Estimating the Frequencies of Maximal Theta-Gamma Coupling in EEG during the N-Back Task: Sensitivity to Methodology and Temporal Instability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Benchmark LFP Data
2.2. Participants
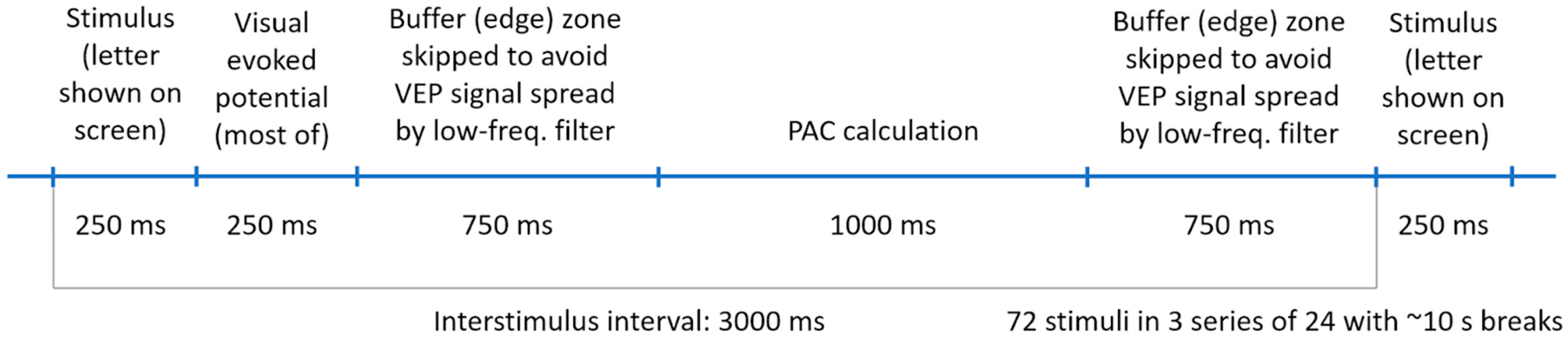
2.3. 3-Back Task
2.4. EEG Recording
2.5. EEG Preprocessing
2.6. Spectrum Calculation
2.7. TGC Computation
2.7.1. Steps of PAC Calculation and Variations in Them
- Selection of electrodes;
- Extraction of oscillations for phase and amplitude;
- Selection of epochs (time intervals) for PAC calculation (optional);
- Calculation of a PAC measure;
- Normalization of the PAC measure (optional);
- Averaging the PAC measure over subsets of the data (optional).
2.7.2. Selection of Electrodes
2.7.3. Extraction of Oscillations for Phase and Amplitude
Band-Pass Filters: Filter Type
Band-Pass Filters: Band Definition
Wavelet Transform
2.7.4. Selection of Epochs for PAC Calculation
2.7.5. Calculation of a PAC Measure
2.7.6. Normalization of the PAC Measure
2.7.7. Averaging the PAC Measure over Subsets of the Data
2.7.8. Algorithms Compared in This Study
Algorithms for Benchmark LFP Data
Algorithms for the EEG Data with the 3-Back Task
2.8. Reproducibility between Measurements: Characteristics and Statistical Tests
3. Results
3.1. Benchmark LFP Data
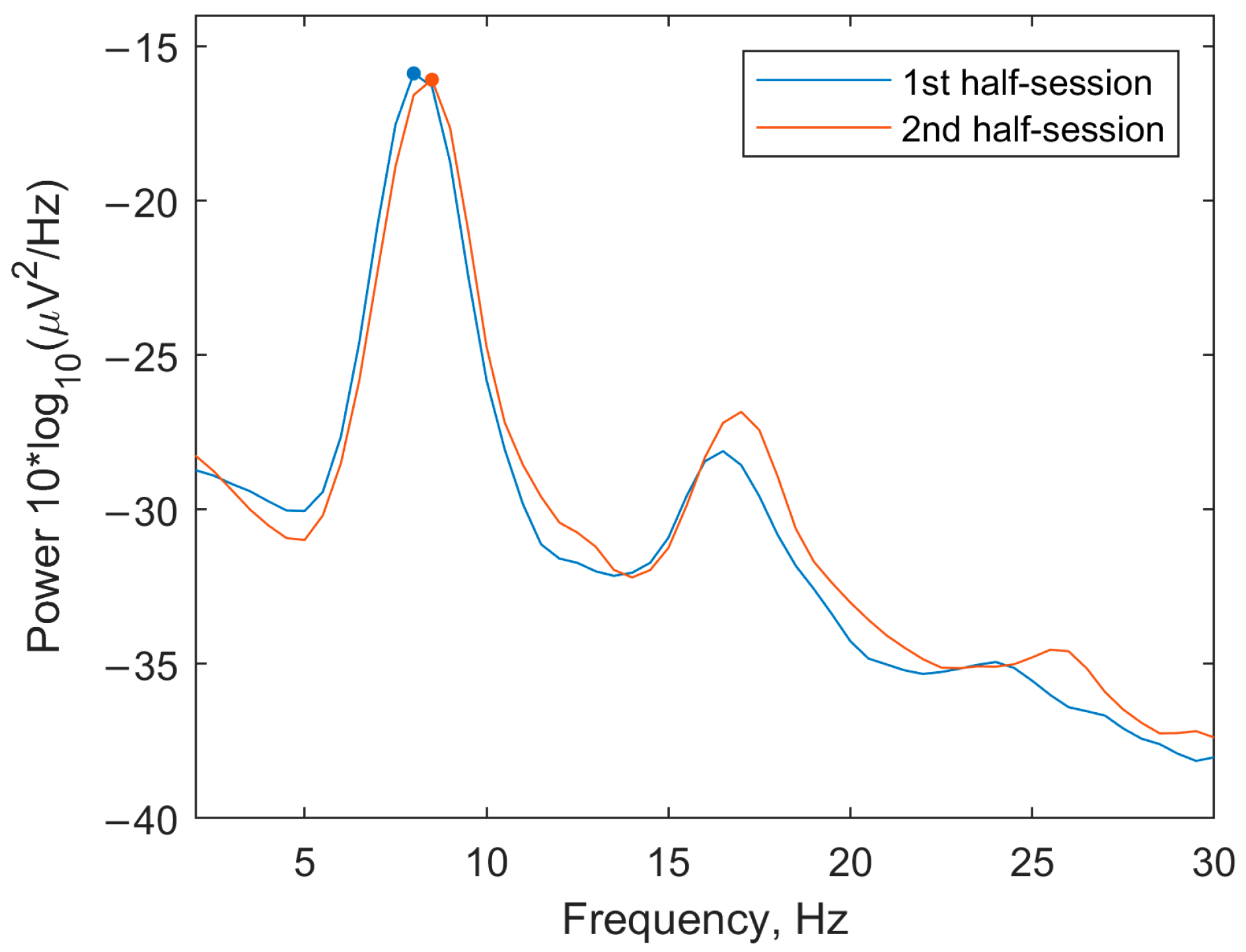
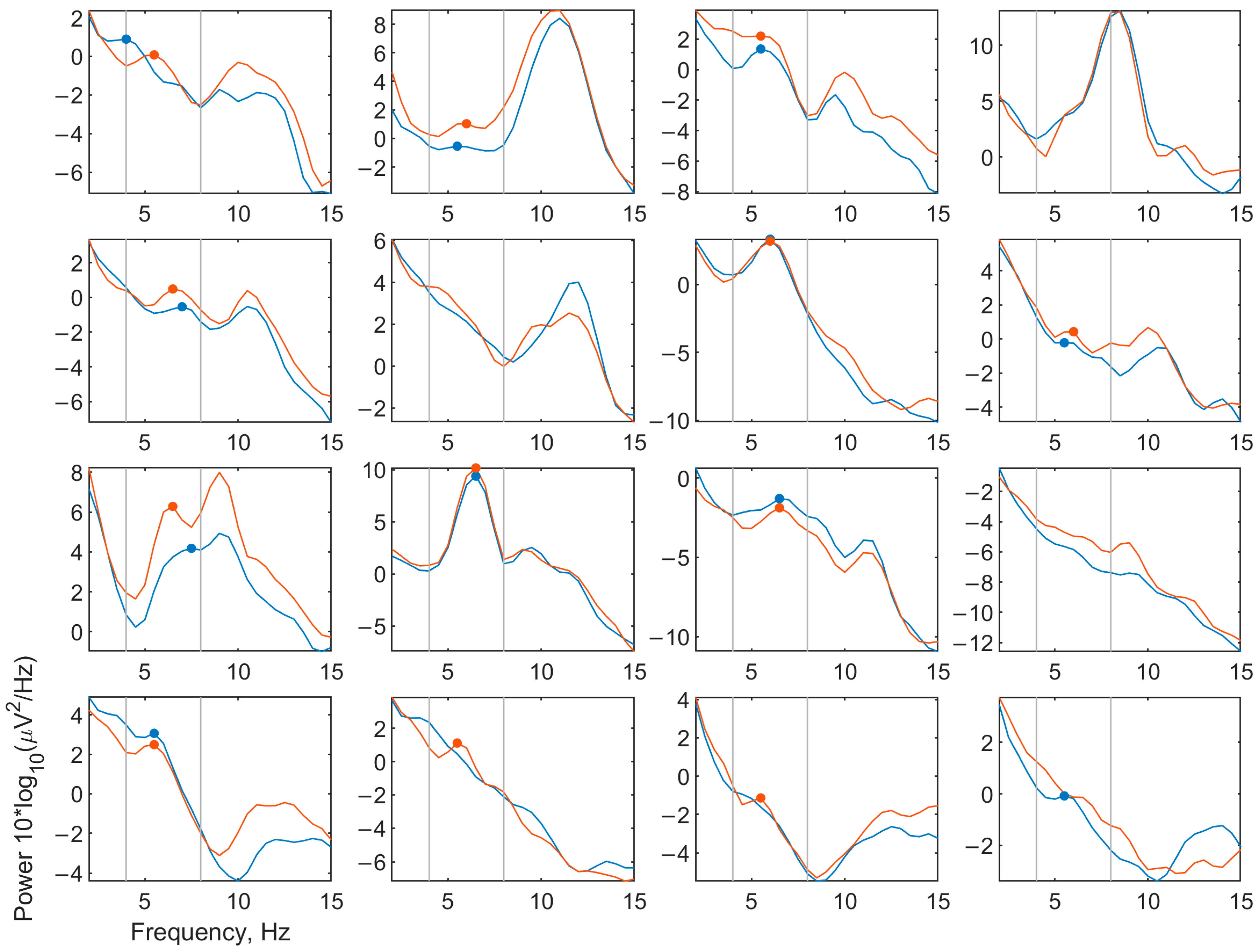
3.1.1. Spectra
3.1.2. Comodulogram Dependence on the Algorithm
3.1.3. Comodulogram and Peak Variability between Half-Sessions
3.2. EEG during the 3-Back Task
3.2.1. Behavioral Statistics for the 3-Back Task
3.2.2. EEG Spectra
3.2.3. Length of EEG for PAC Calculation
3.2.4. Comodulogram Dependence on the Algorithm
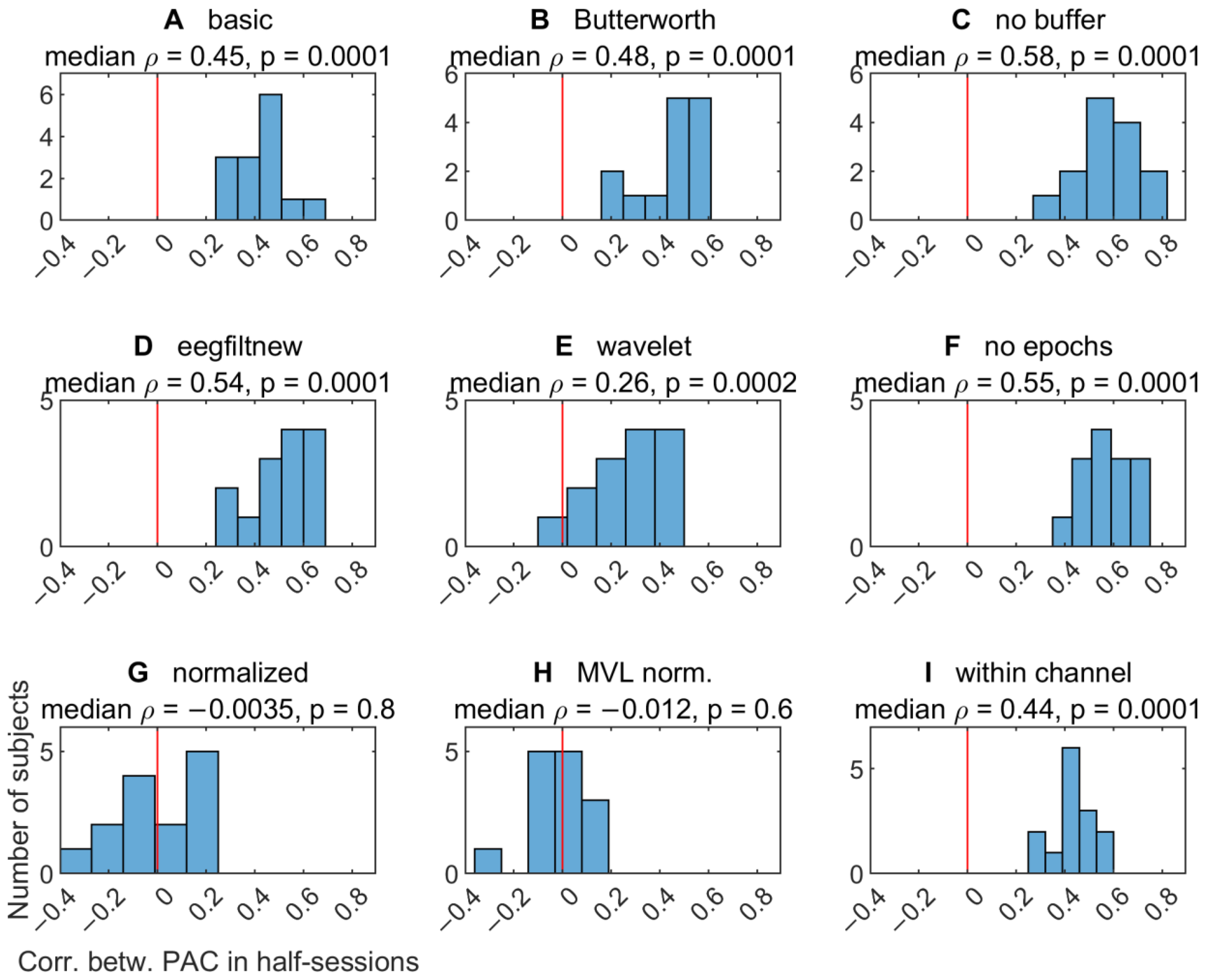
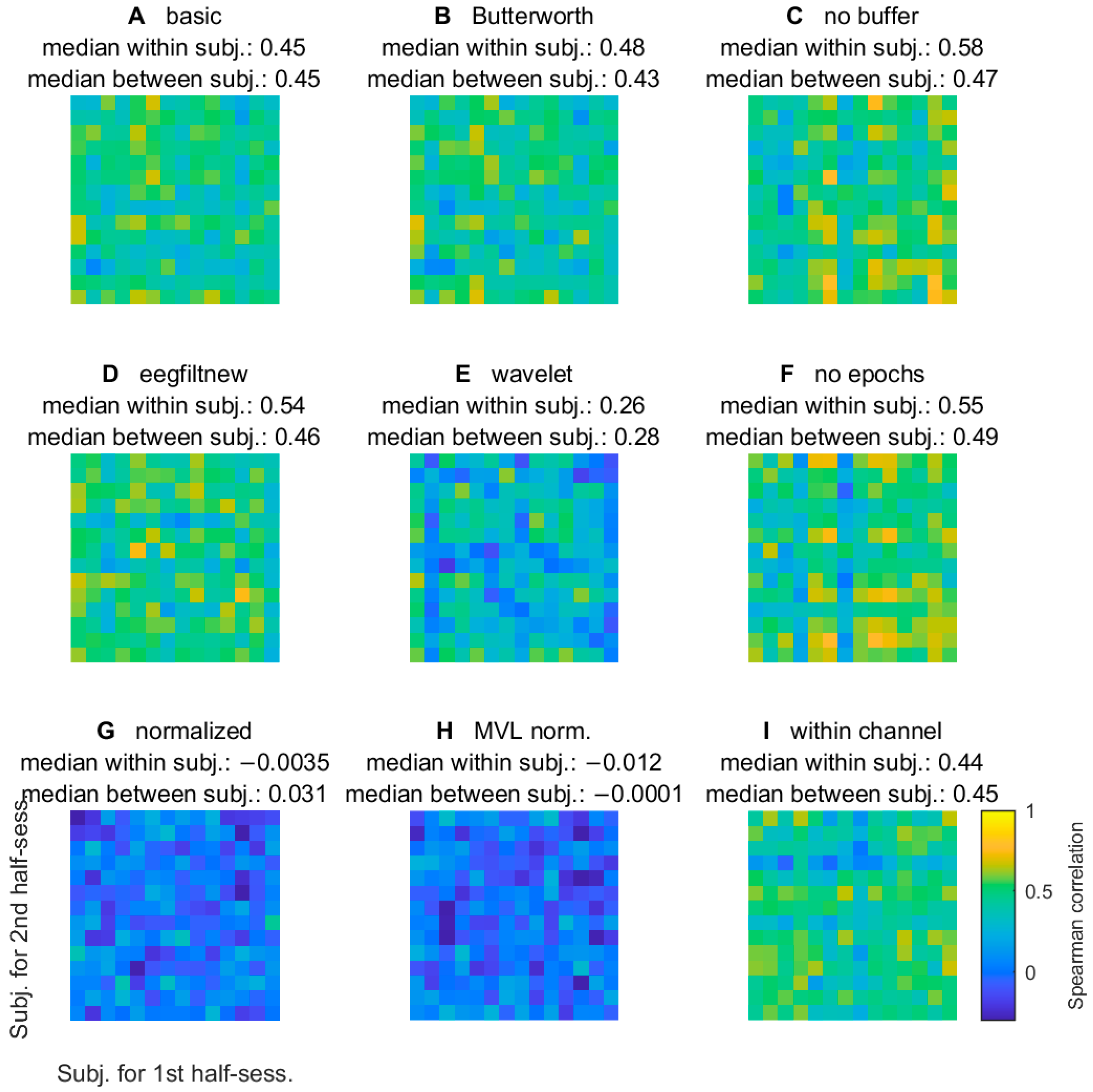
3.2.5. Comodulogram Variability between Half-Sessions
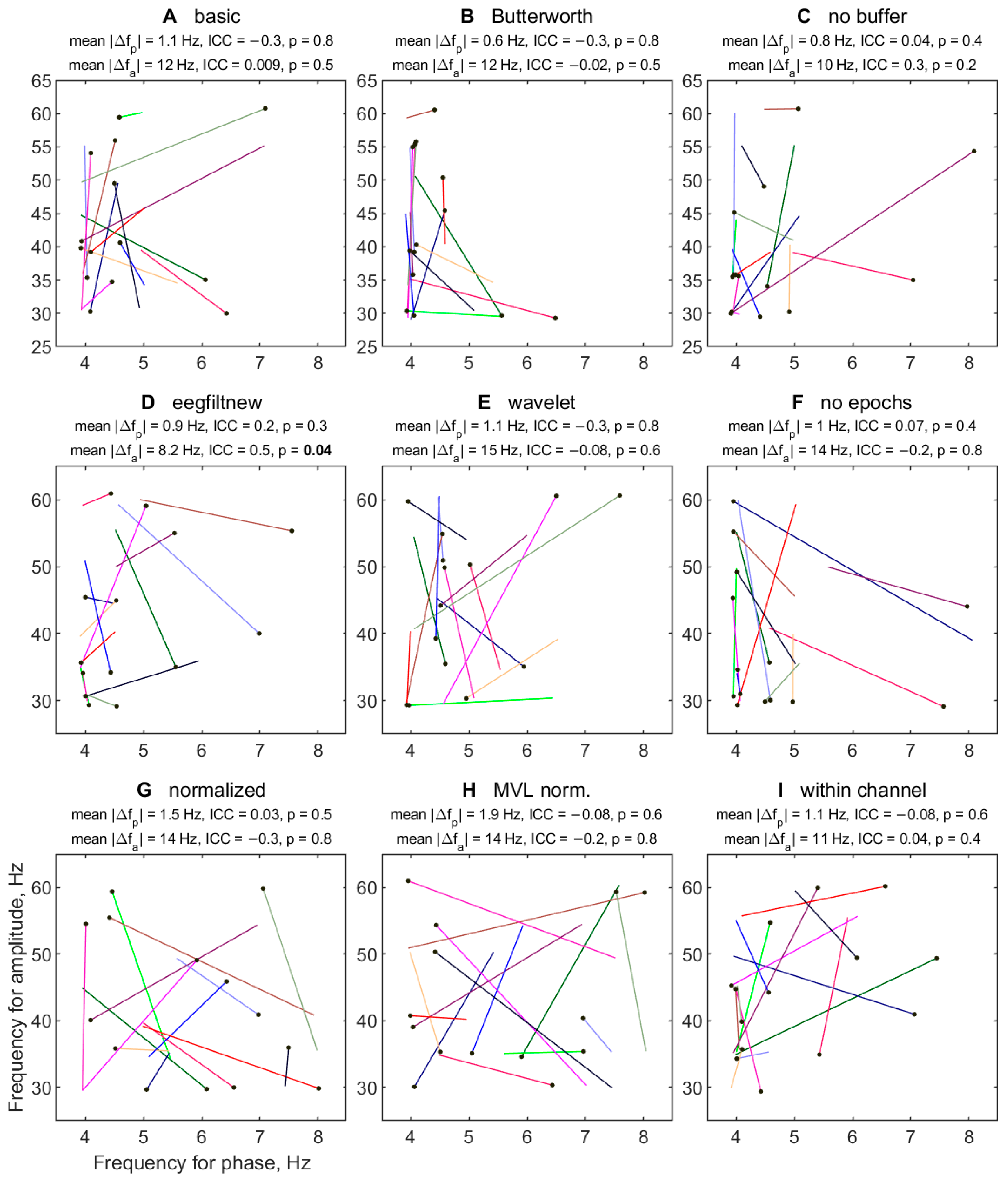
3.2.6. Variability of TGC Peak Frequencies between Half-Sessions
3.2.7. Effect of Half-Session on TGC Peak Frequencies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Buzsáki, G.; Watson, B.O. Brain Rhythms and Neural Syntax: Implications for Efficient Coding of Cognitive Content and Neuropsychiatric Disease. Dialogues Clin. Neurosci. 2012, 14, 345–367. [Google Scholar] [CrossRef] [PubMed]
- Herweg, N.A.; Solomon, E.A.; Kahana, M.J. Theta Oscillations in Human Memory. Trends Cogn. Sci. 2020, 24, 208–227. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Schmitt, O.; Ip, Z.; Rabiller, G.; Omodaka, S.; Tominaga, T.; Yazdan-Shahmorad, A.; Liu, J. Pathological Changes of Brain Oscillations Following Ischemic Stroke. J. Cereb. Blood Flow Metab. 2022, 42, 1753–1776. [Google Scholar] [CrossRef] [PubMed]
- Giustiniani, A.; Danesin, L.; Bozzetto, B.; Macina, A.; Benavides-Varela, S.; Burgio, F. Functional Changes in Brain Oscillations in Dementia: A Review. Rev. Neurosci. 2023, 34, 25–47. [Google Scholar] [CrossRef] [PubMed]
- Bragin, A.; Jandó, G.; Nádasdy, Z.; Hetke, J.; Wise, K.; Buzsáki, G. Gamma (40–100 Hz) Oscillation in the Hippocampus of the Behaving Rat. J. Neurosci. 1995, 15, 47–60. [Google Scholar] [CrossRef]
- Lakatos, P.; Shah, A.S.; Knuth, K.H.; Ulbert, I.; Karmos, G.; Schroeder, C.E. An Oscillatory Hierarchy Controlling Neuronal Excitability and Stimulus Processing in the Auditory Cortex. J. Neurophysiol. 2005, 94, 1904–1911. [Google Scholar] [CrossRef]
- Canolty, R.T.; Edwards, E.; Dalal, S.S.; Soltani, M.; Nagarajan, S.S.; Kirsch, H.E.; Berger, M.S.; Barbaro, N.M.; Knight, R.T. High Gamma Power Is Phase-Locked to Theta Oscillations in Human Neocortex. Science (1979) 2006, 313, 1626–1628. [Google Scholar] [CrossRef]
- Park, J.Y.; Lee, Y.-R.; Lee, J. The Relationship between Theta-Gamma Coupling and Spatial Memory Ability in Older Adults. Neurosci. Lett. 2011, 498, 37–41. [Google Scholar] [CrossRef]
- Abubaker, M.; Al Qasem, W.; Kvašňák, E. Working Memory and Cross-Frequency Coupling of Neuronal Oscillations. Front. Psychol. 2021, 12, 756661. [Google Scholar] [CrossRef]
- Fell, J.; Axmacher, N. The Role of Phase Synchronization in Memory Processes. Nat. Rev. Neurosci. 2011, 12, 105–118. [Google Scholar] [CrossRef]
- Hirano, S.; Nakhnikian, A.; Hirano, Y.; Oribe, N.; Kanba, S.; Onitsuka, T.; Levin, M.; Spencer, K.M. Phase-Amplitude Coupling of the Electroencephalogram in the Auditory Cortex in Schizophrenia. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2018, 3, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Brooks, H.; Goodman, M.S.; Bowie, C.R.; Zomorrodi, R.; Blumberger, D.M.; Butters, M.A.; Daskalakis, Z.J.; Fischer, C.E.; Flint, A.; Herrmann, N.; et al. Theta–Gamma Coupling and Ordering Information: A Stable Brain–Behavior Relationship across Cognitive Tasks and Clinical Conditions. Neuropsychopharmacology 2020, 45, 2038–2047. [Google Scholar] [CrossRef] [PubMed]
- Lisman, J.E.; Idiart, M.A.P. Storage of 7 ± 2 Short-Term Memories in Oscillatory Subcycles. Science (1979) 1995, 267, 1512–1515. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, L.; Idiart, M.; Villavicencio, A.; Lisman, J. Alternating Predictive and Short-Term Memory Modes of Entorhinal Grid Cells. Hippocampus 2012, 22, 1647–1651. [Google Scholar] [CrossRef] [PubMed]
- Lisman, J.E.; Jensen, O. The Theta-Gamma Neural Code. Neuron 2013, 77, 1002–1016. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.A. The Magical Number Seven, plus or Minus Two: Some Limits on Our Capacity for Processing Information. Psychol. Rev. 1956, 63, 81–97. [Google Scholar] [CrossRef] [PubMed]
- Kamiński, J.; Brzezicka, A.; Wróbel, A. Short-Term Memory Capacity (7±2) Predicted by Theta to Gamma Cycle Length Ratio. Neurobiol. Learn. Mem. 2011, 95, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Malenínská, K.; Rudolfová, V.; Šulcová, K.; Koudelka, V.; Brunovský, M.; Horáček, J.; Nekovářová, T. Is Short-Term Memory Capacity (7 ± 2) Really Predicted by Theta to Gamma Cycle Length Ratio? Behav. Brain Res. 2021, 414, 113465. [Google Scholar] [CrossRef]
- Poydasheva, A.G.; Bakulin, I.S.; Sinitsyn, D.O.; Zabirova, A.H.; Lagoda, D.Y.; Suponeva, N.A.; Piradov, M.A. Theta-Gamma Phase-Amplitude Coupling: Physiological Basics, Analysis Methods, and Perspectives of Translation into Clinical Practice. Ann. Clin. Exp. Neurol. 2022, 16, 71–79. [Google Scholar] [CrossRef]
- Vosskuhl, J.; Huster, R.J.; Herrmann, C.S. Increase in Short-Term Memory Capacity Induced by down-Regulating Individual Theta Frequency via Transcranial Alternating Current Stimulation. Front. Hum. Neurosci. 2015, 9, 257. [Google Scholar] [CrossRef]
- Chung, S.W.; Sullivan, C.M.; Rogasch, N.C.; Hoy, K.E.; Bailey, N.W.; Cash, R.F.H.; Fitzgerald, P.B. The Effects of Individualised Intermittent Theta Burst Stimulation in the Prefrontal Cortex: A TMS-EEG Study. Hum. Brain Mapp. 2019, 40, 608–627. [Google Scholar] [CrossRef] [PubMed]
- Penny, W.D.; Duzel, E.; Miller, K.J.; Ojemann, J.G. Testing for Nested Oscillation. J. Neurosci. Methods 2008, 174, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Tort, A.B.L.; Komorowski, R.; Eichenbaum, H.; Kopell, N. Measuring Phase-Amplitude Coupling Between Neuronal Oscillations of Different Frequencies. J. Neurophysiol. 2010, 104, 1195–1210. [Google Scholar] [CrossRef]
- Dvorak, D.; Fenton, A.A. Toward a Proper Estimation of Phase–Amplitude Coupling in Neural Oscillations. J. Neurosci. Methods 2014, 225, 42–56. [Google Scholar] [CrossRef] [PubMed]
- Aru, J.; Aru, J.; Priesemann, V.; Wibral, M.; Lana, L.; Pipa, G.; Singer, W.; Vicente, R. Untangling Cross-Frequency Coupling in Neuroscience. Curr. Opin. Neurobiol. 2015, 31, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Seymour, R.A.; Rippon, G.; Kessler, K. The Detection of Phase Amplitude Coupling during Sensory Processing. Front. Neurosci. 2017, 11, 487. [Google Scholar] [CrossRef] [PubMed]
- Hülsemann, M.J.; Naumann, E.; Rasch, B. Quantification of Phase-Amplitude Coupling in Neuronal Oscillations: Comparison of Phase-Locking Value, Mean Vector Length, Modulation Index, and Generalized-Linear-Modeling-Cross-Frequency-Coupling. Front. Neurosci. 2019, 13, 573. [Google Scholar] [CrossRef]
- Caiola, M.; Devergnas, A.; Holmes, M.H.; Wichmann, T. Empirical Analysis of Phase-Amplitude Coupling Approaches. PLoS ONE 2019, 14, e0219264. [Google Scholar] [CrossRef]
- Combrisson, E.; Nest, T.; Brovelli, A.; Ince, R.A.A.; Soto, J.L.P.; Guillot, A.; Jerbi, K. Tensorpac: An Open-Source Python Toolbox for Tensor-Based Phase-Amplitude Coupling Measurement in Electrophysiological Brain Signals. PLoS Comput. Biol. 2020, 16, e1008302. [Google Scholar] [CrossRef]
- Matlab Routines for Computing the Modulation Index and Comodulogram, as Described in Tort et al., J Neurophysiol 2010. Available online: https://github.com/tortlab/phase-amplitude-coupling (accessed on 2 October 2023).
- Scheffer-Teixeira, R.; Belchior, H.; Caixeta, F.V.; Souza, B.C.; Ribeiro, S.; Tort, A.B.L. Theta Phase Modulates Multiple Layer-Specific Oscillations in the CA1 Region. Cerebral Cortex 2012, 22, 2404–2414. [Google Scholar] [CrossRef]
- Delorme, A.; Makeig, S. EEGLAB: An Open Source Toolbox for Analysis of Single-Trial EEG Dynamics Including Independent Component Analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Friese, U.; Köster, M.; Hassler, U.; Martens, U.; Trujillo-Barreto, N.; Gruber, T. Successful Memory Encoding Is Associated with Increased Cross-Frequency Coupling between Frontal Theta and Posterior Gamma Oscillations in Human Scalp-Recorded EEG. Neuroimage 2013, 66, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Köster, M.; Friese, U.; Schöne, B.; Trujillo-Barreto, N.; Gruber, T. Theta-Gamma Coupling during Episodic Retrieval in the Human EEG. Brain Res. 2014, 1577, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Musaeus, C.S.; Nielsen, M.S.; Musaeus, J.S.; Høgh, P. Electroencephalographic Cross-Frequency Coupling as a Sign of Disease Progression in Patients With Mild Cognitive Impairment: A Pilot Study. Front. Neurosci. 2020, 14, 790. [Google Scholar] [CrossRef] [PubMed]
- Gong, R.; Wegscheider, M.; Mühlberg, C.; Gast, R.; Fricke, C.; Rumpf, J.-J.; Nikulin, V.V.; Knösche, T.R.; Classen, J. Spatiotemporal Features of β-γ Phase-Amplitude Coupling in Parkinson’s Disease Derived from Scalp EEG. Brain 2021, 144, 487–503. [Google Scholar] [CrossRef] [PubMed]
- Rajji, T.K.; Zomorrodi, R.; Barr, M.S.; Blumberger, D.M.; Mulsant, B.H.; Daskalakis, Z.J. Ordering Information in Working Memory and Modulation of Gamma by Theta Oscillations in Humans. Cereb. Cortex 2017, 27, 1482–1490. [Google Scholar] [CrossRef]
- Goodman, M.S.; Kumar, S.; Zomorrodi, R.; Ghazala, Z.; Cheam, A.S.M.; Barr, M.S.; Daskalakis, Z.J.; Blumberger, D.M.; Fischer, C.; Flint, A.; et al. Theta-Gamma Coupling and Working Memory in Alzheimer’s Dementia and Mild Cognitive Impairment. Front. Aging Neurosci. 2018, 10, 101. [Google Scholar] [CrossRef]
- Rustamov, N.; Humphries, J.; Carter, A.; Leuthardt, E.C. Theta-Gamma Coupling as a Cortical Biomarker of Brain-Computer Interface-Mediated Motor Recovery in Chronic Stroke. Brain Commun. 2022, 4, fcac136. [Google Scholar] [CrossRef]
- Park, J.Y.; Jhung, K.; Lee, J.; An, S.K. Theta-Gamma Coupling during a Working Memory Task as Compared to a Simple Vigilance Task. Neurosci. Lett. 2013, 532, 39–43. [Google Scholar] [CrossRef]
- Sun, Y.; Giacobbe, P.; Tang, C.W.; Barr, M.S.; Rajji, T.; Kennedy, S.H.; Fitzgerald, P.B.; Lozano, A.M.; Wong, W.; Daskalakis, Z.J. Deep Brain Stimulation Modulates Gamma Oscillations and Theta-Gamma Coupling in Treatment Resistant Depression. Brain Stimul. 2015, 8, 1033–1042. [Google Scholar] [CrossRef]
- Köster, M.; Finger, H.; Graetz, S.; Kater, M.; Gruber, T. Theta-Gamma Coupling Binds Visual Perceptual Features in an Associative Memory Task. Sci. Rep. 2018, 8, 17688. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.T.; Johnson, E.L.; Berryhill, M.E. Frontoparietal Theta-Gamma Interactions Track Working Memory Enhancement with Training and TDCS. Neuroimage 2020, 211, 116615. [Google Scholar] [CrossRef] [PubMed]
- Swann, N.C.; De Hemptinne, C.; Aron, A.R.; Ostrem, J.L.; Knight, R.T.; Starr, P.A. Elevated Synchrony in Parkinson Disease Detected with Electroencephalography. Ann. Neurol. 2015, 78, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Hammer, M.; Schwale, C.; Brankačk, J.; Draguhn, A.; Tort, A.B.L. Theta-Gamma Coupling during REM Sleep Depends on Breathing Rate. Sleep 2021, 44, zsab189. [Google Scholar] [CrossRef] [PubMed]
- Miyakoshi, M.; Delorme, A.; Mullen, T.; Kojima, K.; Makeig, S.; Asano, E. Automated Detection of Cross-Frequency Coupling in the Electrocorticogram for Clinical Inspection. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; pp. 3282–3285. [Google Scholar]
- Attaheri, A.; Choisdealbha, N.; Di Liberto, G.M.; Rocha, S.; Brusini, P.; Mead, N.; Olawole-Scott, H.; Boutris, P.; Gibbon, S.; Williams, I.; et al. Delta- and Theta-Band Cortical Tracking and Phase-Amplitude Coupling to Sung Speech by Infants. Neuroimage 2022, 247, 118698. [Google Scholar] [CrossRef] [PubMed]
- Widmann, A.; Schröger, E.; Maess, B. Digital Filter Design for Electrophysiological Data-a Practical Approach. J. Neurosci. Methods 2015, 250, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Dupré la Tour, T.; Tallot, L.; Grabot, L.; Doyère, V.; van Wassenhove, V.; Grenier, Y.; Gramfort, A. Non-Linear Auto-Regressive Models for Cross-Frequency Coupling in Neural Time Series. PLoS Comput. Biol. 2017, 13, e1005893. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.X. Analyzing Neural Time Series Data: Theory and Practice; MIT Press: Cambridge, MA, USA, 2014. [Google Scholar]
- Axmacher, N.; Henseler, M.M.; Jensen, O.; Weinreich, I.; Elger, C.E.; Fell, J. Cross-Frequency Coupling Supports Multi-Item Working Memory in the Human Hippocampus. Proc. Natl. Acad. Sci. USA 2010, 107, 3228–3233. [Google Scholar] [CrossRef]
- Papaioannou, O.; Crespo, L.P.; Clark, K.; Ogbuagu, N.N.; Alliende, L.M.; Silverstein, S.M.; Erickson, M.A. Is Cortical Theta-Gamma Phase-Amplitude Coupling Memory-Specific? Brain Sci. 2022, 12, 1131. [Google Scholar] [CrossRef]
- Tallon-Baudry, C.; Bertrand, O.; Delpuech, C.; Pernier, J. Oscillatory γ-Band (30–70 Hz) Activity Induced by a Visual Search Task in Humans. J. Neurosci. 1997, 17, 722–734. [Google Scholar] [CrossRef]
- Martinez-Cancino, R.; Delorme, A.; Kreutz-Delgado, K.; Makeig, S. Computing Phase Amplitude Coupling in EEGLAB: PACTools. In Proceedings of the IEEE 20th International Conference on Bioinformatics and Bioengineering, BIBE 2020, Cincinnati, OH, USA, 26–28 October 2020. [Google Scholar]
- Özkurt, T.E.; Schnitzler, A. A Critical Note on the Definition of Phase-Amplitude Cross-Frequency Coupling. J. Neurosci. Methods 2011, 201, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Heninger, G.R.; Mcdonald, R.K.; Goff, W.R.; Sollberger, A. Diurnal Variations in the Cerebral Evoked Response and EEG: Relations to 17-Hydroxycorticosteroid Levels. Arch. Neurol. 1969, 21, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, K.A.; McArthur, G.M.; Bishop, D.V.M. Is Discrimination Training Necessary to Cause Changes in the P2 Auditory Event-Related Brain Potential to Speech Sounds? Cogn. Brain Res. 2005, 25, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Bhangal, S.; Sharma, S.; Valle-Inclán, F.; Ren, X.; Hackley, S.A. Learning to Deal with Delayed Outcomes: EEG Oscillatory and Slow Potentials during the Prefeedback Interval. Psychophysiology 2021, 58, e13853. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, R.; Wood, G.; Nisar, H.; Yap, V.V. Exploring the Effects of EEG-Based Alpha Neurofeedback on Working Memory Capacity in Healthy Participants. Bioengineering 2023, 10, 200. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, A.; Akbar, A.; Wang, M.; Marks, R.L.; Yu, K.; Park, S.; Foster, B.L.; Noebels, J.L. Persistent Aberrant Cortical Phase–Amplitude Coupling Following Seizure Treatment in Absence Epilepsy Models. J. Physiol. 2017, 595, 7249–7260. [Google Scholar] [CrossRef]
- McGraw, K.O.; Wong, S.P. Forming Inferences about Some Intraclass Correlation Coefficients. Psychol. Methods 1996, 1, 30–46. [Google Scholar] [CrossRef]
- Salarian, A. Intraclass Correlation Coefficient (ICC), MATLAB Central File Exchange. 2023. Available online: https://www.mathworks.com/matlabcentral/fileexchange/22099-intraclass-correlation-coefficient-icc (accessed on 3 October 2023).
- Näpflin, M.; Wildi, M.; Sarnthein, J. Test-Retest Reliability of EEG Spectra during a Working Memory Task. Neuroimage 2008, 43, 687–693. [Google Scholar] [CrossRef]
- Gordon, P.C.; Belardinelli, P.; Stenroos, M.; Ziemann, U.; Zrenner, C. Prefrontal Theta Phase-Dependent RTMS-Induced Plasticity of Cortical and Behavioral Responses in Human Cortex. Brain Stimul. 2022, 15, 391–402. [Google Scholar] [CrossRef]
- Zrenner, C.; Ziemann, U. Closed-Loop Stimulation. Biol. Psychiatry 2023, in press. [Google Scholar] [CrossRef]
- Jia, Z.; Lin, Y.; Wang, J.; Ning, X.; He, Y.; Zhou, R.; Zhou, Y.; Lehman, L.H. Multi-View Spatial-Temporal Graph Convolutional Networks With Domain Generalization for Sleep Stage Classification. IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 29, 1977–1986. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Cai, X.; Jiao, Z. Multi-Modal Physiological Signals Based Squeeze-and-Excitation Network With Domain Adversarial Learning for Sleep Staging. IEEE Sens. J. 2022, 22, 3464–3471. [Google Scholar] [CrossRef]
- Jia, Z.; Ji, J.; Zhou, X.; Zhou, Y. Hybrid Spiking Neural Network for Sleep Electroencephalogram Signals. Sci. China Inf. Sci. 2022, 65, 140403. [Google Scholar] [CrossRef]
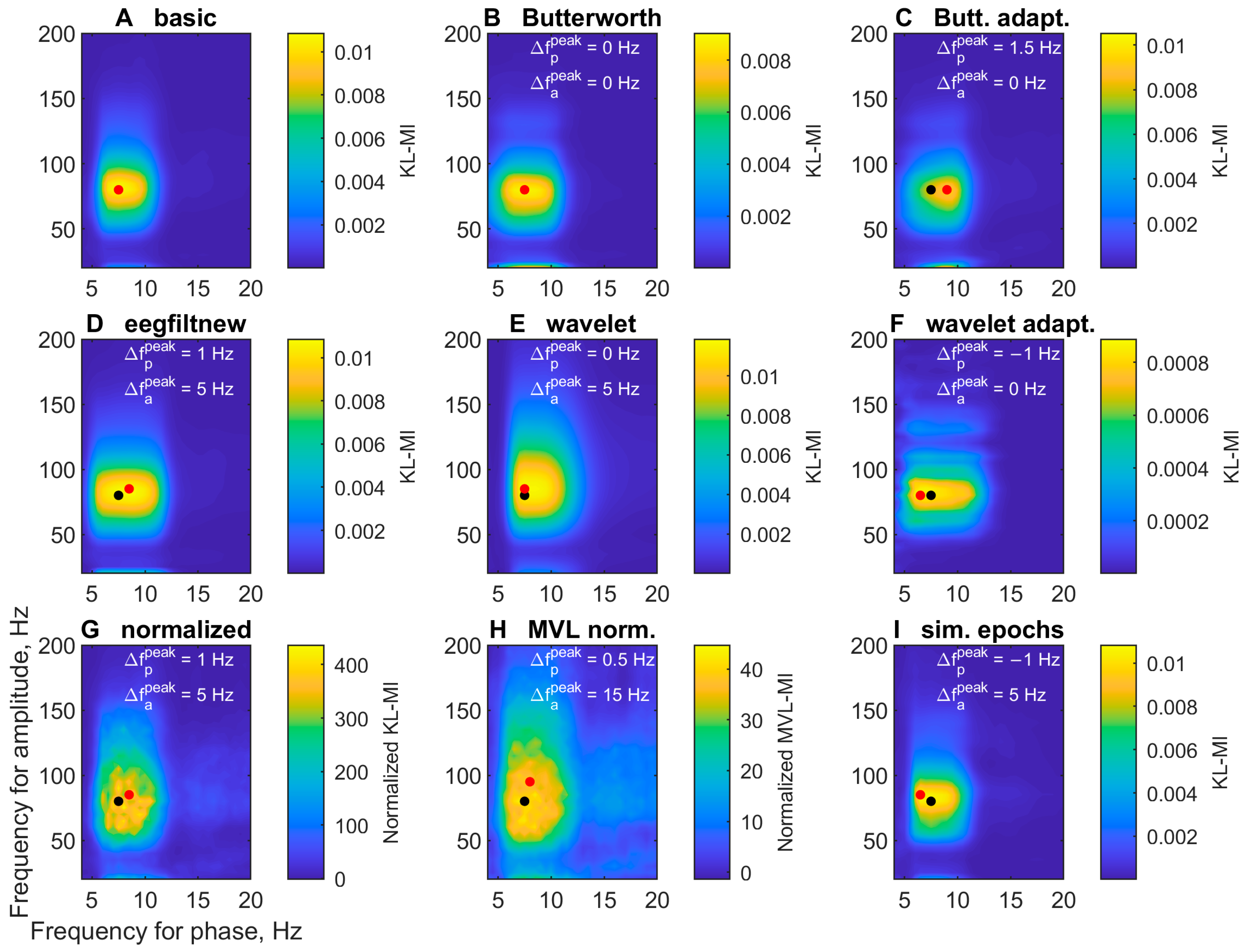
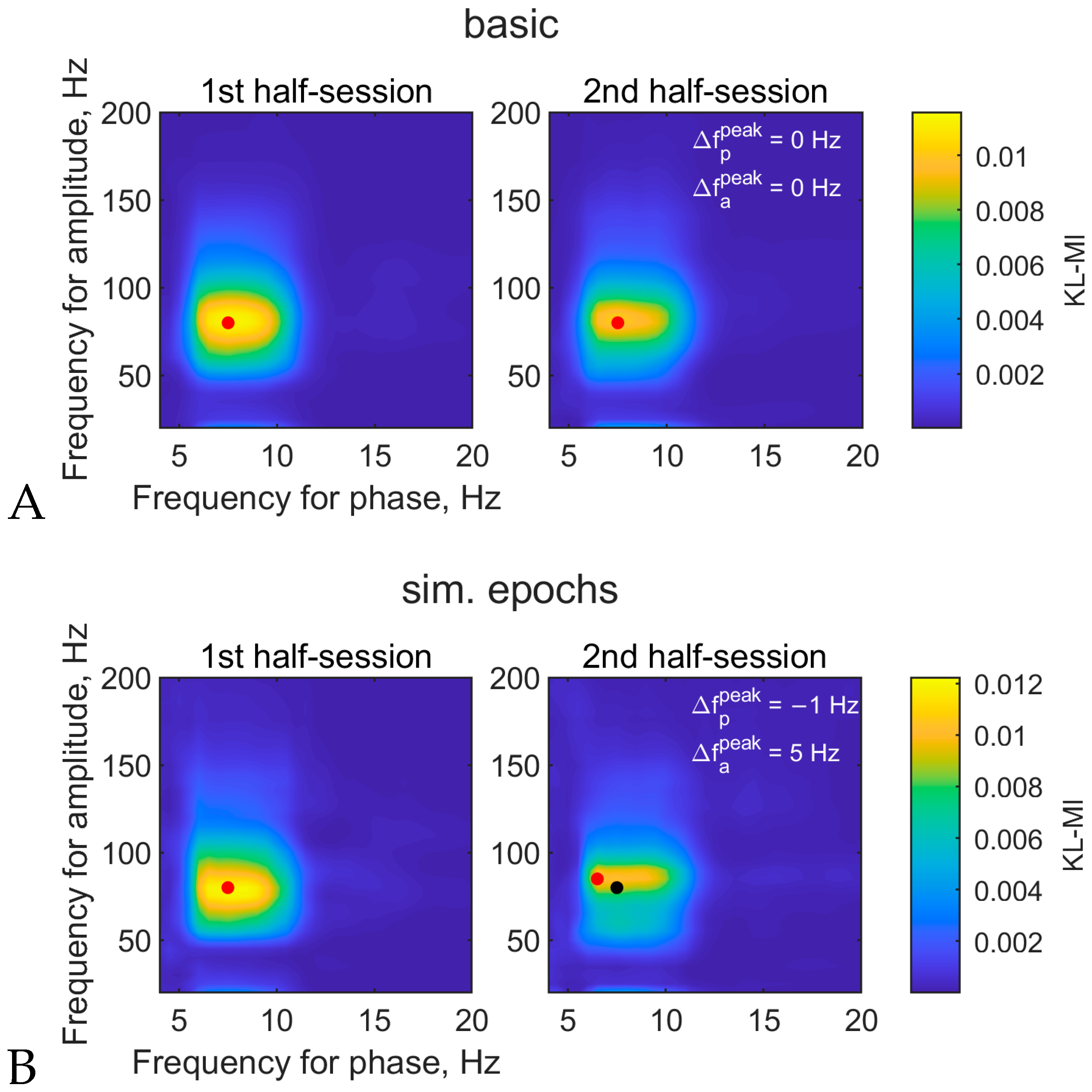
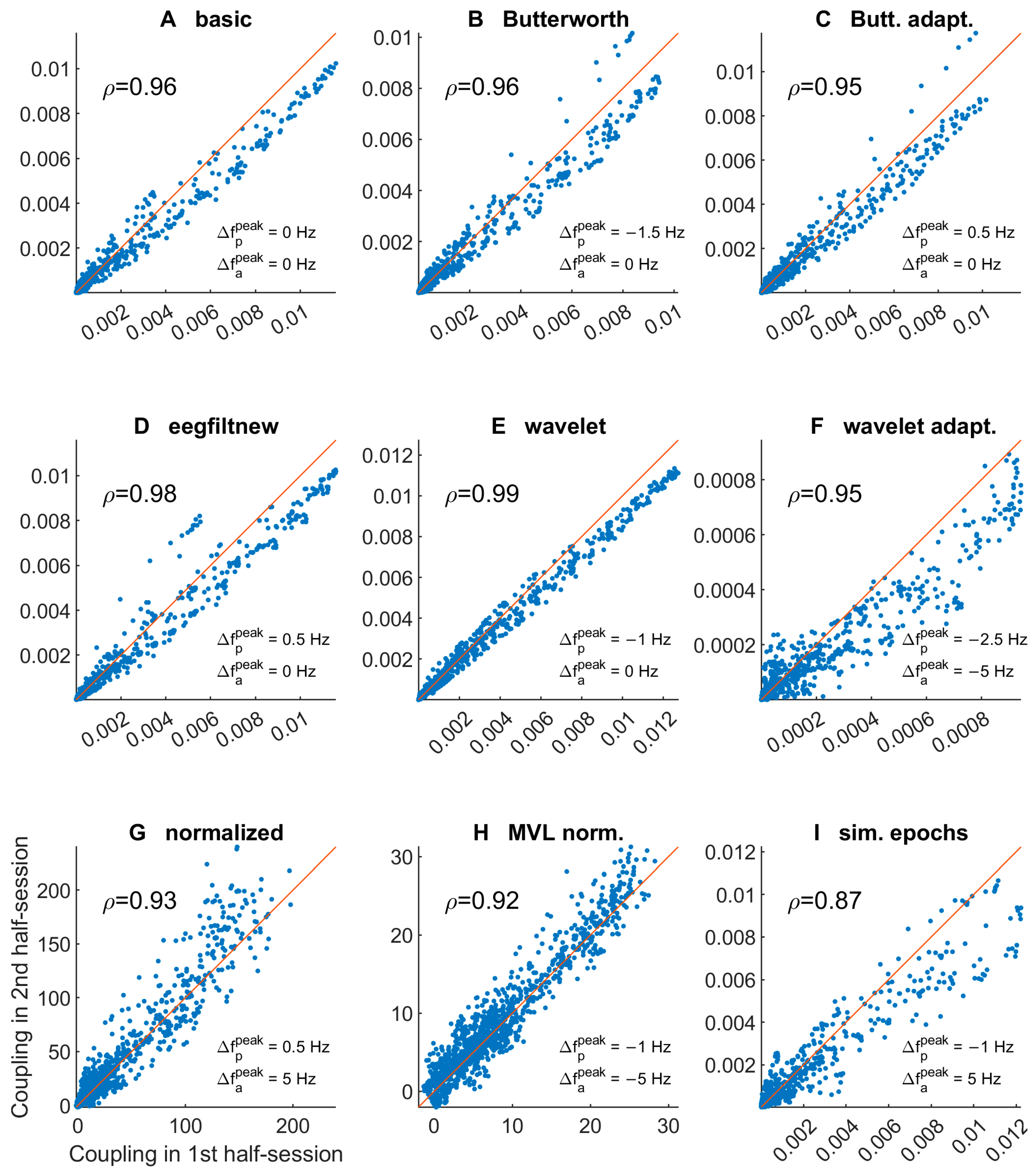
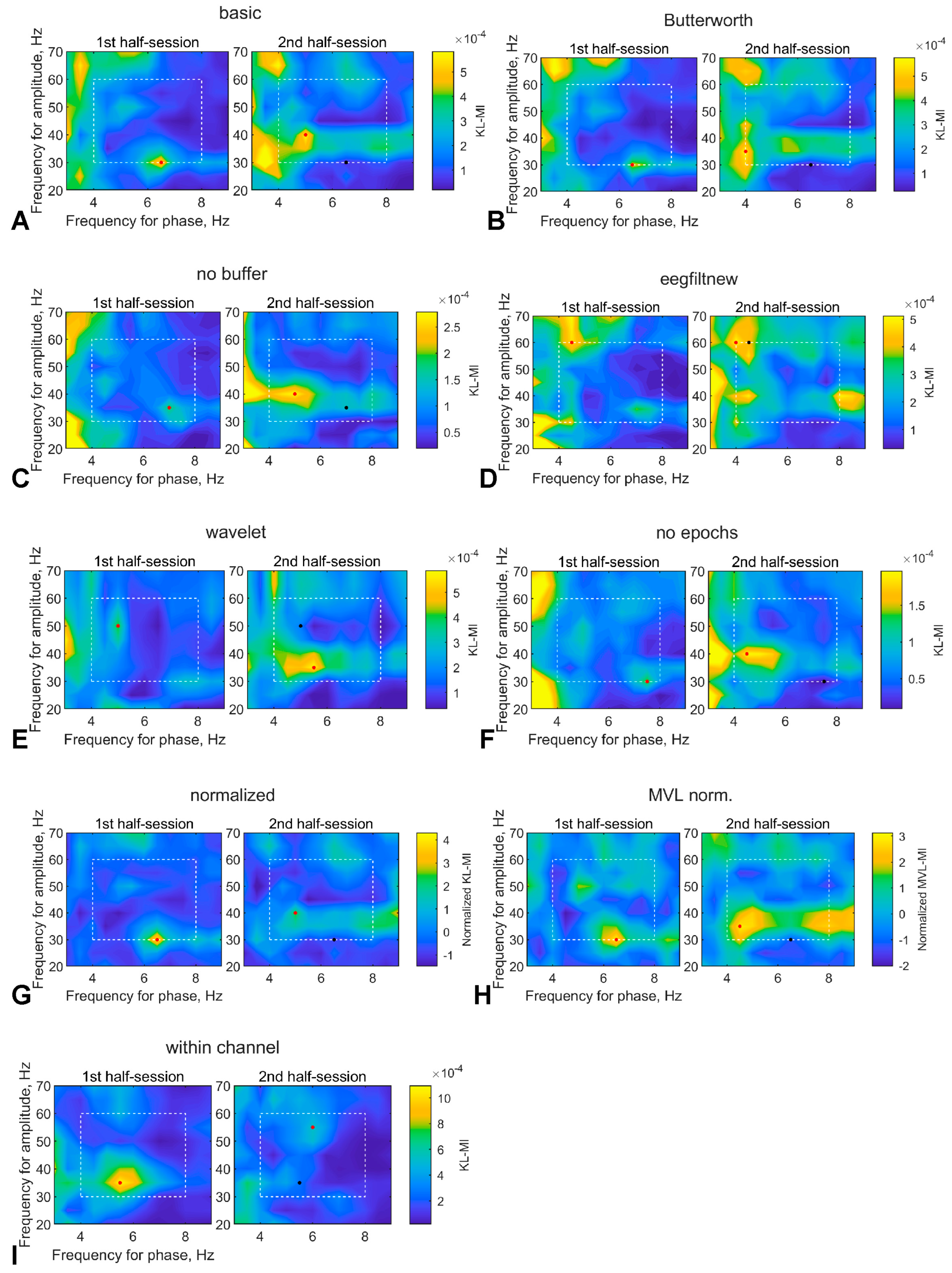
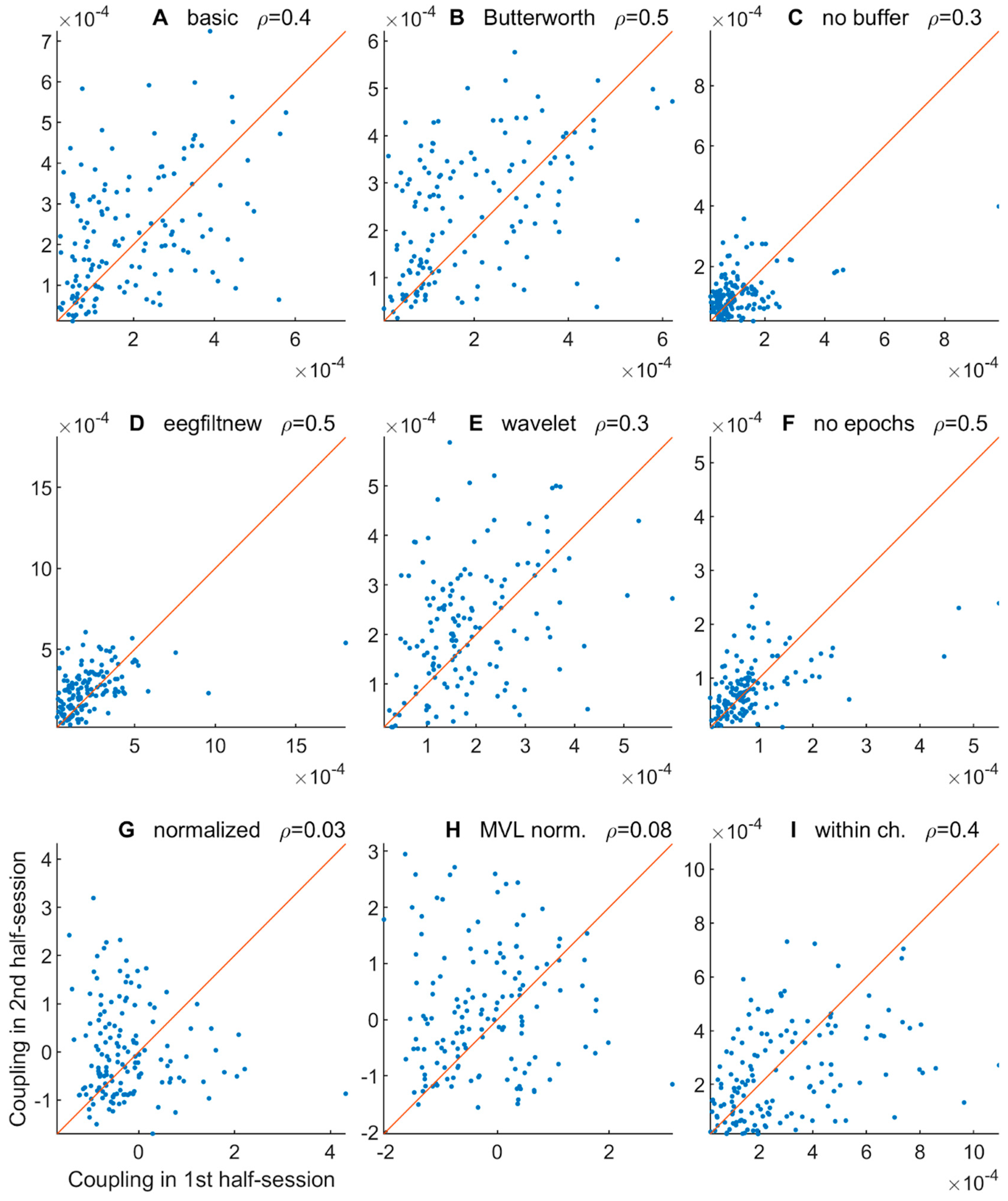
| Letter in Figures | Short Algorithm Name | Extraction of Oscillations | Width Parameters (Phase, Amplitude) | Epoch Selection | PAC Measure | Normalization |
|---|---|---|---|---|---|---|
| A | basic | two-way FIR filter (eegfilt) | 4, 20 Hz | no | KL-MI | no |
| B | Butterworth | two-way Butterworth filter (butter, filtfilt) | 4, 20 Hz | no | KL-MI | no |
| C | Butterworth adaptive | two-way Butterworth filter | 4 Hz, 2 fp + 4 Hz | no | KL-MI | no |
| D | eegfiltnew | delay-corrected one-way FIR filter (pop_eegfiltnew) | 4, 20 Hz | no | KL-MI | no |
| E | wavelet | Morlet wavelet transform | 6, 4 cycles | no | KL-MI | no |
| F | wavelet adaptive | Morlet wavelet transform | adaptive cycles as in PACTools | no | KL-MI | no |
| G | normalized | two-way FIR filter | 4, 20 Hz | no | KL-MI | 200 ‘split-swap’ surrogates |
| H | MVL normalized | two-way FIR filter | 4, 20 Hz | no | MVL-MI | 200 ‘split-swap’ surrogates |
| I | simulated epochs | two-way FIR filter | 4, 20 Hz | from 3-back 1250–2250 ms | KL-MI | no |
| Letter in Figures | Short Algorithm Name | Channels | Extraction of Oscillations | Width Parameters (Phase, Amplitude) | Epoch Selection (from Stimulus Onset) | PAC Measure | Normalization |
|---|---|---|---|---|---|---|---|
| A | basic | Fz-Pz | two-way FIR filter | 4, 20 Hz | 1250–2250 ms | KL-MI | no |
| B | Butterworth | Fz-Pz | two-way Butterworth filter | 4, 20 Hz | 1250–2250 ms | KL-MI | no |
| C | no buffer | Fz-Pz | delay-corrected one-way FIR filter | 4, 20 Hz | 500–3000 ms | KL-MI | no |
| D | eegfiltnew | Fz-Pz | delay-corrected one-way FIR filter | 4, 20 Hz | 1250–2250 ms | KL-MI | no |
| E | wavelet | Fz-Pz | Morlet wavelet transform | 6, 4 cycles | 1250–2250 ms | KL-MI | no |
| F | no epochs | Fz-Pz | delay-corrected one-way FIR filter | 4, 20 Hz | no (all cleaned data) | KL-MI | no |
| G | normalized | Fz-Pz | two-way FIR filter | 4, 20 Hz | 1250–2250 ms | KL-MI | 200 ‘split-swap’ surrogates |
| H | MVL normalized | Fz-Pz | two-way FIR filter | 4, 20 Hz | 1250–2250 ms | MVL-MI | 200 ‘split-swap’ surrogates |
| I | within channel | Fz-Fz | two-way FIR filter | 4, 20 Hz | 1250–2250 ms | KL-MI | no |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sinitsyn, D.O.; Poydasheva, A.G.; Bakulin, I.S.; Zabirova, A.H.; Lagoda, D.Y.; Suponeva, N.A.; Piradov, M.A. Estimating the Frequencies of Maximal Theta-Gamma Coupling in EEG during the N-Back Task: Sensitivity to Methodology and Temporal Instability. Algorithms 2023, 16, 540. https://doi.org/10.3390/a16120540
Sinitsyn DO, Poydasheva AG, Bakulin IS, Zabirova AH, Lagoda DY, Suponeva NA, Piradov MA. Estimating the Frequencies of Maximal Theta-Gamma Coupling in EEG during the N-Back Task: Sensitivity to Methodology and Temporal Instability. Algorithms. 2023; 16(12):540. https://doi.org/10.3390/a16120540
Chicago/Turabian StyleSinitsyn, Dmitry O., Alexandra G. Poydasheva, Ilya S. Bakulin, Alfiia H. Zabirova, Dmitry Yu. Lagoda, Natalia A. Suponeva, and Michael A. Piradov. 2023. "Estimating the Frequencies of Maximal Theta-Gamma Coupling in EEG during the N-Back Task: Sensitivity to Methodology and Temporal Instability" Algorithms 16, no. 12: 540. https://doi.org/10.3390/a16120540
APA StyleSinitsyn, D. O., Poydasheva, A. G., Bakulin, I. S., Zabirova, A. H., Lagoda, D. Y., Suponeva, N. A., & Piradov, M. A. (2023). Estimating the Frequencies of Maximal Theta-Gamma Coupling in EEG during the N-Back Task: Sensitivity to Methodology and Temporal Instability. Algorithms, 16(12), 540. https://doi.org/10.3390/a16120540






