Abstract
Understory vegetation hosts high biodiversity and plays a critical role in the ecosystem processes of boreal forests. However, the drivers of understory plant diversity in this high-latitude ecosystem remain uncertain. To investigate the influences of forest type and latitude on understory beta diversity at different scales, we quantified the species composition of Vaccinium uliginosum Linnaeus communities under broadleaf and coniferous forests at two latitudes at the quadrat (2 × 2 m) and plot (10 × 10 m) scales in the Greater Xing’an Mountains, NE China. At the quadrat scale, species alpha diversity of V. uliginosum communities was higher in broadleaf forests than that in coniferous forests at both latitudes. The differences in species beta diversity (the Sørensen’s dissimilarity) in two forest types depended on the latitude: beta diversity in broadleaf forests was higher than that in coniferous forests at the higher latitude, while beta diversity in coniferous forests was higher at the lower latitude. At the plot scale, alpha and beta diversity of V. uliginosum communities decreased from broadleaf forests to coniferous forests at the higher latitude, and they did not show significant differences between forest types at the lower latitude. These results indicate the interactive effects of forest type and latitude on beta diversity of understory vegetation. Moreover, the influences of forest type and latitude on species alpha and beta diversity were different across the two spatial scales, suggesting that the assembly mechanisms underlying species diversity may be different at different scales. Understanding the maintenance of understory vegetation diversity will benefit the conservation and management of boreal forests.
1. Introduction
The determinants of species diversity in nature as well as its ecological consequences are fundamental and critical questions in ecology []. Species diversity can be partitioned into alpha, beta, and gamma components []. Gamma diversity represents the regional species pool. Differently from alpha diversity, which is the diversity in species within communities, beta diversity is the compositional differences/turnover between communities [,]. Studying beta diversity can aid a better understanding of the patterns of species compositional turnover among communities []. Besides, exploring beta diversity under different environmental conditions can provide insight into the consequences of environmental changes on community structure []. Moreover, species beta diversity can be used to infer ecological processes that determine community assembly [,,,]. Despite these many significances, beta diversity has been relatively much less studied than alpha diversity. Until very recently, the patterns of beta diversity along local and regional environmental gradients were investigated [,]. Meanwhile, a large number of researchers have studied the roles of environment and space in driving species beta diversity [,,,].
Beta diversity of plant communities has been found to vary along biotic or abiotic gradients [,,]. Owing to their distinct environmental conditions, different types of plant communities (e.g., forest types) often had different beta diversity. For example, significant differences in beta diversity of canopy trees were found among forest types in the Brazilian Amazon [], and beta diversity of understory plant communities of secondary forests was significantly higher than that of rubber and eucalypt plantations []. Besides, as with alpha diversity, beta diversity was found to change along large geographic gradients, i.e., latitudinal and elevational gradients [,]. These recent studies tend to support the idea that both biotic and abiotic factors act to form the patterns of beta diversity [,]. However, the mechanism underlying the interaction of multiple factors on beta diversity has not been clear [,].
For beta diversity, the spatial scales of observation are important and they are composed of spatial extent of the sampling area and spatial grain of the sampling unit []. Owing to different study aims, beta diversity has been studied at specific spatial scales, which may have divergent results. Several recent studies found that beta diversity of plant communities varied across different spatial scales [,,,], indicating that different processes determined beta diversity at different scales [,]. On the other hand, the impacts of biotic or abiotic factors on beta diversity probably depend on scales. For example, Sreekar et al. [] found contrasting effects of latitude on tree beta diversity at small and large spatial scales, and Martin and Wilsey [] found that the differences in beta diversity between grassland types changed across spatial scales. Therefore, it seems that the influences of forest types and/or latitude on species beta diversity may vary with scales, which, however, has not been thoroughly studied.
Boreal forest is the largest terrestrial biome, covering 11% of the Earth’s terrestrial surface []. Globally, boreal forests have a circumpolar distribution, while in China they are distributed at the Greater Xing’an Mountains in Heilongjiang Province of NE China [,]. These forests are characterized by cold climate and special vegetation structure, including tree layer, understory shrubs, herbs, graminoids, mosses, and lichens. As the high-latitude ecosystem, boreal forests are expected to be sensitive and vulnerable to climate change [], and therefore should be priority areas for nature conservation. Boreal forests are often classified into two main forest types, i.e., coniferous and broadleaf forests, according to the species composition of the tree layer. Hosting a large number of plant species, fauna, and microflora, understory vegetation was regarded as a forest ecosystem driver in boreal forests []. Many studies have found that the diversity (e.g., alpha and beta diversity) and composition of understory plant communities were strongly influenced by canopy composition or forest type [,]. In boreal forests, Vaccinium uliginosum, one shrub from family Ericaceae, frequently dominates or is subordinate in understory vegetation []. However, the patterns and drivers of beta diversity of understory V. uliginosum communities in boreal forests have not been clear.
In this study, we aimed to investigate the influences of forest type and latitude on beta diversity of understory plant communities in boreal forests at different spatial scales. Broadleaf forest was always thought to have more heterogeneous environmental conditions and higher nutrient availability than coniferous forest [,,]. Thus, our first hypothesis is that species beta diversity of understory V. uliginosum communities in broadleaf forests is higher than that in coniferous forests, and the pattern is consistent at different latitudes. In previous studies, the scale dependence of beta diversity was frequently reported [,]. Thus, our second hypothesis is that species beta diversity of different forest types and latitudes is distinct at different spatial scales. To test these hypotheses, we surveyed the understory V. uliginosum communities of coniferous and broadleaf forests at two latitudes (high vs. low) across the Greater Xing’an Mountains at both quadrat (2 × 2 m) and plot (10 × 10 m) scales. Alpha and beta diversity of understory plant communities was compared between these two forest types and latitudes at two spatial scales.
2. Materials and Methods
2.1. Study Region
We conducted our investigation at the Greater Xing’an Mountains in Heilongjiang Province of NE China. The geographical location of this region ranged from 50.16° to 53.55° N in latitude, from 121.20° to 127.00° E in longitude, and from 300 to 1528 m in altitude. The study region is located in cold temperate zone, where the typical vegetation is coniferous forest []. There also exist large areas of broadleaf and mixed forests due to local environment or disturbances (e.g., logging and fire) []. To examine the effects of latitude on species diversity, we chose two sites across this region: Amuer, which is located in the Mohe country and Nanwenghe, which is located in the Songling area (Figure 1). These two sites were selected mainly because they are distributed in the northern and southern parts of boreal forests in China. The boreal forests in the sites are natural ecosystems. The mean annual temperature of Amuer (52.84° N, 123.42° E) is −5.0 °C and the mean annual precipitation is 479 mm. The mean annual temperature of Nanwenghe (51.13° N, 125.16° E) is −3.0 °C and the mean annual precipitation is 508 mm. Moreover, the regional species pool measured as total species richness of vascular plants in site Amuer (n = 31) is much smaller than that in site Nanwenghe (n = 77).
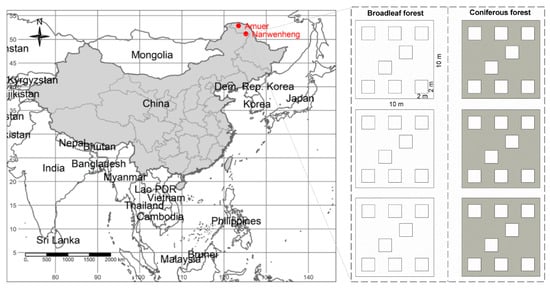
Figure 1.
Geographical location of study sites and sampling methods. Amuer, the site at higher latitude; Nanwenghe, the site at lower latitude.
2.2. Field Investigation
Vaccinium uliginosum, a common shrub at the Greater Xing’an Mountains, was often found to dominate or subordinate in the understory of broadleaf, coniferous, and mixed forests []. In this study, V. uliginosum refers to only V. uliginosum ssp. uliginosum Linnaeus. Because not enough plots were found for the mixed forests with V. uliginosum in the south site Nanwenghe, we only focused on the understory vegetation of broadleaf and coniferous forests. Broadleaf forests were dominated by Betula platyphylla, while Larix gmelinii was the dominant tree species in coniferous forests (Table 1). These two types of forests had similar average coverage of V. uliginosum (broadleaf 23% versus coniferous 31%; Table 1). Other characteristics including soil properties in these forests are given in Table 1.

Table 1.
Vegetation characteristics and soil properties in coniferous and broadleaf forests.
From middle August to early September of 2016, we surveyed the species composition of the understory V. uliginosum communities in the two types of forests in both Amuer and Nanwenghe. Two nested spatial scales were used to investigate the species composition: plot (10 × 10 m) and quadrat (2 × 2 m). In each site, three plots were randomly set for each forest type. Then, eight quadrats, “Z” type of formation, were set within each plot. In total, 12 plots and 96 quadrats were investigated. The occurrence of all vascular plants was carefully recorded within each quadrat. The understory V. uliginosum communities we surveyed had similar forest ages, and obvious logging or recent fire disturbance were avoided.
2.3. Species Alpha and Beta Diversity
We quantified the species alpha and beta diversity of understory communities at the quadrat and plot scales. Alpha diversity was calculated with the number of species within communities, i.e., species richness. Beta diversity was quantified with two pairwise dissimilarity measures: the Sørensen’s dissimilarity [,] and the Jaccard’s dissimilarity [,]. These two dissimilarity measures represented the dissimilarity between each pair of communities using presence-absence data [,]. The Sørensen’s dissimilarity can be calculated as (1 − 2S12/(S1 + S2)), where S1 is the total number of species in Community 1, S2 represents the total number of species in Community 2, and S12 is the number of shared species between Community 1 and Community 2. The Jaccard’s dissimilarity is (1 − S12/(S1 + S2 − S12)), and the meaning of the symbols is as above. For alpha diversity, species richness within each quadrat was used at the quadrat scale, while richness within each plot pooled from the eight quadrats was used at the plot scale. At the quadrat scale, we measured beta diversity as pairwise dissimilarity between quadrats within each plot, while at the plot scale beta diversity was quantified as pairwise dissimilarity between plots. Both species alpha and beta diversity were calculated using the package ‘vegan’ in R and the functions ‘specnumber’ and ‘betadiver’ respectively [].
2.4. Climate and Soil Factors
Mean annual temperature (MAT) and mean annual precipitation (MAP) were accessed from the WorldClim database (http://www.worldclim.org/) according to the geographical coordinate of each site []. For each forest type at each site, soil electrical conductivity (soil EC, µS cm−1) and soil temperature (°C) were measured with a portable soil salinity multimeter (EC-350C; Aquaterr, Costa Mesa, USA) at the soil depth of 15 cm in the field. Both soil EC and temperature could affect plant diversity either by influencing plant growth directly or by changing soil nutrient availability. Within each plot, four soil samples from 0 to 15 cm depth were randomly excavated after the surface litters were removed. Then, all soil samples were brought into the lab, air dried, and passed through a 1-mm sieve before measurement. For soil pH, 5 g subsample of each soil sample was shaken with 12.5 mL demineralized water in a small beaker for 1 min, and measured for pH with a pH Meter (PB-10; Sartorius, Goettingen, German) after standing for 30 min. For soil organic carbon (C), total nitrogen, and total phosphorus content, a small amount of each soil sample which passed through a 0.15-mm sieve was used. We measured soil organic C (mg g−1) by subtracting soil inorganic content from soil total carbon, which were both measured using a total organic carbon analyzer (SSM 5000A; Schimadzu, Kyoto, Japan). Soil total nitrogen content (mg g−1) was determined using an elemental analyzer (vario MICRO cube; Elemental, Hanau, Germany), while soil total phosphorus content (mg g−1) was determined using ascorbic acid colorimetric method after H2SO4 digestion as described by Bao [].
2.5. Data Analysis
All statistical analyses were carried out at both plot and quadrat scales. First of all, two-way ANOVAs were used to examine the influences of forest type and latitude on species alpha and beta diversity. Secondly, independent t-tests were used to explore the differences in alpha and beta diversity between broadleaf and coniferous forests at each latitude, and between the higher and lower latitudes under each forest type. Thirdly, we also performed independent t-tests to investigate the differences in the coverage of V. uliginosum and soil properties between broadleaf and coniferous forests. Lastly, a principal coordinates analysis (PCoA) was performed to investigate the compositional differences between broadleaf and coniferous forests, and between the higher and lower latitudes based on the Sørensen’s or Jaccard’s dissimilarity index [,]. The PCoA with Sørensen’s or Jaccard’s dissimilarity is especially useful for the ordination of presence-absence data []. As the results based on Sørensen’s and Jaccard’s dissimilarity were very similar, we only presented the Sørensen’s index. All data analyses were conducted using R version 3.2.3 [].
3. Results
Across all the quadrats of the current study, 82 understory vascular plants were recorded. Among them, 59 plant species were found in broadleaf forests and 49 species in coniferous forests. These two types of forests differed in some soil properties (Table 1). Soil pH in coniferous forests (mean = 4.58) was significantly lower than that in broadleaf forests (mean = 5.48; t = –6.01, p = 0.007), while soil total nitrogen content (t = –2.48, p = 0.087) and phosphorus content (t = –2.49, p = 0.108) in coniferous forests were slightly lower than that in broadleaf forests (Table 1). The differences in soil temperature, soil EC, and soil organic C between the two forest types were not significant (p > 0.05; Table 1). We found larger variability in soil temperature and soil EC in broadleaf forests (coefficient of variation: temperature, 25.5%; EC, 7.71%) than coniferous forests (temperature, 16.5%; EC, 2.45%) at the higher latitude, and smaller variability in broadleaf forests (temperature, 4.99%; EC, 3.32%) than coniferous forests (temperature, 12.3%; EC, 6.02%) at the lower latitude.
Besides canopy composition, understory plants also had some differences between coniferous and broadleaf forests (Table 1). The dominant shrubs in coniferous forests were V. vitis-idaea, V. uliginosum, Rhododendron palustre, while dominant shrub species in broadleaf forests were V. uliginosum, B. fruticosa, V. vitis-idaea (Table 1). Boreal forests between latitudes had no obvious differences in dominant tree species, but had a few differences in understory shrubs, herbs, and graminoids (Table 2). Although boreal forests at both latitudes shared V. uliginosum and V. vitis-idaea as dominant shrubs, Ledum palustre frequently dominated in forests at the higher latitude and B. fruticosa was a dominant shrub in forests at the lower latitude (Table 2). Moreover, we observed significant larger coverage of V. uliginosum in boreal forests at higher latitudes compared to lower latitudes (Table 2).

Table 2.
Vegetation characteristics and soil properties of boreal forests at higher and lower latitudes.
At the quadrat scale, there is a significant difference in species richness and beta diversity between forest types and between latitudes (Table 3). Species richness of understory V. uliginosum communities was higher in broadleaf forests than that in coniferous forests at both latitudes (Table 3; Figure 2). Moreover, we found significant effects of interaction between forest type and latitude on species beta diversity (Table 3). Beta diversity (the Sørensen’s dissimilarity) in broadleaf forests was higher than that in coniferous forests at the higher latitude, while beta diversity in coniferous forests was higher at the lower latitude (Figure 2). At the plot scale, species richness and beta diversity of V. uliginosum communities decreased from broadleaf forests to coniferous forests at the higher latitude, and they did not show significant differences between forest types at the lower latitude (Figure 2).

Table 3.
Results of the two-way ANOVA for influences of forest type (broadleaf vs. coniferous) and latitude (high vs. low) on species alpha and beta diversity at the quadrat and plot scales.
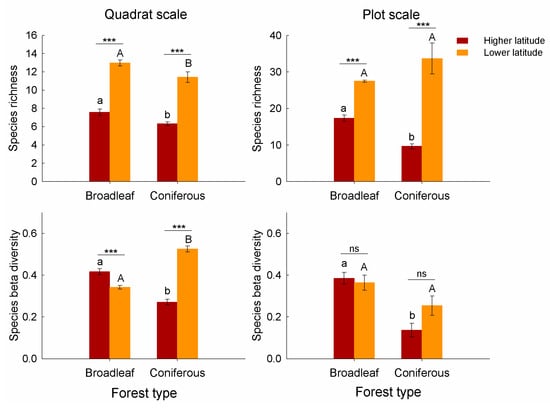
Figure 2.
Species alpha and beta diversity of understory communities under different forest types (broadleaf vs. coniferous) and latitudes (higher vs. lower) at the quadrat and plot scales. Species alpha diversity is measured as species richness, and beta diversity is estimated with the Sørensen dissimilarity. Different small letters (a, b) indicate significant differences (p < 0.05) between broadleaf and coniferous forests at the higher latitude, while different capital letters (A, B) represent significant differences at the lower latitude. The differences in diversity between the two latitudes were either highly significant with p < 0.001 (***) or not significant with p > 0.05 (ns).
The PCoA showed that the first two axes accounted for 54.6% and 67.2% of the total variation at the quadrat and plot scales, respectively. At the quadrat scale, broadleaf forests at the lower latitude were clearly separated from coniferous forest, while at the higher latitude community composition in broadleaf forests is similar to that in coniferous forest (Figure 3). At the plot scale, there was a clear separation in species composition between forest types at both higher and lower latitudes (Figure 3).
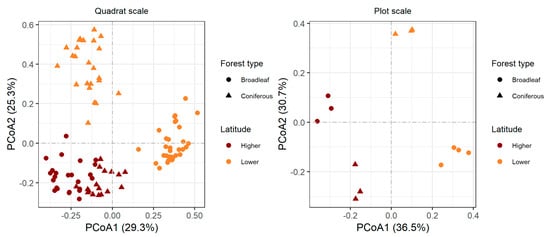
Figure 3.
Ordination of understory plant communities under two forest types and two latitudes using principal coordinates analysis (PCoA). The distance matrix used in PCoA is based on the Sørensen dissimilarity index. The percentage of variation explained by each PCoA axis is in parentheses, and the first two axes accounted for 54.6% and 67.2% of total variation at the quadrat and plot scales, respectively.
4. Discussion
4.1. Interactive Effects of Forest Type and Latitude on Beta Diversity of Understory Communities
In this study, the effects of forest type on beta diversity of understory communities were strong. It indicates the critical role of canopy composition in determining species beta diversity of understory vegetation, which was consistent with previous studies [,]. However, the differences in understory plant beta diversity between coniferous and broadleaf forests depended on latitudes. At the higher latitude, beta diversity of broadleaf forests was higher than that of coniferous forests, while broadleaf forests had lower or similar beta diversity than coniferous forests at the lower latitude. The results at the higher latitude supported our first hypothesis, indicating the higher species turnover in broadleaf forests. Moreover, our result of higher beta diversity of boreal forests at early successional stages (i.e., broadleaf forests) than late successional stages (i.e., coniferous forests) was consistent with the result of Purschke et al. [], which found decreased taxonomic beta diversity during succession. Nevertheless, the results at the lower latitude were contrary to our hypothesis, but consistent with some of previous studies, which found lower beta diversity of understory vegetation in broadleaf forests than coniferous forests [,].
The lower beta diversity in broadleaf forests at lower latitudes may be attributed to species interactions, e.g., competition. Different from the forests at higher latitudes, there were higher species richness and stronger species competition in the forests at lower latitudes (Figure 2). Broadleaf forests tended to have higher soil pH, soil total nitrogen, and phosphorus content, and thus higher species richness than coniferous forests []. Nevertheless, the lower nutrient availability but also relatively high species richness in coniferous forests at lower latitudes probably made the species competition stronger. Species competition tended to cause larger resource heterogeneity and species turnover, i.e., species beta diversity. This suggests that species competition in forests at lower latitudes may also play an important role in explaining the patterns of understory plant beta diversity.
Besides species interaction, environmental heterogeneity may also be an important factor affecting beta diversity of understory communities, which contributed to the interactive effects of forest type and latitude. Previous studies have found that environmental heterogeneity/variability tends to increase beta diversity of plants [,,]. In the current study, there was larger variability in soil temperature and EC in broadleaf than coniferous forests at higher latitudes and smaller variability at lower latitudes, which was consistent with the patterns in beta diversity of understory communities. These results indicate the significant role of environmental heterogeneity.
Our study highlights the critical roles of climate, canopy composition, environmental heterogeneity as well as species interactions in determining the species beta diversity of understory vegetation. Meanwhile, these reflect that deterministic processes instead of random processes structure the species turnover of the understory vegetation of boreal forests []. Multiple biotic and abiotic factors as well as their interactions can play important roles in forming plant communities [,,,,].
4.2. Beta Diversity of Understory Communities at Different Spatial Scales
Contrary to alpha diversity, beta diversity of understory communities was found to decrease from the quadrat scale to the plot scale. It was consistent with the previous studies, which found that tree beta diversity decreased with the increase in cell size of forest plots []. This result suggests the scale dependence of species beta diversity. On the other hand, we found that at the higher latitude the effects of forest type on beta diversity were similar at the quadrat and plot scales, while at the lower latitude there were contrasting impacts of forest type on beta diversity from small to large scales. It seemed that the effects of forest type or latitude on beta diversity of understory vegetation were weaker at larger spatial scales. These results were consistent with a number of previous studies, which found the different effects of biotic or abiotic factors on beta diversity across different spatial scales [,,]. For example, Sreekar et al. [] found contrasting effects of latitude on beta diversity of forests at small and large spatial scales. Martin and Wilsey [] found that the differences in beta diversity between native and exotic grasslands shifted across spatial scales.
Our results of different effects of forest type on beta diversity across spatial scales supported our second hypothesis. It implies that ecological processes that determined species beta diversity were different at different scales [,]. That was because the determinants of beta diversity, e.g., environmental heterogeneity, canopy composition, and species interaction, depended on spatial scales []. Environmental heterogeneity in soil factors, e.g., soil pH, soil moisture, and surface topography, has been reported to change from small to large scales []. Moreover, biotic factors like canopy composition and species interaction tended to have less variability at larger scales []. Furthermore, dispersal processes also operate at different spatial scales, although they may play a minor role in driving species beta diversity relative to environmental factors [].
Our results illustrate the important roles of spatial scales while investigating the patterns and drivers of beta diversity under different conditions. Studies conducted at different spatial scales are likely to have contrasting results. Thus, spatial scales of observation need to be considered when studying species beta diversity. Besides, we may gain some additional information about determinants of beta diversity to consider the different aspects of spatial scales, i.e., extent of sampling area and grain of sampling unit [,], or the nestedness and turnover components of beta diversity [].
5. Conclusions
In the boreal forests, we investigated the effects of forest type and latitude on beta diversity of understory V. uliginosum communities at two spatial scales. We found that differences in beta diversity of understory plants between coniferous and broadleaf forests depended on latitudes, suggesting the interactive effects of forest type and latitude on beta diversity. Furthermore, understory plant beta diversity under different forest types and latitudes was different across spatial scales. These findings indicate the critical roles of climate, canopy composition, environmental heterogeneity as well as species interactions in determining the beta diversity of understory vegetation at different spatial scales. More studies on beta diversity conducted at different spatial scale are needed to understand the mechanism underlying the interaction of multiple factors on understory plant diversity. Examining the patterns and drivers of species alpha and beta diversity of understory vegetation will provide insight into the mechanism of species coexistence within plant communities, which will largely benefit the conservation and management of boreal forests.
Author Contributions
Conceptualization, Y.-S.J. and Y.-K.H.; Data curation, Y.-S.J.; Formal analysis, Y.-K.H.; Funding acquisition, Y.-S.J., Y.-H.L. and Z.-Q.Z.; Investigation, Y.-S.J., Y.-K.H., J.W., D.-D.L., G.L. and Y.-H.Z.; Methodology, Y.-H.L.; Project administration, Y.-H.L.; Resources, Y.-S.J., J.W. and D.-D.L.; Supervision, Y.-H.L.; Validation, Y.-H.L.; Visualization, Y.-K.H.; Writing—original draft, Y.-K.H.; Writing—review and editing, Y.-S.J., Y.-K.H., J.W., D.-D.L., Y.-H.L., G.L., Y.-H.Z. and Z.-Q.Z.
Funding
This research was funded by the National Natural Science Foundation of China (grand number 31570603), the Special Fund for Forestry-Scientific Research in the Public Interest (grant number 201104066) and the Fundamental Research Funds for the Central Universities (grand number 2572017AA22).
Acknowledgments
We are very grateful to Yong-Jie Huang for his lab assistance, Feng-Xin Jin and Dian-Zhong Hou for their great help in the field investigation. We also thank Li-Zhong Wang and Xue-Shuang Liu in the Forest Ecosystem Station of Heilongjiang Nenjiangyuan for their support during our field investigation in the site Nanwenghe. The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sutherland, W.J.; Freckleton, R.P.; Godfray, H.C.J.; Beissinger, S.R.; Benton, T.; Cameron, D.D.; Carmel, Y.; Coomes, D.A.; Coulson, T.; Emmerson, M.C.; et al. Identification of 100 fundamental ecological questions. J. Ecol. 2013, 101, 58–67. [Google Scholar] [CrossRef]
- Whittaker, R.H. Vegetation of the Siskiyou Mountains, Oregon and California. Ecol. Monogr. 1960, 30, 279–338. [Google Scholar] [CrossRef]
- Koleff, P.; Gaston, K.J.; Lennon, J.J. Measuring beta diversity for presence-absence. J. Anim. Ecol. 2003, 72, 367–382. [Google Scholar] [CrossRef]
- Anderson, M.J.; Crist, T.O.; Chase, J.M.; Vellend, M.; Inouye, B.D.; Freestone, A.L.; Sanders, N.J.; Cornell, H.V.; Comita, L.S.; Davies, K.F.; et al. Navigating the multiple meanings of β diversity: A roadmap for the practicing ecologist. Ecol. Lett. 2011, 14, 19–28. [Google Scholar] [CrossRef]
- Gabriel, D.; Roschewitz, I.; Tscharntke, T.; Thies, C. Beta diversity at different spatial scales: Plant communities in organic and conventional agriculture. Ecol. Appl. 2006, 16, 2011–2021. [Google Scholar] [CrossRef]
- Condit, R.; Pitman, N.; Leigh, E.G., Jr.; Chave, J.; Terborgh, J.; Foster, R.B.; Núñez, P.; Aguilar, S.; Valencia, R.; Villa, G.; et al. Beta-diversity in tropical forest trees. Science 2002, 295, 666–669. [Google Scholar] [CrossRef]
- Kraft, N.J.B.; Comita, L.S.; Chase, J.M.; Sanders, N.J.; Swenson, N.G.; Crist, T.O.; Stegen, J.C.; Vellend, M.; Boyle, B.; Anderson, M.J.; et al. Disentangling the drivers of β diversity along latitudinal and elevational gradients. Science 2011, 333, 1755–1758. [Google Scholar] [CrossRef]
- Myers, J.A.; Chase, J.M.; Jiménez, I.; Jørgensen, P.M.; Araujo-Murakami, A.; Paniagua-Zambrana, N.; Seidel, R.; Cornell, H. Beta-diversity in temperate and tropical forests reflects dissimilar mechanisms of community assembly. Ecol. Lett. 2013, 16, 151–157. [Google Scholar] [CrossRef]
- García-Girón, J.; Fernandez-Alaez, C.; Fernandez-Alaez, M.; Alahuhta, J. Untangling the assembly of macrophyte metacommunities by means of taxonomic, functional and phylogenetic beta diversity patterns. Sci. Total Environ. 2019, 693, 133616. [Google Scholar] [CrossRef]
- Harrison, S.; Vellend, M.; Damschen, E.I. ‘Structured’ beta diversity increases with climatic productivity in a classic dataset. Ecosphere 2011, 2, art11. [Google Scholar] [CrossRef]
- Martin, L.M.; Wilsey, B.J. Differences in beta diversity between exotic and native grasslands vary with scale along a latitudinal gradient. Ecology 2015, 96, 1042–1051. [Google Scholar] [CrossRef] [PubMed]
- Duivenvoorden, J.F.; Svenning, J.C.; Wright, S.J. Beta diversity in tropical forests. Science 2002, 295, 636–637. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Ricklefs, R.E. A latitudinal gradient in large-scale beta diversity for vascular plants in North America. Ecol. Lett. 2007, 10, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.H.; Sheng, D.Y.; Xiang, Y.Z.; Yang, Z.J.; Xu, D.P.; Zhang, N.N.; Shi, L.L. The environment, not space, dominantly structures the landscape patterns of the richness and composition of the tropical understory vegetation. PLoS ONE 2013, 8, e81308. [Google Scholar] [CrossRef] [PubMed]
- Emilio, T.; Nelson, B.W.; Schietti, J.; Desmoulière, S.J.M.; Espírito Santo, H.M.V.; Costa, F.R.C. Assessing the relationship between forest types and canopy tree beta diversity in Amazonia. Ecography 2010, 33, 738–747. [Google Scholar] [CrossRef]
- Carmona, C.P.; Azcarate, F.M.; de Bello, F.; Ollero, H.S.; Leps, J.; Peco, B. Taxonomical and functional diversity turnover in Mediterranean grasslands: Interactions between grazing, habitat type and rainfall. J. Appl. Ecol. 2012, 49, 1084–1093. [Google Scholar] [CrossRef]
- Purschke, O.; Schmid, B.C.; Sykes, M.T.; Poschlod, P.; Michalski, S.G.; Durka, W.; Kühn, I.; Winter, M.; Prentice, H.C.; Fridley, J. Contrasting changes in taxonomic, phylogenetic and functional diversity during a long-term succession: Insights into assembly processes. J. Ecol. 2013, 101, 857–866. [Google Scholar] [CrossRef]
- Fernandez-Going, B.M.; Harrison, S.P.; Anacker, B.L.; Safford, H.D. Climate interacts with soil to produce beta diversity in Californian plant communities. Ecology 2013, 94, 2007–2018. [Google Scholar] [CrossRef]
- Barton, P.S.; Cunningham, S.A.; Manning, A.D.; Gibb, H.; Lindenmayer, D.B.; Didham, R.K. The spatial scaling of beta diversity. Glob. Ecol. Biogeogr. 2013, 22, 639–647. [Google Scholar] [CrossRef]
- Dodson, E.K.; Peterson, D.W. Dry coniferous forest restoration and understory plant diversity: The importance of community heterogeneity and the scale of observation. Forest Ecol. Manag. 2010, 260, 1702–1707. [Google Scholar] [CrossRef]
- Chávez, V.; Macdonald, S.E. Partitioning vascular understory diversity in mixedwood boreal forests: The importance of mixed canopies for diversity conservation. Forest Ecol. Manag. 2012, 271, 19–26. [Google Scholar] [CrossRef]
- Carneiro, M.S.; Campos, C.C.F.; Ramos, F.N.; dos Santos, F.A.M. Spatial species turnover maintains high diversities in a tree assemblage of a fragmented tropical landscape. Ecosphere 2016, 7, e01500. [Google Scholar] [CrossRef]
- Sreekar, R.; Katabuchi, M.; Nakamura, A.; Corlett, R.T.; Slik, J.W.F.; Fletcher, C.; He, F.; Weiblen, G.D.; Shen, G.; Xu, H.; et al. Spatial scale changes the relationship between beta diversity, species richness and latitude. R. Soc. Open Sci. 2018, 5, 181168. [Google Scholar] [CrossRef] [PubMed]
- Bonan, G.B.; Shugart, H.H. Environmental factors and ecological processes in boreal forests. Annu. Rev. Ecol. Syst. 1989, 20, 1–28. [Google Scholar] [CrossRef]
- Hou, X.Y. Vegetation Map of the People’s Republic of China (1:4M); Chinese Map Publisher: Beijing, China, 1982. [Google Scholar]
- Zhou, Y. Vegetation in Great Xing’an Mountains of China; Science Press: Beijing, China, 1991. [Google Scholar]
- Fischlin, A.; Midgley, G.F.; Price, J.T.; Leemans, R.; Gopal, B.; Turley, C.; Rounsevell, M.D.A.; Dube, O.P.; Tarazona, J.; Velichko, A.A. Ecosystems, their properties, goods, and services. In Climate Change 2007: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Parry, M.L., Canziani, O.F., Palutikof, J.P., van der Linden, P.J., Hanson, C.E., Eds.; Cambridge University Press: Cambridge, UK, 2007; pp. 211–272. [Google Scholar]
- Nilsson, M.C.; Wardle, D.A. Understory vegetation as a forest ecosystem driver: Evidence from the northern Swedish boreal forest. Front. Ecol. Environ. 2005, 3, 421–428. [Google Scholar] [CrossRef]
- Mestre, L.; Toro-Manríquez, M.; Soler, R.; Huertas-Herrera, A.; Martínez-Pastur, G.; Lencinas, M.V. The influence of canopy-layer composition on understory plant diversity in southern temperate forests. Forest Ecosyst. 2017, 4, 6. [Google Scholar] [CrossRef]
- McGroddy, M.E.; Daufresne, T.; Hedin, L.O. Scaling of C:N:P stoichiometry in forests worldwide: Implications of terrestrial Redfield-type ratios. Ecology 2004, 85, 2390–2401. [Google Scholar] [CrossRef]
- Macdonald, S.E.; Fenniak, T.E. Understory plant communities of boreal mixedwood forests in western Canada: Natural patterns and response to variable-retention harvesting. Forest Ecol. Manag. 2007, 242, 34–48. [Google Scholar] [CrossRef]
- Chávez, V.; Macdonald, S.E. The influence of canopy patch mosaics on understory plant community composition in boreal mixedwood forest. Forest Ecol. Manag. 2010, 259, 1067–1075. [Google Scholar] [CrossRef]
- Sørensen, T.A. A method of establishing groups of equal amplitude in plant sociology based on similarity of species and its application to analyses of the vegetation on Danish commons. Biol. Skr. 1948, 5, 1–34. [Google Scholar]
- Jaccard, P. The distribution of the flora in the alpine zone. New Phytol. 1912, 11, 37–50. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Vegan: Community Ecology Package. R Package Version 2.3–3, 2016. Available online: https://CRAN.R-project.org/package=vegan (accessed on 1 August 2017).
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Bao, S.D. Soil Agricultural Chemistry Analysis; China Agriculture Press: Beijing, China, 2005. [Google Scholar]
- Gotelli, N.J.; Ellison, A.M. A Primer of Ecological Statistics, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2013. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna. 2015. Available online: https://www.R-project.org/ (accessed on 30 November 2017).
- Laliberté, E.; Paquette, A.; Legendre, P.; Bouchard, A. Assessing the scale-specific importance of niches and other spatial processes on beta diversity: A case study from a temperate forest. Oecologia 2009, 159, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Jyrkänkallio-Mikkola, J.; Heino, J.; Soininen, J. Beta diversity of stream diatoms at two hierarchical spatial scales: Implications for biomonitoring. Freshw. Biol. 2016, 61, 239–250. [Google Scholar] [CrossRef]
- De Cáceres, M.; Legendre, P.; Valencia, R.; Cao, M.; Chang, L.W.; Chuyong, G.; Condit, R.; Hao, Z.; Hsieh, C.F.; Hubbell, S.; et al. The variation of tree beta diversity across a global network of forest plots. Glob. Ecol. Biogeogr. 2012, 21, 1191–1202. [Google Scholar] [CrossRef]
- Baselga, A.; Leprieur, F. Comparing methods to separate components of beta diversity. Methods Ecol. Evol. 2015, 6, 1069–1079. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).