Abstract
There is growing interest in the development of non-toxic, natural wood preservation agents to replace conventional chemicals. In this paper, the antifungal activities of silver nanoparticles, chitosan oligomers, and propolis ethanolic extract were evaluated against white-rot fungus Trametes versicolor (L.) Lloyd, with a view to protecting Populus spp. wood. In order to create a more realistic in-service type environment, the biocidal products were assessed according to EN:113 European standard, instead of using routine in vitro antimicrobial susceptibility testing methods. Wood blocks were impregnated with the aforementioned antifungal agents by the vacuum-pressure method in an autoclave, and their biodeterioration was monitored over 16 weeks. The results showed that treatments based on silver nanoparticles, at concentrations ranging from 5 to 20 ppm, presented high antifungal activity, protecting the wood from fungal attack over time, with weight losses in the range of 8.49% to 8.94% after 16 weeks, versus 24.79% weight loss in the control (untreated) samples. This was confirmed by SEM and optical microscopy images, which showed a noticeably higher cell wall degradation in control samples than in samples treated with silver nanoparticles. On the other hand, the efficacy of the treatments based on chitosan oligomers and propolis gradually decreased over time, which would be a limiting factor for their application as wood preservatives. The nanometal-based approach is thus posed as the preferred choice for the industrial treatment of poplar wood aimed at wood-based engineering products (plywood, laminated veneer lumber, cross-laminated timber, etc.).
1. Introduction
Populus spp., part of the Salicaceae family, are amongst the most frequently cultivated trees for industrial purposes. These fast-growing species have a significant economic impact, given that their wood is used for the production of pulp, panels, and many other commercial applications [1,2,3], in addition to being very important from an environmental perspective [4]. According to EN 350:2016, poplar wood is non-durable, and some studies have shown that it is highly susceptible to Trametes versicolor [5,6]. The fungal hyphae propagate through the woody elements, vessels, and tracheids of the sapwood, feeding on the constituents of the cell wall (lignin and structural carbohydrates) through the secretion of enzymes capable of metabolizing these structures [7]. This causes weight and mechanical resistance losses, color changes, and an increase in moisture content.
In recent decades, significant efforts have been devoted to the evaluation of natural products for wood protection applications, since they represent an alternative to traditionally used chemical compounds, which have toxic effects on humans and on the environment [8]. Amongst them, renewable polymers have attracted intense industrial interest, and chitosan in particular has especially promising application prospects [9]. Other products, such as propolis [10,11] and metallic nanoparticles [12] have also been the focus of increasing attention.
Various nano-sized inorganic materials based on metals or metal oxides have been assayed for wood protection, including silver, gold, zinc, copper, boron, titanium, tin, silicon, and cerium [12,13,14,15,16]. Among these, silver has shown the highest toxicity against bacterial and fungal growth [17,18,19,20,21,22]. However, it should be clarified that those studies were conducted against other wood-staining fungi and solely in agar plates. Only Dorau et al. [17] used wood blocks (as in this study), although they tested the activity of ionic silver instead of silver nanoparticles (AgNPs) against three brown-rot fungi (Postia placenta, Tephrophana palustris and Gloeophyllum trabeum). It is worth noting that the toxicity mechanisms of these two silver forms differ, as some biological effects—including the formation of reactive oxygen species (ROS) and extensive membrane damage—have been observed more severely for AgNPs than for ionic Ag+ [23].
Chitosan is a linear aminopolysaccharide biopolymer [24] obtained by the alkaline deacetylation of chitin extracted from the exoskeleton of crustaceans and from the cell walls of some fungi and algae. It stands out for its antimicrobial properties against algae, yeasts, some bacteria, and fungi [25]. With regards to the latter, chitosan not only inhibits their growth (fungistatic), but may also act as a fungicide at high concentrations [26]. Moreover, it has other very interesting properties, including biocompatibility, high biodegradability, bioactivity, and non-toxicity in humans [24,27]. Chitosan can be chemically and/or enzymatically modified [28] to prepare chitosan oligomers (pentamers and heptamers), which present enhanced antifungal behaviors [21,29,30,31].
Propolis is a resinous substance collected and transformed by bees (Apis mellifera L.) for use in honeycomb construction and repair [32]. The use of propolis as an antifungal agent is well known, and its effectiveness has been demonstrated, for instance, against Candida spp. [33,34]. Partially purified propolis extracts have been investigated for wood protection due to their antimicrobial effects against yeasts, molds, bacteria, and parasites [10,32]. Propolis’ activity against wood-decay fungi has been demonstrated in previous works [21,30,31].
The aim of this study was to compare the antifungal capacity of these three products (i.e., AgNPs, chitosan oligomers (CO), and propolis (P)), considered harmless to human health and the environment, against T. versicolor, assaying different concentrations on Populus spp. wood. Treatments were carried out by vacuum-pressure impregnation in an autoclave, according to EN:113 standard. The novelty of this study lies both in the use of a more realistic assessment method of the biocidal efficacy of the candidate wood preservatives (using wood blocks instead of agar plates) and in the fungus against which the products were assayed.
2. Materials and Methods
2.1. Reagents and Biological Material
Medium-molecular-weight chitosan (60–130 kDa; CAS No. 9012-76-4) with 90% deacetylation was acquired from Hangzhou Simit Chemical Technology Co. (Hangzhou, China). Propolis, with a content of ca. 10% w/v of polyphenols and flavonoids, came from the Duero river basin region (Burgos, Spain). Silver nitrate (CAS number 7761-88-8), sodium citrate (CAS 6132-04-3), acetic acid (CAS 64-19-7), and potassium methoxide (CAS 865-33-8) were supplied by Merck Millipore (Darmstadt, Germany).
The Wood Technology Laboratory, Universidad de Valladolid (Spain) supplied 432 wood blocks (50 × 25 × 15 mm3) of Populus × euramericana I-214 clone. The selected isolate of T. versicolor L. Lloyd 1920 (DSM 3086 strain, CECT 863A) was supplied by the Spanish Type Culture Collection (Valencia, Spain) and was cultivated on malt agar (supplied by Scharlau, Barcelona, Spain). Test specimen supports were sterilized by the steam method using an Autester ST DRY PV-II 30 LA autoclave (JP Selecta, Barcelona, Spain).
2.2. Synthesis of the Antifungal Solutions
Silver nanoparticles were synthetized by a sonication method; 50 mL of silver nitrate (50 mM) was mixed with 50 mL of sodium citrate (30 mM) as a reducing agent, and the solution was heated to 90 °C until it turned from colorless to pale yellow, which then became more intense. The yellowish solution was sonicated for 3–5 min with a probe-type UIP1000hdT ultrasonicator (Hielscher, Teltow, Germany; 1000 W, 20 kHz), and finally it was stabilized for at least 24 h in a refrigerator at 5 °C [35]. The resulting AgNPs, characterized by transmission electron microscopy (TEM) with a JEOL (Akishima, Tokyo, Japan) JEM-FS2200 HRP microscope, were spherical in shape with variable sizes, ranging from 10 to 30 nm.
Chitosan oligomers were prepared by oxidative degradation. Commercial medium molecular-weight (MW) chitosan was first dissolved in acetic acid (1%) under constant stirring at 60 °C for 2 h, then the pH was adjusted from 4.5 to 6.5 with potassium methoxide, and the molecular weight was finally reduced by adding hydrogen peroxide (0.3 M) to the solution, under stirring for 1 h at the same temperature. Chitosan oligomers of a viscosity-average MW of ca. 2000 Da were obtained [30] according to the experimental procedure proposed by Costa et al. [36].
Propolis ethanolic extract was prepared by grinding the resin and adding it to an hydroalcoholic solution 7:3 (v/v), followed by stirring for 72 h at room temperature and filtration with a stainless-steel 220 mesh to remove insoluble particles [37].
Every solution was tested at four different concentrations (Table 1). These concentrations were chosen on the basis of previously published work [30]. It should be clarified that, in contrast with that study (in which the wood block samples were only dipped in the preservative solution, and hence the adhesion properties of the bioagents played a major role), the vacuum-pressure impregnation method used herein allowed to use chitosan oligomers instead of medium MW chitosan, taking advantage of their superior minimum inhibitory values and lower viscosity.

Table 1.
Concentrations of the wood preservative substances used in the impregnation treatments.
2.3. Vacuum-Pressure Treatments and Antifungal Tests
The vacuum-pressure treatment and the subsequent antifungal efficacy assessment were carried out based on a modified EN:113 standard, with six replicates for each solution (i.e., antifungal product), concentration, and exposure period, accounting for a total of 288 blocks. The vacuum-pressure cycle can be briefly described as follows: wood blocks were first introduced into a stainless-steel autoclave, and a vacuum was created and maintained for 15 min. The selected product was then introduced by pressure difference, and pressure was increased to 6 kg·cm−2 for 2 h; pressure was finally removed, and the wood blocks were extracted from the remaining liquid. After impregnation, wood blocks were left at 20 °C and 65% relative humidity (RH) for 4 weeks in a Medline Scientific (Oxon, UK) conditioning chamber.
Antifungal activity tests were carried out by placing two treated wood blocks and one control block into a Kolle flask that contained a one-week-old fungal culture of T. versicolor. The samples were incubated in a culture chamber (Incubat 2000944) at 22 °C and 70% RH for 16 weeks. Every 4 weeks, six samples were taken out to measure the fungal attack by the weight loss. Wood blocks were carefully cleaned and dried at 103 ± 2 °C before the final weight was recorded. The weight loss in each sample was calculated according to Equation (1):
where M0 is the oven-dry weight of the sample before impregnation and prior to exposure, and Mf is the oven-dry weight of samples after exposure to fungus. Since the concentrations of the wood preservatives used in this study were very low (in the order of ppm), it was assumed that the dry weight of the impregnated wood was the same as the initial oven-dry weight.
2.4. Wood Degradation Monitoring
Wood degradation was monitored using optical microscopy and scanning electron microscopy (SEM). For the former, 30-µm microtome cuts were taken from the sample using a Leica (Wetzlar, Germany) microtome apparatus. These were analyzed with a Leica DMLM transmission optical microscope. The microtomes were also subjected to SEM examination at the facilities of the Microscopy Unit of the Parque Científico UVa (Spain), using a FE-SEM Leica LEO-1530 microscope. For SEM analyses, samples were adhered to the holder with double-sided carbon tape and covered with gold with a Quorum Emitech K575X sputter coater (Quorum Technologies, Ashford, Kent, UK). To confirm the composition of the AgNPs impregnated into the samples, an FEI QUANTA 200 FEG (Thermo-Fisher Scientific, Waltham, MA, USA) SEM microscope equipped with a Genesis XM 4i energy dispersive X-ray microanalysis unit was used to obtain EDX elemental maps of non-sputter-coated samples.
2.5. Statistical Analyses
All the statistical analyses were performed using R software (v. 3.4.4; R Development Core Team, 2018). Data from 288 treated samples, corresponding to 48 different individual groups (3 preservation agents × 4 concentration values × 4 exposure periods) × 6 replicates, in addition to 144 control samples, were analyzed. Prior to the analyses, the assumptions of independence, normality, and homoscedasticity were checked for all groups. Since all the data met the normality requirement (checked with a Shapiro–Wilks test) and the homoscedasticity requirement (checked with a Bartlett’s test), ANOVA was used. Bootstrapping and robust homogenous groups were used.
3. Results
Table 2 shows the average percentage of weight loss for the untreated samples (control) and for the samples treated with various concentrations of silver nanoparticles after exposure to T. versicolor for 4, 8, 12, and 16 weeks. The results of the analysis of variance verified that there were significant differences (p < 0.01) between the weight loss of the treatment and control samples. A long-term protective effect of the silver nanoparticles was observed during the development of the experiment, since the weight losses for all treatment concentrations oscillated between 8% and 10%, with no statistically significant differences, regardless of the exposure time. On the other hand, controls showed a progressive increase in weight loss over time: 13.7%, 17.5%, 23.8%, and 24.8% for the 4-, 8-, 12-, and 16-week exposure times, respectively.

Table 2.
Weight loss after different incubation periods for the silver nanoparticles treatments.
The weight loss results for the chitosan-oligomers-based treatment, assayed at different concentrations, are summarized in Table 3. As in the case of AgNPs, antifungal behavior was observed even at low concentrations, since all the treatments showed significant differences from the control treatment. In the first sampling, after 4 weeks of exposure, the highest concentration (80 mg·mL−1) showed the best antifungal activity (5% weight loss), showing statistically significant differences vs. the other concentrations (with weight losses in the 7.9%–11.6% range). Nonetheless, the effectiveness of the chitosan treatment clearly decreased over time and, after 12 weeks, there were no significant differences in weight loss between the different protective agent concentrations (all were in the 17.5%–22.3% range).

Table 3.
Effect of chitosan oligomers concentration and fungus exposure time on the protection of poplar wood against Trametes versicolor.
The antifungal activity of the propolis ethanolic extract on wood blocks exposed to T. versicolor for 4, 8, 12, and 16 weeks (Table 4) was more dependent on the concentration as a function of time than the other two protective agents evaluated in this work. A clear relationship between propolis concentration and wood weight loss was observed, as they were inversely proportional in every sampling period (e.g., in the first sampling, the weight loss decreased from of 13.7% to 9.8% as propolis concentration was increased from 5 to 40 mg/mL). Although all propolis ethanolic extract concentrations showed significant differences vs. the control, a gradual decrease in the antifungal activity of these treatments over time was evidenced. The weight loss in the first 8 weeks was higher than that obtained for chitosan (14.3% for propolis vs. 10.1% for chitosan), but after 16 weeks the weight loss values were comparable for the two treatments (21.0% vs. 23.5%).

Table 4.
Effect of treatment concentration of propolis ethanolic extract and time of fungus exposure (sampling) on the protection of poplar wood against T. versicolor.
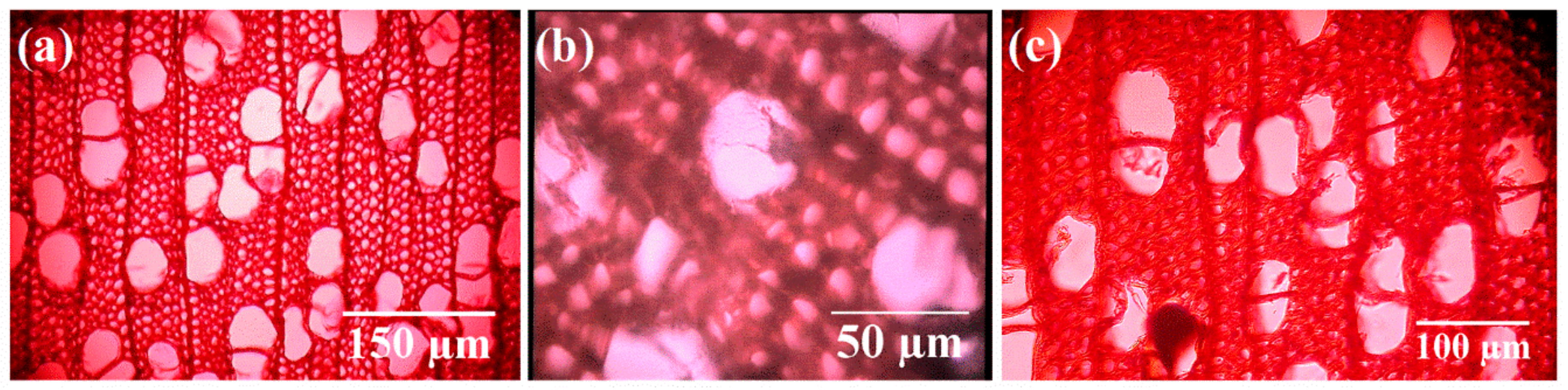
The optical microscopy images confirmed that the wood blocks exposed to the attack of T. versicolor without any protective treatment (control) suffered a progressive degradation over time, with weight loss values ranging from 13.7%–14.5% after 4 weeks to 24.8%–38.1% after 16 weeks. Figure 1 shows the degradation of vessel elements and fibers on untreated wood upon exposure to T. versicolor for 16 weeks, whereas in the case of the sample treated with AgNPs the observed biodeterioration was very small. A micrograph of a healthy poplar wood sample with no degradation is shown for comparison purposes.

Figure 1.
Optical micrographs of (a) undecayed control wood sample; (b) decayed control wood sample; and (c) sample treated with silver nanoparticles (AgNPs) (20 ppm), subjected to the action of T. versicolor for 16 weeks.
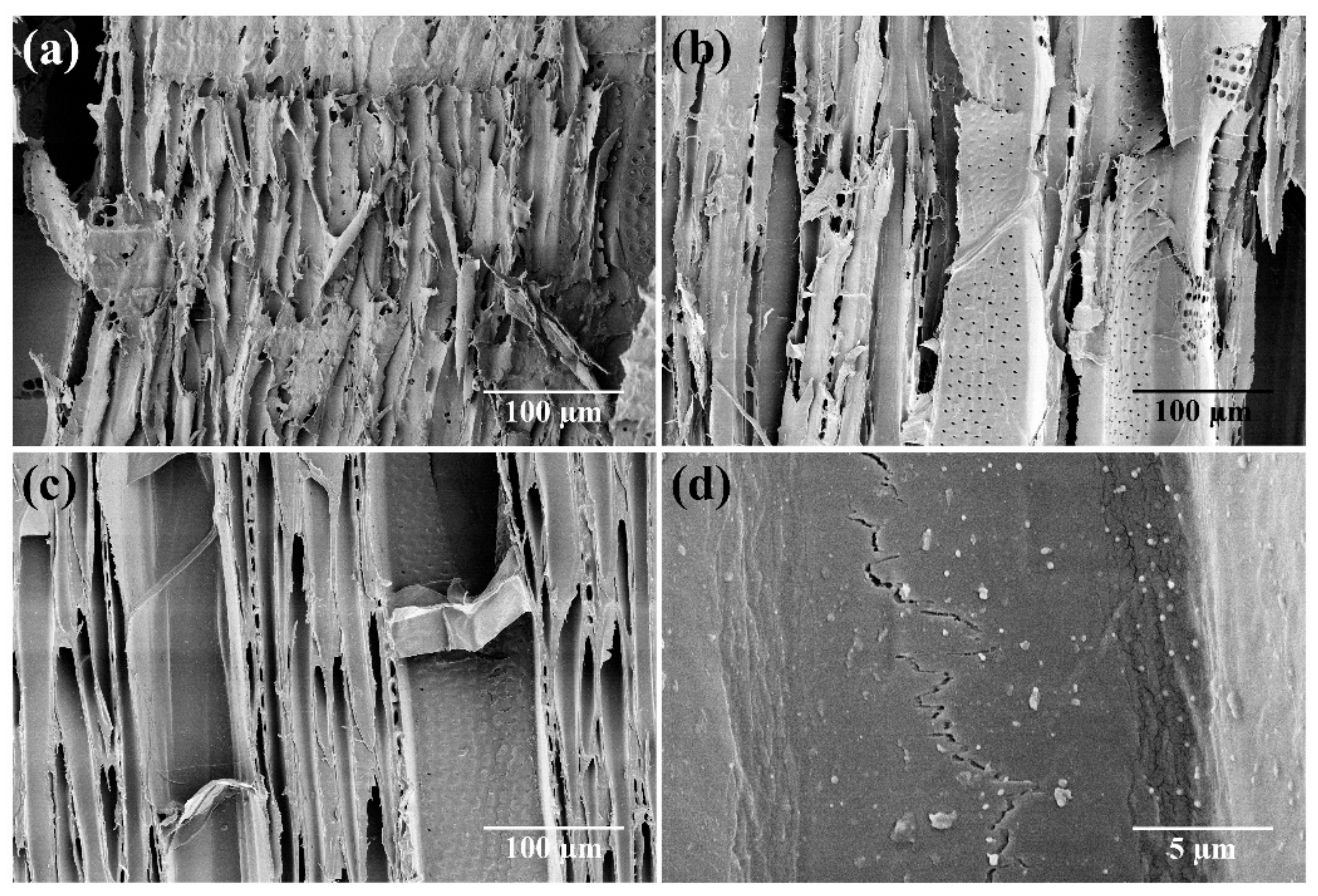
The SEM images (Figure 2) also confirmed that the fungal attack caused severe damage to the wood cell wall after 16 weeks of exposure in control samples (Figure 2a). The damage was reduced in the samples treated with propolis (Figure 2b), suggesting that propolis had a protective activity. However, the best wood preservation was attained for silver nanoparticles: as shown in Figure 2c, the cell wall was practically intact. In Figure 2d, at 5000× magnification, silver nanoparticles can be observed on the cell walls. The composition of AgNPs was confirmed by EDX elemental mapping.
Figure 2.
SEM images of wood samples after 16 weeks of exposure to T. versicolor: (a) control samples, (b) samples treated with propolis (40 mg/mL), (c) samples treated with silver nanoparticles (20 ppm), and (d) zoomed-in view of silver nanoparticles on the cell wall.
4. Discussion
The optical microscopy images showed that wooden blocks exposed to T. versicolor attack without any protective treatment (control) suffered a significant degradation of the cell wall, thus confirming the high susceptibility of poplar wood to white-rot fungus [6]. This was further evidenced in the SEM micrographs, which also showed that the fungal attack caused severe damage to the wood cell wall after 16 weeks of exposure in control samples: the middle lamella was degraded and the fibers were separated due to lignin degradation [38].
In relation to the antifungal effectiveness of the pressure treatment with silver nanoparticles, the obtained results were similar to those of Akhtari et al. [39]. These authors evaluated the effectiveness of aqueous solutions of silver nanoparticles against T. versicolor, albeit at much higher concentrations (400 ppm), and reported a weight loss of only 2.1% for the treated wood 4 weeks after the inoculation. The low weight losses of around 8.5% for the pieces treated with nanosilver are in agreement with Clausen et al. [13] and Matsunaga et al. [14], who suggested that the advantageous behavior of nanometal-based wood protectors would arise from the slow and controlled release of the bioactive metal ions into the wood cells.
Apropos of the observed decrease in the antifungal activity of chitosan oligomers over time, it was also reported by Alfredsen et al. [26], who noted that the protective nature of chitosan in the long term is lower than those of other commercial fungicides, thus confirming that organic compounds are more prone to biodegradation [8]. According to Larnøy et al. [40], the decrease in the effectiveness of chitosan over time could be due to fungal degradation, as it would be affected by the action of enzymes excreted by fungi in cellulose degradation. Other studies that investigated the applicability of chitosan oligomers as a wood protection agent also observed a gradual loss of its antifungal activity over time [30,40,41].
A gradual decrease in the antifungal activity of propolis treatments over time was also noticed, which may be ascribed either to biodegradability or to a lower retention of the propolis ethanolic extract solution in wood, both of which are very common problems in organic biocides [8].
Consequently, although the use of these two organic bioagents may be improved by adding stabilizers to the solution, the nanometal-based approach would be the preferred option for industrial applications.
5. Conclusions
On the basis of biocidal efficacy tests conducted on wood blocks, more realistic than routine antimicrobial susceptibility tests conducted in agar plates, it was evidenced that the three protective agents assayed in the vacuum-pressure treatments (AgNPs, chitosan, and propolis) showed a protective effect on Populus spp. wood. However, the one based on AgNPs featured the highest effectiveness against T. versicolor over time: even at the lowest concentration (5 ppm), it minimized the biodeterioration of poplar wood for over 4 months, albeit higher concentrations (of up to 20 ppm) were more effective. SEM images confirmed the effectiveness of the impregnation with nanosilver and evidenced the severe cell wall degradation by the fungus on untreated samples. Chitosan oligomers and propolis-based treatments also showed results with significant differences vs. the non-treated wood, but a noticeable decrease in their effectiveness over the 16 weeks was observed, pointing to either biodegradation or adsorption issues (to which all organic bioactive agents are susceptible). Hence, AgNPs can be put forward as effective protective agents for poplar-wood-based engineering products.
Author Contributions
Conceptualization, M.M.C.-S., J.M.-G., and L.A.-R.; Formal analysis, P.M.-R. and L.A.-R.; Funding acquisition, M.M.C.-S.; Investigation, M.M.C.-S., I.S.-C., L.P.-H., and J.M.-G.; Methodology, M.M.C.-S., P.M.-R., J.M.-G., and L.A.-R.; Resources, M.M.C.-S., J.M.-G., and L.A.-R.; Supervision, M.M.C.-S., J.M.-G., and L.A.-R.; Validation, I.S.-C., L.P.-H., and L.A.-R.; Visualization, P.M.-R.; Writing—original draft, M.M.C.-S., I.S.-C., L.P.-H., and P.M.-R.; Writing—review & editing, P.M.-R. and L.A.-R.
Funding
This study was funded by JUNTA DE CASTILLA Y LEÓN under project VA258P18, with FEDER co-funding.
Acknowledgments
P.M.-R. acknowledges the support of Universidad de Zaragoza under project UZ2019-TEC-07. I.S.-C. would like to gratefully acknowledge the financial support of the National Council for Science and Technology (CONACYT) of Mexico, through PhD Scholarship ref. no. 329975. The authors also wish to thank Melissa Boyd for kindly revising the use of English language.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Karimi, A.; Taghiyari, H.R.; Fattahi, A.; Karimi, S.; Ebrahimi, G.; Tarmian, A. Effects of wollastonite nanofibers on biological durability of poplar wood (Populus nigra) against Trametes versicolor. BioResources 2013, 8, 4134. [Google Scholar] [CrossRef]
- Casado, M.; Acuña, L.; Basterra, L.-A.; Ramón-Cueto, G.; Vecilla, D. Grading of structural timber of Populus × euramericana clone I-214. Holzforschung 2012, 66, 633. [Google Scholar] [CrossRef]
- Todaro, L.; Russo, D.; Cetera, P.; Milella, L. Effects of thermo-vacuum treatment on secondary metabolite content and antioxidant activity of poplar (Populus nigra L.) wood extracts. Ind. Crop. Prod. 2017, 109, 384–390. [Google Scholar] [CrossRef]
- Kollert, W.; Borodowski, E.D. Situación de las Salicáceas en el Mundo. In Proceedings of the Jornadas de Saliáceas 2014—IV Congreso Internacional de Salicáceas en Argentina, Buenos Aires, Argentina, 18–21 March 2014; p. 10. [Google Scholar]
- Diaz, B.; Murace, M.; Peri, P.; Keil, G.; Luna, L.; Otaño, M.Y. Natural and preservative-treated durability of Populus nigra cv Italica timber grown in Santa Cruz Province, Argentina. Int. Biodeterior. Biodegrad. 2003, 52, 43–47. [Google Scholar] [CrossRef]
- Xing, J.-Q.; Ikuo, M.; Wakako, O. Natural resistance of two plantation woods Populus × canadensis cv. and Cunninghamia lanceolata to decay fungi and termites. For. Stud. China 2005, 7, 36–39. [Google Scholar] [CrossRef]
- Pandey, K.K.; Pitman, A.J. FTIR studies of the changes in wood chemistry following decay by brown-rot and white-rot fungi. Int. Biodeterior. Biodegrad. 2003, 52, 151–160. [Google Scholar] [CrossRef]
- Singh, T.; Singh, A.P. A review on natural products as wood protectant. Wood Sci. Technol. 2011, 46, 851–870. [Google Scholar] [CrossRef]
- Thakur, V.K.; Thakur, M.K. Recent advances in graft copolymerization and applications of chitosan: A review. ACS Sustainable Chem. Eng. 2014, 2, 2637–2652. [Google Scholar] [CrossRef]
- Quiroga, E.N.; Sampietro, D.A.; Soberon, J.R.; Sgariglia, M.A.; Vattuone, M.A. Propolis from the northwest of Argentina as a source of antifungal principles. J. Appl. Microbiol. 2006, 101, 103–110. [Google Scholar] [CrossRef]
- Torlak, E.; Sert, D. Antibacterial effectiveness of chitosan–propolis coated polypropylene films against foodborne pathogens. Int. J. Biol. Macromol. 2013, 60, 52–55. [Google Scholar] [CrossRef]
- Kartal, S.N.; Green, F.; Clausen, C.A. Do the unique properties of nanometals affect leachability or efficacy against fungi and termites? Int. Biodeterior. Biodegrad. 2009, 63, 490–495. [Google Scholar] [CrossRef]
- Clausen, C.A.; Yang, V.W.; Arango, R.A.; Green, F., III. Feasibility of nanozinc oxide as a wood preservative. Proc. Am. Wood Prot. Assoc. 2009, 105, 255–260. [Google Scholar]
- Matsunaga, H.; Kiguchi, M.; Evans, P.D. Microdistribution of copper-carbonate and iron oxide nanoparticles in treated wood. J. Nanopart. Res. 2008, 11, 1087–1098. [Google Scholar] [CrossRef]
- Marzbani, P.; Mohammadnia-afrouzi, Y. Investigation on leaching and decay resistance of wood treated with nano-titanium dioxide. Adv. Environ. Biol. 2014, 8, 974–979. [Google Scholar]
- Nair, S.; Pandey, K.K.; Giridhar, B.N.; Vijayalakshmi, G. Decay resistance of rubberwood (Hevea brasiliensis) impregnated with ZnO and CuO nanoparticles dispersed in propylene glycol. Int. Biodeterior. Biodegrad. 2017, 122, 100–106. [Google Scholar] [CrossRef]
- Dorau, B.; Arango, R.; Green, F. An Investigation into the Potential of Ionic Silver as a Wood Preservative, Proceedings from the Woodframe Housing Durability and Disaster Issues Conference, Las Vegas, NV, USA, 4–6 October 2004; Forest Products Society: Las Vegas, NV, USA, 2004; pp. 133–145.
- Velmurugan, N.; Kumar, G.G.; Han, S.S.; Nahm, K.S.; Lee, Y.S. Synthesis and characterization of potential fungicidal silver nano-sized particles and chitosan membrane containing silver particles. Iran. Polym. J. 2009, 18, 383–392. [Google Scholar]
- Kaur, P.; Thakur, R.; Choudhary, A. An in vitro study of the antifungal activity of silver/chitosan nanoformulations against important seed borne pathogens. Int. J. Sci. Technol. Res. 2012, 1, 83–86. [Google Scholar]
- Narayanan, K.B.; Park, H.H. Antifungal activity of silver nanoparticles synthesized using turnip leaf extract (Brassica rapa L.) against wood rotting pathogens. Eur. J. Plant Pathol. 2014, 140, 185–192. [Google Scholar] [CrossRef]
- Silva-Castro, I.; Martín-García, J.; Diez, J.J.; Flores-Pacheco, J.A.; Martín-Gil, J.; Martín-Ramos, P. Potential control of forest diseases by solutions of chitosan oligomers, propolis and nanosilver. Eur. J. Plant Pathol. 2017, 150, 401–411. [Google Scholar] [CrossRef]
- Kim, S.W.; Jung, J.H.; Lamsal, K.; Kim, Y.S.; Min, J.S.; Lee, Y.S. Antifungal effects of silver nanoparticles (AgNPs) against various plant pathogenic fungi. Mycobiology 2018, 40, 53–58. [Google Scholar] [CrossRef]
- Ivask, A.; ElBadawy, A.; Kaweeteerawat, C.; Boren, D.; Fischer, H.; Ji, Z.; Chang, C.H.; Liu, R.; Tolaymat, T.; Telesca, D.; et al. Toxicity mechanisms in Escherichia coli vary for silver nanoparticles and differ from ionic silver. ACS Nano 2013, 8, 374–386. [Google Scholar] [CrossRef] [PubMed]
- Bin Ahmad, M.; Lim, J.J.; Shameli, K.; Ibrahim, N.A.; Tay, M.Y. Synthesis of silver nanoparticles in chitosan, gelatin and chitosan/gelatin bionanocomposites by a chemical reducing agent and their characterization. Molecules 2011, 16, 7237–7248. [Google Scholar] [CrossRef] [PubMed]
- Goycoolea, F. Monografía XXVIII: Nanotecnología Farmacéutica. Available online: https://www.analesranf.com/index.php/mono/article/view/990/1024 (accessed on 7 October 2019).
- Alfredsen, G.; Eikenes, M.; Militz, H.; Solheim, H. Screening of chitosan against wood-deteriorating fungi. Scand. J. For. Res. 2011, 19, 4–13. [Google Scholar] [CrossRef]
- Xia, W.; Liu, P.; Zhang, J.; Chen, J. Biological activities of chitosan and chitooligosaccharides. Food Hydrocoll. 2011, 25, 170–179. [Google Scholar] [CrossRef]
- Rabea, E.I.; Badawy, M.E.T.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Badawy, M.E.I.; Rabea, E.I. Potential of the biopolymer chitosan with different molecular weights to control postharvest gray mold of tomato fruit. Postharvest Biol. Technol. 2009, 51, 110–117. [Google Scholar] [CrossRef]
- Silva-Castro, I.; Casados-Sanz, M.; Alonso-Cortés, A.; Martín-Ramos, P.; Martín-Gil, J.; Acuña-Rello, L. Chitosan-based coatings to prevent the decay of Populus spp. wood caused by Trametes versicolor. Coatings 2018, 8, 415. [Google Scholar] [CrossRef]
- Silva-Castro, I.; Diez, J.; Martín-Ramos, P.; Pinto, G.; Alves, A.; Martín-Gil, J.; Martín-García, J. Application of bioactive coatings based on chitosan and propolis for Pinus spp. protection against Fusarium circinatum. Forests 2018, 9, 685. [Google Scholar] [CrossRef]
- Burdock, G.A. Review of the biological properties and toxicity of bee propolis (propolis). Food Chem. Toxicol. 1998, 36, 347–363. [Google Scholar] [CrossRef]
- Marcucci, M.C. Propolis: Chemical composition, biological properties and therapeutic activity. Apidologie 1995, 26, 83–99. [Google Scholar] [CrossRef]
- Oryan, A.; Alemzadeh, E.; Moshiri, A. Potential role of propolis in wound healing: Biological properties and therapeutic activities. Biomed. Pharmacother. 2018, 98, 469–483. [Google Scholar] [CrossRef] [PubMed]
- Matei, P.M.; Martin-Ramos, P.; Sanchez-Bascones, M.; Hernandez-Navarro, S.; Correa-Guimaraes, A.; Navas-Gracia, L.M.; Rufino, C.A.; Ramos-Sanchez, M.C.; Martin-Gil, J. Synthesis of chitosan oligomers/propolis/silver nanoparticles composite systems and study of their activity against Diplodia seriata. Int. J. Polym. Sci. 2015, 2015, 864729. [Google Scholar] [CrossRef]
- Costa, C.N.; Teixeira, V.G.; Delpech, M.C.; Souza, J.V.S.; Costa, M.A.S. Viscometric study of chitosan solutions in acetic acid/sodium acetate and acetic acid/sodium chloride. Carbohydr. Polym. 2015, 133, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Araujo-Rufino, C.; Fernandes-Vieira, J.; Martín-Ramos, P.; Silva-Castro, I.; Fernandes-Correa, M.; Matei, P.M.; Sánchez-Báscones, M.; Ramos-Sánchez, M.C.; Martín-Gil, J. Synthesis of chitosan oligomers composite systems and study of their activity against Bipolaris Oryzae. J. Mater. Sci. Eng. Adv. Technol. 2016, 13, 29–52. [Google Scholar]
- Schwarze, F.W.M.R. Wood decay under the microscope. Fungal Biol. Rev. 2007, 21, 133–170. [Google Scholar] [CrossRef]
- Akhtari, M.; Arefkhani, M. Study of microscopy properties of wood impregnated with nanoparticles during exposed to white-rot fungus. Agric. Sci. Dev. 2013, 2, 116–119. [Google Scholar]
- Larnøy, E.; Eikenes, M.; Militz, H. Evaluation of factors that have an influence on the fixation of chitosan in wood. Wood Mater. Sci. Eng. 2006, 1, 138–145. [Google Scholar] [CrossRef]
- El-Gamal, R.; Nikolaivits, E.; Zervakis, G.I.; Abdel-Maksoud, G.; Topakas, E.; Christakopoulos, P. The use of chitosan in protecting wooden artifacts from damage by mold fungi. Electron. J. Biotechnol. 2016, 24, 70–78. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).