Forest Tree Microbiomes and Associated Fungal Endophytes: Functional Roles and Impact on Forest Health
Abstract
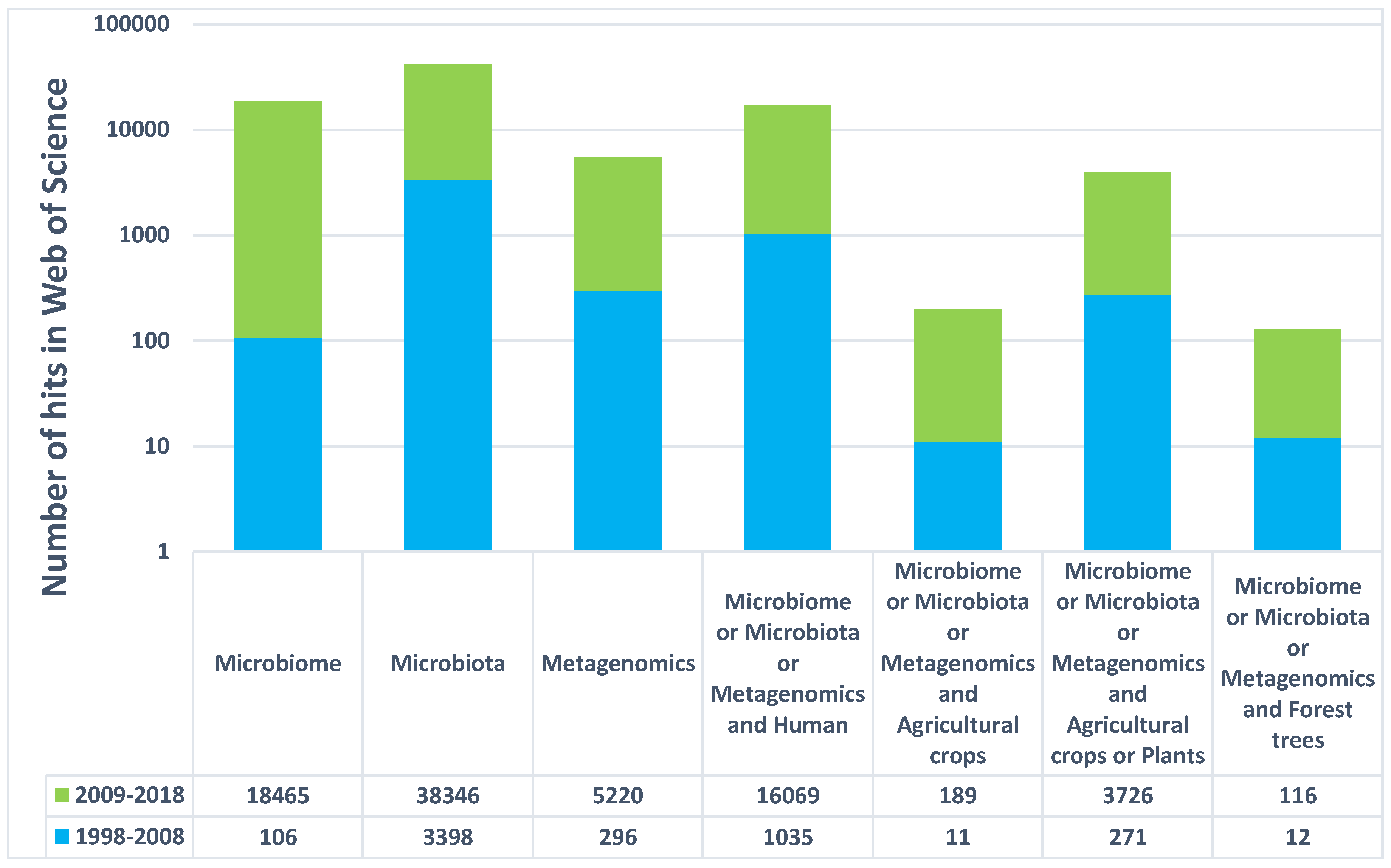
:1. What are Microbiomes
2. Insights from Agricultural Crops and Human Microbiome Projects
3. Phytomicrobiome
3.1. Agricultural Plants Versus Forest Trees Tissues Microbiome
3.2. Soil Microbiome Versus Plant Tissue Microbiome
3.3. Processes Driving Tree Microbiome Dynamics and Diversity: Impact of Host Genotype and Phenotype
3.4. Lifestyle and Phenotypic Plasticity among Phytomycobiomes
4. Endophytes as Part of Tree Microbiomes
5. Nature of Fungal Endophytes
6. Fungal Endophyte Diversity in Forests
6.1. Aerial Endophytes
6.2. Root Endophytes
7. Functional Relevance of Fungal Endophytes of Forest Trees
7.1. Aerial Endophytes and Tree Health
7.2. Root Endophytes and Tree Health
8. New Perceptions of Phytomicrobiomes
8.1. Novel Technological Advances for the Study of Tree Microbiomes and Associated Endophytes
8.2. Technical Limitations
9. Prospects for Translational Applications of the Knowledge for Improvement of Forest Health and Productivity
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Turner, T.R.; James, E.K.; Poole, P.S. The plant microbiome. Genome Biol. 2013, 14, 209. [Google Scholar] [CrossRef] [Green Version]
- Guttman, D.S.; McHardy, A.C.; Schulze-Lefert, P. Microbial genome-enabled insights into plant-microorganism interactions. Nat. Rev. Genet. 2014, 15, 797–813. [Google Scholar] [CrossRef] [PubMed]
- Vandenkoornhuyse, P.; Quaiser, A.; Duhamel, M.; Le Van, A.; Dufresne, A. The importance of the microbiome of the plant holobiont. New Phytol. 2015, 206, 1196–1206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardoim, P.R.; van Overbeek, L.S.; Berg, G.; Pirttila, A.M.; Compant, S.; Campisano, A.; Doring, M.; Sessitsch, A. The hidden world within plants: Ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol. Mol. Biol. Rev. 2015, 79, 293–320. [Google Scholar] [CrossRef] [PubMed]
- Porras-Alfaro, A.; Bayman, P. Hidden fungi, emergent properties: Endophytes and microbiomes. Annu. Rev. Phytopathol. 2011, 49, 291–315. [Google Scholar] [CrossRef]
- Baldrian, P. Forest microbiome: Diversity, complexity and dynamics. FEMS Microbiol. Rev. 2017, 41, 109–130. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.A.; Blaser, M.J.; Caporaso, J.G.; Jansson, J.K.; Lynch, S.V.; Knight, R. Current understanding of the human microbiome. Nat. Med. 2018, 24, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jia, H. Metagenome-wide association studies: Fine-mining the microbiome. Nat. Rev. Microbiol. 2016, 14, 508–522. [Google Scholar] [CrossRef] [PubMed]
- Vorholt, J.A.; Vogel, C.; Carlstrom, C.I.; Muller, D.B. Establishing causality: Opportunities of synthetic communities for plant microbiome research. Cell Host Microbe 2017, 22, 142–155. [Google Scholar] [CrossRef]
- Paredes, S.H.; Lebeis, S.L. Giving back to the community: Microbial mechanisms of plant-soil interactions. Funct. Ecol. 2016, 30, 1043–1052. [Google Scholar] [CrossRef] [Green Version]
- Mueller, U.G.; Sachs, J.L. Engineering microbiomes to improve plant and animal health. Trends Microbiol. 2015, 23, 606–617. [Google Scholar] [CrossRef] [PubMed]
- Bodenhausen, N.; Bortfeld-Miller, M.; Ackermann, M.; Vorholt, J.A. A synthetic community approach reveals plant genotypes affecting the phyllosphere microbiota. PLoS Genet. 2014, 10, e1004283. [Google Scholar] [CrossRef] [PubMed]
- Bulgarelli, D.; Schlaeppi, K.; Spaepen, S.; Ver Loren van Themaat, E.; Schulze-Lefert, P. Structure and functions of the bacterial microbiota of plants. Annu. Rev. Plant. Biol. 2013, 64, 807–838. [Google Scholar] [CrossRef]
- Muller, D.B.; Vogel, C.; Bai, Y.; Vorholt, J.A. The plant microbiota: Systems-level insights and perspectives. Annu. Rev. Genet. 2016, 50, 211–234. [Google Scholar] [CrossRef] [PubMed]
- Vorholt, J.A. Microbial life in the phyllosphere. Nat. Rev. Microbiol. 2012, 10, 828–840. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Kovalchuk, A.; Mukrimin, M.; Liu, M.; Zeng, Z.; Ghimire, R.P.; Kivimaenpaa, M.; Holopainen, J.K.; Sun, H.; Asiegbu, F.O. Tissue microbiome of norway spruce affected by Heterobasidion-induced wood decay. Microb. Ecol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Parniske, M. Arbuscular mycorrhiza: The mother of plant root endosymbioses. Nat. Rev. Microbiol. 2008, 6, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Kovalchuk, A.; Mukrimin, M.; Zeng, Z.; Raffaello, T.; Liu, M.; Kasanen, R.; Sun, H.; Asiegbu, F.O. Mycobiome analysis of asymptomatic and symptomatic norway spruce trees naturally infected by the conifer pathogens Heterobasidion spp. Environ. Microbiol. Rep. 2018, 10, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Lodge, D.J.; Fisher, P.J.; Sutton, B.C. Endophytic fungi of Manilkara bidentata leaves in Puerto Rico. Mycologia 1996, 88, 733–738. [Google Scholar] [CrossRef]
- Sieber, T.N. Endophytic fungi in forest trees: Are they mutualists? Fungal Biol. Rev. 2007, 21, 75–89. [Google Scholar] [CrossRef]
- Lang, C.; Seven, J.; Polle, A. Host preferences and differential contributions of deciduous tree species shape mycorrhizal species richness in a mixed central european forest. Mycorrhiza 2011, 21, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Gallart, M.; Adair, K.L.; Love, J.; Meason, D.F.; Clinton, P.W.; Xue, J.; Turnbull, M.H. Host Genotype and Nitrogen Form Shape the Root Microbiome of Pinus radiata. Microb. Ecol. 2018, 75, 419–433. [Google Scholar] [CrossRef] [PubMed]
- Cregger, M.A.; Veach, A.M.; Yang, Z.K.; Crouch, M.J.; Vilgalys, R.; Tuskan, G.A.; Schadt, C.W. The Populus holobiont: Dissecting the effects of plant niches and genotype on the microbiome. Microbiome 2018, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Terhonen, E.; Kovalchuk, A.; Tuovila, H.; Chen, H.; Oghenekaro, A.O.; Heinonsalo, J.; Kohler, A.; Kasanen, R.; Vasander, H.; et al. Dominant tree species and soil type affect the fungal community structure in a boreal peatland forest. Appl. Environ. Microbiol. 2016, 82, 2632–2643. [Google Scholar] [CrossRef] [PubMed]
- Lebeis, S.L. Greater than the sum of their parts: Characterizing plant microbiomes at the community-level. Curr. Opin. Plant Biol. 2015, 24, 82–86. [Google Scholar] [CrossRef]
- Weinert, N.; Piceno, Y.; Ding, G.C.; Meincke, R.; Heuer, H.; Berg, G.; Schloter, M.; Andersen, G.; Smalla, K. Phylochip hybridization uncovered an enormous bacterial diversity in the rhizosphere of different potato cultivars: Many common and few cultivar-dependent taxa. FEMS Microbiol. Ecol. 2011, 75, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Redford, A.J.; Bowers, R.M.; Knight, R.; Linhart, Y.; Fierer, N. The ecology of the phyllosphere: Geographic and phylogenetic variability in the distribution of bacteria on tree leaves. Environ. Microbiol. 2010, 12, 2885–2893. [Google Scholar] [CrossRef] [PubMed]
- Busby, P.E.; Soman, C.; Wagner, M.R.; Friesen, M.L.; Kremer, J.; Bennett, A.; Morsy, M.; Eisen, J.A.; Leach, J.E.; Dangl, J.L. Research priorities for harnessing plant microbiomes in sustainable agriculture. PLoS Biol. 2017, 15, e2001793. [Google Scholar] [CrossRef] [PubMed]
- Santoyo, G.; Hernandez-Pacheco, C.; Hernandez-Salmeron, J.; Hernandez-Leon, R. The role of abiotic factors modulating the plant-microbe-soil interactions: Toward sustainable agriculture. A review. Span. J. Agric. Res. 2017, 15, e03R01. [Google Scholar] [CrossRef]
- Agler, M.T.; Ruhe, J.; Kroll, S.; Morhenn, C.; Kim, S.T.; Weigel, D.; Kemen, E.M. Microbial hub taxa link host and abiotic factors to plant microbiome variation. PLoS Biol. 2016, 14, e1002352. [Google Scholar] [CrossRef]
- Ardanov, P.; Sessitsch, A.; Haggman, H.; Kozyrovska, N.; Pirttila, A.M. Methylobacterium-induced endophyte community changes correspond with protection of plants against pathogen attack. PLoS ONE 2012, 7, e46802. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.A.; Witzell, J.; Blumenstein, K.; Rozpedowska, E.; Helander, M.; Sieber, T.N.; Gil, L. Resistance to dutch elm disease reduces presence of xylem endophytic fungi in elms (Ulmus spp.). PLoS ONE 2013, 8, e56987. [Google Scholar] [CrossRef] [PubMed]
- Araujo, W.L.; Marcon, J.; Maccheroni, W., Jr.; Van Elsas, J.D.; Van Vuurde, J.W.; Azevedo, J.L. Diversity of endophytic bacterial populations and their interaction with Xylella fastidiosa in citrus plants. Appl. Environ. Microbiol. 2002, 68, 4906–4914. [Google Scholar] [CrossRef] [PubMed]
- Douanla-Meli, C.; Langer, E.; Mouafo, F.T. Fungal endophyte diversity and community patterns in healthy and yellowing leaves of Citrus limon. Fungal Ecol. 2013, 6, 212–222. [Google Scholar] [CrossRef]
- Bulgari, D.; Bozkurt, A.I.; Casati, P.; Caglayan, K.; Quaglino, F.; Bianco, P.A. Endophytic bacterial community living in roots of healthy and ‘Candidatus Phytoplasma mali’-infected apple (Malus domestica, Borkh.) trees. Antonie Van Leeuwenhoek 2012, 102, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Bulgari, D.; Casati, P.; Crepaldi, P.; Daffonchio, D.; Quaglino, F.; Brusetti, L.; Bianco, P.A. Restructuring of endophytic bacterial communities in grapevine yellows-diseased and recovered Vitis vinifera L. plants. Appl. Environ. Microbiol. 2011, 77, 5018–5022. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, B.O.; Taylor, A.F.S.; Finlay, R.D. Defining nutritional constraints on carbon cycling in boreal forests–towards a less ‘phytocentric’ perspective. Plant Soil 2002, 242, 123–135. [Google Scholar] [CrossRef]
- Terhonen, E.; Kovalchuk, A.; Zarsav, A.; Asiegbu, F.O. Biocontrol potential of forest tree endophytes. In Endophytes of Forest Trees; Springer: Berlin, Germany, 2018; pp. 283–318. [Google Scholar]
- Saikkonen, K.; Saari, S.; Helander, M. Defensive mutualism between plants and endophytic fungi? Fungal Divers. 2010, 41, 101–113. [Google Scholar] [CrossRef]
- Boddy, L. Saprotrophic cord-forming fungi: Meeting the challenge of heterogeneous environments. Mycologia 1999, 91, 13–32. [Google Scholar] [CrossRef]
- Asiegbu, F.O.; Adomas, A.; Stenlid, J. Conifer root and butt rot caused by Heterobasidion annosum (Fr.) Bref. s.l. Mol. Plant Pathol. 2005, 6, 395–409. [Google Scholar] [CrossRef]
- Cairney, J.W.G. Evolution of mycorrhiza systems. Naturwissenschaften 2000, 87, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Hibbett, D.S.; Gilbert, L.B.; Donoghue, M.J. Evolutionary instability of ectomycorrhizal symbioses in basidiomycetes. Nature 2000, 407, 506–508. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.C.; Hui, S.; Choi, J.; Asiegbu, F.O.; Valkonen, J.P.T.; Lee, Y.H. Secret lifestyles of Neurospora crassa. Sci. Rep. 2014, 4, 5135. [Google Scholar] [CrossRef] [PubMed]
- Vasiliauskas, R.; Menkis, A.; Finlay, R.D.; Stenlid, J. Wood-decay fungi in fine living roots of conifer seedlings. New Phytol. 2007, 174, 441–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaber, E.; Xiao, C.; Asiegbu, F.O. Comparative pathobiology of Heterobasidion annosum during challenge on Pinus sylvestris and Arabidopsis roots: An analysis of defensin gene expression in two pathosystems. Planta 2014, 239, 717–733. [Google Scholar] [CrossRef] [PubMed]
- Cooke, R.C.; Whipps, J.M. Ecophysiology of Fungi; Blackwell Scientific Publications: Hoboken, NJ, USA, 1993. [Google Scholar]
- Kikuchi, G.; Higuchi, M.; Morota, T.; Nagasawa, E.; Suzuki, A. Fungal symbiont and cultivation test of Gastrodia elata Blume (Orchidaceae). J. Jpn. Bot. 2008, 83, 88–95. [Google Scholar]
- Kikuchi, G.; Higuchi, M.; Yoshimura, H.; Morota, T.; Suzuki, A. In vitro symbiosis between Gastrodia elata Blume (Orchidaceae) and Armillaria Kummer (Tricholomataceae) species isolated from orchid tuber. J. Jpn. Bot. 2008, 83, 77–87. [Google Scholar]
- Martin, F.; Kohler, A.; Murat, C.; Veneault-Fourrey, C.; Hibbett, D.S. Unearthing the roots of ectomycorrhizal symbioses. Nat. Rev. Microbiol. 2016, 14, 760–773. [Google Scholar] [CrossRef]
- Stergiopoulos, I.; Gordon, T.R. Cryptic fungal infections: The hidden agenda of plant pathogens. Front. Plant Sci. 2014, 5, 506. [Google Scholar] [CrossRef]
- Rodriguez, R.J.; White, J.F., Jr.; Arnold, A.E.; Redman, R.S. Fungal endophytes: Diversity and functional roles. New Phytol. 2009, 182, 314–330. [Google Scholar] [CrossRef]
- De Bary, A. Die Erscheinung der Symbiose. Verlag von Karl J. Trübner: Strassburg, Germany, 1879. [Google Scholar]
- Carroll, G.C.; Carroll, F.E. Studies on the incidence of coniferous needle endophytes in the pacific northwest. Can. J. Bot. 1978, 56, 3034–3043. [Google Scholar] [CrossRef]
- Wilson, D. Endophyte—The evolution of a term, and clarification of its use and definition. Oikos 1995, 73, 274–276. [Google Scholar] [CrossRef]
- Petrini, O. Fungal endophytes of tree leaves. In Microbial Ecology of Leaves; Springer: Berlin, Germany, 1991; pp. 179–197. [Google Scholar]
- Saikkonen, K.; Faeth, S.H.; Helander, M.; Sullivan, T.J. Fungal endophytes: A continuum of interactions with host plants. Annu. Rev. Ecol. Syst. 1998, 29, 319–343. [Google Scholar] [CrossRef]
- Bußkamp, J. Schadenserhebung, Kartierung und Charakterisierung des “Diplodia-Triebsterbens” der Kiefer, Insbesondere des Endophytischen Vorkommens in den klimasensiblen Räumen und Identifikation von den in Kiefer (Pinus sylvestris) Vorkommenden Endophyten. Ph.D. Thesis, Universität Kassel, Kassel, Germany, 2018. [Google Scholar]
- Reininger, V.; Grünig, C.R.; Sieber, T.N. Host species and strain combination determine growth reduction of spruce and birch seedlings colonized by root-associated dark septate endophytes. Environ. Microbiol. 2012, 14, 1064–1076. [Google Scholar] [CrossRef] [PubMed]
- Rajala, T.; Velmala, S.M.; Tuomivirta, T.; Haapanen, M.; Muller, M.; Pennanen, T. Endophyte communities vary in the needles of Norway spruce clones. Fungal Biol. 2013, 117, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Jumpponen, A.; Trappe, J.M. Dark septate endophytes: A review of facultative biotrophic root-colonizing fungi. New Phytol. 1998, 140, 295–310. [Google Scholar] [CrossRef]
- Sieber, T.N.; Grünig, C.R. Fungal root endophytes. In Plant Roots—The Hidden Half, 4th ed.; CRC Press: Boca Raton, FL, USA, 2013; pp. 38–49. [Google Scholar]
- Rodriguez, R.; Redman, R. More than 400 million years of evolution and some plants still can’t make it on their own: Plant stress tolerance via fungal symbiosis. J. Exp. Bot. 2008, 59, 1109–1114. [Google Scholar] [CrossRef]
- Eyles, A.; Bonello, P.; Ganley, R.; Mohammed, C. Induced resistance to pests and pathogens in trees. New Phytol. 2010, 185, 893–908. [Google Scholar] [CrossRef]
- Albrectsen, B.; Witzell, J. Disentangling functions of fungal endophytes in forest trees. In Fungi: Types, Environmental Impact and Role in Disease; Nova Science Publishers Incorporated: New York, NY, USA, 2012; pp. 235–246. [Google Scholar]
- Blumenstein, K.; Albrectsen, B.R.; Martin, J.A.; Hultberg, M.; Sieber, T.N.; Helander, M.; Witzell, J. Nutritional niche overlap potentiates the use of endophytes in biocontrol of a tree disease. BioControl 2015, 60, 655–667. [Google Scholar] [CrossRef]
- Witzell, J.; Martín, J.A.; Blumenstein, K. Ecological aspects of endophyte-based biocontrol of forest diseases. In Advances in Endophytic Research; Verma, V.C., Gange, A.C., Eds.; Springer-Verlag: Berlin, Germany, 2014; pp. 321–333. ISBN 9788132215752. [Google Scholar]
- Schulz, B.; Boyle, C.; Draeger, S.; Rommert, A.K.; Krohn, K. Endophytic fungi: A source of novel biologically active secondary metabolites. Mycol. Res. 2002, 106, 996–1004. [Google Scholar] [CrossRef]
- Stierle, A.A.; Stierle, D.B. Bioactive secondary metabolites produced by the fungal endophytes of conifers. Nat. Prod. Commun. 2015, 10, 1671–1682. [Google Scholar] [PubMed]
- Tanney, J.B.; McMullin, D.R.; Miller, J.D. Toxigenic foliar endophytes from the Acadian forest. In Endophytes of Forest Trees; Springer: Berlin, Germany, 2018; pp. 343–381. [Google Scholar]
- Schulz, B.; Haas, S.; Junker, C.; Andree, N.; Schobert, M. Fungal endophytes are involved in multiple balanced antagonisms. Curr. Sci. India 2015, 109, 39–45. [Google Scholar]
- Schulz, B.; Rommert, A.K.; Dammann, U.; Aust, H.J.; Strack, D. The endophyte-host interaction: A balanced antagonism? Mycol. Res. 1999, 103, 1275–1283. [Google Scholar] [CrossRef]
- Saikkonen, K.; Lehtonen, P.; Helander, M.; Koricheva, J.; Faeth, S.H. Model systems in ecology: Dissecting the endophyte-grass literature. Trends Plant Sci. 2006, 11, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Saikkonen, K.; Ion, D.; Gyllenberg, M. The persistence of vertically transmitted fungi in grass metapopulations. Proc. R. Soc. B Biol. Sci. 2002, 269, 1397–1403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clay, K. Fungal endophytes of grasses—A defensive mutualism between plants and fungi. Ecology 1988, 69, 10–16. [Google Scholar] [CrossRef]
- Clay, K. Endophytes as antagonists of plant pests. In Microbial Ecology of Leaves; Springer: Berlin, Germany, 1991; pp. 331–357. [Google Scholar]
- Patterson, C.G.; Potter, D.A.; Fannin, F.F. Feeding deterrency of alkaloids from endophyte-infected grasses to japanese-beetle grubs. Entomol. Exp. Appl. 1991, 61, 285–289. [Google Scholar] [CrossRef]
- Riedell, W.E.; Kieckhefer, R.E.; Petroski, R.J.; Powell, R.G. Naturally-occurring and synthetic loline alkaloid derivatives—Insect feeding-behavior modification and toxicity. J. Entomol. Sci. 1991, 26, 122–129. [Google Scholar] [CrossRef]
- Saikkonen, K.; Helander, M.; Faeth, S.H.; Schulthess, F.; Wilson, D. Endophyte-grass-herbivore interactions: The case of Neotyphodium endophytes in Arizona fescue populations. Oecologia 1999, 121, 411–420. [Google Scholar] [CrossRef]
- Faeth, S.H.; Gardner, D.R.; Hayes, C.J.; Jani, A.; Wittlinger, S.K.; Jones, T.A. Temporal and spatial variation in alkaloid levels in Achnatherum robustum, a native grass infected with the endophyte Neotyphodium. J. Chem. Ecol. 2006, 32, 307–324. [Google Scholar] [CrossRef]
- Faeth, S.H.; Sullivan, T.J. Mutualistic asexual endophytes in a native grass are usually parasitic. Am. Nat. 2003, 161, 310–325. [Google Scholar] [CrossRef] [PubMed]
- Sieber, T.; Riesen, T.K.; Muller, E.; Fried, P.M. Endophytic fungi in 4 winter-wheat cultivars (Triticum-aestivum L.) differing in resistance against Stagonospora-nodorum (Berk) Cast and Germ = Septoria nodorum (Berk) Berk. J. Phytopathol. 1988, 122, 289–306. [Google Scholar] [CrossRef]
- Rodriguez, R.J.; Henson, J.; Van Volkenburgh, E.; Hoy, M.; Wright, L.; Beckwith, F.; Kim, Y.; Redman, R.S. Stress tolerance in plants via habitat-adapted symbiosis. ISME J. 2008, 2, 404–416. [Google Scholar] [CrossRef] [Green Version]
- Arnold, A.E.; Lutzoni, F. Diversity and host range of foliar fungal endophytes: Are tropical leaves biodiversity hotspots? Ecology 2007, 88, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Arnold, A.E. Understanding the diversity of foliar endophytic fungi: Progress, challenges, and frontiers. Fungal Biol. Rev. 2007, 21, 51–66. [Google Scholar] [CrossRef]
- Nguyen, D.; Boberg, J.; Ihrmark, K.; Stenstrom, E.; Stenlid, J. Do foliar fungal communities of Norway spruce shift along a tree species diversity gradient in mature European forests? Fungal Ecol. 2016, 23, 97–108. [Google Scholar] [CrossRef] [Green Version]
- Siddique, A.; Khokon, A.M.; Unterseher, M. What do we learn from cultures in the omics age? High-throughput sequencing and cultivation of leaf-inhabiting endophytes from beech (Fagus sylvatica L.) revealed complementary community composition but similar correlations with local habitat conditions. Mycokeys 2017, 20, 1–16. [Google Scholar] [CrossRef]
- Saikkonen, K.; Helander, M.; Faeth, S.H. Fungal endophytes: Hitch-hikers of the green world. In Plant Microbiology; Taylor & Francis: Didcot, UK, 2004; pp. 93–113. [Google Scholar]
- Saikkonen, K.; Wali, P.; Helander, M.; Faeth, S.H. Evolution of endophyte-plant symbioses. Trends Plant Sci. 2004, 9, 275–280. [Google Scholar] [CrossRef]
- Osono, T. Role of phyllosphere fungi of forest trees in the development of decomposer fungal communities and decomposition processes of leaf litter. Can. J. Microbiol. 2006, 52, 701–716. [Google Scholar] [CrossRef]
- Vaz, A.B.M.; Fonseca, P.L.C.; Badotti, F.; Skaltsas, D.; Tome, L.M.R.; Silva, A.C.; Cunha, M.C.; Soares, M.A.; Santos, V.L.; Oliveira, G.; et al. A multiscale study of fungal endophyte communities of the foliar endosphere of native rubber trees in Eastern Amazon. Sci. Rep. 2018, 8, 16151. [Google Scholar] [CrossRef]
- Saikkonen, K. Forest structure and fungal endophytes. Fungal Biol. Rev. 2007, 21, 67–74. [Google Scholar] [CrossRef] [Green Version]
- Mandyam, K.; Jumpponen, A. Seeking the elusive function of the root-colonising dark septate endophytic fungi. Stud. Mycol. 2005, 173–189. [Google Scholar] [CrossRef]
- Bonfim, J.A.; Vasconcellos, R.L.F.; Baldesin, L.F.; Sieber, T.N.; Cardoso, E.J.B.N. Dark septate endophytic fungi of native plants along an altitudinal gradient in the Brazilian Atlantic forest. Fungal Ecol. 2016, 20, 202–210. [Google Scholar] [CrossRef]
- Ahlich, K.; Sieber, T.N. The profusion of dark septate endophytic fungi in non-ectomycorrhizal fine roots of forest trees and shrubs. New Phytol. 1996, 132, 259–270. [Google Scholar] [CrossRef] [Green Version]
- Grünig, C.R.; Duo, A.; Sieber, T.N.; Holdenrieder, O. Assignment of species rank to six reproductively isolated cryptic species of the Phialocephala fortinii s.l.-Acephala applanata species complex. Mycologia 2008, 100, 47–67. [Google Scholar] [CrossRef] [PubMed]
- Ahlich, K.; Rigling, D.; Holdenrieder, O.; Sieber, T.N. Dark septate hyphomycetes in Swiss conifer forest soils surveyed using Norway spruce seedlings as bait. Soil Biol. Biochem. 1998, 30, 1069–1075. [Google Scholar] [CrossRef]
- Tejesvi, M.V.; Ruotsalainen, A.L.; Markkola, A.M.; Pirttilä, A.M. Root endophytes along a primary succession gradient in northern Finland. Fungal Divers. 2010, 41, 125–134. [Google Scholar] [CrossRef]
- Grünig, C.R.; Sieber, T.N.; Rogers, S.O.; Holdenrieder, O. Spatial distribution of dark septate endophytes in a confined forest plot. Mycol. Res. 2002, 106, 832–840. [Google Scholar] [CrossRef]
- Queloz, V.; Duo, A.; Sieber, T.N.; Grünig, C.R. Microsatellite size homoplasies and null alleles do not affect species diagnosis and population genetic analysis in a fungal species complex. Mol. Ecol. Resour. 2010, 10, 348–367. [Google Scholar] [CrossRef]
- Queloz, V.; Grünig, C.R.; Sieber, T.N.; Holdenrieder, O. Monitoring the spatial and temporal dynamics of a community of the tree-root endophyte Phialocephala fortinii sl. New Phytol. 2005, 168, 651–660. [Google Scholar] [CrossRef]
- Queloz, V.; Duo, A.; Grünig, C.R. Isolation and characterization of microsatellite markers for the tree-root endophytes Phialocephala subalpina and Phialocephala fortinii s.s. Mol. Ecol. Resour. 2008, 8, 1322–1325. [Google Scholar] [CrossRef] [PubMed]
- Queloz, V.; Sieber, T.N.; Holdenrieder, O.; McDonald, B.A.; Grünig, C.R. No biogeographical pattern for a root-associated fungal species complex. Glob. Ecol. Biogeogr. 2011, 20, 160–169. [Google Scholar] [CrossRef]
- Tejesvi, M.V.; Sauvola, T.; Pirttila, A.M.; Ruotsalainen, A.L. Neighboring Deschampsia flexuosa and Trientalis europaea harbor contrasting root fungal endophytic communities. Mycorrhiza 2013, 23, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Terhonen, E.; Kerio, S.; Sun, H.; Asiegbu, F.O. Endophytic fungi of Norway spruce roots in boreal pristine mire, drained peatland and mineral soil and their inhibitory effect on Heterobasidion parviporum in vitro. Fungal Ecol. 2014, 9, 17–26. [Google Scholar] [CrossRef]
- Sietiö, O.M.; Tuomivirta, T.; Santalahti, M.; Kiheri, H.; Timonen, S.; Sun, H.; Fritze, H.; Heinonsalo, J. Ericoid plant species and Pinus sylvestris shape fungal communities in their roots and surrounding soil. New Phytol. 2018, 218, 738–751. [Google Scholar] [CrossRef] [PubMed]
- Addy, H.D.; Piercey, M.M.; Currah, R.S. Microfungal endophytes in roots. Can. J. Bot. 2005, 83, 1–13. [Google Scholar] [CrossRef]
- Singh, D.K.; Sharma, V.K.; Kumar, J.; Mishra, A.; Verma, S.K.; Sieber, T.N.; Kharwar, R.N. Diversity of endophytic mycobiota of tropical tree Tectona grandis Linn.f.: Spatiotemporal and tissue type effects. Sci. Rep. 2017, 7, 3745. [Google Scholar] [CrossRef]
- Arnold, A.E.; Mejia, L.C.; Kyllo, D.; Rojas, E.I.; Maynard, Z.; Robbins, N.; Herre, E.A. Fungal endophytes limit pathogen damage in a tropical tree. Proc. Natl. Acad. Sci. USA 2003, 100, 15649–15654. [Google Scholar] [CrossRef] [Green Version]
- Arnold, A.E.; Maynard, Z.; Gilbert, G.S. Fungal endophytes in dicotyledonous neotropical trees: Patterns of abundance and diversity. Mycol. Res. 2001, 105, 1502–1507. [Google Scholar] [CrossRef]
- Petrini, O. Taxonomy of endophytic fungi of aerial plant tissues. In Microbiology of the Phyllosphere; Cambridge University Press: Cambridge, UK; New York, NY, USA, 1986. [Google Scholar]
- Sieber, T. Endophytic fungi in needles of healthy-looking and diseased Norway spruce (Picea-abies [L] Karsten). Eur. J. For. Pathol. 1988, 18, 321–342. [Google Scholar] [CrossRef]
- Siebercanavesi, F.; Sieber, T.N. Successional patterns of fungal communities in needles of European silver fir (Abies-alba Mill). New Phytol. 1993, 125, 149–161. [Google Scholar] [CrossRef]
- Kowalski, T. Fungi in living symptomless needles of Pinus-sylvestris with respect to some observed disease processes. J. Phytopathol. 1993, 139, 129–145. [Google Scholar] [CrossRef]
- Fisher, P.J.; Graf, F.; Petrini, L.E.; Sutton, B.C.; Wookey, P.A. Fungal endophytes of Dryas-octopetala from a high arctic polar semidesert and from the Swiss Alps. Mycologia 1995, 87, 319–323. [Google Scholar] [CrossRef]
- Davis, E.C.; Franklin, J.B.; Shaw, A.J.; Vilgalys, R. Endophytic Xylaria (Xylariaceae) among liverworts and angiosperms: Phylogenetics, distribution, and symbiosis. Am. J. Bot. 2003, 90, 1661–1667. [Google Scholar] [CrossRef]
- Davis, E.C.; Shaw, A.J. Biogeographic and phylogenetic patterns in diversity of liverwort-associated endophytes. Am. J. Bot. 2008, 95, 914–924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, K.L.; Arnold, A.E.; Miadlikowska, J.; Sarvate, S.D.; Lutzoni, F. Phylogenetic relationships, host affinity, and geographic structure of boreal and arctic endophytes from three major plant lineages. Mol. Phylogenet. Evol. 2007, 42, 543–555. [Google Scholar] [CrossRef]
- Murali, T.S.; Suryanarayanan, T.S.; Venkatesan, G. Fungal endophyte communities in two tropical forests of Southern India: Diversity and host affiliation. Mycol. Prog. 2007, 6, 191–199. [Google Scholar] [CrossRef]
- Muller, M.M.; Hallaksela, A.M. Diversity of Norway spruce needle endophytes in various mixed and pure Norway spruce stands. Mycol. Res. 1998, 102, 1183–1189. [Google Scholar] [CrossRef]
- Muller, M.M.; Hallaksela, A.M. Fungal diversity in Norway spruce: A case study. Mycol. Res. 2000, 104, 1139–1145. [Google Scholar] [CrossRef]
- Terhonen, E.; Marco, T.; Sun, H.; Jalkanen, R.; Kasanen, R.; Vuorinen, M.; Asiegbu, F. The effect of latitude, season and needle-age on the mycota of Scots pine (Pinus sylvestris) in Finland. Silva Fenn. 2011, 45, 301–317. [Google Scholar] [CrossRef]
- Millberg, H.; Boberg, J.; Stenlid, J. Changes in fungal community of Scots pine (Pinus sylvestris) needles along a latitudinal gradient in Sweden. Fungal Ecol. 2015, 17, 126–139. [Google Scholar] [CrossRef]
- Helander, M.L.; Sieber, T.N.; Petrini, O.; Neuvonen, S. Endophytic fungi in Scots pine needles—Spatial variation and consequences of simulated acid-rain. Can. J. Bot. 1994, 72, 1108–1113. [Google Scholar] [CrossRef]
- Hata, K.; Futai, K.; Tsuda, M. Seasonal and needle age-dependent changes of the endophytic mycobiota in Pinus thunbergii and Pinus densiflora needles. Can. J. Bot. 1998, 76, 245–250. [Google Scholar] [CrossRef]
- Guo, L.D.; Huang, G.R.; Wang, Y. Seasonal and tissue age influences on endophytic fungi of Pinus tabulaeformis (Pinaceae) in the Dongling mountains, Beijing. J. Integr. Plant Biol. 2008, 50, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Carroll, F.; Muller, E.; Sutton, B.C. Preliminary studies on the incidence of needle endophytes in some european conifers. Sydowia 1977, 29, 87–103. [Google Scholar]
- Arnold, A.E.; Henk, D.A.; Eells, R.L.; Lutzoni, F.; Vilgalys, R. Diversity and phylogenetic affinities of foliar fungal endophytes in loblolly pine inferred by culturing and environmental PCR. Mycologia 2007, 99, 185–206. [Google Scholar] [CrossRef] [PubMed]
- Ihrmark, K.; Bodeker, I.T.M.; Cruz-Martinez, K.; Friberg, H.; Kubartova, A.; Schenck, J.; Strid, Y.; Stenlid, J.; Brandstrom-Durling, M.; Clemmensen, K.E.; et al. New primers to amplify the fungal ITS2 region—Evaluation by 454-sequencing of artificial and natural communities. FEMS Microbiol. Ecol. 2012, 82, 666–677. [Google Scholar] [CrossRef]
- Delhomme, N.; Sundstrom, G.; Zamani, N.; Lantz, H.; Lin, Y.C.; Hvidsten, T.R.; Hoppner, M.P.; Jern, P.; Van de Peer, Y.; Lundeberg, J.; et al. Serendipitous meta-transcriptomics: The fungal community of Norway spruce (Picea abies). PLoS ONE 2015, 10, e0139080. [Google Scholar] [CrossRef]
- Antonio, V. Sanz-Ros, A.V.; Müller, M.M.; San Martín, R.; Diez, J.J. Fungal endophytic communities on twigs of fast and slow growing Scots pine (Pinus sylvestris L.) in northern Spain. Fungal Biol. 2015, 119, 870–883. [Google Scholar]
- Gazis, R.; Chaverri, P. Diversity of fungal endophytes in leaves and stems of wild rubber trees (Hevea brasiliensis) in Peru. Fungal Ecol. 2010, 3, 240–254. [Google Scholar] [CrossRef]
- Gonzalez-Teuber, M. The defensive role of foliar endophytic fungi for a south american tree. AoB Plants 2016, 8, plw050. [Google Scholar] [CrossRef] [PubMed]
- Saucedo-García, A.; Anaya, A.L.; Espinosa-García, F.J.; González, M.C. Diversity and communities of foliar endophytic fungi from different agroecosystems of Coffea arabica L. in two regions of Veracruz, Mexico. PLoS ONE 2014, 9, e98454. [Google Scholar] [CrossRef]
- U’Ren, J.M.; Lutzoni, F.; Miadlikowska, J.; Laetsch, A.; Arnold, A.E. Host and geographic structure of endophytic and endolichenic fungi at a continental scale. Am. J. Bot. 2012, 99, 898–914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlegel, M.; Queloz, V.; Sieber, T.N. The endophytic mycobiome of European ash and Sycamore maple leaves—Geographic patterns, host specificity and influence of Ash Dieback. Front. Microbiol. 2018, 9, 2345. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.; Boberg, J.; Cleary, M.; Bruelheide, H.; Hönig, L.; Koricheva, J.; Stenlid, J. Foliar fungi of Betula pendula: Impact of tree species mixtures and assessment methods. Sci. Rep. 2017, 9, 41801. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.B.; Weblen, G.D.; May, G. Host associations and beta diversity of fungal endophyte communities in New Guinea rainforest trees. Mol. Ecol. 2016, 25, 825–84. [Google Scholar] [CrossRef] [PubMed]
- Chen, J. Fungal Community Survey of Fraxinus excelsior in New Zealand. 2012. Available online: http://stud.epsilon.slu.se/4172/ (accessed on 11 November 2018).
- Ibrahim, M.; Sieber, T.N.; Schlegel, M. Communities of fungal endophytes in leaves of Fraxinus ornus are highly diverse. Fungal Ecol. 2017, 29, 10–19. [Google Scholar] [CrossRef]
- Ibrahim, M.; Schlegel, M.; Sieber, T.N. Venturia orni sp. nov., a species distinct from Venturia fraxini, living in the leaves of Fraxinus ornus. Mycol. Prog. 2016, 15. [Google Scholar] [CrossRef]
- Schlegel, M.; Dubach, V.; von Buol, L.; Sieber, T.N. Effects of endophytic fungi on the ash dieback pathogen. FEMS Microbiol. Ecol. 2016, 92, fiw142. [Google Scholar] [CrossRef] [PubMed]
- Helander, M.L.; Wäli, P.; Kuuluvainen, T.; Saikkonen, K. Birch leaf endophytes in managed and natural boreal forests. Can. J. For. Res. 2006, 53, 20–29. [Google Scholar] [CrossRef]
- Taudière, A.; Bellanger, J.M.; Carcaillet, C.; Hugot, L.; Kjellberg, F.; Lecanda, A.; Lesne, A.; Moreau, P.A.; Scharmann, K.; Leidel, S.; Richard, F. Diversity of foliar endophytic ascomycetes in the endemic Corsican pine forests. Fungal Ecol. 2018, 36, 128–140. [Google Scholar]
- Koukol, O.; Kolarik, M.; Kolarova, Z.; Baldrian, P. Diversity of foliar endophytes in wind-fallen Picea abies trees. Fungal Divers. 2012, 54, 69–77. [Google Scholar] [CrossRef]
- Albrectsen, B.R.; Siddique, A.B.; Decker, V.; Unterseher, M.; Robinson, K.M. Both plant genotype and herbivory shape aspen endophyte communities. Oecologia 2018, 187, 535–545. [Google Scholar] [CrossRef]
- Melin, E. On the mycorrhizas of Pinus sylvestris L. and Picea abies Karst. A preliminary note. J. Ecol. 1922, 9, 254–257. [Google Scholar] [CrossRef]
- Melin, E. Experimentelle Untersuchungen über die Konstitution und Ökologie der Mykorrhizen von Pinus silvestris und Picea abies. In Mykologische Untersuchungen und Berichte, 2; Falck, R., Ed.; Gottheilt: Kassel, Germany, 1923; pp. 73–334. [Google Scholar]
- Menkis, A.; Allmer, J.; Vasiliauskas, R.; Lygis, V.; Stenlid, J.; Finlay, R. Ecology and molecular characterization of dark septate fungi from roots, living stems, coarse and fine woody debris. Mycol. Res. 2004, 108, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Grünig, C.R.; Duò, A.; Sieber, T.N. Population genetic analysis of Phialocephala fortinii s.l. and Acephala applanata in two undisturbed forests in Switzerland and evidence for new cryptic species. Fungal Genet. Biol. 2006, 43, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Kernaghan, G.; Patriquin, G. Host associations between fungal root endophytes and boreal trees. Microbiol. Ecol. 2011, 62, 460–473. [Google Scholar] [CrossRef] [PubMed]
- Reininger, V.; Sieber, T.N. Mitigation of antagonistic effects on plant growth due to root co-colonization by dark septate endophytes (DSE) and ectomycorrhiza (ECM). Environ. Microbiol. Rep. 2013, 5, 892–898. [Google Scholar] [CrossRef] [PubMed]
- Mandyam, K.; Jumpponen, A. Seasonal and temporal dynamics of arbuscular mycorrhizal and dark septate endophytic fungi in a tallgrassprairie ecosystem are minimally affected by nitrogen enrichment. Mycorrhiza 2008, 18, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Bougoure, D.S.; Parkin, P.I.; Cairney, J.W.G.; Alexander, I.J.; Anderson, I.C. Diversity of fungi in hair roots of Ericaceae varies along a vegetation gradient. Mol. Ecol. 2007, 16, 4624–4636. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T.A.; Nordin, A. No evidence that nitrogen enrichment affect fungal communities of Vaccinium roots in two contrasting boreal forest types. Soil Biol. Biochem. 2010, 42, 234–243. [Google Scholar] [CrossRef]
- Kjøller, R.; Olsrud, M.; Michelsen, A. Co-existing ericaceous plant species in a subarctic mire community share fungal root endophytes. Fungal Ecol. 2010, 3, 205–214. [Google Scholar] [CrossRef]
- Toju, H.; Yamamoto, S.; Sato, H.; Tanabe, A.S. Sharing of diverse mycorrhizal and root-endophytic fungi among plant species in an oak-dominated cool–temperate forest. PLoS ONE 2013, 8, e78248. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.F.; Aldrich-Wolfe, L.; Riffel, A.; Barbare, H.; Simpson, N.B.; Trowbridge, J.; Jumpponen, A. Diverse Helotiales associated with the roots of three species of Arctic Ericaceae provide no evidence for host specificity. New Phytol. 2011, 191, 515–527. [Google Scholar] [CrossRef]
- Grünig, C.R.; McDonald, B.A.; Sieber, T.N.; Rogers, S.O.; Holdenrieder, O. Evidence for subdivision of the root-endophyte Phialocephala fortinii into cryptic species and recombination within species. Fung Genet. Biol. 2004, 41, 676–687. [Google Scholar] [CrossRef] [PubMed]
- Stroheker, S.; Dubach, V.; Queloz, V.; Sieber, T.N. Resilience of Phialocephala fortinii s.l.—Acephala applanata communities—Effects of disturbance and strain introduction. Fungal Ecol. 2018, 31, 19–28. [Google Scholar] [CrossRef]
- Stroheker, S.; Queloz, V.; Sieber, T.N. Spatial and temporal dynamics in the Phialocephala fortinii s.l.—Acephala applanata species complex (PAC). Plant Soil 2016, 407, 231e241. [Google Scholar] [CrossRef]
- Morrison, D.; Mallett, K. Silvicultural management of Armillaria root disease in western Canadian forests. Can. J. Plant Pathol. 2016, 18, 194–199. [Google Scholar] [CrossRef]
- Stenlid, J.; Redfern, D.B. Spread within the tree and stand. In Heterobasidion Annosum: Biology, Ecology, Impact and Control; Woodward, S., Stenlid, J., Karjalainen, R., Hüttermann, A., Eds.; Blackwell Science Ltd.: Hoboken, NJ, USA, 1998; pp. 125–141. [Google Scholar]
- Wilson, B.J.; Addy, H.D.; Tsuneda, A.; Hambleton, S.; Currah, R.S. Phialocephala sphaeroides sp. nov., a new species among the dark septate endophytes from a boreal wetland in Canada. Can. J. Bot. 2004, 82, 607–617. [Google Scholar] [CrossRef]
- Grünig, C.R.; Queloz, V.; Sieber, T.N.; Holdenrieder, O. Dark septate endophytes (DSE) of the Phialocephala fortinii s.l.—Acephala applanata species complex in tree roots: Classification, population biology, and ecology. Botany 2008, 86, 1355–1369. [Google Scholar] [CrossRef]
- Zijlstra, J.D.; Van’t Hof, P.; Baar, J.; Verkley, G.J.M.; Summerbell, R.C.; Paradi, I.; Braakhekke, W.G.; Berendse, F. Diversity of symbiotic root endophytes of the Helotiales in ericaceous plants and the grass, Deschampsia flexuosa. Stud. Mycol. 2005, 53, 147–162. [Google Scholar] [CrossRef]
- Maciá-Vicente, J.G.; Jansson, H.-B.; Abdullah, S.K.; Descals, E.; Salinas, J.; Lopez-Llorca, L.V. Fungal root endophytes from natural vegetation in Mediterranean environments with special reference to Fusarium spp. FEMS Microbiol. Ecol. 2008, 64, 90–105. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson, J.; Nilsson, S.G.; Franc, A.; Menozzi, P. Biodiversity, disturbances, ecosystem function and management of European forests. For. Ecol. Manag. 2000, 132, 39–50. [Google Scholar] [CrossRef]
- McCann, K.S. The diversity-stability debate. Nature 2002, 405, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.E.; Tilman, D.; Groth, J.V. Effects of grassland plant species diversity, abundance, and composition on foliar fungal disease. Ecology 2002, 83, 1713–1716. [Google Scholar] [CrossRef]
- Pautasso, M.; Holdenrieder, O.; Stenlid, J. Susceptibility to fungal pathogens of forests differing in tree diversity. In Forest Diversity and Function; Scherer-Lorenzen, M., Körner, C., Schulze, E.-D., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 263–289. [Google Scholar]
- Yachi, S.; Loreau, M. Biodiversity and ecosystem productivity in a fluctuating environment: The insurance hypothesis. Proc. Natl. Acad. Sci. USA 1999, 96, 57–64. [Google Scholar] [CrossRef]
- Tudzynski, B.; Sharon, A. Biosynthesis, biological role and application of fungal phytohormones. In The Mycota (A Comprehensive Treatise on Fungi as Experimental Systems for Basic and Applied Research); Osiewacz, H.D., Ed.; Industrial Applications; Springer: Berlin/Heidelberg, Germany, 2002; Volume 10, pp. 183–21. [Google Scholar]
- Campanile, G.; Ruscelli, A.; Luisi, N. Antagonistic activity of endophytic fungi towards Diplodia corticola assessed by in vitro and in planta tests. Eur. J. Plant Pathol. 2007, 117, 237–246. [Google Scholar] [CrossRef]
- Danielsen, S.; Jensen, D.F. Fungal endophytes from stalks of tropical maize and grasses: Isolation, identification, and screening for antagonism against Fusarium verticillioides in maize stalks. Biocontrol Sci. Technol. 1999, 9, 545–553. [Google Scholar] [CrossRef]
- Narisawa, K.; Kawamata, H.; Currah, R.S.; Hashiba, T. Suppression of Verticillium wilt in eggplant by some fungal root endophytes. Eur. J. Plant Pathol. 2002, 108, 103–109. [Google Scholar] [CrossRef]
- Hussain, H.; Root, N.; Jabeen, F.; Al-Harrasi, A.; Al-Rawahi, A.; Ahmad, M.; Hassan, Z.; Abbas, G.; Mabood, F.; Shah, A.; et al. Seimatoric acid and colletonoic acid: Two new compounds from the endophytic fungi, Seimatosporium sp. and Colletotrichum sp. Chin. Chem. Lett. 2014, 25, 1577–1579. [Google Scholar] [CrossRef]
- Samuels, G.J.; Pardo-Schultheiss, R.; Hebbar, K.P.; Lumsden, R.D.; Bastos, C.N.; Costa, J.C.; Bezerra, J.L. Trichoderma stromaticum sp. nov., a parasite of the cacao witches broom pathogen. Mycol. Res. 2000, 104, 760–764. [Google Scholar] [CrossRef]
- Vu, T.T.; Hauschild, R.; Sikora, R.A. Fusarium oxysporum endophytes induced systemic resistance against Radopholus similis on banana. Nematology 2006, 8, 847–852. [Google Scholar] [CrossRef]
- Korkama-Rajala, T.; Müller, M.; Pennanen, T. Decomposition and fungi of needle litter from slow- and fast-growing Norway spruce (Picea abies) clones. Microb. Ecol. 2008, 56, 76–89. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.M.; Valjakka, R.; Suokko, A.; Hantula, J. Diversity of endophytic fungi of single Norway spruce needles and their role as pioneer decomposers. Mol. Ecol. 2001, 10, 1801–1810. [Google Scholar] [CrossRef] [PubMed]
- Sumarah, M.W.; Puniani, E.; Blackwell, B.A.; Miller, J.D. Characterization of polyketide metabolites from foliar endophytes of Picea glauca. J. Nat. Prod. 2008, 71, 1393–1398. [Google Scholar] [CrossRef] [PubMed]
- Mejía, L.C.; Herre, E.A.; Sparks, J.P.; Winter, K.; Garcia, M.N.; Van Bael, S.A.; Stitt, J.; Shi, Z.; Zhang, Y.; Guiltinan, M.J.; et al. Pervasive effects of a dominant foliar endophytic fungus on host genetic and phenotypic expression in a tropical tree. Front. Microbiol. 2014, 5, 479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gazis, R.; Chaverri, P. Wild trees in the Amazon basin harbor a great diversity of beneficial endosymbiotic fungi: Is this evidence of protective mutualism? Fungal Ecol. 2015, 17, 18–29. [Google Scholar] [CrossRef]
- Evans, H.; Holmes, K.; Thomas, S. Endophytes and mycoparasites associated with an indigenous forest tree, Theobroma gileri, in Ecuador and a preliminary assessment of their potential as biocontrol agents of cocoa diseases. Mycol. Prog. 2003, 2, 149–160. [Google Scholar] [CrossRef]
- Holmes, K.A.; Schroers, H.-J.; Thomas, S.E.; Evans, H.C.; Samuels, G.J. Taxonomy and biocontrol potential of a new species of Trichoderma from the Amazon basin of South America. Mycol. Prog. 2004, 3, 199–210. [Google Scholar] [CrossRef]
- Mejía, L.C.; Rojas, E.I.; Maynard, Z.; Van Bael, S.; Arnold, A.E.; Hebbar, P.; Samuels, G.J.; Robbins, N.; Herre, E.A. Endophytic fungi as biocontrol agents of Theobroma cacao pathogens. Biol. Control 2008, 46, 4–14. [Google Scholar] [CrossRef]
- Rubini, M.R.; Silva-Ribeiro, R.T.; Pomella, A.W.V.; Maki, C.S.; Araujo, W.L.; Santos, D.R.; Azevedo, J.L. Diversity of endophytic fungal community of cacao (Theobroma cacao L.) and biological control of Crinipellis perniciosa, causal agent of witches’ broom disease. Int. J. Biol. Sci. 2005, 1, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Tondje, P.R.; Roberts, D.P.; Bon, M.C.; Widmer, T.; Samuels, G.J.; Ismaiel, A.; Begoude, A.D.; Tchana, T.; Nyemb-Tshomb, E.; Ndoumbe-Nkeng, M.; et al. Isolation and identification of mycoparasitic isolates of Trichoderma asperellum with potential for suppression of black pod disease of cacao in Cameroon. Biol. Control 2007, 43, 202–212. [Google Scholar] [CrossRef]
- Ridout, M.; Newcombe, G. The frequency of modification of Dothistroma pine needle blight severity by fungi withion the native range. For. Ecol. Manag. 2015, 337, 153–160. [Google Scholar] [CrossRef]
- Preszler, R.W.; Gaylord, E.S.; Boecklen, W.J. Reduced parasitism of a leaf-mining moth on trees with high infection frequencies of an endophytic fungus. Oecologia 1996, 108, 159–166. [Google Scholar] [CrossRef]
- Miller, J.D.; Sumarah, M.W.; Adams, G.W. Effect of a rugulosin-producing endophyte in Picea glauca on Choristoneura fumiferana. J. Chem. Ecol. 2008, 34, 362–368. [Google Scholar] [CrossRef]
- Calhoun, L.A.; Findlay, J.A.; Miller, J.D.; Whitney, N.J. Metabolites toxic to spruce budworm from balsam fir needle endophytes. Mycol. Res. 1992, 96, 281–286. [Google Scholar] [CrossRef]
- Miller, J.D.; Mackenzie, S.; Foto, M.; Adams, G.W.; Findlay, J.A. Needles of white spruce inoculated with rugulosin-producing endophytes contain rugulosin reducing spruce budworm growth rate. Mycol. Res. 2002, 106, 471–479. [Google Scholar] [CrossRef]
- Sumarah, M.W.; Adams, G.W.; Bergout, J.; Slack, G.J.; Wilson, A.M.; Miller, J.D. Spread and persistence of a rugulosin-producing endophyte in white spruce seedlings. Mycol. Res. 2008, 112, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.D. Foliar endophytes of spruce species found in the Acadian forest: Basis and potential for improving the tolerance of the forest to spruce budworm. In Endophytes of Forest Trees: Biology and Applications; Pirttilä, A.M., Carolin, F.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 237–257. [Google Scholar]
- Frasz, S.L.; Walker, A.K.; Nsiama, T.K.; Adams, G.W.; Miller, J.D. Distribution of the foliar fungal endophyte Phialocephala scopiformis and its toxin in the crown of a mature white spruce tree as revealed by chemical and qPCR analyses. Can. J. For. Res. 2014, 44, 1138–1143. [Google Scholar] [CrossRef]
- McMullin, D.R.; Nguyen, H.D.T.; Daly, G.J.; Menard, B.S.; Miller, J.D. Detection of foliar endophytes and their metabolites in Picea and Pinus seedling needles. Fungal Ecol. 2018, 31, 1–8. [Google Scholar] [CrossRef]
- McMullin, D.R.; Green, B.D.; Prince, N.C.; Tanney, J.B.; Miller, J.D. Natural products of Picea endophytes from the Acadian forest. J. Nat. Prod. 2017, 80, 1475–1483. [Google Scholar] [CrossRef] [PubMed]
- Tanney, J.B.; McMullin, D.R.; Green, B.D.; Miller, J.D.; Seifert, K.A. Production of antifungal and antiinsectan metabolites by the Picea endophyte Diaporthe maritima sp. nov. Fungal Biol. 2016, 120, 1448–1457. [Google Scholar] [CrossRef] [PubMed]
- Sumarah, M.W.; Walker, A.K.; Seifert, K.A.; Todorov, A.; Miller, J.D. Screening of fungal endophytes isolated from eastern white pine needles. Recent Adv. Phytochem. 2015, 45, 195–206. [Google Scholar]
- Richardson, S.N.; Walker, A.K.; Nsiama, T.K.; McFarlane, J.; Sumarah, M.W.; Ibrahim, A.; Miller, J.D. Griseofulvin-producing Xylaria endophytes of Pinus strobus and Vaccinium angustifolium: Evidence for a conifer-understory species endophyte ecology. Fungal Ecol. 2014, 11, 107–113. [Google Scholar] [CrossRef]
- Sumarah, M.W.; Kesting, J.R.; Sørensen, D.; Miller, J.D. Antifungal metabolites from fungal endophytes of Pinus strobus. Phytochemistry 2011, 72, 14–15. [Google Scholar] [CrossRef]
- Ganley, R.J.; Sniezko, R.A.; Newcombe, G. Endophyte-mediated resistance against white pine blister rust in Pinus monticola. For. Ecol. Manag. 2008, 255, 2751–2760. [Google Scholar] [CrossRef]
- Raghavendra, A.K.H.; Newcombe, G. The contribution of foliar endophytes to quantitative resistance to Melampsora rust. New Phytol. 2013, 197, 909–918. [Google Scholar] [CrossRef]
- Christian, N.; Herre, E.A.; Mejía, L.C.; Clay, K. Exposure to the leaf litter microbiome of healthy adults protects seedlings from pathogen damage. Proc. R. Soc. B Biol. Sci. 2017, 12, 284. [Google Scholar] [CrossRef]
- Jumpponen, A.; Trappe, J.M. Performance of Pinus contorta inoculated with two strains of root endophytic fungus, Phialocephala fortinii: Effects of synthesis system and glucose concentration. Can. J. Bot. 1998, 76, 1205–1213. [Google Scholar]
- Terhonen, E.; Sipari, S.; Asiegbu, F.O. Inhibition of phytopathogens by fungal root endophytes of Norway spruce. Biol. Control 2016, 99, 53–63. [Google Scholar] [CrossRef]
- Vohnik, M.; Lukan, S.; Bahor, E.; Regvap, M.; Vositka, M.; Vodnik, D. Inoculation of Rhododendron cv. belle-heller with two strains of Phialocephala fortinii in two different substrates. Folia Geobot. 2003, 38, 191–200. [Google Scholar] [CrossRef]
- Tellenbach, C.; Grünig, C.R.; Sieber, T.N. Negative effects on survival and performance of Norway spruce seedlings colonized by dark septate root endophytes are primarily isolate-dependent. Environ. Microbiol. 2011, 13, 2508–2517. [Google Scholar] [CrossRef] [PubMed]
- Mayerhofer, M.S.; Kernaghan, G.; Harper, K.A. The effects of fungal root endophytes on plant growth: A meta-analysis. Mycorrhiza 2013, 23, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Newsham, K.K. A meta-analysis of plant responses to dark septate root endophytes. New Phytol. 2011, 190, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Fernando, A.A.; Currah, R.S. A comparative study of the effects of the root endophytes Leptodontidium orchidicola and Phialocephala fortinii (Fungi imperfecti) on the growth of some subalpine plants in culture. Can. J. Bot. 1996, 74, 1071–1078. [Google Scholar] [CrossRef]
- Currah, R.S.; Tsuneda, A.; Murakami, S. Morphology and ecology of Phialocephala fortinii in roots of Rhododendron brachycarpum. Can. J. Bot. 1993, 71, 1639–1644. [Google Scholar] [CrossRef]
- Jumpponen, A.; Mattson, K.G.; Trappe, J.M. Mycorrhizal functioning of Phialocephala fortinii with Pinus contorta on glacier forefront soil: Interactions with soil nitrogen and organic matter. Mycorrhiza 1998, 7, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, H.E.; Wang, C.J.K. Ectomycorrhizal and ectendomycorrhizal associations of Phialophora finlandia with Pinus resinosa, Picea rubens, and Betula alleghaensis. Can. J. For. Res. 1987, 17, 976–990. [Google Scholar] [CrossRef]
- Brundrett, M.C. Understanding the roles of multifunctional mycorrhizal and endophytic fungi. In Microbial Root Endophytes; Schulz, B.J.E., Boyle, C.J.C., Sieber, T.N., Eds.; Springer: Berlin/Heidelberg, Germeny; New York, NY, USA, 2006; pp. 281–298. [Google Scholar]
- O’Dell, T.E.; Massicotte, H.B.; Trappe, J.M. Root colonization of Lupinus latifolius Agardh. and Pinus contorta Dougl. by Phialocephala fortinii Wang & Wilcox. New Phytol. 1993, 124, 93–100. [Google Scholar]
- Wilcox, H.E.; Wang, C.J.K. Mycorrhizal and pathological associations of dematiaceous fungi in roots of 7-month-old tree seedlings. Can. J. For. Res. 1987, 17, 884–889. [Google Scholar] [CrossRef]
- Jumpponen, A. Dark septate endophytes—Are they mycorrhizal? Mycorrhiza 2001, 11, 207–211. [Google Scholar] [CrossRef]
- Lukešová, T.; Kohout, P.; Větrovský, T.; Vohník, M. The potential of dark septate endophytes to form root symbioses with ectomycorrhizal and ericoid mycorrhizal middle European forest plants. PLoS ONE 2015, 10, e0124752. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Elsevier, Academic Press: New York, NY, USA, 2008. [Google Scholar]
- Haselwandter, K.; Read, D.J. The significance of a root-fungus association in two Carex species of high alpine communities. Oecologia 1982, 53, 352–354. [Google Scholar] [CrossRef] [PubMed]
- Varma, A.; Rai, M.K.; Sahay, N.S. Microbial biotechnology: New paradigms and role in sustainable agriculture. In Microbial biotechnology for Sustainable Development and Productivity; Rajak, R.C., Ed.; Scientific Publishers: Jodhpur, India, 2000; pp. 22–37. [Google Scholar]
- Hiruma, K.; Gerlach, N.; Sacristan, S.; Nakano, R.T.; Hacquard, S.; Kracher, B.; Neumann, U.; Ramirez, D.; Bucher, M.; O’Connel, R.J.; et al. Root endophyte Colletotrichum tofieldiae confers plant fitness benefits that are phosphate status dependent. Cell 2016, 165, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Surono; Narisawa, K. The dark septate endophytic fungus Phialocephala fortinii is a potential decomposer of soil organic compounds and a promoter of Asparagus officinalis growth. Fungal Ecol. 2017, 28, 1–10. [Google Scholar] [CrossRef]
- Della Monica, I.F.; Saparrat, M.C.N.; Godeas, A.M.; Scervino, J.M. The co-existence between DSE and AMF symbionts affects P pools through P mineralization and solubilization processes. Fungal Ecol. 2015, 17, 10–17. [Google Scholar] [CrossRef]
- Heinonsalo, J.; Buée, M.; Vaario, L.-M. Root-endophytic fungi cause morphological and functional differences in Scots pine roots in contrast to ectomycorrhizal fungi. Botany 2017, 95, 203–210. [Google Scholar] [CrossRef] [Green Version]
- Schulz, B.J.E.; Guske, S.; Dammann, U.; Boyle, C. Endophyte-host interactions II. Defining symbiosis of the endophyte-host interaction. Symbiosis 1998, 25, 213–227. [Google Scholar]
- Schultz, B.J.E.; Boyle, C.J.C. The endophytic continuum. Mycol. Res. 2005, 109, 661–686. [Google Scholar] [CrossRef]
- Tellenbach, C.; Sieber, T.N. Do colonization by dark septate endophytes and elevated temperature affect pathogenicity of oomycetes? FEMS Microbiol. Ecol. 2012, 82, 157–168. [Google Scholar] [CrossRef] [Green Version]
- Tellenbach, C.; Sumarah, M.W.; Grünig, C.R.; Miller, D.J. Inhibition of Phytophthora species by secondary metabolites produced by the dark septate endophyte Phialocephala europaea. Fungal Ecol. 2012, 6, 12–18. [Google Scholar] [CrossRef]
- Bogner, C.W.; Kamdem, R.S.; Sichtermann, G.; Matthäus, C.; Hölscher, D.; Popp, J.; Proksch, P.; Grundler, F.M.; Schouten, A. Bioactive secondary metabolites with multiple activities from a fungal endophyte. Microb. Biotechnol. 2017, 10, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Martinuz, A.; Schouten, A.; Menjivar, R.D.; Sikora, R.A. Effectiveness of systemic resistance toward Aphis gossypii (Hom., Aphididae) as induced by combined applications of the endophytes Fusarium oxysporum Fo162 and Rhizobium etli G12. Biol. Control 2012, 62, 206–212. [Google Scholar] [CrossRef]
- Singh, U.B.; Sahu, A.; Sahu, N.; Singh, B.P.; Singh, R.K.; Singh, D.P.; Jaiswal, R.K.; Sarma, B.K.; Singh, H.B.; Manna, M.C.; et al. Can endophytic Arthrobotrys oligospora modulate accumulation of defence related biomolecules and induced systemic resistance in tomato (Lycopersicon esculentum Mill.) against root knot disease caused by Meloidogyne incognita. Appl. Soil Ecol. 2013, 63, 45–56. [Google Scholar] [CrossRef]
- Martínez-Medina, A.; Fernandez, I.; Lok, G.B.; Pozo, M.J.; Pieterse, C.M.J.; Van Wees, S.C.M. Shifting from priming of salicylic acid- to jasmonic acid-regulated defences by Trichoderma protects tomato against the root knot nematode Meloidogyne incognita. New Phytol. 2017, 213, 1363–1377. [Google Scholar] [CrossRef] [PubMed]
- Roylawar, P.; Panda, S.; Kamble, A. Comparative analysis of BABA and Piriformospora indica mediated priming of defence-related genes in tomato against early blight. Physiol. Mol. Plant Pathol. 2015, 91, 88–95. [Google Scholar] [CrossRef]
- Redman, R.S.; Sheehan, K.B.; Stout, R.G.; Rodriguez, R.J.; Henson, J.M. Thermotolerance conferred to plant host and fungal endophyte during mutualistic symbiosis. Science 2002, 298, 1581. [Google Scholar] [CrossRef]
- Waller, F.; Achatz, B.; Baltruschat, H.; Fodor, J.; Becker, K.; Fischer, M.; Heier, T.; Hückelhoven, R.; Neumann, C.; von Wettstein, D.; et al. The endophytic fungus Piriformospora indica reprograms barley to salt-stress tolerance, disease resistance, and higher yield. Proc. Natl. Acad. Sci. USA 2005, 102, 13386–13391. [Google Scholar] [CrossRef]
- Baltruschat, H.; Fodor, J.; Harrach, B.D.; Niemczyk, E.; Barna, B.; Gullner, G.; Janeczko, A.; Kogel, K.H.; Schafer, P.; Schwarczinger, I.; et al. Salt tolerance of barley induced by the root endophyte Piriformospora indica is associated with a strong increase in antioxidants. New Phytol. 2008, 180, 501–510. [Google Scholar] [CrossRef]
- Yamaji, K.; Watanabe, Y.; Masuya, H.; Shigeto, A.; Yui, H.; Haruma, T. Root fungal endophytes enhance heavy-metal stress tolerance of Clethra barbinervis growing naturally at mining sites via growth enhancement, promotion of nutrient uptake and decrease of heavy-metal concentration. PLoS ONE 2016, 11, e0169089. [Google Scholar] [CrossRef]
- Wang, J.L.; Li, T.; Liu, G.Y.; Smith, J.M.; Zhao, Z.W. Unraveling the role of dark septate endophyte (DSE) colonizing maize (Zea mays) under cadmium stress: Physiological, cytological and genic aspects. Sci. Rep. 2016, 6, 22028. [Google Scholar] [CrossRef] [PubMed]
- Likar, M.; Regvar, M. Isolates of dark septate endophytes reduce metal uptake and improve physiology of Salix caprea L. Plant Soil 2016, 370, 593–604. [Google Scholar] [CrossRef]
- Lacercat-Didier, L.; Berthelot, C.; Foulon, J.; Errard, A.; Martino, E.; Chalot, M.; Blaudez, D. New mutualistic fungal endophytes isolated from poplar roots display high metal tolerance. Mycorrhiza 2016, 26, 657–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schultz, B.J.E.; Boyle, C.J.C. What are endophytes. In Microbial Root Endophytes; Schulz, B.J.E., Boyle, C.J.C., Sieber, T., Eds.; Springer-Verlag: Berlin/Heidelberg, Germany, 2006; pp. 1–13. [Google Scholar]
- Van der Heijden, M.G.; Hartmann, M. Networking in the plant microbiome. PLoS Biol. 2016, 14, e1002378. [Google Scholar] [CrossRef] [PubMed]
- Poudel, R.; Jumpponen, A.; Schlatter, D.C.; Paulitz, T.C.; Gardener, B.B.; Kinkel, L.L.; Garrett, K.A. Microbiome networks: A systems framework for identifying candidate microbial assemblages for disease management. Phytopathology 2016, 106, 1083–1096. [Google Scholar] [CrossRef] [PubMed]
- Desprez-Loustau, M.L.; Aguayo, J.; Dutech, C.; Hayden, K.J.; Husson, C.; Jakushkin, B.; Marcais, B.; Piou, D.; Robin, C.; Vacher, C. An evolutionary ecology perspective to address forest pathology challenges of today and tomorrow. Ann. For. Sci. 2016, 73, 45–67. [Google Scholar] [CrossRef] [Green Version]
- Castrillo, G.; Teixeira, P.J.; Paredes, S.H.; Law, T.F.; de Lorenzo, L.; Feltcher, M.E.; Finkel, O.M.; Breakfield, N.W.; Mieczkowski, P.; Jones, C.D.; et al. Root microbiota drive direct integration of phosphate stress and immunity. Nature 2017, 543, 513–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, B.; Paulson, J.N.; Zheng, X.; Kolter, R. Simplified and representative bacterial community of maize roots. Proc. Natl. Acad. Sci. USA 2017, 114, E2450–E2459. [Google Scholar] [CrossRef]
- Timm, C.M.; Pelletier, D.A.; Jawdy, S.S.; Gunter, L.E.; Henning, J.A.; Engle, N.; Aufrecht, J.; Gee, E.; Nookaew, I.; Yang, Z.; et al. Two poplar-associated bacterial isolates induce additive favorable responses in a constructed plant-microbiome system. Front. Plant Sci. 2016, 7, 497. [Google Scholar] [CrossRef]
- Martin, J.A.; Macaya-Sanz, D.; Witzell, J.; Blumenstein, K.; Gil, L. Strong in vitro antagonism by elm xylem endophytes is not accompanied by temporally stable in planta protection against a vascular pathogen under field conditions. Eur. J. Plant Pathol. 2015, 142, 185–196. [Google Scholar] [CrossRef]
- Ahlholm, J.U.; Helander, M.; Henriksson, J.; Metzler, M.; Saikkonen, K. Environmental conditions and host genotype direct genetic diversity of Venturia ditricha, a fungal endophyte of birch trees. Evolution 2002, 56, 1566–1573. [Google Scholar] [CrossRef]
- Balint, M.; Tiffin, P.; Hallstrom, B.; O’Hara, R.B.; Olson, M.S.; Fankhauser, J.D.; Piepenbring, M.; Schmitt, I. Host genotype shapes the foliar fungal microbiome of balsam poplar (Populus balsamifera). PLoS ONE 2013, 8, e53987. [Google Scholar] [CrossRef] [PubMed]
- Pautasso, M.; Schlegel, M.; Holdenrieder, O. Forest health in a changing world. Microb. Ecol. 2015, 69, 826–842. [Google Scholar] [CrossRef]
- Newton, A.C.; Gravouil, C.; Fountaine, J.M. Managing the ecology of foliar pathogens: Ecological tolerance in crops. Ann. Appl. Biol. 2010, 157, 343–359. [Google Scholar] [CrossRef]
- Chakraborty, S.; Newton, A.C. Climate change, plant diseases and food security: An overview. Plant Pathol 2011, 60, 2–14. [Google Scholar] [CrossRef]
- Schlaeppi, K.; Bulgarelli, D. The plant microbiome at work. Mol. Plant Microbe Interact. 2015, 28, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Witzell, J.; Martín, J.A. Endophytes and Forest Health. In Endophytes of Forest Trees; Pirttilä, A., Frank, A., Eds.; Springer: Cham, Switzerland, 2018; pp. 261–282. [Google Scholar]
- Martín, J.A.; Solla, A.; Venturas, M.; Collada, C.; Domínguez, J.; Miranda, E.; Fuentes, P.; Burón, M.; Iglesias, S.; Gil, L. Seven Ulmus minor clones tolerant to Ophiostoma novo-ulmi registered as forest reproductive material in Spain. IForest 2014, 8, 172–180. [Google Scholar] [CrossRef]
- Newcombe, G. Endophytes in Forest Management: Four Challenges. In Endophytes of Forest Trees; Pirttilä, A., Frank, A., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 251–262. [Google Scholar]
- Carroll, G. Forest endophytes: Pattern and process. Can. J. Bot. 1995, 73, S1316–S1324. [Google Scholar] [CrossRef]
- Sieber, T. Fungal Root Endophytes. In Plant Roots: The Hidden Half; Waisel, Y., Eshel, A., Kafkafi, U., Eds.; Marcel Dekker: New York, NY, USA; Basel, Switzerland, 2002; pp. 887–917. ISBN 9780203909423. [Google Scholar]
- Stone, J.; Petrini, O. Endophytes of Forest Trees: A Model for Fungus-Plant Interactions. In Plant Relationships Part B. The Mycota (A Comprehensive Treatise on Fungi as Experimental Systems for Basic and Applied Research); Carroll, G.C., Tudzynski, P., Eds.; Springer: Berlin/Heidelberg, Germany, 1997; Volume 5B, ISBN 978-3-642-60647-2. [Google Scholar]
- Wilson, D.; Carroll, G.C. Avoidance of high-endophyte space by gall-forming insects. Ecology 1997, 78, 2153–2163. [Google Scholar] [CrossRef]
- Martínez-Arias, C.; Macaya-Sanz, D.; Witzell, J.; Martín, J.A. Enhancement of Populus alba tolerance to Venturia tremulae upon inoculation with endophytes showing in vitro biocontrol potential. Eur. J. Plant Pathol. 2018, 1–12. [Google Scholar] [CrossRef]
- Tellenbach, C.; Grünig, C.R.; Sieber, T.N. Suitability of quantitative real-time PCR to estimate the biomass of fungal root endophytes. Appl. Environ. Microbiol. 2010, 76, 5764–5772. [Google Scholar] [CrossRef] [PubMed]
- Chaparro, J.M.; Sheflin, A.M.; Manter, D.K.; Vivanco, J.M. Manipulating the soil microbiome to increase soil health and plant fertility. Biol. Fertil. Soils 2012, 48, 489–499. [Google Scholar] [CrossRef]
- Alabouvette, C.; Olivain, C.; Migheli, Q.; Steinberg, C. Microbiological control of soil-borne phytopathogenic fungi with special emphasis on wilt-inducing Fusarium oxysporum. New Phytol. 2009, 184, 529–544. [Google Scholar] [CrossRef] [PubMed]
- Postma, J.; Goossen-van de Geijn, H. Twenty-four years of Dutch Trig® application to control Dutch elm disease. BioControl 2016, 61, 305–312. [Google Scholar] [CrossRef]
- Scheffer, R.J. Mechanisms Involved in Biological Control of Dutch Elm Disease. J. Phytopathol. 1990, 130, 265–276. [Google Scholar] [CrossRef]
- Blodgett, J.T.; Eyles, A.; Bonello, P. Organ-dependent induction of systemic resistance and systemic susceptibility in Pinus nigra inoculated with Sphaeropsis sapinea and Diplodia scrobiculata. Tree Physiol. 2007, 27, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Onello, P.; Gordon, T.R.; Herms, D.A.; Wood, D.L.; Erbilgin, N. Nature and ecological implications of pathogen-induced systemic resistance in conifers: A novel hypothesis. Physiol. Mol. Plant Pathol. 2006, 68, 95–104. [Google Scholar] [CrossRef]
- Blumenstein, K.; Macaya-Sanz, D.; Martín, J.A.J A.; Albrectsen, B.R.B.R.; Witzell, J. Phenotype microarrays as a complementary tool to next generation sequencing for characterization of tree endophytes. Front. Microbiol. 2015, 6, 1033. [Google Scholar] [CrossRef]
- Stanosz, G.R.; Smith, D.R.; Guthmiller, M.A.; Stanosz, J.C. Persistence of Sphaeropsis sapinea on or in asymptomatic shoots of red and Jack pines. Mycologia 1997, 89, 525–530. [Google Scholar] [CrossRef]
- Stone, J.K. Initiation and development of latent infections by Rhabdocline parkeri on Douglas-fir. Can. J. Bot. 1987, 65, 2614–2621. [Google Scholar] [CrossRef]



| Main Group | Class | Transmission Type | Endophyte Classification | Host Range | Tissue Type | Functional Roles/Characteristics of Endophytes | Bioactive Compounds/Function | Literature Reference Number 1 |
|---|---|---|---|---|---|---|---|---|
| C 2 | 1 | vertical | Ascomycota | Grasses | Enhance drought tolerance, increase plant biomass, decrease herbivory | Yes 2 | [39,52,73,74,75,76,77,78] | |
| NC | 2 | most horizontal, few vertical | Dikarya (Ascomycota, Basidiomycota) | Forest trees 3 | Shoot, root and rhizome | Enhance stress tolerance of host plants (salinity, pH, temperature), increase host root and shoot biomass | E.g., phytohormones, seimatoric acid, colletonoic acid | [52,72,82,83,173,177] |
| 3 | horizontal | Dikarya (Ascomycota, Basidiomycota) | Aboveground tissues, especially leaves | Localized infections, pioneer decomposers, protection against herbivores or pathogens, niche competition, induction of systemic resistance, inhibition of insect and pathogen growth | Rugulosin, skyrin, emodin, vermiculin; homodimeric macrolide pyrenophorol, griseofulvin | [52,57,66,78,84,85,86,87,180,181,182,183,192,193,194,195,196,197,198] | ||
| 4 | horizontal | Ascomycota and Basidiomycota; mainly DSEs | Roots | Growth inhibition of pathogens, host plant growth promoti0n | Sclerin, sclerotinin, phytohormones | [52,61,68,229,230,232] | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terhonen, E.; Blumenstein, K.; Kovalchuk, A.; Asiegbu, F.O. Forest Tree Microbiomes and Associated Fungal Endophytes: Functional Roles and Impact on Forest Health. Forests 2019, 10, 42. https://doi.org/10.3390/f10010042
Terhonen E, Blumenstein K, Kovalchuk A, Asiegbu FO. Forest Tree Microbiomes and Associated Fungal Endophytes: Functional Roles and Impact on Forest Health. Forests. 2019; 10(1):42. https://doi.org/10.3390/f10010042
Chicago/Turabian StyleTerhonen, Eeva, Kathrin Blumenstein, Andriy Kovalchuk, and Fred O. Asiegbu. 2019. "Forest Tree Microbiomes and Associated Fungal Endophytes: Functional Roles and Impact on Forest Health" Forests 10, no. 1: 42. https://doi.org/10.3390/f10010042
APA StyleTerhonen, E., Blumenstein, K., Kovalchuk, A., & Asiegbu, F. O. (2019). Forest Tree Microbiomes and Associated Fungal Endophytes: Functional Roles and Impact on Forest Health. Forests, 10(1), 42. https://doi.org/10.3390/f10010042






