′ AGGGAATTCTGGTTGGTCCTCT 3′ |
| MIA | 5′ AAAGGGGTCATCGTAACAGG 3′ | 5′ GGGAAGTCGAACCTCTTCTG 3′ |
| PSMB 2 | 5′ GCTGCCAGGTAGTCCATGTAA 3′ | 5′ CGAAACCTGGCTGACTGTCT 3′ |
| REEP 5 | 5′ AGGTCAGCCACTGGGTATCA 3′ | 5′ CCTCTCTCCTCTGCAACCTG 3′ |
| VPS 29 | 5′ AGCTGGCAAACTGTTGCAC 3′ | 5′ GACGGTGGTGGTGACTGAG 3′ |

References
- Basad, E.; Ishaque, B.; Bachmann, G.; Sturz, H.; Steinmeyer, J. Matrix-induced autologous chondrocyte implantation versus microfracture in the treatment of cartilage defects of the knee: A 2-year randomised study. Knee Surg. Sports Traumatol. Arthrosc. 2010, 18, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Peterson, L.; Minas, T.; Brittberg, M.; Lindahl, A. Treatment of osteochondritis dissecans of the knee with autologous chondrocyte transplantation: Results at two to ten years. J. Bone Jt. Surg. Am. 2003, 85 (Suppl. 2), 17–24. [Google Scholar]
- Mithoefer, K.; McAdams, T.; Williams, R.J.; Kreuz, P.C.; Mandelbaum, B.R. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: An evidence-based systematic analysis. Am. J. Sports Med. 2009, 37, 2053–2063. [Google Scholar] [CrossRef] [PubMed]
- Brittberg, M.; Lindahl, A.; Nilsson, A.; Ohlsson, C.; Isaksson, O.; Peterson, L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N. Engl. J. Med. 1994, 331, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.D.; Siston, R.A.; Brophy, R.H.; Lattermann, C.; Carey, J.L.; Flanigan, D.C. Failures, re-operations, and complications after autologous chondrocyte implantation—A systematic review. Osteoarthr. Cartil. 2011, 19, 779–791. [Google Scholar] [CrossRef] [PubMed]
- Niemeyer, P.; Pestka, J.M.; Kreuz, P.C.; Erggelet, C.; Schmal, H.; Suedkamp, N.P.; Steinwachs, M. Characteristic complications after autologous chondrocyte implantation for cartilage defects of the knee joint. Am. J. Sports Med. 2008, 36, 2091–2099. [Google Scholar] [CrossRef] [PubMed]
- Pietschmann, M.F.; Niethammer, T.R.; Horng, A.; Gulecyuz, M.F.; Feist-Pagenstert, I.; Jansson, V.; Muller, P.E. The incidence and clinical relevance of graft hypertrophy after matrix-based autologous chondrocyte implantation. Am. J. Sports Med. 2012, 40, 68–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnstone, B.; Alini, M.; Cucchiarini, M.; Dodge, G.R.; Eglin, D.; Guilak, F.; Madry, H.; Mata, A.; Mauck, R.L.; Semino, C.E.; et al. Tissue engineering for articular cartilage repair—The state of the art. Eur. Cell Mater. 2013, 25, 248–267. [Google Scholar] [PubMed]
- Zellner, J.; Krutsch, W.; Pfeifer, C.G.; Koch, M.; Nerlich, M.; Angele, P. Autologous chondrocyte implantation for cartilage repair: Current perspectives. Orthop. Res. Rev. 2015, 7, 149–158. [Google Scholar] [CrossRef]
- Johnstone, B.; Hering, T.M.; Caplan, A.I.; Goldberg, V.M.; Yoo, J.U. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp. Cell Res. 1998, 238, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Nejadnik, H.; Hui, J.H.; Feng Choong, E.P.; Tai, B.C.; Lee, E.H. Autologous bone marrow-derived mesenchymal stem cells versus autologous chondrocyte implantation: An observational cohort study. Am. J. Sports Med. 2010, 38, 1110–1116. [Google Scholar] [CrossRef] [PubMed]
- Rutjes, A.W.; Juni, P.; da Costa, B.R.; Trelle, S.; Nuesch, E.; Reichenbach, S. Viscosupplementation for osteoarthritis of the knee: A systematic review and meta-analysis. Ann. Intern. Med. 2012, 157, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Kujawa, M.J.; Caplan, A.I. Hyaluronic acid bonded to cell-culture surfaces stimulates chondrogenesis in stage 24 limb mesenchyme cell cultures. Dev. Biol. 1986, 114, 504–518. [Google Scholar] [CrossRef]
- Kim, I.L.; Mauck, R.L.; Burdick, J.A. Hydrogel design for cartilage tissue engineering: A case study with hyaluronic acid. Biomaterials 2011, 32, 8771–8782. [Google Scholar] [CrossRef] [PubMed]
- Lisignoli, G.; Cristino, S.; Piacentini, A.; Zini, N.; Noel, D.; Jorgensen, C.; Facchini, A. Chondrogenic differentiation of murine and human mesenchymal stromal cells in a hyaluronic acid scaffold: Differences in gene expression and cell morphology. J. Biomed. Mater. Res. A 2006, 77, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Mitsuhashi, N.; Klein, A.; Barsky, L.W.; Weinberg, K.; Barr, M.L.; Demetriou, A.; Wu, G.D. The role of the hyaluronan receptor CD44 in mesenchymal stem cell migration in the extracellular matrix. Stem Cells 2006, 24, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Erickson, I.E.; Kestle, S.R.; Zellars, K.H.; Farrell, M.J.; Kim, M.; Burdick, J.A.; Mauck, R.L. High mesenchymal stem cell seeding densities in hyaluronic acid hydrogels produce engineered cartilage with native tissue properties. Acta Biomater. 2012, 8, 3027–3034. [Google Scholar] [CrossRef] [PubMed]
- Filardo, G.; Kon, E.; Roffi, A.; Di Martino, A.; Marcacci, M. Scaffold-based repair for cartilage healing: A systematic review and technical note. Arthroscopy 2013, 29, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.D.; Tirelli, N.; Hubbell, J.A. Photopolymerized hyaluronic acid-based hydrogels and interpenetrating networks. Biomaterials 2003, 24, 893–900. [Google Scholar] [CrossRef]
- Burdick, J.A.; Chung, C.; Jia, X.; Randolph, M.A.; Langer, R. Controlled degradation and mechanical behavior of photopolymerized hyaluronic acid networks. Biomacromolecules 2005, 6, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Bian, L.; Guvendiren, M.; Mauck, R.L.; Burdick, J.A. Hydrogels that mimic developmentally relevant matrix and N-cadherin interactions enhance MSC chondrogenesis. Proc. Natl. Acad. Sci. USA 2013, 110, 10117–10122. [Google Scholar] [CrossRef] [PubMed]
- Mow, V.C.; Ratcliffe, A.; Poole, A.R. Cartilage and diarthrodial joints as paradigms for hierarchical materials and structures. Biomaterials 1992, 13, 67–97. [Google Scholar] [CrossRef]
- Angele, P.; Muller, R.; Schumann, D.; Englert, C.; Zellner, J.; Johnstone, B.; Yoo, J.; Hammer, J.; Fierlbeck, J.; Angele, M.K.; et al. Characterization of esterified hyaluronan-gelatin polymer composites suitable for chondrogenic differentiation of mesenchymal stem cells. J. Biomed. Mater. Res. A 2009, 91, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Bosserhoff, A.K.; Buettner, R. Establishing the protein MIA (melanoma inhibitory activity) as a marker for chondrocyte differentiation. Biomaterials 2003, 24, 3229–3234. [Google Scholar] [CrossRef]
- Mueller, M.B.; Tuan, R.S. Functional characterization of hypertrophy in chondrogenesis of human mesenchymal stem cells. Arthritis Rheum. 2008, 58, 1377–1388. [Google Scholar] [CrossRef] [PubMed]
- Angele, P.; Kujat, R.; Nerlich, M.; Yoo, J.; Goldberg, V.; Johnstone, B. Engineering of osteochondral tissue with bone marrow mesenchymal progenitor cells in a derivatized hyaluronan-gelatin composite sponge. Tissue Eng. 1999, 5, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Zellner, J.; Mueller, M.; Berner, A.; Dienstknecht, T.; Kujat, R.; Nerlich, M.; Hennemann, B.; Koller, M.; Prantl, L.; Angele, M.; et al. Role of mesenchymal stem cells in tissue engineering of meniscus. J. Biomed. Mater. Res. A 2010, 94, 1150–1161. [Google Scholar] [CrossRef] [PubMed]
- Zellner, J.; Hierl, K.; Mueller, M.; Pfeifer, C.; Berner, A.; Dienstknecht, T.; Krutsch, W.; Geis, S.; Gehmert, S.; Kujat, R.; et al. Stem cell-based tissue-engineering for treatment of meniscal tears in the avascular zone. J. Biomed. Mater. Res. B Appl. Biomater. 2013, 101, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Al-Munajjed, A.A.; Hien, M.; Kujat, R.; Gleeson, J.P.; Hammer, J. Influence of pore size on tensile strength, permeability and porosity of hyaluronan-collagen scaffolds. J. Mater. Sci. Mater. Med. 2008, 19, 2859–2864. [Google Scholar] [CrossRef] [PubMed]
- Matsiko, A.; Gleeson, J.P.; O’Brien, F.J. Scaffold mean pore size influences mesenchymal stem cell chondrogenic differentiation and matrix deposition. Tissue Eng. Part. A 2015, 21, 486–497. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, L.; Cortivo, R.; Berti, T.; Berti, A.; Pea, F.; Mazzo, M.; Moras, M.; Abatangelo, G. Biocompatibility and biodegradation of different hyaluronan derivatives (Hyaff) implanted in rats. Biomaterials 1993, 14, 1154–1160. [Google Scholar] [CrossRef]
- Huang, Y.; Onyeri, S.; Siewe, M.; Moshfeghian, A.; Madihally, S.V. In vitro characterization of chitosan-gelatin scaffolds for tissue engineering. Biomaterials 2005, 26, 7616–7627. [Google Scholar] [CrossRef] [PubMed]
- Bergemann, C.; Elter, P.; Lange, R.; Weissmann, V.; Hansmann, H.; Klinkenberg, E.D.; Nebe, B. Cellular Nutrition in Complex Three-Dimensional Scaffolds: A Comparison between Experiments and Computer Simulations. Int. J. Biomater. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Grigolo, B.; Roseti, L.; Fiorini, M.; Fini, M.; Giavaresi, G.; Aldini, N.N.; Giardino, R.; Facchini, A. Transplantation of chondrocytes seeded on a hyaluronan derivative (hyaff-11) into cartilage defects in rabbits. Biomaterials 2001, 22, 2417–2424. [Google Scholar] [CrossRef]
- Erickson, I.E.; Huang, A.H.; Sengupta, S.; Kestle, S.; Burdick, J.A.; Mauck, R.L. Macromer density influences mesenchymal stem cell chondrogenesis and maturation in photocrosslinked hyaluronic acid hydrogels. Osteoarthr. Cartil. 2009, 17, 1639–1648. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.L.; Pfeifer, C.G.; Fisher, M.B.; Saxena, V.; Meloni, G.R.; Kwon, M.Y.; Kim, M.; Steinberg, D.R.; Mauck, R.L.; Burdick, J.A. Fibrous Scaffolds with Varied Fiber Chemistry and Growth Factor Delivery Promote Repair in a Porcine Cartilage Defect Model. Tissue Eng. Part. A 2015, 21, 2680–2690. [Google Scholar] [CrossRef] [PubMed]
- Nuernberger, S.; Cyran, N.; Albrecht, C.; Redl, H.; Vecsei, V.; Marlovits, S. The influence of scaffold architecture on chondrocyte distribution and behavior in matrix-associated chondrocyte transplantation grafts. Biomaterials 2011, 32, 1032–1040. [Google Scholar] [CrossRef] [PubMed]
- Angele, P.; Johnstone, B.; Kujat, R.; Zellner, J.; Nerlich, M.; Goldberg, V.; Yoo, J. Stem cell based tissue engineering for meniscus repair. J. Biomed. Mater. Res. A 2008, 85, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Dienstknecht, T.; Ehehalt, K.; Jenei-Lanzl, Z.; Zellner, J.; Muller, M.; Berner, A.; Nerlich, M.; Angele, P. Resazurin dye as a reliable tool for determination of cell number and viability in mesenchymal stem cell culture. Bull. Exp. Biol. Med. 2010, 150, 157–159. [Google Scholar] [CrossRef] [PubMed]








© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pfeifer, C.G.; Berner, A.; Koch, M.; Krutsch, W.; Kujat, R.; Angele, P.; Nerlich, M.; Zellner, J. Higher Ratios of Hyaluronic Acid Enhance Chondrogenic Differentiation of Human MSCs in a Hyaluronic Acid–Gelatin Composite Scaffold. Materials 2016, 9, 381. https://doi.org/10.3390/ma9050381
Pfeifer CG, Berner A, Koch M, Krutsch W, Kujat R, Angele P, Nerlich M, Zellner J. Higher Ratios of Hyaluronic Acid Enhance Chondrogenic Differentiation of Human MSCs in a Hyaluronic Acid–Gelatin Composite Scaffold. Materials. 2016; 9(5):381. https://doi.org/10.3390/ma9050381
Chicago/Turabian StylePfeifer, Christian G., Arne Berner, Matthias Koch, Werner Krutsch, Richard Kujat, Peter Angele, Michael Nerlich, and Johannes Zellner. 2016. "Higher Ratios of Hyaluronic Acid Enhance Chondrogenic Differentiation of Human MSCs in a Hyaluronic Acid–Gelatin Composite Scaffold" Materials 9, no. 5: 381. https://doi.org/10.3390/ma9050381
APA StylePfeifer, C. G., Berner, A., Koch, M., Krutsch, W., Kujat, R., Angele, P., Nerlich, M., & Zellner, J. (2016). Higher Ratios of Hyaluronic Acid Enhance Chondrogenic Differentiation of Human MSCs in a Hyaluronic Acid–Gelatin Composite Scaffold. Materials, 9(5), 381. https://doi.org/10.3390/ma9050381