Self-Assembly and Drug Release Capacities of Organogels via Some Amide Compounds with Aromatic Substituent Headgroups
Abstract
:1. Introduction
2. Results and Discussion
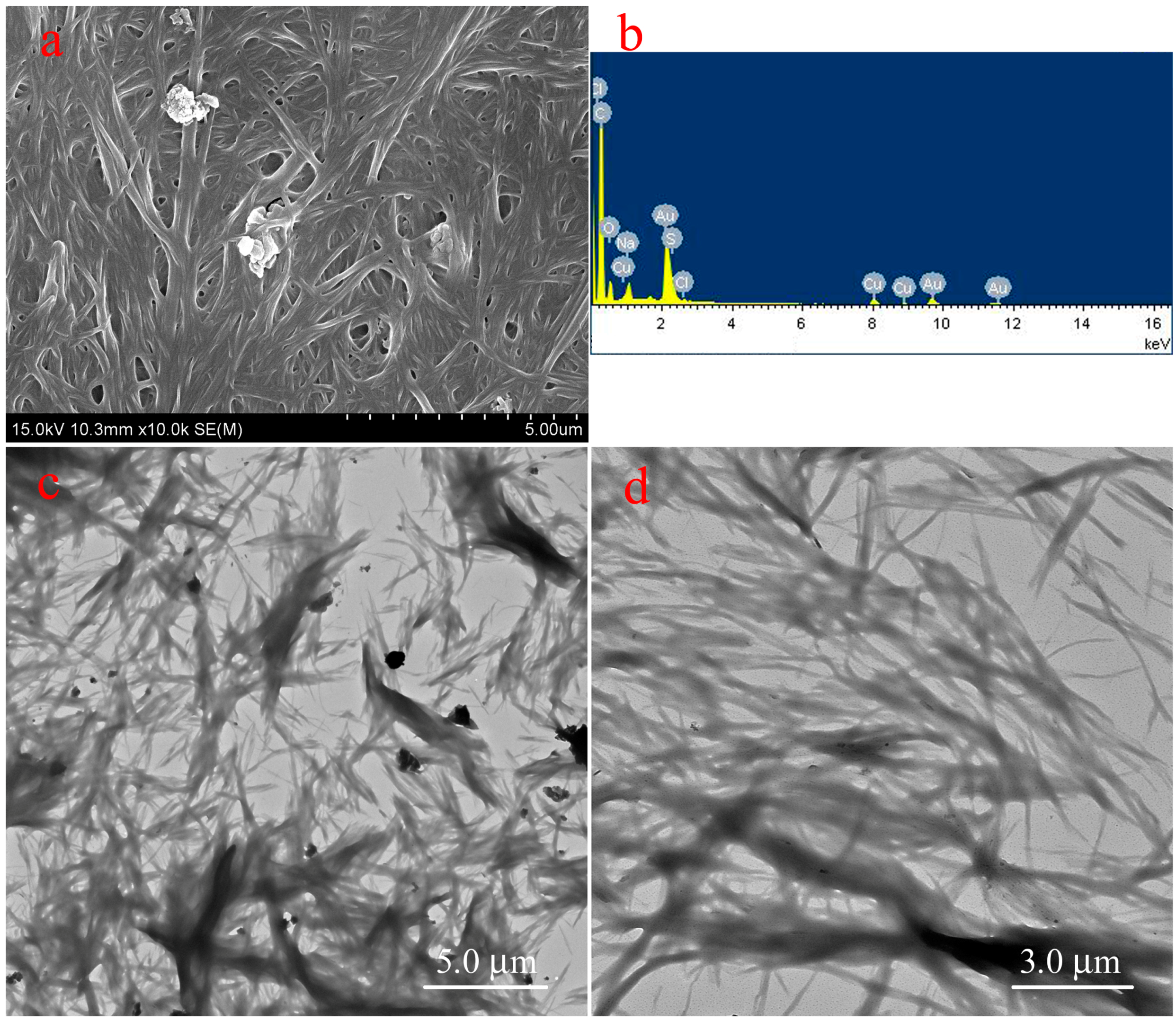
2.1. Preparation and Characterization of Supramolecular Gels
2.2. Drug Release Properties of Supramolecular Gels
3. Experimental Section
3.1. Materials and Reagents
3.2. Gelation Preparation and Drug Delivery
3.3. Measurements
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Delbecq, F.; Tsujimoto, K.; Ogue, Y.; Endo, H.; Kawai, T. N-stearoyl amino acid derivatives: Potent biomimetic hydro/organogelators as templates for preparation of gold nanoparticles. J. Colloid Interface Sci. 2013, 390, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.; Jung, B.M.; Lee, H.P.; Chang, J.Y. Dispersion of single walled carbon nanotubes in organogels by incorporation into organogel fibers. J. Colloid Interface Sci. 2010, 352, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Jiao, T.; Zhang, Q.; Luo, X.; Hu, J.; Chen, Y.; Peng, Q.; Yan, X.; Li, B. Hydrothermal synthesis of hierarchical core–shell manganese oxide nanocomposites as efficient dye adsorbents for wastewater treatment. RSC Adv. 2015, 5, 56279–56285. [Google Scholar] [CrossRef]
- Basrur, V.R.; Guo, J.; Wang, C.; Raghavan, S.R. Synergistic gelation of silica nanoparticles and a sorbitol-based molecular gelator to yield highly-conductive free-standing gel electrolytes. ACS Appl. Mater. Interfaces 2013, 5, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Xing, R.; Jiao, T.; Yan, L.; Ma, G.; Liu, L.; Dai, L.; Li, J.; Möhwald, H.; Yan, X. A colloidal gold-collagen protein core-shell nanoconjugate: One-step biomimetic synthesis, layer-by-layer assembled film and controlled cell growth. ACS Appl. Mater. Interfaces 2015, 7, 24733–24740. [Google Scholar] [CrossRef] [PubMed]
- Yan, N.; Xu, Z.; Diehn, K.K.; Raghavan, S.R.; Fang, Y.; Weiss, R.G. Pyrenyl-linker-glucono gelators. correlations of gel properties with gelator structures and characterization of solvent effects. Langmuir 2013, 29, 793–805. [Google Scholar] [CrossRef] [PubMed]
- George, S.J.; Ajayaghosh, A. Self-assembled nanotapes of oligo(p-phenylene vinylene)s: Sol-gel-controlled optical properties in fluorescent π-electronic gels. Chem. Eur. J. 2005, 11, 3217–3227. [Google Scholar] [CrossRef] [PubMed]
- Ajayaghosh, A.; Chithra, P.; Varghese, R. Self-assembly of tripodal squaraines: Cation-assisted expression of molecular chirality and change from spherical to helical morphology. Angew. Chem. Int. Ed. 2007, 46, 230–233. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, Z.; Wurthner, F. Morphology control of fluorescent nanoaggregates by co-self-assembly of wedge- and dumbbell-shaped amphiphilic perylene bisimides. J. Am. Chem. Soc. 2007, 129, 4886–4887. [Google Scholar] [CrossRef] [PubMed]
- Boerakker, M.J.; Botterhuis, N.E.; Bomans, P.H.H.; Frederik, P.M.; Meijer, E.M.; Nolte, R.J.M.; Sommerdijk, N.A.J.M. Aggregation behavior of giant amphiphiles prepared by cofactor reconstitution. Chem. Eur. J. 2006, 12, 6071–6080. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.C.; Yan, X.Z.; Han, C.Y.; Huang, F.H. Characterization of supramolecular gels. Chem. Soc. Rev. 2013, 42, 6697–6722. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.F.; Shi, B.B.; Wang, H.; Xia, D.Y.; Jie, K.C.; Wu, Z.L.; Huang, F.H. Supramolecular construction of multifluorescent gels: Interfacial assembly of discrete fluorescent gels through multiple hydrogen bonding. Adv. Mater. 2015, 27, 8062–8066. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.F.; Jie, K.C.; Zimmerman, S.C.; Huang, F.H. A double supramolecular crosslinked polymer gel exhibiting macroscale expansion and contraction behavior and multistimuli responsiveness. Polym. Chem. UK 2015, 6, 1912–1917. [Google Scholar] [CrossRef]
- Dong, S.Y.; Zheng, B.; Wang, F.; Huang, F.H. Supramolecular polymers constructed from macrocycle-based host-guest molecular recognition motifs. Acc. Chem. Res. 2014, 47, 1982–1994. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.Y.; Yuan, J.Y.; Huang, F.H. A pillar[5]arene/imidazolium [2]rotaxane: Solvent-and thermo-driven molecular motions and supramolecular gel formation. Chem. Sci. 2014, 5, 247–252. [Google Scholar] [CrossRef]
- Dong, S.Y.; Zheng, B.; Xu, D.H.; Yan, X.Z.; Zhang, M.M.; Huang, F.H. A crown ether appended super gelator with multiple stimulus responsiveness. Adv. Mater. 2012, 24, 3191–3195. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Chen, Q.; Qin, H.; Guan, Y.; Shen, D.; Hua, Y.; Tang, Y.; Xu, J. A temperature-responsive copolymer hydrogel in controlled drug delivery. Macromolecules 2006, 39, 6584–6589. [Google Scholar] [CrossRef]
- Kuroiwa, K.; Shibata, T.; Takada, A.; Nemoto, N.; Kimizuka, N. Heat-set gel-like networks of lipophilic Co(II) triazole complexes in organic media and their thermochromic structural transitions. J. Am. Chem. Soc. 2004, 126, 2016–2021. [Google Scholar] [CrossRef] [PubMed]
- Aldred, M.P.; Eastwood, A.J.; Kelly, S.M.; Vlachos, P.; Contoret, A.E.A.; Farrar, S.R.; Mansoor, B.; O’Neill, M.; Tsoi, W.C. Light-emitting fluorene photoreactive liquid crystals for organic electroluminescence. Chem. Mater. 2004, 16, 4928–4936. [Google Scholar] [CrossRef]
- Guo, H.; Jiao, T.; Zhang, Q.; Guo, W.; Peng, Q.; Yan, X. Preparation of graphene oxide-based hydrogels as efficient dye adsorbents for wastewater treatment. Nanoscale Res. Lett. 2015, 10, 272. [Google Scholar] [CrossRef] [PubMed]
- Xin, F.; Zhang, H.; Hao, B.; Sun, T.; Kong, L.; Li, Y.; Hou, Y.; Li, S.; Zhang, Y.; Hao, A. Controllable transformation from sensitive and reversible heat-set organogel to stable gel induced by sodium acetate. Colloid Surf. A Physicochem. Eng. Asp. 2012, 410, 18–22. [Google Scholar] [CrossRef]
- Iwanaga, K.; Sumizawa, T.; Miyazaki, M.; Kakemi, M. Characterization of organogel as a novel oral controlled release formulation for lipophilic compounds. Int. J. Pharm. 2010, 388, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Lofman, M.; Koivukorpi, J.; Noponen, V.; Salo, H.; Sievanen, E. Bile acid alkylamide derivatives as low molecular weight organogelators: Systematic gelation studies and qualitative structural analysis of the systems. J. Colloid Interface Sci. 2011, 360, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Jiao, T.; Wang, Y.; Zhang, Q.; Yan, X.; Zhao, X.; Zhou, J.; Gao, F. Self-assembly and headgroup effect in nanostructured organogels via cationic amphiphile-graphene oxide composites. PLoS ONE 2014, 9, e101620. [Google Scholar] [CrossRef] [PubMed]
- Bastiat, G.; Plourde, F.; Motulsky, A.; Furtos, A.; Dumont, Y.; Quirion, R.; Fuhrmann, G.; Leroux, J.C. Tyrosine-based rivastigmine-loaded organogels in the treatment of Alzheimer’s disease. Biomaterials 2010, 31, 6031–6038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyamoto, K.; Jintoku, H.; Sawada, T.; Takafuji, M.; Sagawa, T.; Ihara, H. Informative secondary chiroptics in binary molecular organogel systems for donor-acceptor energy transfer. Tetrahedron Lett. 2011, 52, 4030–4035. [Google Scholar] [CrossRef]
- Slowing, I.I.; Vivero-Escoto, J.L.; Wu, C.W.; Lin, V.S.Y. Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Adv. Drug Deliv. Rev. 2008, 60, 1278–1288. [Google Scholar] [CrossRef] [PubMed]
- Eeckman, F.; Moës, A.J.; Amighi, K. Evaluation of a new controlled-drug delivery concept based on the use of thermoresponsive polymers. Int. J. Pharm. 2002, 241, 113–125. [Google Scholar] [CrossRef]
- Xing, R.; Jiao, T.; Liu, Y.; Ma, K.; Zou, Q.; Ma, G.; Yan, X. Co-assembly of graphene oxide and albumin/photosensitizer nanohybrids towards enhanced photodynamic therapy. Polymers 2016, 8, 181. [Google Scholar] [CrossRef]
- Kumar, C.S.S.R.; Mohammad, F. Magnetic nanomaterials for hyperthermia-based therapy and controlled drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 789–808. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Xing, R.; Jiao, T.; Ma, K.; Chen, C.; Ma, G.; Yan, X. Carrier-free, chemo-photodynamic dual nanodrugs via self-assembly for synergistic antitumor therapy. ACS Appl. Mater. Interfaces 2016, 13262–13269. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Jiao, T.; Shen, X.; Zhang, Q.; Li, X.; Gao, F. Binary organogels via some aminobenzimidazole/benzothiazole compounds and fatty acids with different alkyl lengths: Self-assembly and drug release properties. Integr. Ferroelectr. 2015, 160, 38–48. [Google Scholar] [CrossRef]
- Karbarz, M.; Hyk, W.; Stojek, Z. Swelling ratio driven changes of probe concentration in pH-and ionic strength-sensitive poly (acrylic acid) hydrogels. Electrochem. Commun. 2009, 11, 1217–1220. [Google Scholar] [CrossRef]
- Ang, K.L.; Venkatraman, S.; Ramanujan, R.V. Magnetic PNIPA hydrogels for hyperthermia applications in cancer therapy. Mater. Sci. Eng. C 2007, 27, 347–351. [Google Scholar] [CrossRef]
- Wang, L.; Liu, M.; Gao, C.; Ma, L.; Cui, D. A pH-, thermo-, and glucose-, triple-responsive hydrogels: Synthesis and controlled drug delivery. React. Funct. Polym. 2010, 70, 159–167. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, L.; Zhao, C.; Bai, Y.; Deng, M.; Shan, H.; Zhuang, X.; Chen, X.; Jing, X. Thermo-and pH-responsive HPC-g-AA/AA hydrogels for controlled drug delivery applications. Polymer 2011, 52, 676–682. [Google Scholar] [CrossRef]
- Jiao, T.F.; Wang, Y.J.; Gao, F.Q.; Zhou, J.X.; Gao, F.M. Photoresponsive organogel and organized nanostructures of cholesterol imide derivatives with azobenzene substituent groups. Prog. Nat. Sci. 2012, 22, 64–70. [Google Scholar] [CrossRef]
- Jiao, T.F.; Gao, F.Q.; Wang, Y.J.; Zhou, J.X.; Gao, F.M.; Luo, X.Z. Supramolecular gel and nanostructures of bolaform and trigonal cholesteryl derivatives with different aromatic spacers. Curr. Nanosci. 2012, 8, 111–116. [Google Scholar] [CrossRef]
- Jiao, T.F.; Gao, F.Q.; Shen, X.H.; Zhang, Q.R.; Zhang, X.F.; Zhou, J.X.; Gao, F.M. Self-assembly and nanostructures in organogels based on a bolaform cholesteryl imide compound with conjugated aromatic spacer. Materials 2013, 6, 5893–5906. [Google Scholar] [CrossRef]
- Jiao, T.; Huang, Q.; Zhang, Q.; Xiao, D.; Zhou, J.; Gao, F. Self-assembly of organogels via new luminol imide derivatives: Diverse nanostructures and substituent chain effect. Nanoscale Res. Lett. 2013, 8, 278. [Google Scholar] [CrossRef] [PubMed]
- Jiao, T.F.; Wang, Y.J.; Zhang, Q.R.; Zhou, J.X.; Gao, F.M. Regulation of substituent groups on morphologies and self-assembly of organogels based on some azobenzene imide derivatives. Nanoscale Res. Lett. 2013, 8, 160. [Google Scholar] [CrossRef] [PubMed]
- Jiao, T.F.; Gao, F.Q.; Zhang, Q.R.; Zhou, J.X.; Gao, F.M. Spacer effect on nanostructures and self-assembly in organogels via some bolaform cholesteryl imide derivatives with different spacers. Nanoscale Res. Lett. 2013, 8, 406. [Google Scholar] [CrossRef] [PubMed]
- Xing, R.; Liu, K.; Jiao, T.; Zhang, N.; Ma, K.; Zhang, R.; Zou, Q.; Ma, G.; Yan, X. An injectable self-assembling collagen-gold hybrid hydrogel for combinatorial antitumor photothermal/photodynamic therapy. Adv. Mater. 2016, 28, 3669–3676. [Google Scholar] [CrossRef] [PubMed]
- Jiao, T.; Zhao, H.; Zhou, J.; Zhang, Q.; Luo, X.; Hu, J.; Peng, Q.; Yan, X. The self-assembly reduced graphene oxide nanosheet hydrogel fabrication by anchorage of chitosan/silver and its potential efficient application toward dyes degradation for wastewater treatments. ACS Sustain. Chem. Eng. 2015, 3, 3130–3139. [Google Scholar] [CrossRef]
- Zhu, X.; Duan, P.; Zhang, L.; Liu, M. Regulation of the chiral twist and supramolecular chirality in co-assemblies of amphiphilic L-glutamic acid with bipyridines. Chem. Eur. J. 2011, 17, 3429–3437. [Google Scholar] [CrossRef] [PubMed]
- Duan, P.; Qin, L.; Zhu, X.; Liu, M. Hierarchical self-assembly of amphiphilic peptide dendrons: Evolution of diverse chiral nanostructures through hydrogel formation over a wide pH range. Chem. Eur. J. 2011, 17, 6389–6395. [Google Scholar] [CrossRef] [PubMed]
- Nayak, M.K. Functional organogel based on a hydroxyl naphthanilide derivative and aggregation induced enhanced fluorescence emission. J. Photochem. Photobiol. A Chem. 2011, 217, 40–48. [Google Scholar] [CrossRef]
- Atsbeha, T.; Bussotti, L.; Cicchi, S.; Foggi, P.; Ghini, G.; Lascialfari, L.; Marcelli, A. Photophysical characterization of low-molecular weight organogels for energy transfer and light harvesting. J. Mol. Struct. 2011, 993, 459–463. [Google Scholar] [CrossRef]
- Shimizu, T.; Masuda, M. Stereochemical effect of even-odd connecting links on supramolecular assemblies made of 1-glucosamide bolaamphiphiles. J. Am. Chem. Soc. 1997, 119, 2812–2818. [Google Scholar] [CrossRef]
- Kogiso, M.; Ohnishi, S.; Yase, K.; Masuda, M.; Shimizu, T. Dicarboxylic oligopeptide bola-amphiphiles: Proton-triggered self-assembly of microtubes with loose solid surfaces. Langmuir 1998, 14, 4978–4986. [Google Scholar] [CrossRef]
- Wang, T.Y.; Li, Y.G.; Liu, M.H. Gelation and self-assembly of glutamate bolaamphiphiles with hybrid linkers: Effect of the aromatic ring and alkyl linkers. Soft Matter 2009, 5, 1066–1073. [Google Scholar] [CrossRef]
- Zhao, W.; Li, Y.; Sun, T.; Yan, H.; Hao, A.; Xin, F.; Zhang, H.; An, W.; Kong, L.; Li, Y. Heat-set supramolecular organogels composed of β-cyclodextrin and substituted aniline in N,N-dimethylformamide. Colloid Surf. A-Physicochem. Eng. Asp. 2011, 374, 115–120. [Google Scholar] [CrossRef]
- Li, Y.G.; Wang, T.Y.; Liu, M.H. Ultrasound induced formation of organogel from a glutamic dendron. Tetrahedron 2007, 63, 7468–7473. [Google Scholar] [CrossRef]
- He, P.; Liu, J.; Liu, K.; Ding, L.; Yan, J.; Gao, D.; Fang, Y. Preparation of novel organometallic derivatives of cholesterol and their gel-formation properties. Colloid Surf. A Physicochem. Eng. Asp. 2010, 362, 127–134. [Google Scholar] [CrossRef]
- Wu, J.C.; Yi, T.; Xia, Q.; Zou, Y.; Liu, F.; Dong, J.; Shu, T.M.; Li, F.Y.; Huang, C.H. Tunable gel formation by both sonication and thermal processing in a cholesterol-based self-assembly system. Chem. Eur. J. 2009, 15, 6234–6243. [Google Scholar] [CrossRef] [PubMed]
- Xing, R.; Jiao, T.; Ma, K.; Ma, G.; Möhwald, H.; Yan, X. Regulating cell apoptosis on layer-by-layer assembled multilayers of photosensitizer-coupled polypeptides and gold nanoparticles. Sci. Rep. UK 2016, 6, 26506. [Google Scholar] [CrossRef] [PubMed]
- Jiao, T.; Liu, M. Supramolecular assemblies of a new series of gemini-type Schiff base amphiphiles at the air/water interface: In situ coordination, interfacial nanoarchitectures, and spacer effect. Langmuir 2006, 22, 5005–5012. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, N.; Gunn, J.; Zhang, M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv. Drug Deliv. Rev. 2010, 62, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yi, T.; Zhou, Z.; Zhou, Y.; Wu, J.; Xu, M.; Li, F.; Huang, C. Switchable fluorescent organogels and mesomorphic superstructure based on naphthalene derivatives. Langmuir 2007, 23, 8224–8230. [Google Scholar] [CrossRef] [PubMed]











| Solvents | TC16-Ben | TC16-Np | TC16-Fl |
|---|---|---|---|
| Nitrobenzene | S | S | G (2.0) |
| Acetone | PS | S | G (2.0) |
| DMF | PS | G (2.5) | G (2.5) |
| Aniline | G (1.5) | G (2.5) | G (2.0) |
| Pyridine | PS | S | G (2.5) |
| Petroleum ether | G (1.5) | PS | G (2.0) |
| Ethanolamine | I | PS | I |
| n-Hexane | G (1.5) | PS | G (2.0) |
| Ethyl acetate | PS | PS | PS |
| Ethanol | G (2.0) | S | G (2.0) |
| DMSO | S | S | S |
| n-Propanol | G (1.5) | G (2.0) | G (2.5) |
| Isopropanol | G (1.5) | PS | G (2.5) |
| Isooctanol | S | PS | G (2.0) |
| n-Butanol | G (1.5) | G (2.0) | G (2.0) |
| n-Butyl acrylate | I | PS | G (2.5) |
| Cyclohexanone | S | S | G (2.5) |
| n-Pentanol | G (1.5) | G (2.0) | G (2.0) |
| 1,4-Dioxane | PS | G (2.0) | G (2.0) |
| Cyclopentanone | PS | PS | G (2.0) |
| THF | PS | PS | PS |
| Isopentanol | G (2.0) | G (2.5) | G (2.5) |
| TC16-Fl Organogels in n-Pentanol | Pseudo-First-Order Model | Pseudo-Second-Order Model | ||||
|---|---|---|---|---|---|---|
| qe (mg/mL) | R2 | k1 (min−1) | qe (mg/mL) | R2 | k2 (mL/mg·min) | |
| CR concern. (2 mg/mL) | 1.8468 | 0.94511 | 2.76 × 10−3 | 2.24 | 0.7117 | 2.86 × 10−3 |
| CR concern. (4 mg/mL) | 4.1351 | 0.94472 | 2.30 × 10−3 | 7.68 | 0.24702 | 2.55 × 10−4 |
| CR concern. (6 mg/mL) | 6.6846 | 0.94878 | 1.84 × 10−4 | 16.83 | 0.03573 | 5.86 × 10−5 |
| CR concern. (8 mg/mL) | 9.8288 | 0.96162 | 9.21 × 10−4 | 41.19 | 0.01986 | 1.27 × 10−5 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Jiao, T.; Ma, K.; Xing, R.; Liu, Y.; Xiao, Y.; Zhou, J.; Zhang, Q.; Peng, Q. Self-Assembly and Drug Release Capacities of Organogels via Some Amide Compounds with Aromatic Substituent Headgroups. Materials 2016, 9, 541. https://doi.org/10.3390/ma9070541
Zhang L, Jiao T, Ma K, Xing R, Liu Y, Xiao Y, Zhou J, Zhang Q, Peng Q. Self-Assembly and Drug Release Capacities of Organogels via Some Amide Compounds with Aromatic Substituent Headgroups. Materials. 2016; 9(7):541. https://doi.org/10.3390/ma9070541
Chicago/Turabian StyleZhang, Lexin, Tifeng Jiao, Kai Ma, Ruirui Xing, Yamei Liu, Yong Xiao, Jingxin Zhou, Qingrui Zhang, and Qiuming Peng. 2016. "Self-Assembly and Drug Release Capacities of Organogels via Some Amide Compounds with Aromatic Substituent Headgroups" Materials 9, no. 7: 541. https://doi.org/10.3390/ma9070541
APA StyleZhang, L., Jiao, T., Ma, K., Xing, R., Liu, Y., Xiao, Y., Zhou, J., Zhang, Q., & Peng, Q. (2016). Self-Assembly and Drug Release Capacities of Organogels via Some Amide Compounds with Aromatic Substituent Headgroups. Materials, 9(7), 541. https://doi.org/10.3390/ma9070541







