Myocardial Infarction Quantification from Late Gadolinium Enhancement MRI Using Top-Hat Transforms and Neural Networks
Abstract
:1. Introduction
2. Background and Related Works
2.1. Myocardial Damage Detection
2.2. Myocardial Infarction Segmentation
3. Material and Methods
3.1. Data Acquisition
3.2. Proposed Method
3.2.1. Data Pre-Processing
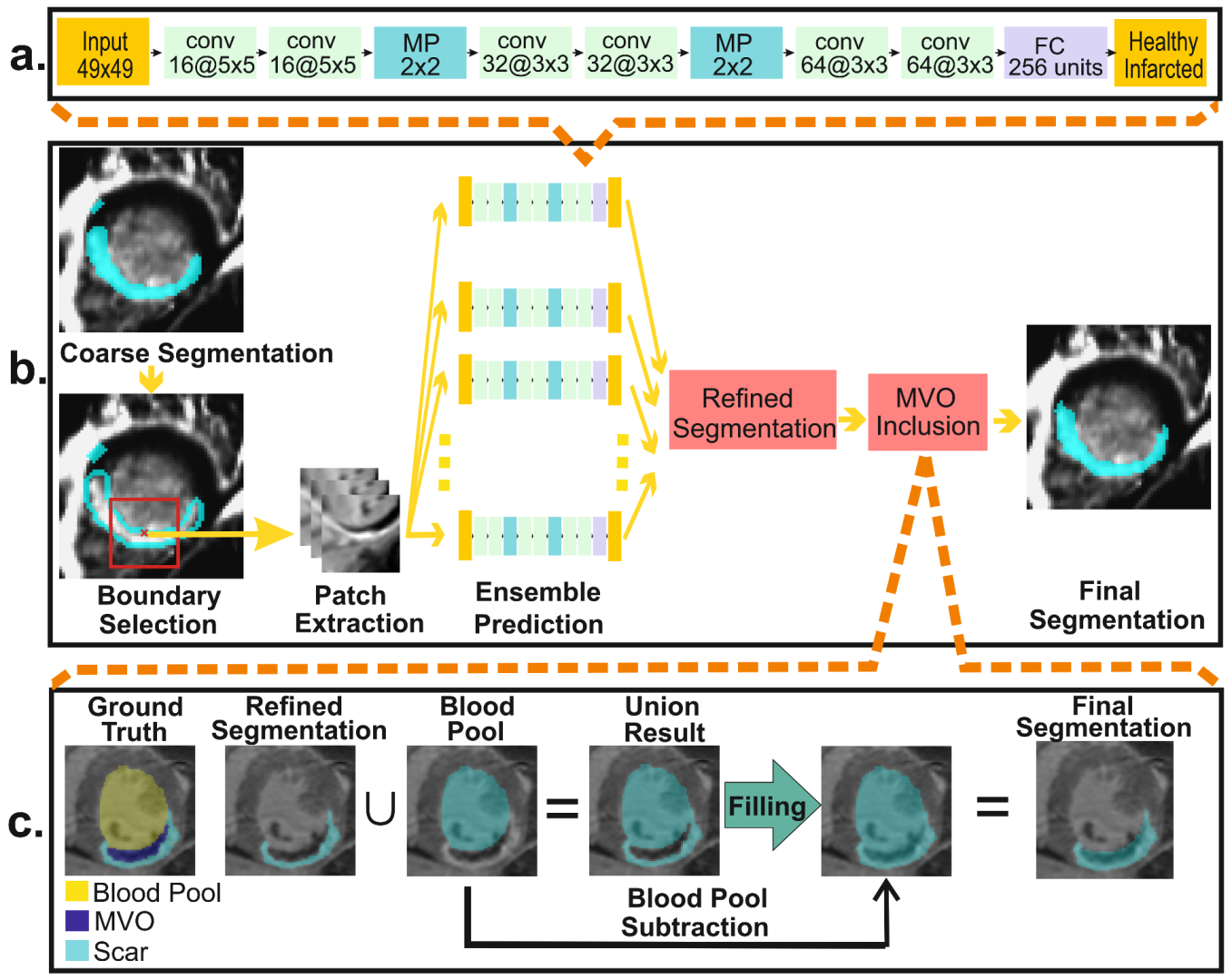
3.2.2. Myocardial Abnormality Detection
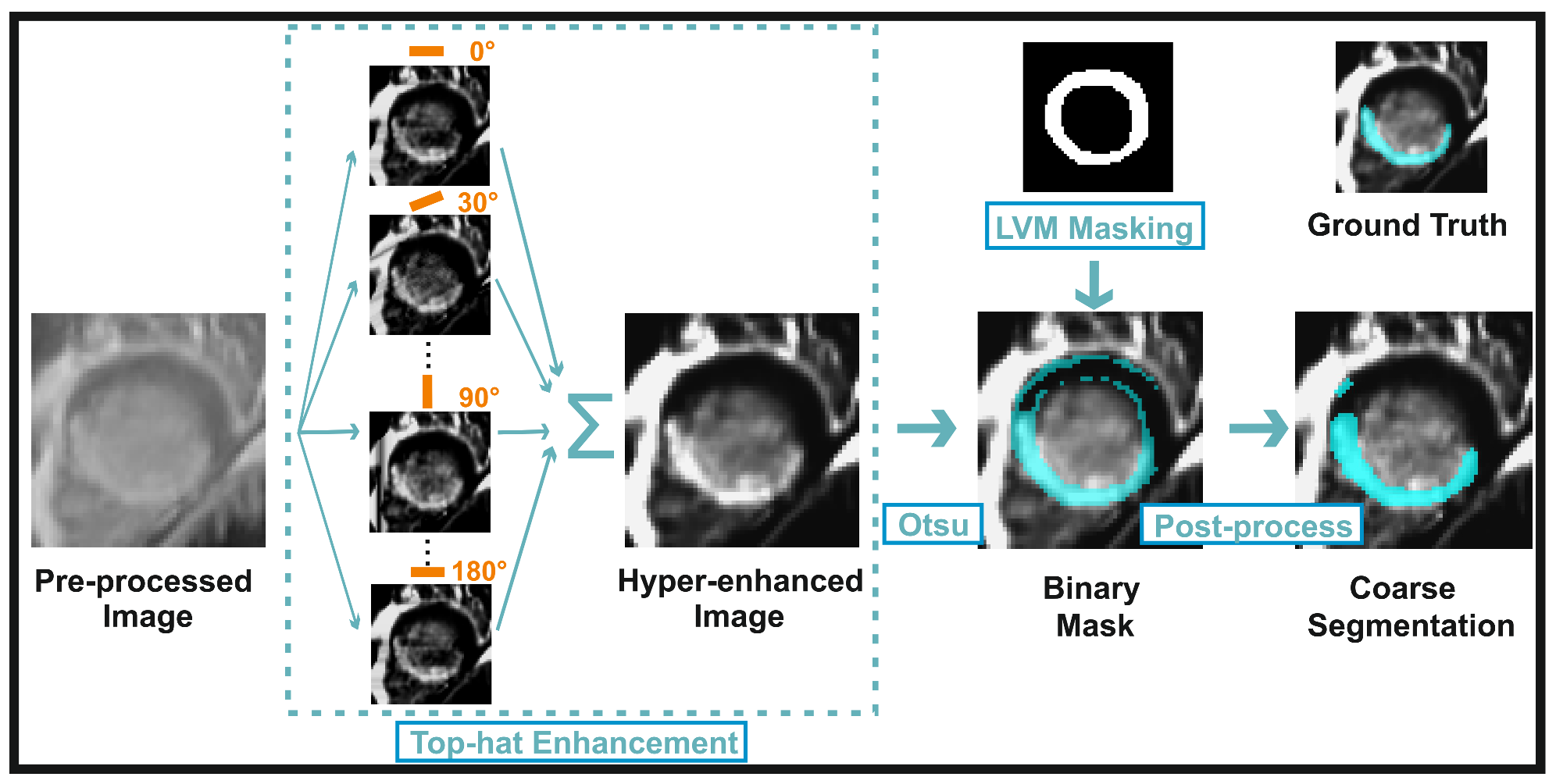
3.2.3. Myocardial Scar Quantification: Coarse Segmentation
3.2.4. Myocardial Scar Quantification: Refined Segmentation
3.2.5. Myocardial Scar Quantification: MVO Inclusion
3.2.6. Comparison against Previous Methods
4. Results
4.1. Myocardial Abnormality Detection
4.1.1. Model Selection
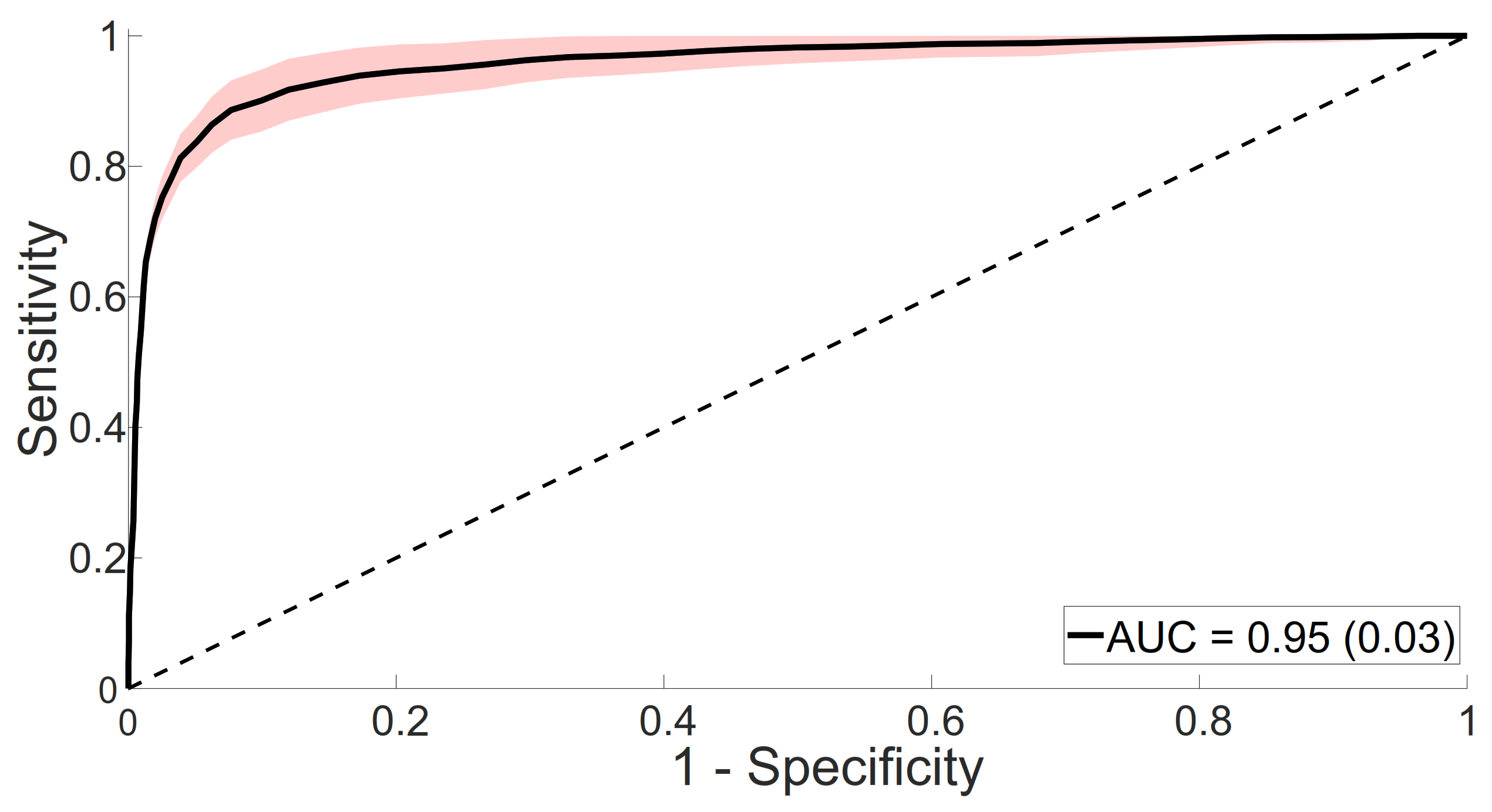
4.1.2. Classifier Evaluation
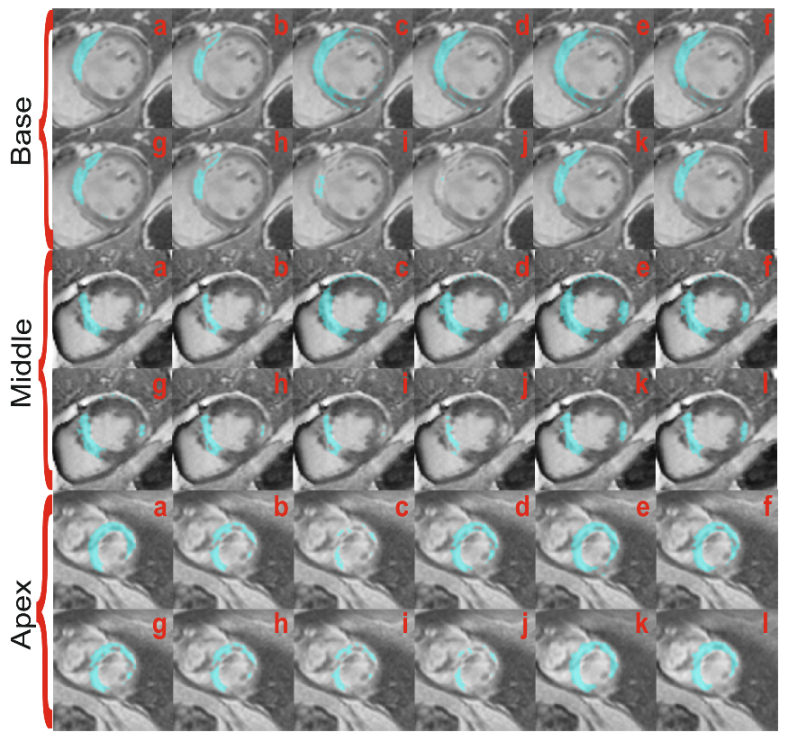
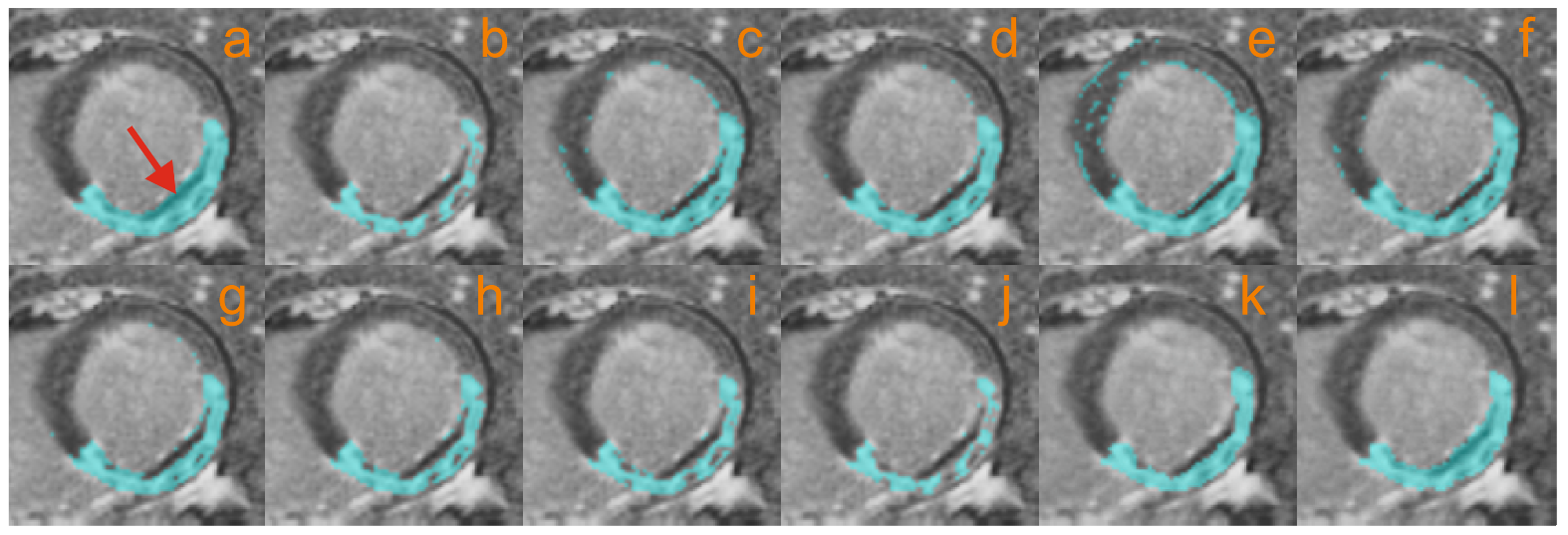
4.2. Myocardial Scar Segmentation and Quantification
4.2.1. Ensemble Size Selection
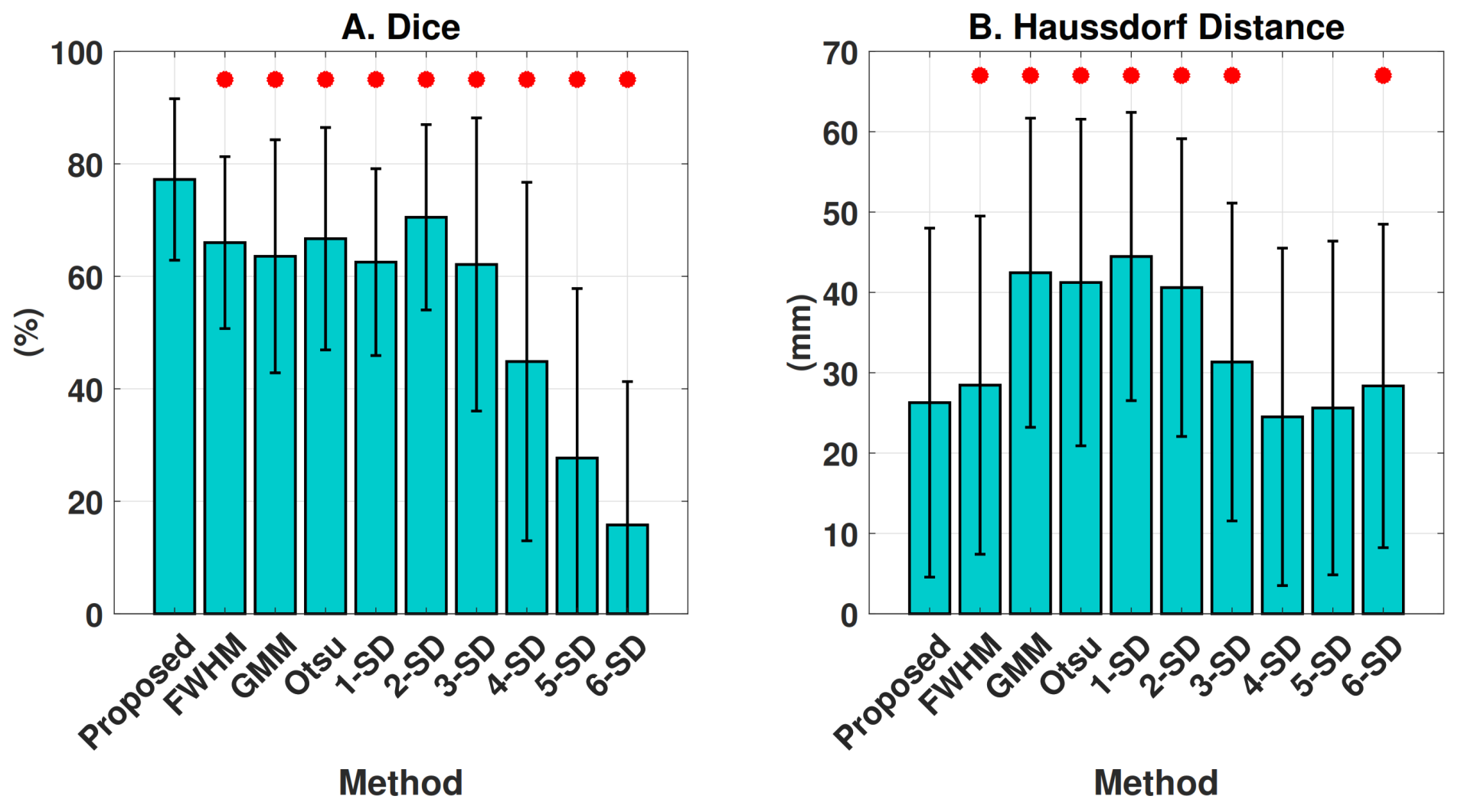
4.2.2. Segmentation Performance
4.2.3. Microvascular Obstruction Inclusion
4.3. Computational Performance
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dastidar, A.G.; Rodrigues, J.C.; Baritussio, A.; Bucciarelli-Ducci, C. MRI in the assessment of ischaemic heart disease. Heart 2016, 102, 239–252. [Google Scholar] [CrossRef]
- Puntmann, V.O.; Valbuena, S.; Hinojar, R.; Petersen, S.E.; Greenwood, J.P.; Kramer, C.M.; Kwong, R.Y.; McCann, G.P.; Berry, C.; Nagel, E. Society for Cardiovascular Magnetic Resonance (SCMR) expert consensus for CMR imaging endpoints in clinical research: PartI-analytical validation and clinical qualification. J. Cardiovasc. Magn. Reson. 2018, 20, 67. [Google Scholar] [CrossRef] [Green Version]
- Rajiah, P.; Desai, M.Y.; Kwon, D.; Flamm, S.D. MR imaging of myocardial infarction. Radiographics 2013, 33, 1383–1412. [Google Scholar] [CrossRef]
- Kim, R.J.; Fieno, D.S.; Parrish, T.B.; Harris, K.; Chen, E.L.; Simonetti, O.; Bundy, J.; Finn, J.P.; Klocke, F.J.; Judd, R.M. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation 1999, 100, 1992–2002. [Google Scholar] [CrossRef] [Green Version]
- Cochet, A.A.; Lorgis, L.; Lalande, A.; Zeller, M.; Beer, J.C.; Walker, P.M.; Touzery, C.; Wolf, J.E.; Brunotte, F.; Cottin, Y. Major prognostic impact of persistent microvascular obstruction as assessed by contrast-enhanced cardiac magnetic resonance in reperfused acute myocardial infarction. Eur. Radiol. 2009, 19, 2117–2126. [Google Scholar] [CrossRef]
- Hamirani, Y.S.; Wong, A.; Kramer, C.M.; Salerno, M. Effect of microvascular obstruction and intramyocardial hemorrhage by CMR on LV remodeling and outcomes after myocardial infarction: A systematic review and meta-analysis. JACC Cardiovasc. Imaging 2014, 7, 940–952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbas, A.; Matthews, G.; Brown, I.; Shambrook, J.; Peebles, C.; Harden, S. Cardiac MR assessment of microvascular obstruction. Br. J. Radiol. 2015, 88, 20140470. [Google Scholar] [CrossRef] [PubMed]
- Engblom, H.; Tufvesson, J.; Jablonowski, R.; Carlsson, M.; Aletras, A.H.; Hoffmann, P.; Jacquier, A.; Kober, F.; Metzler, B.; Erlinge, D.; et al. A new automatic algorithm for quantification of myocardial infarction imaged by late gadolinium enhancement cardiovascular magnetic resonance: Experimental validation and comparison to expert delineations in multi-center, multi-vendor patient data. J. Cardiovasc. Magn. Reson. 2016, 18, 27. [Google Scholar] [CrossRef] [PubMed]
- Pattanayak, P.; Bleumke, D.A. Tissue characterization of the myocardium: State of the art characterization by magnetic resonance and computed tomography imaging. Radiol. Clin. 2015, 53, 413–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amado, L.C.; Gerber, B.L.; Gupta, S.N.; Rettmann, D.W.; Szarf, G.; Schock, R.; Nasir, K.; Kraitchman, D.L.; Lima, J.A. Accurate and objective infarct sizing by contrast-enhanced magnetic resonance imaging in a canine myocardial infarction model. J. Am. Coll. Cardiol. 2004, 44, 2383–2389. [Google Scholar] [CrossRef] [Green Version]
- Flett, A.S.; Hasleton, J.; Cook, C.; Hausenloy, D.; Quarta, G.; Ariti, C.; Muthurangu, V.; Moon, J.C. Evaluation of techniques for the quantification of myocardial scar of differing etiology using cardiac magnetic resonance. JACC Cardiovasc. Imaging 2011, 4, 150–156. [Google Scholar] [CrossRef] [Green Version]
- Spiewak, M.; Malek, L.A.; Misko, J.; Chojnowska, L.; Milosz, B.; Klopotowski, M.; Petryka, J.; Dabrowski, M.; Kepka, C.; Ruzyllo, W. Comparison of different quantification methods of late gadolinium enhancement in patients with hypertrophic cardiomyopathy. Eur. J. Radiol. 2010, 74, e149–e153. [Google Scholar] [CrossRef]
- Zhang, L.; Huttin, O.; Marie, P.y.; Felblinger, J.; Beaumont, M.; de Chillou, C.; Girerd, N.; Mandry, D. Myocardial infarct sizing by late gadolinium-enhanced MRI: Comparison of manual, full-width at half-maximum, and n-standard deviation methods. J. Magn. Reson. Imaging 2016, 44, 1206–1217. [Google Scholar] [CrossRef]
- Karim, R.; Claus, P.; Chen, Z.; Housden, R.J.; Obom, S.; Gill, H.; Ma, Y.; Acheampong, P.; O’Neill, M.; Razavi, R.; et al. Infarct segmentation challenge on delayed enhancement MRI of the left ventricle. In International Workshop on Statistical Atlases and Computational Models of the Heart; Springer: Berlin/Heidelberg, Germany, 2012; pp. 97–104. [Google Scholar]
- Hennemuth, A.; Seeger, A.; Friman, O.; Miller, S.; Klumpp, B.; Oeltze, S.; Peitgen, H.O. A comprehensive approach to the analysis of contrast enhanced cardiac MR images. IEEE Trans. Med. Imaging 2008, 27, 1592–1610. [Google Scholar] [CrossRef] [PubMed]
- Tao, Q.; Milles, J.; Zeppenfeld, K.; Lamb, H.J.; Bax, J.J.; Reiber, J.H.; van der Geest, R.J. Automated segmentation of myocardial scar in late enhancement MRI using combined intensity and spatial information. Magn. Reson. Med. 2010, 64, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Sun, Y.; Ong, S.H.; Chai, P.; Teo, L.L.; Low, A.F. A comprehensive 3-D framework for automatic quantification of late gadolinium enhanced cardiac magnetic resonance images. IEEE Trans. Biomed. Eng. 2013, 60, 1499–1508. [Google Scholar]
- Carminati, M.C.; Boniotti, C.; Fusini, L.; Andreini, D.; Pontone, G.; Pepi, M.; Caiani, E.G. Comparison of image processing techniques for nonviable tissue quantification in late gadolinium enhancement cardiac magnetic resonance images. J. Thorac. Imaging 2016, 31, 168–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pop, M.; Ghugre, N.R.; Ramanan, V.; Morikawa, L.; Stanisz, G.; Dick, A.J.; Wright, G.A. Quantification of fibrosis in infarcted swine hearts by late gadolinium-enhancement and diffusion-weighted MRI methods. Phys. Med. Biol. 2013, 58, 5009. [Google Scholar] [CrossRef] [PubMed]
- Valindria, V.V.; Angue, M.; Vignon, N.; Walker, P.M.; Cochet, A.; Lalande, A. Automatic quantification of myocardial infarction from delayed enhancement MRI. In Proceedings of the 2011 Seventh International Conference on Signal-Image Technology and Internet-Based Systems (SITIS), Dijon, France, 28 November–1 December 2011; IEEE: Piscataway, NJ, USA, 2011; pp. 277–283. [Google Scholar]
- Zreik, M.; Lessmann, N.; van Hamersvelt, R.W.; Wolterink, J.M.; Voskuil, M.; Viergever, M.A.; Leiner, T.; Išgum, I. Deep learning analysis of the myocardium in coronary CT angiography for identification of patients with functionally significant coronary artery stenosis. Med. Image Anal. 2018, 44, 72–85. [Google Scholar] [CrossRef] [Green Version]
- Kolipaka, A.; Chatzimavroudis, G.P.; White, R.D.; O’Donnell, T.P.; Setser, R.M. Segmentation of non-viable myocardium in delayed enhancement magnetic resonance images. Int. J. Cardiovasc. Imaging 2005, 21, 303–311. [Google Scholar] [CrossRef]
- Otsu, N. A threshold selection method from gray-level histograms. IEEE Trans. Syst. Man, Cybern. 1979, 9, 62–66. [Google Scholar] [CrossRef] [Green Version]
- Andreu, D.; Berruezo, A.; Ortiz-Pérez, J.T.; Silva, E.; Mont, L.; Borràs, R.; de Caralt, T.M.; Perea, R.J.; Fernández-Armenta, J.; Zeljko, H.; et al. Integration of 3D electroanatomic maps and magnetic resonance scar characterization into the navigation system to guide ventricular tachycardia ablation clinical perspective. Circ. Arrhythmia Electrophysiol. 2011, 4, 674–683. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, A.; Azevedo, C.F.; Cheng, A.; Gupta, S.N.; Bluemke, D.A.; Foo, T.K.; Gerstenblith, G.; Weiss, R.G.; Marbán, E.; Tomaselli, G.F.; et al. Infarct tissue heterogeneity by magnetic resonance imaging identifies enhanced cardiac arrhythmia susceptibility in patients with left ventricular dysfunction. Circulation 2007, 115, 2006–2014. [Google Scholar] [CrossRef]
- Hsu, L.Y.; Natanzon, A.; Kellman, P.; Hirsch, G.A.; Aletras, A.H.; Arai, A.E. Quantitative myocardial infarction on delayed enhancement MRI. Part I: Animal validation of an automated feature analysis and combined thresholding infarct sizing algorithm. J. Magn. Reson. Imaging 2006, 23, 298–308. [Google Scholar] [CrossRef]
- Detsky, J.S.; Paul, G.; Dick, A.J.; Wright, G.A. Reproducible classification of infarct heterogeneity using fuzzy clustering on multicontrast delayed enhancement magnetic resonance images. IEEE Trans. Med Imaging 2009, 28, 1606–1614. [Google Scholar] [CrossRef]
- Positano, V.; Pingitore, A.; Giorgetti, A.; Favilli, B.; Santarelli, M.F.; Landini, L.; Marzullo, P.; Lombardi, M. A fast and effective method to assess myocardial necrosis by means of contrast magnetic resonance imaging. J. Cardiovasc. Magn. Reson. 2005, 7, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Dikici, E.; O’Donnell, T.; Setser, R.; White, R.D. Quantification of delayed enhancement MR images. In Proceedings of the International Conference on Medical Image Computing and Computer-Assisted Intervention, Saint-malo, France, 26–29 September 2004; Springer: Berlin/Heidelberg, Germany, 2004; pp. 250–257. [Google Scholar]
- O’Donnell, T.P.; Xu, N.; Setser, R.M.; White, R.D. Semi-automatic segmentation of nonviable cardiac tissue using cine and delayed enhancement magnetic resonance images. In Medical Imaging 2003: Physiology and Function: Methods, Systems, and Applications; International Society for Optics and Photonics: Bellingham, WA, USA, 2003; Volume 5031, pp. 242–252. [Google Scholar]
- Lu, Y.; Yang, Y.; Connelly, K.A.; Wright, G.A.; Radau, P.E. Automated quantification of myocardial infarction using graph cuts on contrast delayed enhanced magnetic resonance images. Quant. Imaging Med. Surg. 2012, 2, 81. [Google Scholar] [PubMed]
- Kruk, D.; Boucher, A.; Lalande, A.; Cochet, A.; Sliwa, T. Segmentation Integrating Watershed and Shape Priors Applied to Cardiac Delayed Enhancement MR Images. IRBM 2017, 38, 224–227. [Google Scholar] [CrossRef]
- Ukwatta, E.; Arevalo, H.; Li, K.; Yuan, J.; Qiu, W.; Malamas, P.; Wu, K.C.; Trayanova, N.A.; Vadakkumpadan, F. Myocardial infarct segmentation from magnetic resonance images for personalized modeling of cardiac electrophysiology. IEEE Trans. Med. Imaging 2016, 35, 1408–1419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajchl, M.; Yuan, J.; White, J.A.; Ukwatta, E.; Stirrat, J.; Nambakhsh, C.M.; Li, F.P.; Peters, T.M. Interactive hierarchical-flow segmentation of scar tissue from late-enhancement cardiac MR images. IEEE Trans. Med. Imaging 2014, 33, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Moccia, S.; Banali, R.; Martini, C.; Moscogiuri, G.; Pontone, G.; Pepi, M.; Caiani, E.G. Automated Scar Segmentation From CMR-LGE Images Using a Deep Learning Approach. In Proceedings of the 2018 Computing in Cardiology Conference (CinC), Maastricht, The Netherlands, 23–26 September 2018; IEEE: Piscataway, NJ, USA, 2018; Volume 45, pp. 1–4. [Google Scholar]
- Moccia, S.; Banali, R.; Martini, C.; Muscogiuri, G.; Pontone, G.; Pepi, M.; Caiani, E.G. Development and testing of a deep learning-based strategy for scar segmentation on CMR-LGE images. Magn. Reson. Mater. Phys. Biol. Med. 2019, 32, 187–195. [Google Scholar] [CrossRef] [Green Version]
- Zabihollahy, F.; White, J.A.; Ukwatta, E. Myocardial scar segmentation from magnetic resonance images using convolutional neural network. In Medical Imaging 2018: Computer-Aided Diagnosis; Petrick, N., Mori, K., Eds.; International Society for Optics and Photonics, SPIE: San Diego, CA, USA, 2018; Volume 10575, pp. 663–670. [Google Scholar]
- Zabihollahy, F.; White, J.A.; Ukwatta, E. Convolutional neural network-based approach for segmentation of left ventricle myocardial scar from 3D late gadolinium enhancement MR images. Med. Phys. 2019, 46, 1740–1751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, G.; Zhuang, X.; Khan, H.; Haldar, S.; Nyktari, E.; Ye, X.; Slabaugh, G.; Wong, T.; Mohiaddin, R.; Keegan, J.; et al. A fully automatic deep learning method for atrial scarring segmentation from late gadolinium-enhanced MRI images. In Proceedings of the 2017 IEEE 14th International Symposium on Biomedical Imaging (ISBI 2017), Melbourne, Australia, 18–21 April 2017; IEEE: Piscataway, NJ, USA, 2017; pp. 844–848. [Google Scholar]
- Yang, G.; Zhuang, X.; Khan, H.; Haldar, S.; Nyktari, E.; Li, L.; Wage, R.; Ye, X.; Slabaugh, G.; Mohiaddin, R.; et al. Fully automatic segmentation and objective assessment of atrial scars for long-standing persistent atrial fibrillation patients using late gadolinium-enhanced MRI. Med. Phys. 2018, 45, 1562–1576. [Google Scholar] [CrossRef]
- Paszke, A.; Chaurasia, A.; Kim, S.; Culurciello, E. ENet: A Deep Neural Network Architecture for Real-Time Semantic Segmentation. arXiv 2016, arXiv:1606.02147. [Google Scholar]
- Zabihollahy, F.; Rajchl, M.; White, J.A.; Ukwatta, E. Fully automated segmentation of left ventricular scar from 3D late gadolinium enhancement magnetic resonance imaging using a cascaded multi-planar U-Net (CMPU-Net). Med. Phys. 2020, 47, 1645–1655. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, A.S.; Rowin, E.J.; Chan, R.H.; Manning, W.J.; Maron, M.S.; Nezafat, R. Improved quantification of myocardium scar in late gadolinium enhancement images: Deep learning based image fusion approach. J. Magn. Reson. Imaging 2021. [Google Scholar] [CrossRef] [PubMed]
- Lalande, A.; Garreau, M.; Frouin, F. Evaluation of cardiac structure segmentation in cine magnetic resonance imaging. In Multi-Modality Cardiac Imaging: Processing and Analysis; Wiley Online Library: Hoboken, NJ, USA, 2015; pp. 169–215. [Google Scholar]
- Lalande, A.; Chen, Z.; Decourselle, T.; Qayyum, A.; Pommier, T.; Lorgis, L.; de la Rosa, E.; Cochet, A.; Cottin, Y.; Ginhac, D.; et al. Emidec: A database usable for the automatic evaluation of myocardial infarction from delayed-enhancement cardiac MRI. Data 2020, 5, 89. [Google Scholar] [CrossRef]
- Manjón, J.V.; Coupé, P.; Martí-Bonmatí, L.; Collins, D.L.; Robles, M. Adaptive non-local means denoising of MR images with spatially varying noise levels. J. Magn. Reson. Imaging 2010, 31, 192–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simonyan, K.; Zisserman, A. Very Deep Convolutional Networks for Large-Scale Image Recognition. arXiv 2014, arXiv:1409.1556. [Google Scholar]
- Antropova, N.; Huynh, B.Q.; Giger, M.L. A deep feature fusion methodology for breast cancer diagnosis demonstrated on three imaging modality datasets. Med. Phys. 2017, 44, 5162–5171. [Google Scholar] [CrossRef]
- Jia, H.; Xia, Y.; Song, Y.; Cai, W.; Fulham, M.; Feng, D.D. Atlas registration and ensemble deep convolutional neural network-based prostate segmentation using magnetic resonance imaging. Neurocomputing 2018, 275, 1358–1369. [Google Scholar] [CrossRef]
- Johnson, J.M.; Khoshgoftaar, T.M. Survey on deep learning with class imbalance. J. Big Data 2019, 6, 27. [Google Scholar] [CrossRef]
- Haixiang, G.; Yijing, L.; Shang, J.; Mingyun, G.; Yuanyue, H.; Bing, G. Learning from class-imbalanced data: Review of methods and applications. Expert Syst. Appl. 2017, 73, 220–239. [Google Scholar] [CrossRef]
- Srivastava, N.; Hinton, G.; Krizhevsky, A.; Sutskever, I.; Salakhutdinov, R. Dropout: A simple way to prevent neural networks from overfitting. J. Mach. Learn. Res. 2014, 15, 1929–1958. [Google Scholar]
- Ram, S.; Rodríguez, J.J.; Bosco, G. Segmentation and detection of fluorescent 3D spots. Cytom. Part A 2012, 81, 198–212. [Google Scholar] [CrossRef] [PubMed]
- BahadarKhan, K.; Khaliq, A.A.; Shahid, M. A morphological hessian based approach for retinal blood vessels segmentation and denoising using region based otsu thresholding. PLoS ONE 2016, 11, e0158996. [Google Scholar] [CrossRef] [Green Version]
- Savelli, B.; Marchesi, A.; Bria, A.; Marrocco, C.; Molinara, M.; Tortorella, F. Retinal Vessel Segmentation Through Denoising and Mathematical Morphology. In Proceedings of the International Conference on Image Analysis and Processing, Catania, Italy, 11–15 September 2017; Springer: Berlin/Heidelberg, Germany, 2017; pp. 267–276. [Google Scholar]
- Krizhevsky, A.; Sutskever, I.; Hinton, G.E. Imagenet classification with deep convolutional neural networks. Adv. Neural Inf. Process. Syst. 2012, 25, 1097–1105. [Google Scholar] [CrossRef]
- Bernal, J.; Kushibar, K.; Cabezas, M.; Valverde, S.; Oliver, A.; Lladó, X. Quantitative Analysis of Patch-Based Fully Convolutional Neural Networks for Tissue Segmentation on Brain Magnetic Resonance Imaging. IEEE Access 2019, 7, 89986–90002. [Google Scholar] [CrossRef]
- Durante, A.; Camici, P.G. Novel insights into an “old” phenomenon: The no reflow. Int. J. Cardiol. 2015, 187, 273–280. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 327, 307–310. [Google Scholar] [CrossRef]
- Ronneberger, O.; Fischer, P.; Brox, T. U-net: Convolutional networks for biomedical image segmentation. In Proceedings of the International Conference on Medical Image Computing and Computer-Assisted Intervention, Munich, Germany, 5–9 October 2015; Springer: Berlin/Heidelberg, Germany, 2015; pp. 234–241. [Google Scholar]
- Milletari, F.; Navab, N.; Ahmadi, S.A. V-net: Fully convolutional neural networks for volumetric medical image segmentation. In Proceedings of the 2016 Fourth International Conference on 3D Vision (3DV), Stanford, CA, USA, 25–28 October 2016; IEEE: Piscataway, NJ, USA, 2016; pp. 565–571. [Google Scholar]
- Badrinarayanan, V.; Kendall, A.; Cipolla, R. Segnet: A deep convolutional encoder-decoder architecture for image segmentation. IEEE Trans. Pattern Anal. Mach. Intell. 2017, 39, 2481–2495. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.; Mercan, E.; Bartlett, J.; Weaver, D.; Elmore, J.G.; Shapiro, L. Y-Net: Joint segmentation and classification for diagnosis of breast biopsy images. In Proceedings of the International Conference on Medical Image Computing and Computer-Assisted Intervention, Granada, Spain, 16–20 September 2018; Springer: Berlin/Heidelberg, Germany, 2018; pp. 893–901. [Google Scholar]
- Rundo, L.; Tangherloni, A.; Cazzaniga, P.; Nobile, M.S.; Russo, G.; Gilardi, M.C.; Vitabile, S.; Mauri, G.; Besozzi, D.; Militello, C. A novel framework for MR image segmentation and quantification by using MedGA. Comput. Methods Programs Biomed. 2019, 176, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Haaf, P.; Garg, P.; Messroghli, D.R.; Broadbent, D.A.; Greenwood, J.P.; Plein, S. Cardiac T1 mapping and extracellular volume (ECV) in clinical practice: A comprehensive review. J. Cardiovasc. Magn. Reson. 2017, 18, 89. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Xu, L.; Gao, Z.; Zhao, S.; Zhang, H.; Zhang, Y.; Du, X.; Zhao, S.; Ghista, D.; Li, S. Direct detection of pixel-level myocardial infarction areas via a deep-learning algorithm. In Proceedings of the International Conference on Medical Image Computing and Computer-Assisted Intervention, Quebec City, QC, Canada, 11–13 September 2017; Springer: Berlin/Heidelberg, Germany, 2017; pp. 240–249. [Google Scholar]
- Zhang, N.; Yang, G.; Gao, Z.; Xu, C.; Zhang, Y.; Shi, R.; Keegan, J.; Xu, L.; Zhang, H.; Fan, Z.; et al. Deep Learning for Diagnosis of Chronic Myocardial Infarction on Nonenhanced Cardiac Cine MRI. Radiology 2019, 291, 606–617. [Google Scholar] [CrossRef] [Green Version]
| Goal | Net | Patch | Loss | Optimizer | LR | M | MB | L2 | Epochs |
|---|---|---|---|---|---|---|---|---|---|
| Disease Detection | VGG19 [47] | 89 × 89 | CE | SGDM | 0.9 | 16 | 20 | ||
| Scar Segmentation | [21] * | 49 × 49 | CE | SGDM | 0.75 | 256 | 50 |
| Method | Se | Sp | Acc |
|---|---|---|---|
| Fine-tuned VGG19 | 84.41 (11.02) | 93.89 (6.79) | 89.15 (5.36) |
| Our method | 88.11 (6.54) | 93.15 (4.84) | 90.63 (4.32) |
| Method | Dice (%) | HD (mm) |
|---|---|---|
| Coarse | 73.0 (14.5) | 27.5 (21.7) |
| Coarse + Ensemble 1 | 76.3 (14.9) | 26.0 (21.8) |
| Coarse + Ensemble 3 | 76.9 (14.7) | 26.3 (21.8) |
| Coarse + Ensemble 5 | 77.1 (14.4) | 26.4 (21.8) |
| Coarse + Ensemble 7 | 77.2 (14.3) | 26.3 (21.7) |
| Coarse + Ensemble 9 | 77.2 (14.3) | 26.2 (21.8) |
| Myocardial Location | |||
|---|---|---|---|
| Metric | Base | Middle | Apex |
| Dice (%) | 75.1 (16.2) | 78.1 (13.0) | 77.9 (14.6) |
| HD (mm) | 36.1 (23.4) | 25.2 (21.2) | 17.4 (15.6) |
| Scar Volume (cm) | % Infarcted Myocardium Volume | |||||||
|---|---|---|---|---|---|---|---|---|
| Method | Value | BA Bias | p-Value | Value | BA Bias | p-Value | ||
| Manual | 25.7 (19.4) | 18.5 (12.7) | ||||||
| FWHM | 17.6 (12.9) | 0.937 | −8.1 (8.7) | 12.7 (8.1) | 0.934 | −5.8 (5.9) | ||
| GMM | 32.7 (17.2) | 0.807 | 7.1 (14.6) | 24.2 (11.0) | 0.777 | 5.6 (9.5) | ||
| Otsu | 39.3 (20.8) | 0.907 | 13.6 (10.1) | 28.6 (12.1) | 0.885 | 10.1 (6.6) | ||
| 1-SD | 44.0 (24.4) | 0.932 | 18.3 (10.8) | 32.3 (15.8) | 0.923 | 13.8 (7.2) | ||
| 2-SD | 28.6 (18.7) | 0.927 | 3.0 (9.4) | 21.1 (13.0) | 0.916 | 2.6 (6.4) | ||
| 3-SD | 18.4 (14.5) | 0.875 | −7.2 (12.6) | 13.7 (10.4) | 0.847 | −4.9 (7.7) | ||
| 4-SD | 11.2 (11.1) | 0.756 | −14.5 (15.3) | 8.4 (8.0) | 0.723 | −10.2 (9.4) | ||
| 5-SD | 6.4 (8.4) | 0.567 | −19.3 (17.0) | 4.8 (6.0) | 0.565 | −13.7 (10.6) | ||
| 6-SD | 3.7 (6.4) | 0.458 | −21.9 (17.7) | 2.8 (4.4) | 0.471 | −15.8 (11.3) | ||
| Proposed | 26.6 (18.5) | 0.945 | 1.0 (6.9) | 0.196 | 19.1 (11.0) | 0.945 | 0.5 (4.6) | 0.314 |
| FWHM | GMM | Otsu | 1-SD | 2-SD | 3-SD | 4-SD | 5-SD | 6-SD | Proposed |
|---|---|---|---|---|---|---|---|---|---|
| 18.8 * | 54.6 * | 57.7 * | 63.6 | 46.5 * | 27.9 * | 14.1 * | 5.7 * | 2.8 * | 66.9 |
| (23.0) | (37.1) | (35.0) | (36.4) | (38.9) | (34.0) | (25.1) | (14.6) | (9.1) | (40.5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de la Rosa, E.; Sidibé, D.; Decourselle, T.; Leclercq, T.; Cochet, A.; Lalande, A. Myocardial Infarction Quantification from Late Gadolinium Enhancement MRI Using Top-Hat Transforms and Neural Networks. Algorithms 2021, 14, 249. https://doi.org/10.3390/a14080249
de la Rosa E, Sidibé D, Decourselle T, Leclercq T, Cochet A, Lalande A. Myocardial Infarction Quantification from Late Gadolinium Enhancement MRI Using Top-Hat Transforms and Neural Networks. Algorithms. 2021; 14(8):249. https://doi.org/10.3390/a14080249
Chicago/Turabian Stylede la Rosa, Ezequiel, Désiré Sidibé, Thomas Decourselle, Thibault Leclercq, Alexandre Cochet, and Alain Lalande. 2021. "Myocardial Infarction Quantification from Late Gadolinium Enhancement MRI Using Top-Hat Transforms and Neural Networks" Algorithms 14, no. 8: 249. https://doi.org/10.3390/a14080249
APA Stylede la Rosa, E., Sidibé, D., Decourselle, T., Leclercq, T., Cochet, A., & Lalande, A. (2021). Myocardial Infarction Quantification from Late Gadolinium Enhancement MRI Using Top-Hat Transforms and Neural Networks. Algorithms, 14(8), 249. https://doi.org/10.3390/a14080249







