Drought Superimposes the Positive Effect of Silver Fir on Water Relations of European Beech in Mature Forest Stands
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Site Conditions and Experimental Setup
2.2. Meteorological and Soil Data
2.3. Tree Water Use
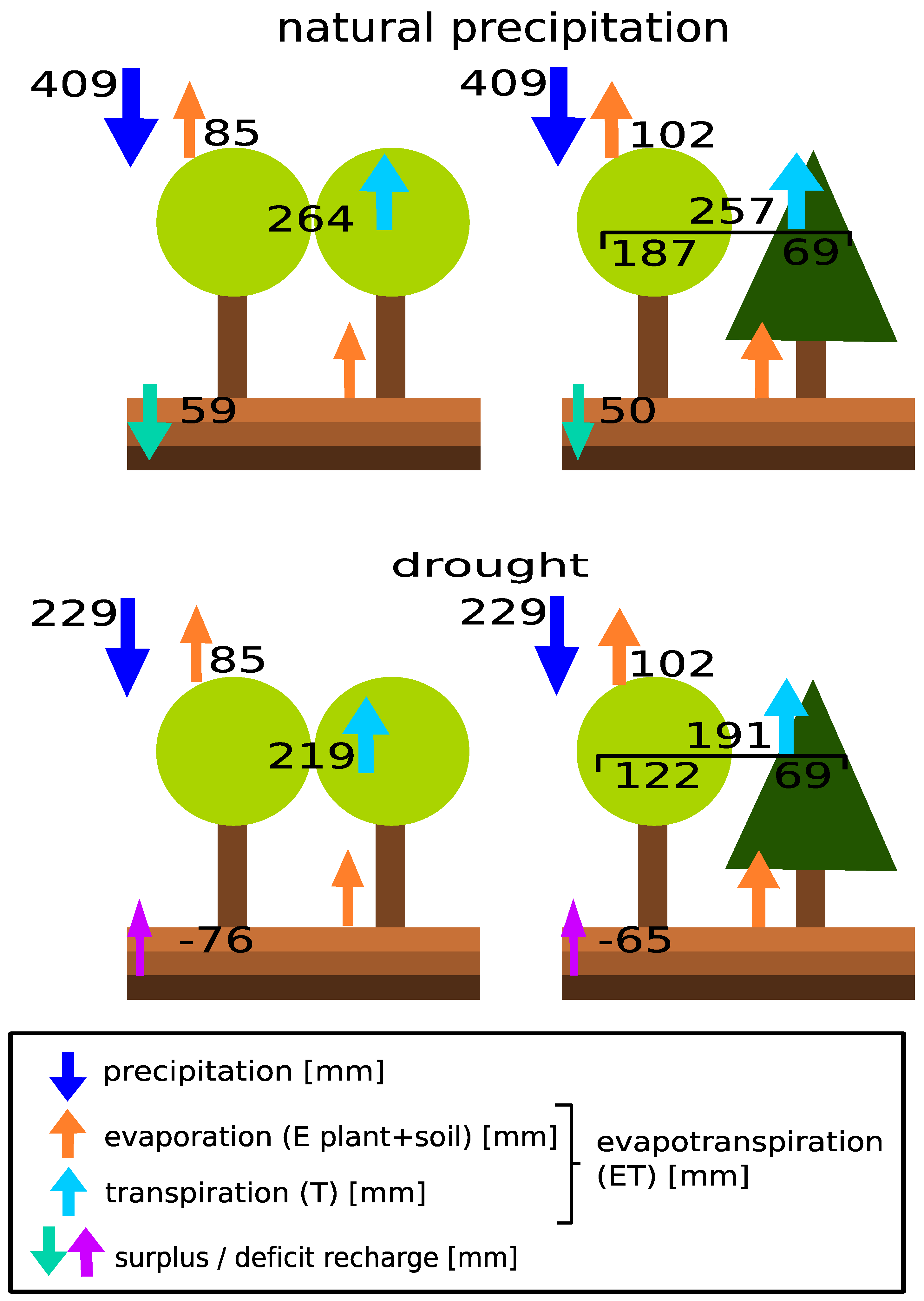
2.4. Upscaling Approach
2.5. Stand Water Budget
2.6. Statistics
3. Results
3.1. Meteorological and Soil Moisture Conditions
3.2. Individual Tree Sap Flow Rates and Correlation with Environmental Parameters
3.3. Mean Sap Flow Density
3.4. Stand Water Use
4. Discussion
4.1. Sap Flow of Individual Trees Provide a Good Estimate for Upscaling to Stand Transpiration
4.2. Sap Flow of Beech Trees in Pure and Mixed Stands Differs Considerably
4.3. Rain Exclusion Superimposes Facilitation in Mixed Stands
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Keenan, R.J. Climate Change Impacts and Adaptation in Forest Management: A Review. Ann. For. Sci. 2015, 72, 145–167. [Google Scholar] [CrossRef]
- Seppala, R.; Buck, A.; Katila, P. (Eds.) Adaption of Forests and People to Climate Change—A Global Assessment Report; International Union of Forest Research Organizations (IUFRO): Helsinki, Finland, 2009; Volume 22. [Google Scholar]
- Kliwa. Klimawandel in Suddeutschland Veranderungen von Meteorologischen Und Hydrologischen Kenngroßen Klimamonitoring Im Rahmen Der Kooperation; KLIWA: Berlin, Germany, 2016. [Google Scholar]
- Rennenberg, H.; Seiler, W.; Matyssek, R.; Gessler, A.; Kreuzwieser, J. European Beech (Fagus sylvatica L.)—A Forest Tree without Future in the South of Central Europe. Allg. For. Und Jagdztg. 2004, 175, 210–224. [Google Scholar]
- Lindner, M.; Maroschek, M.; Netherer, S.; Kremer, A.; Barbati, A.; Garcia-Gonzalo, J.; Seidl, R.; Delzon, S.; Corona, P.; Kolström, M.; et al. Climate Change Impacts, Adaptive Capacity, and Vulnerability of European Forest Ecosystems. For. Ecol. Manag. 2010, 259, 698–709. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change (IPCC). Climate Change 2013—The Physical Science Basis; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Millar, C.I.; Stephenson, N.L.; Stephens, S.L. Climate Change and Forest of the Future: Managing in the Face of Uncertanity. Ecol. Appl. 2007, 17, 2145–2151. [Google Scholar] [CrossRef] [PubMed]
- Griess, V.C.; Knoke, T. Bioeconomic Modeling of Mixed Norway Spruce-European Beech Stands: Economic Consequences of Considering Ecological Effects. Eur. J. For. Res. 2013, 132, 511–522. [Google Scholar] [CrossRef]
- Griess, V.C.; Acevedo, R.; Härtl, F.; Staupendahl, K.; Knoke, T. Does Mixing Tree Species Enhance Stand Resistance against Natural Hazards? A Case Study for Spruce. For. Ecol. Manag. 2012, 267, 284–296. [Google Scholar] [CrossRef]
- Lebourgeois, F.; Gomez, N.; Pinto, P.; Mérian, P. Mixed Stands Reduce Abies Alba Tree-Ring Sensitivity to Summer Drought in the Vosges Mountains, Western Europe. For. Ecol. Manag. 2013, 303, 61–71. [Google Scholar] [CrossRef]
- Pretzsch, H.; Schütze, G.; Uhl, E. Resistance of European Tree Species to Drought Stress in Mixed versus Pure Forests: Evidence of Stress Release by Inter-Specific Facilitation. Plant Biol. 2013, 15, 483–495. [Google Scholar] [CrossRef]
- Bundesministerium Für Ernährung Und Landwirtschaft (BMEL). Der Wald in Deutschland: Ausgewählte Ergebnisse Der Dritten Bundeswaldinventur; Bundesministerium für Ernährung und Landwirtschaft: Berlin, Germany, 2014. [Google Scholar]
- Puettmann, K.J.; Coates, K.D.; Messier, C. A Critique of Silviculture. Managing for Complexity; Island Press: Washington, DC, USA, 2008. [Google Scholar]
- Holling, C.S. Resilience and Stability of Ecological Systems. Annu. Rev. Ecol. Syst. 1973, 4, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Paluch, J.G.; Gruba, P. Effect of Local Species Composition on Topsoil Properties in Mixed Stands with Silver Fir (Abies alba Mill.). Forestry 2012, 85, 413–426. [Google Scholar] [CrossRef]
- Pretzsch, H.; Bielak, K.; Block, J.; Bruchwald, A.; Dieler, J.; Ehrhart, H.P.; Kohnle, U.; Nagel, J.; Spellmann, H.; Zasada, M.; et al. Productivity of Mixed versus Pure Stands of Oak (Quercus petraea Matt.) Liebl. and Quercus robur L.) and European Beech (Fagus sylvatica L.) along an Ecological Gradient. Eur. J. For. Res. 2013, 132, 263–280. [Google Scholar] [CrossRef]
- Zang, C.; Hartl-Meier, C.; Dittmar, C.; Rothe, A.; Menzel, A. Patterns of Drought Tolerance in Major European Temperate Forest Trees: Climatic Drivers and Levels of Variability. Glob. Chang. Biol. 2014, 20, 3767–3779. [Google Scholar] [CrossRef] [PubMed]
- Fritz, P. Ökologischer Waldumbau in Deutschland: Fragen, Antworten, Perspektiven; Oekom: München, Germany, 2006; Volume 153. [Google Scholar]
- Robakowski, P.; Wyka, T.; Samardakiewicz, S.; Kierzkowski, D. Growth, Photosynthesis, and Needle Structure of Silver Fir (Abies alba Mill.) Seedlings under Different Canopies. For. Ecol. Manag. 2004, 201, 211–227. [Google Scholar] [CrossRef]
- Mérian, P.; Lebourgeois, F. Size-Mediated Climate-Growth Relationships in Temperate Forests: A Multi-Species Analysis. For. Ecol. Manag. 2011, 261, 1382–1391. [Google Scholar] [CrossRef]
- Ellenberg, H.; Leuschner, C. Vegetation Mitteleuropas Mit Den Alpen: In Ökologischer, Dynamischer Und Historischer Sicht; UTB Ulmer: Stuttgart, Germany, 2010. [Google Scholar]
- Meier, I.C.; Leuschner, C. Belowground Drought Response of European Beech: Fine Root Biomass and Carbon Partitioning in 14 Mature Stands across a Precipitation Gradient. Glob. Chang. Biol. 2008, 14, 2081–2095. [Google Scholar] [CrossRef]
- Kölling, C.; Ewald, J.; Walentowski, H. Lernen von Der Natur: Die Tanne in Den Natürlichen Waldgesellschaften Bayerns. Ber. Bayer. Landesanst. Wald Forstwirtsch. 2004, 45, 24–29. [Google Scholar]
- Köstler, J.; Brückner, E.; Bibelriether, H. Die Wurzeln Der Waldbäume: Untersuchungen Zur Morphologie Der Waldbäume in Mitteleropa; Parey: Hamburg, Germany, 1968. [Google Scholar]
- Granier, A.; Bobay, V.; Gash, J.H.C.; Gelpe, J.; Saugier, B.; Shuttleworth, W.J. Vapour Flux Density and Transpiration Rate Comparisons in a Stand of Maritime Pine (Pinus pinaster Ait.) in Les Landes Forest. Agric. For. Meteorol. 1990, 51, 309–319. [Google Scholar] [CrossRef]
- Granier, A.; Biron, P.; Köstner, B.; Gay, L.W.; Najjar, G. Comparisons of Xylem Sap Flow and Water Vapour Flux at the Stand Level and Derivation of Canopy Conductance for Scots Pine. Theor. Appl. Climatol. 1996, 53, 115–122. [Google Scholar] [CrossRef]
- Kostner, B.M.M.; Schulze, E.D.; Kelliher, F.M.; Hollinger, D.Y.; Byers, J.N.; Hunt, J.E.; McSeveny, T.M.; Meserth, R.; Weir, P.L. Transpiration and Canopy Conductance in a Pristine Broad-Leaved Forest of Nothofagus: An Analysis of Xylem Sap Flow and Eddy Correlation Measurements. Oecologia 1992, 91, 350–359. [Google Scholar] [CrossRef]
- Köstner, B.; Granier, A.; Cermak, J. Sapflow Measurements in Forest Stands: Methods and Uncertainties. Ann. For. Sci. 1998, 55, 13–27. [Google Scholar] [CrossRef]
- Magh, R.-K.; Yang, F.; Rehschuh, S.; Burger, M.; Dannenmann, M.; Pena, R.; Burzlaff, T.; Ivankovic, M.; Rennenberg, H. Nitrogen Nutrition of European Beech Is Maintained at Sufficient Water Supply in Mixed Beech-Fir Stands. Forests 2018, 9, 733. [Google Scholar] [CrossRef]
- Alduchov, O.A.; Eskridge, R.E. Improved Magnus Form Approximation of Saturation Vapor Pressure. J. Appl. Meteorol. 1996, 35, 601–609. [Google Scholar] [CrossRef]
- Burgess, S.S.O.; Adams, M.A.; Bleby, T.M. Measurement of Sap Flow in Roots of Woody Plants: A Commentary. Tree Physiol. 2000, 20, 909–913. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.S.O.; Adams, M.A.; Turner, N.C.; Beverly, C.R.; Ong, C.K.; Khan, A.A.H.; Bleby, T.M. An Improved Heat Pulse Method to Measure Low and Reverse Rates of Sap Flow in Woody Plants. Tree Physiol. 2001, 21, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Kutscha, N.P.; Sachs, I.B. Color Tests for Differentiating Heartwood and Sapwood in Certain Softwood Tree Species (Report 2246); United States Department of Agriculture and Forest Service: Madison WI, USA, 1962. [Google Scholar]
- Meinzer, F.C.; Goldstein, G.; Andrade, J.L. Regulation of Water Flux through Tropical Forest Canopy Trees: Do Universal Rules Apply? Tree Physiol. 2001, 21, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, T.; Horna, V.; Leuschner, C. Variability in Radial Sap Flux Density Patterns and Sapwood Area among Seven Co-Occurring Temperate Broad-Leaved Tree Species. Tree Physiol. 2008, 28, 1821–1830. [Google Scholar] [CrossRef] [PubMed]
- Haas, E.; Klatt, S.; Fröhlich, A.; Kraft, P.; Werner, C.; Kiese, R.; Grote, R.; Breuer, L.; Butterbach-Bahl, K. LandscapeDNDC: A Process Model for Simulation of Biosphere–Atmosphere–Hydrosphere Exchange Processes at Site and Regional Scale. Landsc. Ecol. 2013, 28, 615–636. [Google Scholar] [CrossRef]
- Li, C.; Aber, J.; Stange, F.; Butterbach-bahl, K.; Papen, H. A Process-Oriented Model of and NO. 1 Model Development. J. Geophys. Res. 2000, 105, 4369–4384. [Google Scholar] [CrossRef]
- Kiese, R.; Li, C.; Hilbert, D.W.; Papen, H.; Butterbach-Bahl, K. Regional Application of PnET-N-DNDC for Estimating the N2O Source Strength of Tropical Rainforests in the Wet Tropics of Australia. Glob. Chang. Biol. 2005, 11, 128–144. [Google Scholar] [CrossRef]
- Holst, J.; Grote, R.; Offermann, C.; Ferrio, J.P.; Gessler, A.; Mayer, H.; Rennenberg, H. Water Fluxes within Beech Stands in Complex Terrain. Int. J. Biometeorol. 2010, 54, 23–36. [Google Scholar] [CrossRef]
- Grote, R.; Lehmann, E.; Brummer, C.; Bruggemann, N.; Szarzynski, J.; Kunstmann, H. Modelling and Observation of Biosphere–Atmosphere Interactions in Natural Savannah in Burkina Faso, West Africa. Phys. Chem. Earth Parts A/B/C 2009, 34, 251–260. [Google Scholar] [CrossRef]
- Grote, R.; Kiese, R.; Grunwald, T.; Ourcival, J.-M.; Granier, A. Modelling Forest Carbon Balances Considering Tree Mortality and Removal. Agric. For. Meteorol. 2011, 151, 179–190. [Google Scholar] [CrossRef]
- Grote, R.; Korhonen, J.; Mammarella, I. Challenges for Evaluating Process-Based Models of Gas Exchange. For. Syst. 2011, 20, 389. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Hentschel, R.; Bittner, S.; Janott, M.; Biernath, C.; Holst, J.; Ferrio, J.P.; Gessler, A.; Priesack, E. Simulation of Stand Transpiration Based on a Xylem Water Flow Model for Individual Trees. Agric. For. Meteorol. 2013, 182–183, 31–42. [Google Scholar] [CrossRef]
- Lüttschwager, D.; Remus, R. Radial Distribution of Sap Flux Density in Trunks of a Mature Beech Stand. Ann. For. Sci. 2007, 64, 431–438. [Google Scholar] [CrossRef]
- Gessler, A.; Rienks, M.; Dopatka, T.; Rennenberg, H. Radial Variation of Sap Flow Densities in the Sap-Wood of Beech Trees (Fagus sylvatica L.). Phyton Ann. Rei Bot. 2005, 45, 257–266. [Google Scholar]
- Schipka, F.; Heimann, J.; Leuschner, C. Regional Variation in Canopy Transpiration of Central European Beech Forests. Oecologia 2005, 143, 260–270. [Google Scholar] [CrossRef]
- Lyr, H.; Fiedler, J.; Tranquillini, W. Wachstum Und Umwelt. Physiologie Und Ökologie Der Gehölze; Gustav Fischer Verlag: Jena, Germany, 1992. [Google Scholar]
- Nourtier, M.; Chanzy, A.; Granier, A.; Huc, R. Sap Flow Measurements by Thermal Dissipation Method Using Cyclic Heating: A Processing Method Accounting for the Non-Stationary Regime. Ann. For. Sci. 2011, 68, 1255–1264. [Google Scholar] [CrossRef]
- Nourtier, M.; Chanzy, A.; Cailleret, M.; Yingge, X.; Huc, R.; Davi, H. Transpiration of Silver Fir (Abies alba Mill.) during and after Drought in Relation to Soil Properties in a Mediterranean Mountain Area. Ann. For. Sci. 2014, 71, 683–695. [Google Scholar] [CrossRef]
- Cermak, J.; Nadezhdina, N.; Meiresonne, L.; Ceulemans, R. Scots Pine Root Distribution Derived from Radial Sap Flow Patterns in Stems of Large Leaning Trees. Plant Soil 2008, 305, 61–75. [Google Scholar] [CrossRef]
- Nadezhdina, N.; Cermak, J.; Meiresonne, L.; Ceulemans, R. Transpiration of Scots Pine in Flanders Growing on Soil with Irregular Substratum. For. Ecol. Manag. 2007, 243, 1–9. [Google Scholar] [CrossRef]
- Jonard, F.; Andre, F.; Ponette, Q.; Vincke, C.; Jonard, M. Sap Flux Density and Stomatal Conductance of European Beech and Common Oak Trees in Pure and Mixed Stands during the Summer Drought of 2003. J. Hydrol. 2011, 409, 371–381. [Google Scholar] [CrossRef]
- Gebauer, T.; Horna, V.; Leuschner, C. Canopy Transpiration of Pure and Mixed Forest Stands with Variable Abundance of European Beech. J. Hydrol. 2012, 442–443, 2–14. [Google Scholar] [CrossRef]
- Pretzsch, H.; Biber, P. A Re-Evaluation of Reineke’s Rule and Stand Density Index. For. Sci. 2005, 51, 304–320. [Google Scholar]
- Pretzsch, H.; Dieler, J.; Seifert, T.; Rotzer, T. Climate Effects on Productivity and Resource-Use Efficiency of Norway Spruce (Picea abies [L.] Karst.) and European Beech (Fagus sylvatica [L.]) in Stands with Different Spatial Mixing Patterns. Trees 2012, 26, 1343–1360. [Google Scholar] [CrossRef]
- Kelty, M.J. Comparative Productivity of Monocultures and Mixed-Species Stands. In The Ecology and Silviculture of Mixed-Species Forests: A Festschrift for David M. Smith; Kelty, M.J., Larson, B.C., Oliver, C.D., Eds.; Springer: Dordrecht, The Netherlands, 1992; pp. 125–141. [Google Scholar]
- Otto, H.-J. Waldökologie; Ulmer, UTB für Wissenschaft: Stuttgart, Germany, 1994. [Google Scholar]
- Potępa, B.; Szynkiewicz, A.; Udyrysz-Krawec, M. GPR Survey for Fir (Abies alba) and Spruce (Picea abies) Root Systems in Different Locations in the Western Carpathians Mts. (Poland). J. Geol. Resour. Eng. 2018, 6, 194–209. [Google Scholar]
- Schmid, I.; Kazda, M. Vertical Distribution and Radial Growth of Coarse Roots in Pure and Mixed Stands of Fagus sylvatica and Picea abies. Can. J. For. Res. 2001, 31, 539–548. [Google Scholar] [CrossRef]
- Leuschner, C.; Hertel, D.; Schmid, I.; Koch, O.; Muhs, A.; Hölscher, D. Stand Fine Root Biomass and Fine Root Morphology in Old-Growth Beech Forests as a Function of Precipitation and Soil Fertility: Plant and Soil. Plant Soil 2004, 258, 43–56. [Google Scholar] [CrossRef]
- Grossiord, C.; Gessler, A.; Granier, A.; Berger, S.; Brechet, C.; Hentschel, R.; Hommel, R.; Scherer-Lorenzen, M.; Bonal, D. Impact of Interspecific Interactions on the Soil Water Uptake Depth in a Young Temperate Mixed Species Plantation. J. Hydrol. 2014, 519, 3511–3519. [Google Scholar] [CrossRef]
- Bello, J.; Hasselquist, N.J.; Vallet, P.; Kahmen, A.; Perot, T.; Korboulewsky, N. Complementary Water Uptake Depth of Quercus petraea and Pinus sylvestris in Mixed Stands during an Extreme Drought. Plant Soil 2019, 437, 93–115. [Google Scholar] [CrossRef]
- Zapater, M.; Hossann, C.; Bréda, N.; Brechet, C.; Bonal, D.; Granier, A. Evidence of Hydraulic Lift in a Young Beech and Oak Mixed Forest Using 18O Soil Water Labelling. Trees 2011, 25, 885–894. [Google Scholar] [CrossRef]
- Camarero, J.J.; Gazol, A.; Sangüesa-Barreda, G.; Oliva, J.; Vicente-Serrano, S.M. To Die or Not to Die: Early Warnings of Tree Dieback in Response to a Severe Drought. J. Ecol. 2015, 103, 44–57. [Google Scholar] [CrossRef]
- Caldwell, M.M.; Dawson, T.E.; Richards, J.H. Hydraulic Lift: Consequences of Water Efflux from the Roots of Plants. Oecologia 1998, 113, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Magh, R.-K.; Grün, M.; Knothe, V.E.; Stubenazy, T.; Tejedor, J.; Dannenmann, M.; Rennenberg, H. Silver-Fir (Abies alba Mill.) Neighbors Improve Water Relations of European Beech (Fagus sylvatica L.), but Do Not Affect N Nutrition. Trees 2018, 32, 337–348. [Google Scholar] [CrossRef]
- Rotzer, T.; Haberle, K.H.; Kallenbach, C.; Matyssek, R.; Schutze, G.; Pretzsch, H. Tree Species and Size Drive Water Consumption of Beech/Spruce Forests—A Simulation Study Highlighting Growth under Water Limitation. Plant Soil 2017, 418, 337–356. [Google Scholar] [CrossRef]
- Mitscherlich, G. Waldklima Und Wasserhaushalt. In Wald, Wachstum Und Umwelt; Sauerländer Verlag: Frankfurt, Germany, 1998; p. 365. [Google Scholar]
- Oke, T. Boundary Layer Climates, 2nd ed.; Routledge: London, UK, 1996. [Google Scholar]
- Meinzer, F.C.; Clearwater, M.J.; Goldstein, G. Water Transport in Trees: Current Perspectives, New Insights and Some Controversies. Environ. Exp. Bot. 2001, 45, 239–262. [Google Scholar] [CrossRef]
- Rennenberg, H. Communities and Ecosystem Functioning. In Ecological Biochemistry—Environmental and Interspecies Interactions; Krauss, G., Nies, D., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015; pp. 77–91. [Google Scholar]
- Huxman, T.E.; Smith, M.D.; Fay, P.A.; Knapp, A.K.; Shaw, M.R.; Loik, M.E.; Smith, S.D.; Tissue, D.T.; Zak, J.C.; Weltzin, J.F.; et al. Convergence across Biomes to a Common Rain-Use Efficiency. Nature 2004, 429, 651–654. [Google Scholar] [CrossRef]
- Breda, N.; Huc, R.; Granier, A.; Dreyer, E. Temperate Forest Trees and Stands under Severe Drought: A Review of Ecophysiological Responses, Adaptation Processes and Long-Term Consequences. Ann. For. Sci. 2006, 63, 625–644. [Google Scholar] [CrossRef]
- Morin, X.; Fahse, L.; Scherer-Lorenzen, M.; Bugmann, H. Tree Species Richness Promotes Productivity in Temperate Forests through Strong Complementarity between Species. Ecol. Lett. 2011, 14, 1211–1219. [Google Scholar] [CrossRef]
- Pretzsch, H.; Block, J.; Dieler, J.; Dong, P.H.; Kohnle, U.; Nagel, J.; Spellmann, H.; Zingg, A. Comparison between the Productivity of Pure and Mixed Stands of Norway Spruce and European Beech along an Ecological Gradient. Ann. For. Sci. 2010, 67, 712. [Google Scholar] [CrossRef]




| 2016 | 2017 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| ID | Species | DBH (cm) | Sw (cm) | As (cm²) | ID | Species | DBH (cm) | Sw (cm) | As (cm²) |
| 209 | Beech | 25.4 | 6.6 | 376.6 | 209 | Beech | 26.4 | 6.8 | 405.7 |
| 208 | Beech | 30.9 | 7.8 | 547.7 | 208 | Beech | 32.1 | 8.0 | 591.1 |
| 6 | Beech | 29.8 | 7.5 | 512.5 | 6 | Beech | 31.2 | 7.8 | 559.4 |
| 207 | Beech | 33.7 | 8.4 | 648.6 | 207 | Beech | 35.6 | 8.8 | 723.0 |
| 202 | Beech | 27.5 | 7.0 | 437.6 | 201 | Beech | 36.3 | 8.9 | 750.1 |
| 203 | Beech | 31.8 | 8.0 | 576.1 | 203 | Beech | 33.9 | 8.4 | 652.4 |
| 102 | Beech | 21.2 | 5.7 | 269.7 | 102 | Beech | 22.6 | 6.0 | 304.6 |
| 101 | Fir | 40.1 | 6.5 | 661.6 | 101 | Fir | 41.4 | 6.5 | 687.8 |
| 15 | Beech | 25.1 | 6.5 | 368.1 | 15 | Beech | 26.9 | 6.9 | 420.0 |
| 16 | Fir | 34.8 | 6.0 | 508.9 | 16 | Fir | 36.4 | 6 | 539.9 |
| 302 | Beech | 22.3 | 5.9 | 296.2 | 302 | Beech | 22.9 | 6.0 | 311.6 |
| 301 | Fir | 25.8 | 4.4 | 280.1 | 301 | Fir | 27.7 | 4.4 | 306.0 |
| 24 | Fir | 28.7 | 4.2 | 310.6 | 24 | Fir | 30.1 | 4.3 | 333.4 |
| 103 | Fir | 37.1 | 6.1 | 548.1 | 19 | Beech | 40.6 | 9.8 | 926.1 |
| 99 | Fir | 21.5 | 3.6 | 192.5 | 20 | Fir | 40.7 | 8.5 | 815.5 |
| 105 | Beech | 28.6 | 7.3 | 474.7 | |||||
| 106 | Beech | 30.1 | 7.6 | 519.5 | |||||
| 104 | Fir | 47.4 | 4.7 | 595.3 | |||||
| 2016 | Tree Water Use (L day−1) | |||
|---|---|---|---|---|
| May | min | mean | median | max |
| pure beech | 3.59 | 61.48 (a) | 61.10 | 118.14 |
| mix beech | 2.66 | 42.14 (b) | 41.09 | 79.34 |
| mix fir | 5.20 | 42.65 (c) | 47.80 | 77.89 |
| June | ||||
| pure beech | 5.78 | 76.95 (a) | 71.67 | 158.97 |
| mix beech | 3.28 | 50.17 (b) | 46.81 | 104.23 |
| mix fir | 2.21 | 25.76 (c) | 25.68 | 46.72 |
| July | ||||
| pure beech | 16.61 | 107.48 (a) | 110.62 | 157.33 |
| mix beech | 9.90 | 73.82 (b) | 82.36 | 102.57 |
| mix fir | 3.09 | 28.79 (c) | 31.99 | 40.67 |
| August | ||||
| pure beech | 19.17 | 81.09 (a) | 91.00 | 106.07 |
| mix beech | 12.40 | 63.46 (b) | 70.87 | 83.95 |
| mix fir | 3.99 | 21.73 (c) | 23.72 | 29.75 |
| September | ||||
| pure beech | 2.22 | 55.66 (a) | 59.21 | 83.01 |
| mix beech | 1.65 | 46.83 (b) | 47.22 | 69.35 |
| mix fir | 2.16 | 13.00 (c) | 13.69 | 21.62 |
| October | ||||
| pure beech | 1.05 | 21.60 (a) | 19.11 | 43.63 |
| mix beech | 1.55 | 18.74 (a) | 16.87 | 36.83 |
| mix fir | 2.09 | 6.51 (b) | 6.46 | 10.44 |
| 2017 | Tree Water Use (L day−1) | |||
|---|---|---|---|---|
| June | min | mean | median | max |
| pure beech treatment | 49.07 | 103.00 (A) | 107.40 | 136.43 |
| pure beech control | NA | NA | NA | NA |
| mix beech treatment | 26.39 | 65.91 (aB) | 68.30 | 87.87 |
| mix beech control | 62.53 | 142.91(bA) | 148.34 | 189.48 |
| mix fir treatment | 15.21 | 31.33 (C) | 33.02 | 39.77 |
| July | ||||
| pure beech treatment | 18.12 | 76.76 (aA) | 79.80 | 120.65 |
| pure beech control | 20.53 | 92.56 (aA) | 95.36 | 143.43 |
| mix beech treatment | 8.11 | 44.39 (aB) | 44.92 | 76.29 |
| mix beech control | 24.19 | 114.77 (bA) | 116.15 | 181.91 |
| mix fir treatment | 4.88 | 36.16 (B) | 34.39 | 75.01 |
| August | ||||
| pure beech treatment | 4.75 | 68.41 (aA) | 78.26 | 96.21 |
| pure beech control | 5.88 | 82.98 (bA) | 90.72 | 118.18 |
| mix beech treatment | 3.05 | 37.86 (aB) | 41.45 | 54.71 |
| mix beech control | 8.04 | 111.38 (bB) | 123.16 | 155.43 |
| mix fir treatment | 1.83 | 40.63 (B) | 44.20 | 59.12 |
| September | ||||
| pure beech treatment | 11.09 | 47.06 (aA) | 48.19 | 83.69 |
| pure beech control | 10.08 | 46.91 (aA) | 50.44 | 86.12 |
| mix beech treatment | 4.30 | 22.08 (aB) | 23.23 | 41.48 |
| mix beech control | 16.65 | 69.72 (bB) | 73.82 | 126.23 |
| mix fir treatment | 6.15 | 27.50 (C) | 29.77 | 45.26 |
| October | ||||
| pure beech treatment | 5.79 | 32.55 (aA) | 29.23 | 69.33 |
| pure beech control | 4.10 | 28.38 (aA) | 24.09 | 62.74 |
| mix beech treatment | 2.04 | 12.67 (aB) | 12.37 | 29.04 |
| mix beech control | 6.32 | 47.93 (bB) | 40.97 | 114.72 |
| mix fir treatment | 2.34 | 21.65 (C) | 20.19 | 41.39 |
| Site | Species | Sapwood Area (m2 ha−1) | Xylem Sap Flow (m3 ha−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| May | June | July | August | September | October | ||||
| pure | Fagus sylvatica | 14.99 | |||||||
| sum | 445.58 | 651.97 | 934.3 | 688.77 | 455.14 | 181.63 | |||
| daily mean | 17.14 (a) | 21.73 (a) | 30.14 (a) | 22.22 (a) | 15.17 (a) | 5.86 (a) | |||
| sd | 11.1 | 11.41 | 11.21 | 6.74 | 4.89 | 3.47 | |||
| mixed | Fagus sylvatica | 8.23 | |||||||
| sum | 290.07 | 385.7 | 587.93 | 506.69 | 363.77 | 150.95 | |||
| daily mean | 10.74 | 12.86 | 18.97 | 16.34 | 12.13 | 4.87 | |||
| sd | 6.97 | 7.07 | 6.6 | 4.87 | 4.09 | 2.88 | |||
| Abies alba | 7.51 | ||||||||
| sum | 176.19 | 148.93 | 180.91 | 135.16 | 76.59 | 39.9 | |||
| daily mean | 6.53 | 4.96 | 5.84 | 4.36 | 2.55 | 1.29 | |||
| sd | 3.85 | 2.38 | 2.07 | 1.28 | 0.93 | 0.49 | |||
| total sum | 15.75 | 466.26 | 534.63 | 768.84 | 641.85 | 440.36 | 190.85 | ||
| daily mean | 17.27 (a) | 17.82 (a) | 24.8 (b *) | 20.7 (a *) | 14.68 (a *) | 6.16 (a) | |||
| total sd | 7.96 | 7.46 | 6.91 | 5.03 | 4.19 | 2.92 | |||
| precipitation | 1654 | 2048 | 575 | 549 | 454 | 630.1 | |||
| evaporation pure | 219 | 302 | 133 | 159 | 86 | 75 | |||
| evaporation mixed | 246 | 360 | 164 | 185 | 102 | 108 | |||
| surplus/deficit pure | 989 | 1094 | −493 | −299 | −87 | 374 | |||
| surplus/deficit mixed | 942 | 1153 | −358 | −278 | −88 | 332 | |||
| Site/Treatment | Species | Sapwood Area (m2 ha−1) | Xylem Sap Flow (m3 ha−1) | |||||
|---|---|---|---|---|---|---|---|---|
| June | July | August | September | October | ||||
| pure control | Fagus sylvatica | 14.99 | ||||||
| sum | 750 ° | 500.56 | 733.42 | 402.31 | 258.02 | |||
| daily mean | 26.34 (aA) | 23.65 (aA) | 13.41 (aA) | 8.32 (aA) | ||||
| sd | 10.22 | 8.73 | 5.31 | 4.86 | ||||
| pure treatment | Fagus sylvatica | 14.99 | ||||||
| sum | 577.47 | 559.14 | 495.2 | 326.6 | 233.91 | |||
| daily mean | 25.1 (a) | 18.03 (aB) | 15.97 (aB) | 10.88 (aA) | 7.54 (aA) | |||
| sd | 5.38 | 7.6 | 5.77 | 4.22 | 4.06 | |||
| mixed control | Fagus sylvatica | 8.23 | ||||||
| sum | 440.73 | 488.18 | 469.62 | 282.08 | 197.66 | |||
| daily mean | 19.16 | 15.74 | 15.14 | 9.4 | 6.37 | |||
| Abies alba | 7.51 | |||||||
| sum | 111.13 | 168.53 | 192.07 | 124.5 | 100.22 | |||
| daily mean | 5.05 | 5.43 | 6.19 | 4.15 | 3.23 | |||
| total sum | 15.75 | 547.44 | 656.73 | 661.70 | 406.59 | 297.89 | ||
| daily mean | 23.8 (A) | 21.2 (bA *) | 21.35 (bA *) | 13.55 (aA *) | 9.61 (aA) | |||
| sd | 5.12 | 7.31 | 5.91 | 3.94 | 4.32 | |||
| mixed treatment | Fagus sylvatica | 8.23 | ||||||
| sum | 351.04 | 336.98 | 286.03 | 157.59 | 94.08 | |||
| daily mean | 15.95 | 10.87 | 9.22 | 5.25 | 3.03 | |||
| Abies alba | 7.51 | |||||||
| sum | 106.70 | 168.53 | 192.07 | 124.5 | 100.22 | |||
| daily mean | 4.63 | 5.43 | 6.19 | 4.15 | 3.23 | |||
| total sum | 15.75 | 457.75 | 505.52 | 478.11 | 282.10 | 194.31 | ||
| daily mean | 20.6 (bB) | 16.33 (aA) | 15.42 (aB) | 9.4 (aA) | 6.27 (aA) | |||
| sd | 4.03 | 5.68 | 3.95 | 2.7 | 2.41 | |||
| precipitation | 436 | 1576 | 794 | 660 | 631 | |||
| precipitation treatment | 0 | 0 | 1000 | 660 | 631 | |||
| evaporation pure | 155 | 271 | 154 | 181 | 99 | |||
| evaporation mixed | 172 | 331 | 177 | 211 | 134 | |||
| surplus/deficit pure control | −469 ° | 804 | −93 | 76 | 273 | |||
| surplus/deficit pure treatment | −732 | −829 | 350 | 152 | 297 | |||
| surplus/deficit mixed control | −283 | 588 | −45 | 42 | 198 | |||
| surplus/deficit mixed treatment | −629 | −836 | 344 | 167 | 302 | |||
| Plot/Association | Sum of Transpiration (mm) Measured (May–October) | Sum of Transpiration (mm) Simulated (May–October) |
|---|---|---|
| pure beech | 337 | 328 |
| mixed beech-fir | 304 | 301 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magh, R.-K.; Bonn, B.; Grote, R.; Burzlaff, T.; Pfautsch, S.; Rennenberg, H. Drought Superimposes the Positive Effect of Silver Fir on Water Relations of European Beech in Mature Forest Stands. Forests 2019, 10, 897. https://doi.org/10.3390/f10100897
Magh R-K, Bonn B, Grote R, Burzlaff T, Pfautsch S, Rennenberg H. Drought Superimposes the Positive Effect of Silver Fir on Water Relations of European Beech in Mature Forest Stands. Forests. 2019; 10(10):897. https://doi.org/10.3390/f10100897
Chicago/Turabian StyleMagh, Ruth-Kristina, Boris Bonn, Rüdiger Grote, Tim Burzlaff, Sebastian Pfautsch, and Heinz Rennenberg. 2019. "Drought Superimposes the Positive Effect of Silver Fir on Water Relations of European Beech in Mature Forest Stands" Forests 10, no. 10: 897. https://doi.org/10.3390/f10100897
APA StyleMagh, R.-K., Bonn, B., Grote, R., Burzlaff, T., Pfautsch, S., & Rennenberg, H. (2019). Drought Superimposes the Positive Effect of Silver Fir on Water Relations of European Beech in Mature Forest Stands. Forests, 10(10), 897. https://doi.org/10.3390/f10100897








