Whitebark Pine in Crater Lake and Lassen Volcanic National Parks: Assessment of Stand Structure and Condition in a Management and Conservation Perspective
Abstract
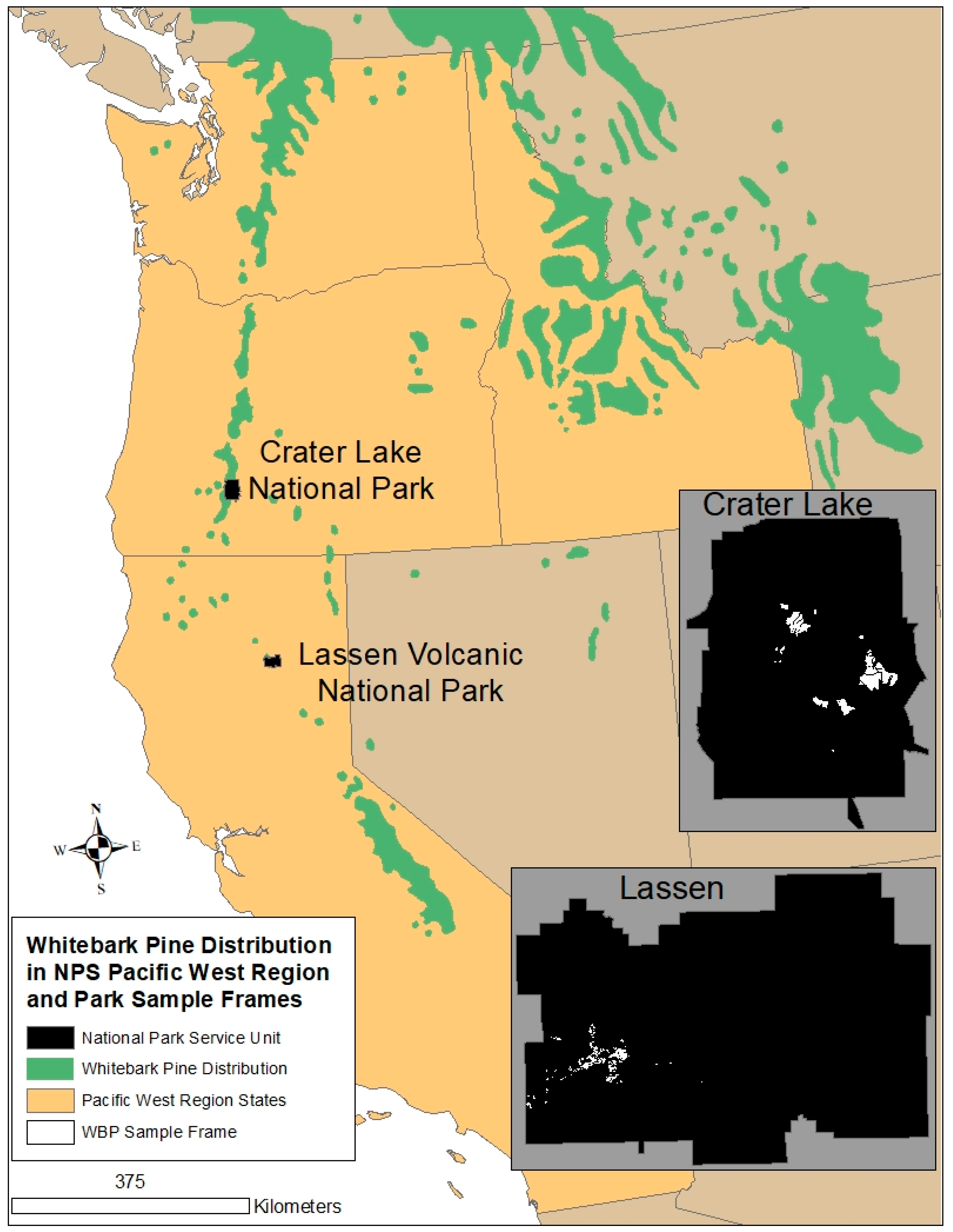
:1. Introduction
2. Materials and Methods
3. Results
3.1. Crater Lake National Park
3.2. Lassen Volcanic National Park
4. Discussion
4.1. Crater Lake National Park
4.2. Lassen Volcanic National Park
4.3. Regional Patterns
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tomback, D.F.; Achuff, P. Blister rust and western forest biodiversity: Ecology, values, and outlook for white pines. For. Pathol. 2010, 40, 186–225. [Google Scholar] [CrossRef]
- Ellison, A.M.; Bank, M.S.; Clinton, B.D.; Colburn, E.A.; Elliott, K.; Ford, C.R.; Foster, D.R.; Kloeppel, B.D.; Knoepp, J.D.; Lovett, G.M.; et al. Loss of foundation species: consequences for the structure and dynamics of forested ecosystems. Front. Ecol. Environ. 2005, 3, 479–486. [Google Scholar] [CrossRef]
- Keane, R.E.; Tomback, D.F.; Aubry, C.A.; Bower, A.D.; Campbell, E.M.; Cripps, C.L.; Jenkins, M.B.; Mahalovich, M.F.; Manning, M.; McKinney, S.T.; et al. A Range-wide Restoration Strategy for Whitebark Pine (Pinus albicaulis); Gen. Tech. Rep. RMRS-GTR-279; U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2012; p. 118.
- Tomback, D.F. Dispersal of whitebark pine seeds by Clark’s nutcracker: A mutualism hypothesis. J. Anim. Ecol. 1982, 51, 451–467. [Google Scholar] [CrossRef]
- Barringer, L.E.; Tomback, D.F.; Wunder, W.B. The Relationship Between whitebark pine health, cone production, and nutcracker occurrence across four National parks. In The future of high-Elevation, Five-Needle White Pines in Western North America: Proceedings of the High Five Symposium, Missoula, MT USA 28-30 June 2010; Keane, R.E., Tomback, D.F., Murray, M.P., Eds.; U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station RMRS-P-63: Fort Collins, CO, USA, 2010; pp. 45–46. [Google Scholar]
- McDonald, G.I.; Hoff, R.J. Blister rust: An introduced plague. In Whitebark Pine Communities: Ecology and Restoration; Tomback, D.F., Arno, S.F., Keane, R.E., Eds.; Island Press: Washington, DC, USA, 2001; pp. 193–220. [Google Scholar]
- McDonald, G.I.; Richardson, B.A.; Zambino, P.J.; Klopfenstein, N.B.; Kim, M.S. Pedicularis and Castilleja are natural hosts of Cronartium ribicola in North America: A first report. For. Pathol. 2006, 36, 73–82. [Google Scholar] [CrossRef]
- Beck, J.S.; Holm, G.W. Crater Lake National Park Whitebark Pine Conservation Plan; Crater Lake, OR, USA, Unpublished Report; 2014. [Google Scholar]
- Millar, C.I.; Westfall, R.D.; Delany, D.L.; Bokach, M.J.; Flint, A.L.; Flint, L.E. Forest mortality in high-elevation whitebark pine (Pinus albicaulis) forests of eastern California, USA; influence of environmental context, bark beetles, climatic water deficit, and warming. Can. J. For. Res. 2012, 42, 749–765. [Google Scholar] [CrossRef]
- Goheen, E.M.; Goheen, D.J.; Marshall, K.; Danchok, R.S.; Petrick, J.A.; White, D.E. The Status of Whitebark Pine along the Pacific Crest National Scenic Trail on the Umpqua National Forest; U.S. Department Agriculture, Forest Service, Pacific Northwest Research Station: Portland, OR, USA, 2002; p. 21.
- Murray, M.P.; Rasmussen, M.C. Non-native blister rust disease on whitebark pine at Crater Lake National Park. Northwest Sci. 2003, 77, 87–90. [Google Scholar]
- Murray, M. Will whitebark pine not fade away? Insight from CRLA National Park (2003–2009). Park Sci. 2010, 27, 64–67. [Google Scholar]
- Smith, S.B.; Odion, D.C.; Sarr, D.A.; Irvine, K.M. Monitoring direct and indirect climate effects on whitebark pine ecosystems at CRLA National Park. Park Sci. 2011, 28, 82–84. [Google Scholar]
- Keane, R.E.; Holsinger, L.M.; Mahalovich, M.F.; Tomback, D.F. Restoring Whitebark Pine Ecosystems in the Face of Climate Change; Gen. Tech. Rep. RMRS-GTR-361; U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2017; p. 123.
- Aubry, C.; Goheen, D.; Shoal, R.; Ohlson, T.; Lorenz, T.; Bower, A.; Mehmel, C.; Sniezko, R. Whitebark Pine Restoration Strategy for the Pacific Northwest Region: 2009–2013, Region 6 Report; USDA Forest Service, Pacific Northwest Region: Portland, Oregon, USA, 2008; pp. 1–96.
- Sniezko, R.A.; Mahalovich, M.F.; Schoettle, A.W.; Vogler, D.R. Past and current investigations of the genetic resistance to Cronartium ribicola. In The Future of High-elevation, Five-needle White Pines in Western North America: Proceedings of the High Five Symposium, Missoula, MT USA 28-30 June 2010; Keane, R.E., Tomback, D.F., Murray, M.P., Smith, C.M., Eds.; U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station RMRS-P-63: Fort Collins, CO, USA, 2011; pp. 246–264. [Google Scholar]
- Sniezko, R.A.; Kegley, A.J. Whitebark Pine Blister Rust Screening and Experimental Restoration at Crater Lake National Park: March 2012 Update; Dorena, OR, USA, Unpublished Report; 2012. [Google Scholar]
- Bentz, B.J.; Kegley, S.; Gibson, K.; Their, R. A test of high-dose verbenone for stand-level protection of lodgepole and whitebark pine from mountain pine beetle (Coleoptera: Curculionidae: Scolytinae) attacks. J. Econ. Entomol. 2005, 98, 1614–1621. [Google Scholar] [CrossRef]
- Kegley, S.; Gibson, K. Protecting Whitebark Pine Trees from Mountain Pine Beetle Attack Using Verbenone; Forest Health Protection Report 04-8; Department of Agriculture, Forest Service, Northern Region: Missoula, MT, USA, 2004.
- Arno, S.F. Community types and natural disturbance processes. In Whitebark Pine Communities: Ecology and Restoration; Tomback, D.F., Arno, S.F., Keane, R.E., Eds.; Island Press: Washington, DC, USA, 2001; pp. 74–88. [Google Scholar]
- Shannahan, E.; Irvine, K.M.; Thoma, D.; Wilmoth, S.; Ray, A.; Legg, K.; Shovic, H. Whitebark pine mortality related to white pine blister rust, mountain pine beetle outbreak, and water availability. Ecosphere 2016, 7, 1–18. [Google Scholar] [CrossRef]
- Chang, T.; Hansen, A.J.; Piekielek, N. Patterns and variability of project bioclimatic habitat for Pinus albicaulis in the Greater Yellowstone Area. PLoS ONE 2014, 9, e111669. [Google Scholar] [CrossRef] [PubMed]
- Diggins, C.; Fulé, P.Z.; Kaye, J.P.; Covington, W.W. Future climate affects management strategies for maintaining forest restoration treatments. Int. J. Wildland Fire 2010, 19, 903–913. [Google Scholar] [CrossRef]
- McKinney, S.T.; Rodhouse, T.; Chow, L.; Chung-Mac Coubrey, A.; Dicus, G.; Garrett, L.; Irvine, K.; Mohren, S.; Odion, D.; Sarr, D.; et al. Monitoring White Pine (Pinus albicaulis, P. balfouriana, P. flexilis) Community Dynamics in the Pacific West Region—Klamath, Sierra Nevada, and Upper Columbia Basin Networks: Narrative Version 1.0; Natural Resource Report NPS/PWR/–2012/532; National Park Service: Fort Collins, CO, USA, 2012.
- Stevens, D.L.; Olsen, A.R. Spatially balanced sampling of natural resources. J. Am. Stat. Assoc. 2004, 99, 262–278. [Google Scholar] [CrossRef]
- Daly, C.; Neilson, R.P.; Phillips, D.L. A statistical-topographic model for mapping climatological precipitation over mountainous terrain. J. Appl. Meteorol. 1994, 33, 140–158. [Google Scholar] [CrossRef]
- Daly, C.; Taylor, G.H.; Gibson, W.P.; Parzybok, T.W.; Johnson, G.L.; Pasteris, P.A. High-quality spatial climate data sets for the United States and beyond. Trans. ASAE 2000, 43, 1957–1962. [Google Scholar] [CrossRef]
- Daly, C.; Gibson, W.P.; Taylor, G.H.; Johnson, G.L.; Pasteris, P. A knowledge-based approach to the statistical mapping of climate. Clim. Res. 2002, 22(2), 99–113. [Google Scholar] [CrossRef] [Green Version]
- PRISM Climate Group. Available online: http://prism.oregonstate.edu (accessed on 4 February 2014).
- Soil Survey Staff. Natural Resources Conservation Service, United States Department of Agriculture. Web Soil Survey, 2016. Available online: http://websoilsurvey.nrcs.usda.gov/ (accessed on 6 January 2016).
- Thornthwaite, C.W. An approachtowards a rational classification of climate. Geogr. Rev. 1948, 38, 55–102. [Google Scholar] [CrossRef]
- Thornthwaite, C.W.; Mather, J.R. The water balance. Publicat. Climatol. 1955, 8, 1–104. [Google Scholar]
- Hamon, W.R. Computation of direct runoff amounts from storm rainfall. Int. Assoc. Sci. Hydrol. 1963, 63, 52–62. [Google Scholar]
- Willmott, C.T.; Rowe, C.M.; Mintz, Y. Climatology of the terrestrial seasonal water cycle. Int. J. Climatol. 1985, 5(6), 589–606. [Google Scholar] [CrossRef]
- Dingman, S.L. Physical Hydrology; Prentice Hall: Upper Saddle River, NJ, USA, 2002; pp. 272–325. [Google Scholar]
- Vorosmarty, C.J.; Federer, C.A.; Schloss, A.L. Evaporation functions compared on US watersheds: Possible implications for global-scale water balance and terrestrial ecosystem modeling. J. Hydrol. 1998, 207, 147–169. [Google Scholar] [CrossRef]
- Lutz, J.A.; van Wagtendonk, J.W.; Franklin, J.F. Climatic water deficit, tree species ranges, and climate change in Yosemite National Park. J. Biogeogr. 2010, 37, 936–950. [Google Scholar] [CrossRef]
- McCune, B.; Keon, D. Equations for potential annual direct incident radiation and heat load. J. Veg. Sci. 2002, 13, 603–606. [Google Scholar] [CrossRef]
- Smithson, M.; Verkuilen, J. A better lemon squeezer? Maximum-Likelihood Regression with beta-distributed dependent variables. Psychol. Methods 2006, 11, 54–71. [Google Scholar] [CrossRef] [PubMed]
- Schreuder, H.T.; Gregoire, T.G.; Weyer, J.P. The use of probability and non-probability sampling. Environ. Monit. Assess. 2001, 66, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Jules, E.S.; Jackson, J.I.; Smith, S.B.; Nesmith, J.C.B.; Starcevich, L.A.; Sarr, D.A. Whitebark Pine in Crater Lake and Lassen Volcanic National Parks: Initial Assessment of Stand Structure and Condition; Natural Resource Report NPS/KLMN/NRR—2017/1459; National Park Service: Fort Collins, CO, USA, 2017.
- Särndal, C.-E.; Swensson, B.; Wretman, J. Model Assisted Survey Sampling; Springer: New York, NY, USA, 1992. [Google Scholar]
- Horvitz, D.G.; Thompson, D.T. A generalization of sampling without replacement from a finite universe. J. Am. Stat. Assoc. 1952, 47, 663–685. [Google Scholar] [CrossRef]
- Gelman, A. Scaling regression inputs by dividing by two standard deviations. Stat. Med. 2008, 27, 2865–2873. [Google Scholar] [CrossRef] [PubMed]
- Saveland, J.M.; Neuenschwander, L.F. A signal detection framework to evaluate models of tree mortality following fire damage. Forest Sci. 1990, 36, 66–76. [Google Scholar]
- Hosmer, D.W.; Lemeshow, S. Applied Logistic Regression; John Wiley & Sons: New York, NY, USA, 2000; pp. 160–164. [Google Scholar]
- Nakagawa, S.; Schielzeth, H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 2013, 4, 133–142. [Google Scholar] [CrossRef]
- Ferrari, S.; Cribari-Neto, F. Beta regression for modelling rates and proportions. J. Appl. Stat. 2004, 31, 799–815. [Google Scholar] [CrossRef]
- Hilbe, J.M. Negative Binomial Regression; Cambridge University Press: New York, USA, 2011; pp. 141–180. ISBN 9780521198158. [Google Scholar]
- R Developement Core Team. R: A Language and Environment for Statistical Computing; R Developement Core Team: Vienna, Austria, 2016. [Google Scholar]
- Fournier, D.A.; Skaug, H.J.; Ancheta, J.; Ianelli, J.; Magnusson, A.; Maunder, M.N.; Nielsen, A.; Sibert, J. AD Model Builder: Using automatic differentiation for statistical inference of highly parameterized complex nonlinear models. Optim. Methods Softw. 2012, 27, 233–250. [Google Scholar] [CrossRef]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Zeileis, A.; Kleiber, C.; Jackman, S. Regression models for count data in R. J. Stat. Softw. 2008, 27, 1–25. [Google Scholar] [CrossRef]
- Six, D.L.; Adams, J. White pine blister rust severity and selection of individual whitebark pine by the mountain pine beetle (Coleoptera: Curculionidae, Scolytinae). J. Entomol. Sci. 2006, 42, 345–353. [Google Scholar] [CrossRef]
- Larson, E.R. Influences of the biophysical environment on blister rust and mountain pine beetle, and their interactions, in whitebark pine forests. J. Biogeogr. 2011, 38, 453–470. [Google Scholar] [CrossRef]
- Logan, J.; Powell, J. Ghost forests, global warming, and the mountain pine beetle (Coleoptera: Scolytidae). Am. Entomol. 2001, 47, 160–173. [Google Scholar] [CrossRef]
- Mote, P.W.; Salathé, E.P., Jr. Future climate in the Pacific Northwest. Clim. Change 2010, 102. [Google Scholar] [CrossRef]
- Bachelet, D.; Johnson, B.R.; Bridgham, S.D.; Dunn, P.V.; Anderson, H.E.; Rogers, B.M. Climate change impacts on western Pacific Northwest prairies and savannas. Northwest Sci. 2011, 85, 411–429. [Google Scholar] [CrossRef]
- Dobrowski, S.Z. A climatic basis for microrefugia: the influence of terrain on climate. Glob. Chang. Biol. 2011, 17, 1022–1035. [Google Scholar] [CrossRef]
- Campbell, E.M.; Antos, J.A. Distribution and severity of white pine blister rust and mountain pine beetle on whitebark pine in British Columbia. Can. J. For. Res. 2000, 30, 1051–1059. [Google Scholar] [CrossRef]
- Smith, J.P.; Hoffman, J.T. Site and stand characteristics related to white pine blister rust in high-elevation forests of southern Idaho and western Wyoming. West. N. Am. Nat. 2001, 61, 409–416. [Google Scholar]
- Kearns, H.S.J.; Jacobi, W.R.; Reich, R.M.; Burns, K.S.; Geils, B.W. Risk of white pine blister rust to limber pine in Colorado and Wyoming, USA. For. Path. 2014, 44, 21–38. [Google Scholar] [CrossRef]
- Zeglen, S. Whitebark pine and white pine blister rust in British Columbia, Canada. Can. J. For. Res. 2002, 32, 1265–1274. [Google Scholar] [CrossRef]
- Smith, C.M.; Wilson, B.; Rasheed, S.; Walker, R.C.; Carolin, T.; Shepherd, B. Whitebark pine and white pine blister rust in the Rocky Mountains of Canada and northern Montana. Can. J. For. Res. 2008, 38, 982–995. [Google Scholar] [CrossRef]
- Bockino, N.K.; Tinker, D.B. Interactions of white pine blister rust and mountain pine beetle in whitebark pine ecosystems in the Southern Yellowstone Area. Nat. Area. J. 2012, 32, 31–40. [Google Scholar] [CrossRef]
- Perkins, D.L.; Jorgensen, C.L.; Rinelle, M.J. Verbenone Decreases Whitebark Pine Mortality Throughout a Mountain Pine Beetle Outbreak. For. Sci. 2015, 61, 747–752. [Google Scholar] [CrossRef] [Green Version]
- McKinney, S.T.; Fiedler, C.E.; Tomback, D.F. Invasive pathogen threatens bird-pine mutualism: Implications for sustaining a high-elevation ecosystem. Ecol. Appl. 2009, 19, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.P.; Siderius, J. Historic Frequency and Severity of Fire in Whitebark Pine Forests of the Cascade Mountain Range, USA. Forests 2018, 9, 78. [Google Scholar] [CrossRef]
- Keane, R.E.; Gray, K.L.; Dickinson, L.J. Whitebark Pine Diameter Growth Response to Removal of Competition; Res. Note RMRS-RN-32; U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2007; pp. 1–9.
- Kauffmann, M.; Taylor, S.; Sikes, K.; Evens, J. Lassen National Forest: Whitebark Pine Pilot Fieldwork Report; Unpublished report; California Native Plant Society Vegetation Program: Sacramento, CA, USA, 2014. [Google Scholar]
- Naficy, C.E.; Keeling, E.G.; Landres, P.; Hessburg, P.F.; Veblen, T.T.; Sala, A. Wilderness in the 21st century: A framework for testing assumptions about ecological intervention in wilderness using a case study of fire ecology in the Rocky Mountains. J. For. 2015, 114, 384–395. [Google Scholar] [CrossRef]
- Morgan, P.; Murray, M. Implications for Conservation of Whitebark Pine. In Whitebark Pine Communities: Ecology and Restoration; Tomback, D.F., Arno, S.F., Keane, R.E., Eds.; Island Press: Washington, DC, USA, 2001; pp. 289–309. [Google Scholar]
- Weed, A.S.; Bentz, B.J.; Ayres, M.P.; Holmes, T.P. Geographically variable response of Dendroctonus ponderosae to winter warming in the western United States. Landsc. Ecol. 2015, 30, 1075–1093. [Google Scholar] [CrossRef]
- McCaughey, W.W.; Tomback, D.F. The natural regeneration process. In Whitebark Pine Communities: Ecology and Restoration; Tomback, D.F., Arno, S.F., Keane, R.E., Eds.; Island Press: Washington, DC, USA, 2001; pp. 105–120. [Google Scholar]
- Maloney, P.E. Incidence and distribution of white pine blister rust in the high-elevation forests of California. For. Pathol. 2011, 41, 308–316. [Google Scholar] [CrossRef]
- Nesmith, J.C.B.; Wright, M.; Jules, E.S.; McKinney, S.T. Whitebark and Foxtail Pine in Yosemite, Sequoia, and Kings Canyon National Parks: Initial Assessment of Stand Structure and Condition. Forests 2019, 10, 35. [Google Scholar] [CrossRef]
- Preisler, H.K.; Hicke, J.A.; Ager, A.A.; Hayes, J.L. Climate and weather influences on spatial temporal patterns of mountain pine beetle populations in Washington and Oregon. Ecology 2012, 93, 2421–2434. [Google Scholar] [CrossRef] [PubMed]
- Meigs, G.W.; Kennedy, R.E.; Gray, A.N.; Gregory, M.J. Spatiotemporal dynamics of recent mountain pine beetle and western spruce budworm outbreaks across the Pacific Northwest Region, USA. For. Ecol. Manage. 2015, 339, 71–86. [Google Scholar] [CrossRef] [Green Version]
- Meyer, M.; Bulaon, B.; MacKenzie, M.; Safford, H. Mortality structure and regeneration in whitebark pine stands impacted by mountain pine beetle in the southern Sierra Nevada, Can. J. For. Res. 2016, 46, 572–581. [Google Scholar]
- Ganz, D.J.; Dahlsten, D.L.; Shea, P.J. The post-burning response of bark beetles to prescribed burning treatments. In Fire, Fuels and Ecological Restoration: Conference Proceedings, Fort Collins, CO, USA, 16–18 April 2002; U.S. Department of Agriculture, Forest Service Rocky Mountain Research Station, RMRS-P-29: Fort Collins, CO, USA, 2003; pp. 143–158. [Google Scholar]







| Code | Description |
|---|---|
| Plot-level variables | |
| BaOther | Summed basal area of all non-WBP individuals in a plot (m2/ha) |
| BaWBP | Summed basal area of all WBP individuals in a plot (m2/ha). |
| Cwd | Average climatic water deficit (potential evapotranspiration-actual evapotranspiration, mm) |
| Tree-level variables | |
| Dbh | Diameter at breast height (cm) |
| Wpbr | Blister rust infection (Active and inactive cankers) (y/n) |
| Mpb | Mountain pine beetle infestation (y/n) |
| Cones | Female cones (y/n) |
| Parameter | CRLA | LAVO | ||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Live Trees (# individuals/ha) | ||||
| mountain hemlock | 312.53 | 444.57 | 525.87 | 505.55 |
| whitebark pine | 126.93 | 218.24 | 87.60 | 86.30 |
| lodgepole pine | 34.53 | 55.67 | 0.13 | 0.73 |
| red fir | 9.73 | 45.90 | 5.73 | 10.85 |
| western white pine | 0.27 | 1.46 | 0.13 | 0.73 |
| Live Trees (basal area, m2/ha) | ||||
| mountain hemlock | 20.01 | 25.69 | 14.47 | 14.52 |
| whitebark pine | 2.60 | 3.73 | 2.79 | 3.73 |
| lodgepole pine | 1.33 | 2.46 | <0.01 | 0 |
| red fir | 0.26 | 1.37 | 0.16 | 0.43 |
| western white pine | <0.01 | 0.01 | <0.01 | 0 |
| Dead whitebark pine (# individuals/ha) | 7.08 | 9.01 | 10 | 18.66 |
| Dead whitebark pine (basal area, m2/ha) | 1.85 | 3.49 | 0.40 | 1.06 |
| Proportion blister rust infected trees (basal area, m2/ha) | 0.51 | 0.32 | 0.54 | 0.35 |
| Proportion mountain pine beetle infested trees (basal area, m2/ha) | 0.02 | 0.07 | 0.01 | 0.03 |
| Covariate | CRLA | LAVO |
|---|---|---|
| BaWBP (m2/ha) | 4.45 (5.55) | 3.19 (3.99) |
| BaOther (m2/ha) | 29.76 (28.76) | 16.99 (15.2) |
| Cwd (mm) | 8.04 (2.32) | 8.81 (1.74) |
| Dbh (cm) | 14.11 (7.04) | 15.32 (13.33) |
| Characteristic | Model | Est | Std. Error | p | 95% CI |
|---|---|---|---|---|---|
| Incidence of white pine blister rust | Dbh | 0.372 | 0.086 | <0.001 | (0.203, 0.540) |
| Cwd | 0.596 | 0.252 | <0.05 | (0.101, 1.091) | |
| BaWBP | −0.365 | 0.321 | 0.255 | (−0.994, 0.263) | |
| BaOther | −0.069 | 0.185 | 0.707 | (−0.434, 0.294) | |
| Incidence of mpb | Wpbr | 2.359 | 0.754 | <0.01 | (0.881, 3.387) |
| Cwd | 0.384 | 0.263 | 0.144 | (−0.131, 0.900) | |
| Dbh | −0.273 | 0.268 | 0.308 | (−0.798, 0.252) | |
| BaOther | 0.160 | 0.231 | 0.488 | (−0.293, 0.614) | |
| BaWBP | −0.127 | 0.263 | 0.628 | (−0.644, 0.389) | |
| Production of cones | Dbh | 1.425 | 0.151 | <0.001 | (1.128, 1.721) |
| BaOther | −1.24 | 0.449 | <0.01 | (−2.123, −0.362) | |
| Cwd | 0.717 | 0.410 | 0.080 | (−0.086, 1.521) | |
| Wpbr | 0.157 | 0.279 | 0.572 | (−0.389, 0.704) | |
| Mpb | 0.645 | 0.749 | 0.389 | (−0.823, 2.114) | |
| BaWPB | −0.077 | 0.374 | 0.836 | (−0.811, 0.657) | |
| Proportion of average canopy killed | Dbh | 0.243 | 0.031 | <0.001 | (0.182, 0.304) |
| Wpbr | 0.228 | 0.063 | <0.001 | (0.104, 0.351) | |
| BaOther | 0.185 | 0.063 | <0.01 | (0.061, 0.310) | |
| BaWBP | 0.303 | 0.107 | <0.01 | (0.092, 0.515) | |
| Mpb | 0.433 | 0.178 | <0.05 | (0.083, 0.782) | |
| Cwd | −0.028 | 0.085 | 0.739 | (−0.194, 0.138) |
| Characteristic | Model | Est | Std. Error | p | 95% CI |
|---|---|---|---|---|---|
| Incidence of white pine blister rust | Dbh | −0.049 | 0.106 | 0.644 | (−0.257, 0.159) |
| Cwd | 0.312 | 0.357 | 0.383 | (−0.375, 1.005) | |
| BaWBP | −0.604 | 0.451 | 0.180 | (−1.475, 0.261) | |
| BaOther | −0.161 | 0.347 | 0.642 | (−0.830, 0.508) | |
| Production of cones | Dbh | 1.087 | 0.121 | <0.001 | (0.849, 1.325) |
| BaOther | −0.325 | 0.177 | 0.067 | (−0.672, 0.022) | |
| Cwd | 0.079 | 0.176 | 0.651 | (−0.264, 0.424) | |
| Wpbr | −0.102 | 0.236 | 0.664 | (−0.566, 0.361) | |
| BaWPB | 0.199 | 0.206 | 0.333 | (−0.202, 0.603) | |
| Mpb | −0.712 | 0.871 | 0.413 | (−2.41, 1.00) | |
| Proportion of average canopy killed | Wpbr | 0.464 | 0.074 | <0.001 | (0.293, 0.635) |
| Cwd | −0.232 | 0.106 | 0.030 | (−0.441, −0.023) | |
| Mpb | 0.509 | 0.265 | 0.055 | (−0.011, 1.030) | |
| Dbh | −0.031 | 0.038 | 0.421 | (−0.107, 0.044) | |
| BaOther | 0.074 | 0.102 | 0.471 | (−0.127, 0.275) | |
| Ba_WBP | 0.064 | 0.131 | 0.623 | (−0.322, 0.193) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jackson, J.I.; Smith, S.B.; Nesmith, J.C.B.; Starcevich, L.A.; Hooke, J.S.; Buckley, S.; Jules, E.S. Whitebark Pine in Crater Lake and Lassen Volcanic National Parks: Assessment of Stand Structure and Condition in a Management and Conservation Perspective. Forests 2019, 10, 834. https://doi.org/10.3390/f10100834
Jackson JI, Smith SB, Nesmith JCB, Starcevich LA, Hooke JS, Buckley S, Jules ES. Whitebark Pine in Crater Lake and Lassen Volcanic National Parks: Assessment of Stand Structure and Condition in a Management and Conservation Perspective. Forests. 2019; 10(10):834. https://doi.org/10.3390/f10100834
Chicago/Turabian StyleJackson, Jenell I., Sean B. Smith, Jonathan C.B. Nesmith, Leigh Ann Starcevich, Jennifer S. Hooke, Steve Buckley, and Erik S. Jules. 2019. "Whitebark Pine in Crater Lake and Lassen Volcanic National Parks: Assessment of Stand Structure and Condition in a Management and Conservation Perspective" Forests 10, no. 10: 834. https://doi.org/10.3390/f10100834
APA StyleJackson, J. I., Smith, S. B., Nesmith, J. C. B., Starcevich, L. A., Hooke, J. S., Buckley, S., & Jules, E. S. (2019). Whitebark Pine in Crater Lake and Lassen Volcanic National Parks: Assessment of Stand Structure and Condition in a Management and Conservation Perspective. Forests, 10(10), 834. https://doi.org/10.3390/f10100834





