Compliance Study of Endovascular Stent Grafts Incorporated with Polyester and Polyurethane Graft Materials in both Stented and Unstented Zones
Abstract
:1. Introduction
2. Materials and Methods
2.1. Grafts
2.2. Nitinol Stent
2.3. Stent Grafts
2.4. Compliance Tests
2.4.1. Test Instruments
2.4.2. Test Methods
2.5. Statistical Analysis
3. Results and Discussion
3.1. Diameter Changes of Both PET and PU Grafts
3.2. Compliance of Both PET and PU Grafts
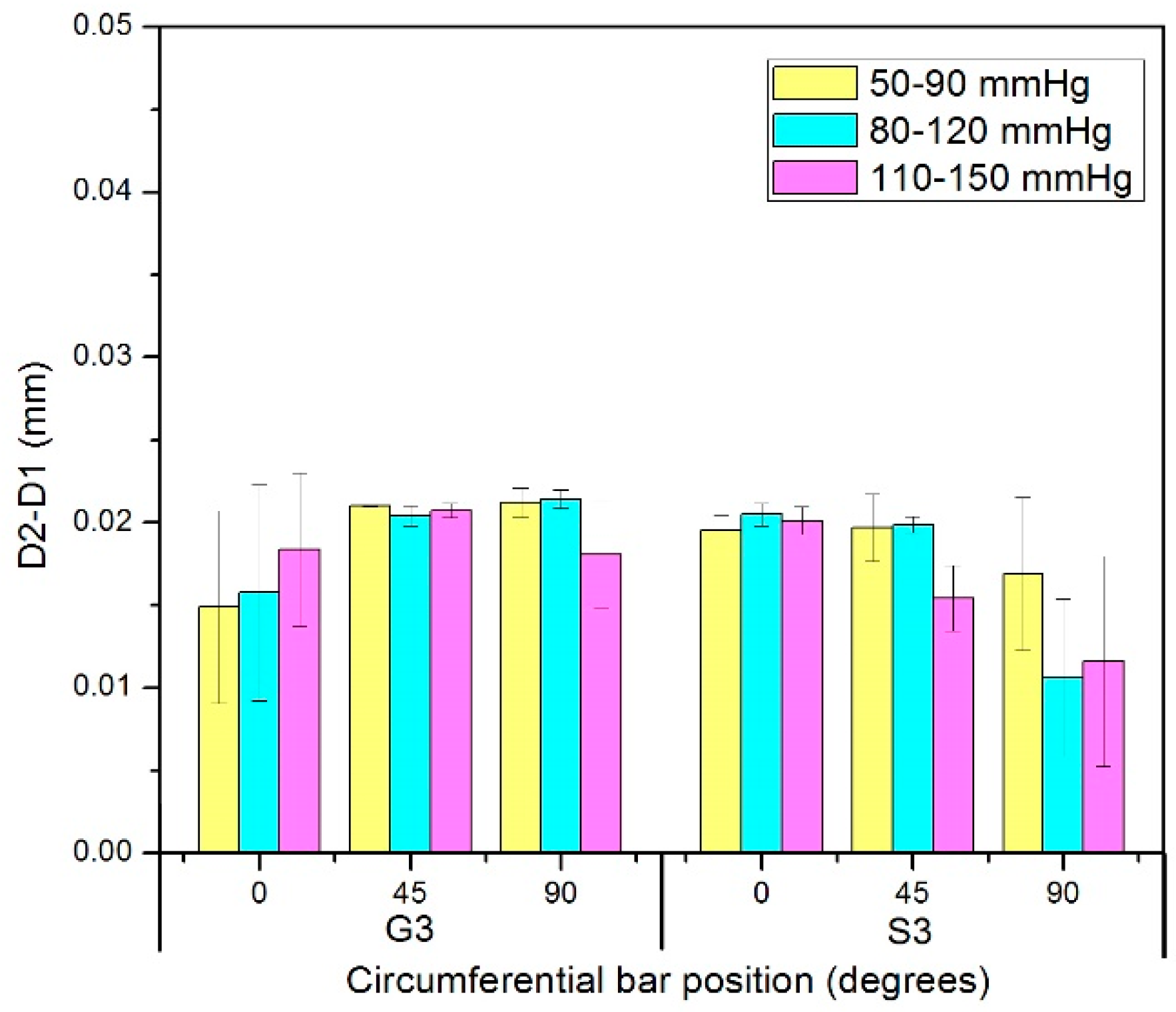
3.3. Diameter Changes of Nitinol-PET and Nitinol-PU Stent Grafts
3.4. Compliance of Nitinol-PET and Nitinol-PU Stent Grafts
4. Conclusions
- (1)
- The PU graft material showed significantly greater compliance than the PET graft material. The difference was at least 17 times higher.
- (2)
- Analysis of the nitinol-PET stent graft showed no difference in the amount of compliance between the stented and unstented zones regardless of the applied pressure range.
- (3)
- For the nitinol-PU stent graft, significant differences were observed in the amount of compliance in the stented and unstented regions. Thus, the compliance of the unstented PU region was approximately twice that of the stented region along the length of the stent graft. In addition, the pressure range was observed to influence the compliance of the stented region, with hypertensive pressures being associated with the highest compliance values in the stented zone S3 of the nitinol-PU stent graft.
- (4)
- Furthermore, because the stent graft was constructed with a longitudinal bar connecting the stents, the nitinol-PU stent graft was associated with a different kind of non-uniformity along its length, different from that of nitinol-PET stent graft. The shape of the unstented PU cross-section was found to be non-circular and rather “heart-shaped”, which was significantly different from the circular cross-sectional shape observed in the PU-stented region. Further work needs to be undertaken so as to translate these cross-sectional size and shape changes into disruptions of laminar flow through the stent graft.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Arko, F.R.; Murphy, E.H.; Boyes, C.; Nussbaum, T.; Lalka, S.G.; Holleman, J.; Roush, T.S. Current status of endovascular aneurysm repair: 20 years of learning. Semin. Vasc. Surg. 2012, 25, 131–135. [Google Scholar]
- Teigen, C.; Stanley, J.R.; Johnsont, P.; Gross, C. Preclinical evaluation of the InCraft” aortic endograft in a sheep model. Vascular 2014, 22, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Becker, G.J.; Kovacs, M.; Mathison, M.N.; Katzen, B.T.; Benenati, J.F.; Zemel, G.; Powell, A.; Almeida, J.I.; Alvarez, J.; Coello, A.A.; et al. Risk stratification and outcomes of transluminal endografting for abdominal aortic aneurysm: 7-year experience and long-term follow-up. J. Vasc. Interv. Radiol. 2001, 12, 1033–1046. [Google Scholar] [CrossRef]
- Blum, U.; Hauer, M.; Pfammatter, T.; Voshage, G. Percutaneous endoprosthesis for treatment of aortic aneurysms. World J. Surg. 2001, 25, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.L.; Vallabhaneni, S.R.; Desgranges, P.; Bacquemin, J.P.; van Marrewijk, C.; Laheij, R.J.F. Incidence and risk factors of late rupture, conversion, and death after endovascular repair of infrarenal aortic aneurysms: The EUROSTAR experience. J. Vasc. Surg. 2000, 32, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Hinchliffe, R.J.; Ivancev, K. Endovascular aneurysm repair: Current and future status. Cardiovasc. Interv. Radiol. 2008, 31, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Zarins, C.K.; Bloch, D.A.; Crabtree, T.; Matsumoto, A.H.; White, R.A.; Fogarty, T.J. Stent graft migration after endovascular aneurysm repair: Importance of proximal fixation. J. Vasc. Surg. 2003, 38, 1264–1272. [Google Scholar] [CrossRef]
- Lin, J.; Guidoin, R.; Wang, L.; Zhang, Z.; Nutley, M.; Paynter, R.; Wei, D.H.; How, T.; Crepeau, H.; Douville, Y.; et al. Fatigue and/or failure phenomena observed in the fabric of stent-grafts explanted after adverse events. J. Long Term Eff. Med. Implants 2013, 23, 67–86. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Wang, L.; Guidoin, R.; Nutley, M.; Song, G.; Zhang, Z.; Du, J.; Douville, Y. Stent fabric fatigue of grafts supported by Z-stents versus ringed stents: An in vitro buckling test. J. Biomater. Appl. 2014, 28, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Guidoin, R.; Du, J.; Wang, L.; Douglas, G.; Zhu, D.; Nutley, M.; Perron, L.; Zhang, Z.; Douville, Y. An in vitro twist fatigue test of fabric stent-grafts supported by z-stents vs. ringed stents. Materials 2016, 9, 113. [Google Scholar] [CrossRef]
- Rutherford, R.B. Structural failures in abdominal aortic aneurysm stentgrafts: Threat to durability and challenge to technology. Semin. Vasc. Surg. 2004, 17, 294–297. [Google Scholar] [CrossRef] [PubMed]
- Greenhalgh, R.M.; Powell, J.T. Endovascular repair of abdominal aortic aneurysm. N. Engl. J. Med. 2008, 358, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Laturnus, J.; Oliveira, N.; Goncalves, F.B.; Schurink, G.W.; Verhagen, H.; Jacobs, M.J.; Mees, B.M.E. Towards individualized follow-up protocols after endovascular aortic aneurysm repair. J. Cardiovasc. Surg. 2016, 57, 242–247. [Google Scholar]
- Mehta, M.; Sternbach, Y.; Taggert, J.B.; Kreienberg, P.B.; Roddy, S.P.; Paty, P.S.; Ozsvath, K.J.; Darling, R.C. Long-term outcomes of secondary procedures after endovascular aneurysm repair. J. Vasc. Surg. 2010, 52, 1442–1449. [Google Scholar] [CrossRef] [PubMed]
- Cao, P.; Verzini, F.; Zannetti, S.; De Rango, P.; Parlani, G.; Lupattelli, L.; Maselli, A. Device migration after endoluminal abdominal aortic aneurysm repair: Analysis of 113 cases with a minimum follow-up period of 2 years. J. Vasc. Surg. 2002, 35, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.; Wang, X.G. A biomimetic approach for designing stent-graft structures: Caterpillar cuticle as design model. J. Mech. Behav. Biomed. 2014, 30, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Alderson, H.; Zamir, M. Effects of stent stiffness on local haemodynamics with particular reference to wave reflections. J. Biomech. 2004, 37, 339–348. [Google Scholar] [CrossRef]
- Matsumoto, T.; Naiki, T.; Hayashi, K. Flow visualization analysis in a model of artery-graft anastomosis. Biomed. Mater. Eng. 1992, 2, 171–183. [Google Scholar] [PubMed]
- Morris, L.; Stefanov, F.; McGloughlin, T. Stent graft performance in the treatment of abdominal aortic aneurysms: The influence of compliance and geometry. J. Biomech. 2013, 46, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Tortoriello, A.; Pedrizzetti, G. Flow-tissue interaction with compliance mismatch in a model stented artery. J. Biomech. 2004, 37, 1–11. [Google Scholar] [CrossRef]
- Walsh, P.W.; Chin-Quee, S.; Moore, J.E. Flow changes in the aorta associated with the deployment of a AAA stent graft. Med. Eng. Phys. 2003, 25, 299–307. [Google Scholar] [CrossRef]
- Rolland, P.H.; Mekkaoui, C.; Vidal, V.; Berry, J.L.; Moore, J.E.; Moreno, M.; Amabile, P.; Bartoli, J.M. Compliance matching stent placement in the carotid artery of the swine promotes optimal blood flow and attenuates restenosis. Eur. J. Vasc. Endovasc. 2004, 28, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Yazdani, S.K.; Moore, J.E.; Berry, J.L.; Vlachos, P.P. DPIV measurements of flow disturbances in stented artery models: Adverse affects of compliance mismatch. J. Biomech. Eng. 2004, 126, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Berry, J.L.; Manoach, E.; Mekkaoui, C.; Rolland, P.H.; Moore, J.E.; Rachev, A. Hemodynamics and wall mechanics of a compliance matching stent: In vitro and in vivo analysis. J. Vasc. Interv. Radiol. 2002, 13, 97–105. [Google Scholar] [CrossRef]
- Kadoglou, N.P.E.; Moulakakis, K.G.; Papadakis, I.; Ikonomidis, I.; Alepaki, M.; Spathis, A.; Karakitsos, P.; Lekakis, J.; Liapis, C.D. Differential effects of stent-graft fabrics on arterial stiffness in patients undergoing endovascular aneurysm repair. J. Endovasc. Ther. 2014, 21, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.; Bakhshi, R.; Zhou, X.; Odlyha, M.; You, Z.; Seifalian, A.M.; Hamilton, G. A sutureless aortic stent-graft based on a nitinol scaffold bonded to a compliant nanocomposite polymer is durable for 10 years in a simulated in vitro model. J. Endovasc. Ther. 2012, 19, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Wang, N.; Wang, L.; Hou, L.; Ma, L.; Liu, J.; Chen, Y.; Fan, B.; Zhao, Y. An electrospun strong PCL/PU composite vascular graft with mechanical anisotropy and cyclic stability. J. Mater. Chem. A 2015, 3, 4782–4787. [Google Scholar] [CrossRef]
- Ajalloueian, F.; Lim, M.L.; Lemon, G.; Haag, J.C.; Gustafsson, Y.; Sjoqvist, S.; Beltran-Rodriguez, A.; Del Gaudio, C.; Baiguera, S.; Bianco, A.; et al. Biomechanical and biocompatibility characteristics of electrospun polymeric tracheal scaffolds. Biomaterials 2014, 35, 5307–5315. [Google Scholar] [CrossRef] [PubMed]
- Santerre, J.P.; Woodhouse, K.; Laroche, G.; Labow, R.S. Understanding the biodegradation of polyurethanes: From classical implants to tissue engineering materials. Biomaterials 2005, 26, 7457–7470. [Google Scholar] [CrossRef] [PubMed]
- Thomas, V.; Jayabalan, M. A new generation of high flex life polyurethane urea for polymer heart valve-studies on in vivo biocompatibility and biodurability. J. Biomed. Mater. Res. A 2009, 89, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Lee, B.S.; Park, K.; Han, D.K.; Park, K.D.; Kim, Y.H. Beneficial effect of sulfonated PEO-grafted polyurethanes on calcification and lipid adsorption of vascular implants. Macromol. Res. 2010, 18, 1133–1136. [Google Scholar] [CrossRef]
- Gu, P.; Nishida, T.; Fan, Z.H. The use of polyurethane as an elastomer in thermoplastic microfluidic devices and the study of its creep properties. Electrophoresis 2014, 35, 289–297. [Google Scholar] [CrossRef] [PubMed]




 represents the vertical bar.
represents the vertical bar.
 represents the vertical bar.
represents the vertical bar.











| Pressure/mmHg | 50 | 90 | 80 | 120 | 110 | 150 |
|---|---|---|---|---|---|---|
| /MPa | 0.276 | 0.472 | 0.421 | 0.630 | 0.579 | 0.787 |
| Test Positions | Bar Positions | Pressure Range/mmHg | ||
|---|---|---|---|---|
| 50–90 | 80–120 | 110–150 | ||
| G3 | 0° | 0.380 ± 0.023 | 0.373 ± 0.024 | 0.398 ± 0.034 |
| 45° | 0.385 ± 0.031 | 0.376 ± 0.023 | 0.404 ± 0.029 | |
| 90° | 0.258 ± 0.007 | 0.301 ± 0.012 | 0.304 ± 0.013 | |
| S3 | 0° | 0.192 ± 0.025 | 0.218 ± 0.009 | 0.238 ± 0.003 |
| 45° | 0.165 ± 0.019 | 0.204 ± 0.026 | 0.214 ± 0.021 | |
| 90° | 0.196 ± 0.012 | 0.235 ± 0.013 | 0.239 ± 0.013 | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, Y.; Wang, L.; Lin, J.; King, M.W. Compliance Study of Endovascular Stent Grafts Incorporated with Polyester and Polyurethane Graft Materials in both Stented and Unstented Zones. Materials 2016, 9, 658. https://doi.org/10.3390/ma9080658
Guan Y, Wang L, Lin J, King MW. Compliance Study of Endovascular Stent Grafts Incorporated with Polyester and Polyurethane Graft Materials in both Stented and Unstented Zones. Materials. 2016; 9(8):658. https://doi.org/10.3390/ma9080658
Chicago/Turabian StyleGuan, Ying, Lu Wang, Jing Lin, and Martin W. King. 2016. "Compliance Study of Endovascular Stent Grafts Incorporated with Polyester and Polyurethane Graft Materials in both Stented and Unstented Zones" Materials 9, no. 8: 658. https://doi.org/10.3390/ma9080658






